Bamboo-like MnO2/TiO2 Nanotube Arrays with Enhanced Photocatalytic Degradation
Abstract
:1. Introduction
2. Experiment Details
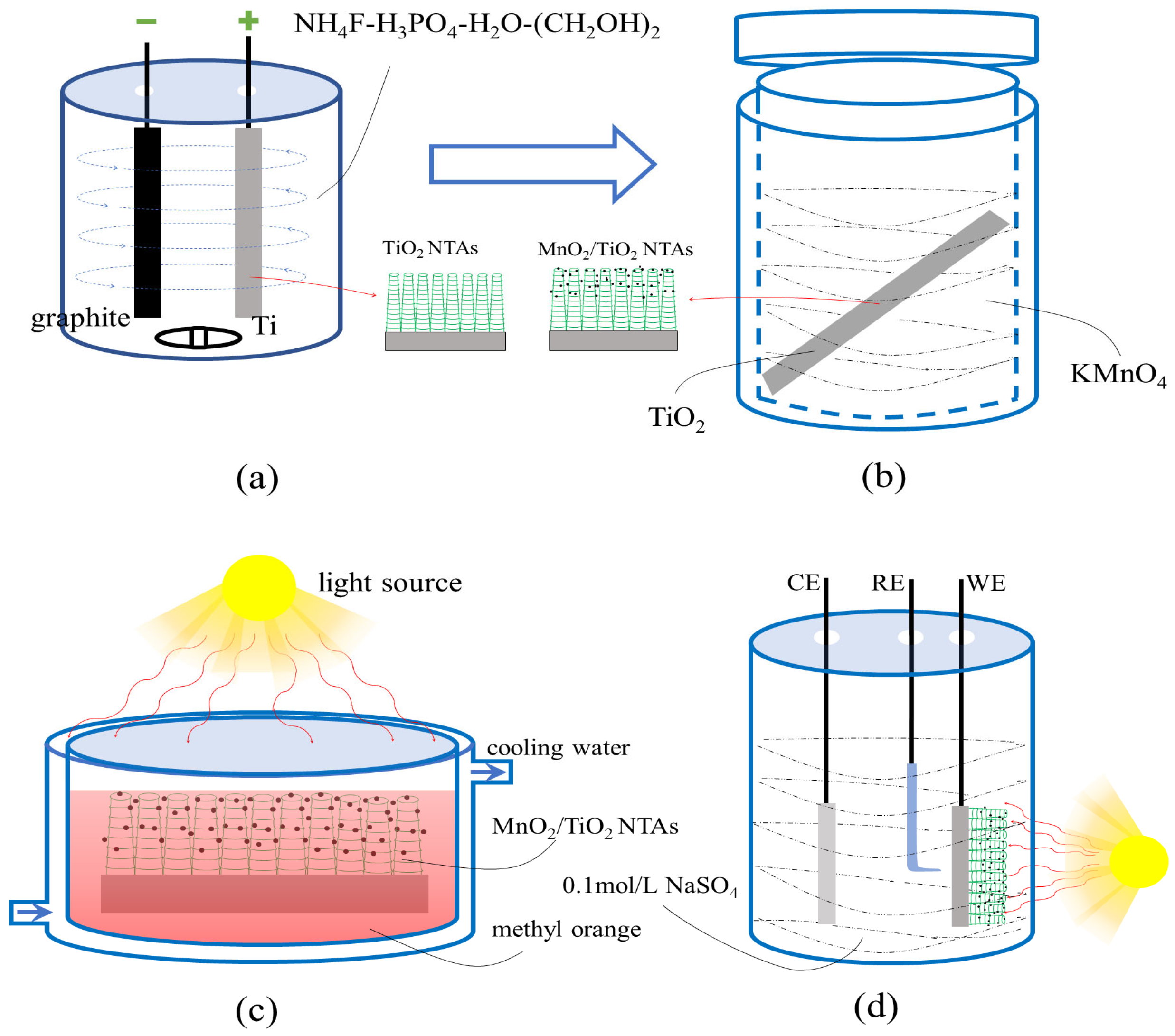
2.1. Preparation of Bamboo-like TiO2 NTAs
2.2. Preparation of MnO2/TiO2 NTAs
2.3. Characterization and Activity Analysis
2.3.1. Characterization
2.3.2. Photocatalytic Testing
2.3.3. Photocurrent Response Test
3. Results and Discussion
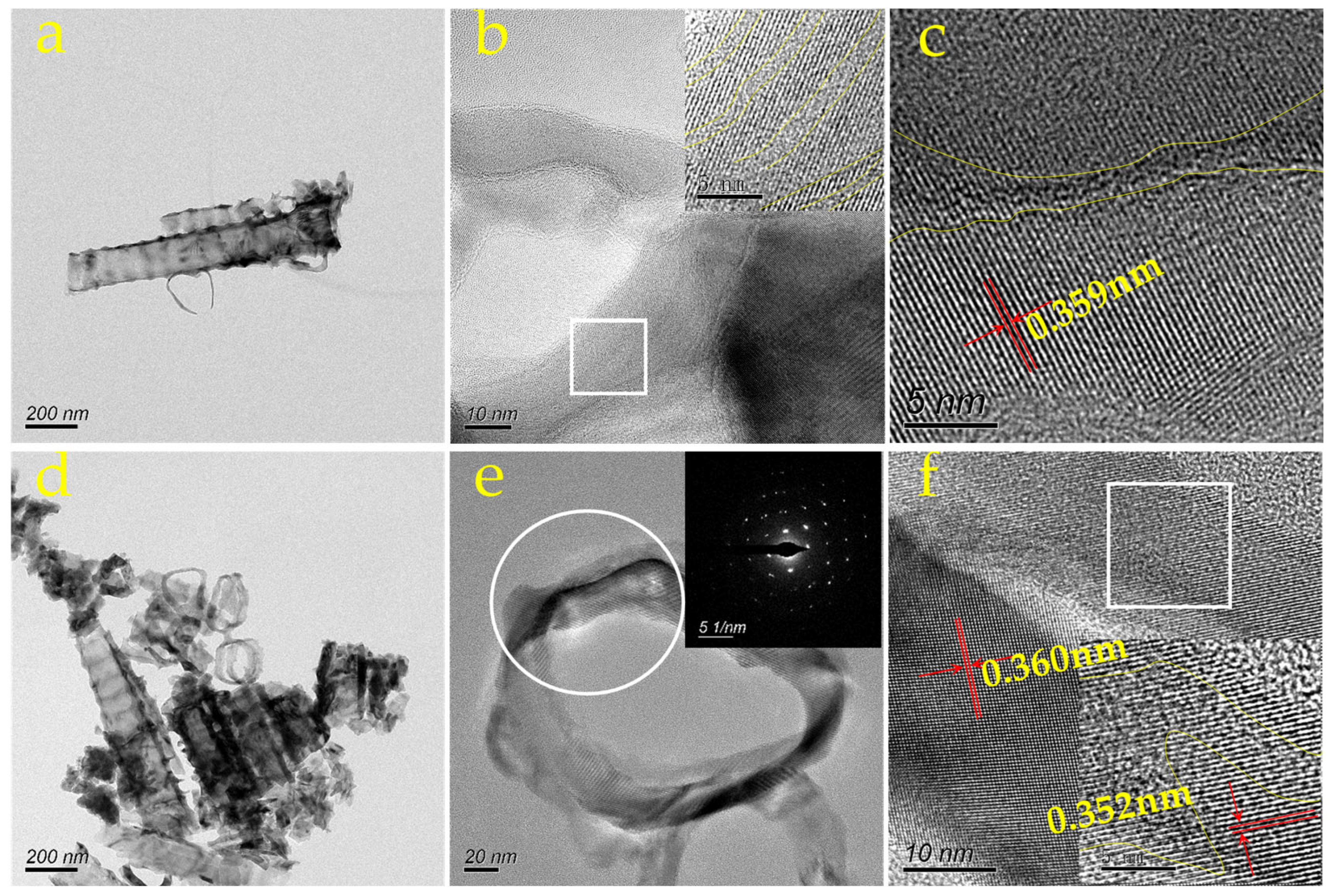
3.1. Characterization Results
3.2. Photocatalytic Activity
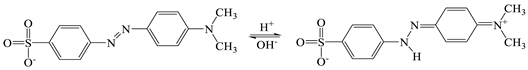
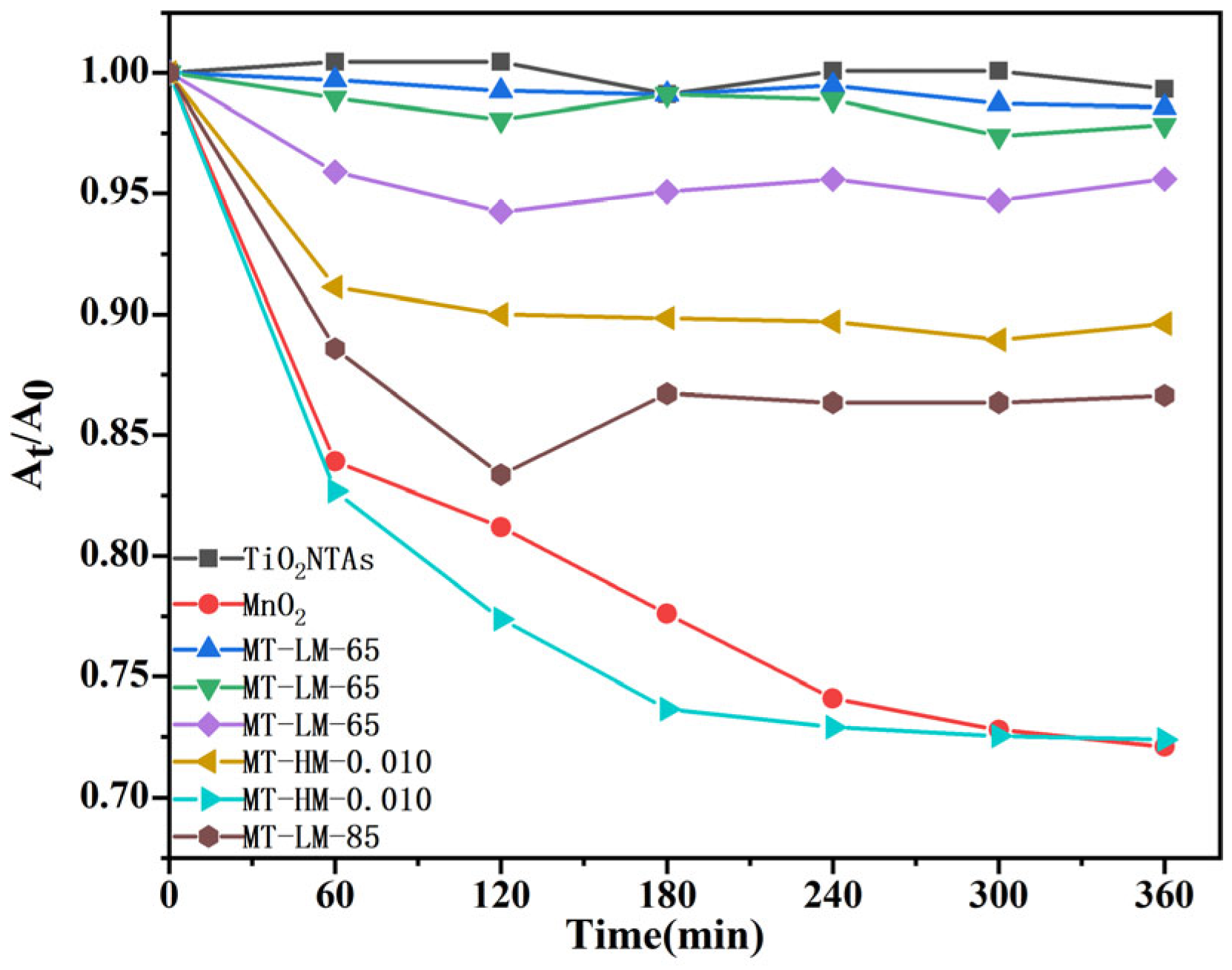
3.2.1. Adsorption and Degradation in the Absence of Light
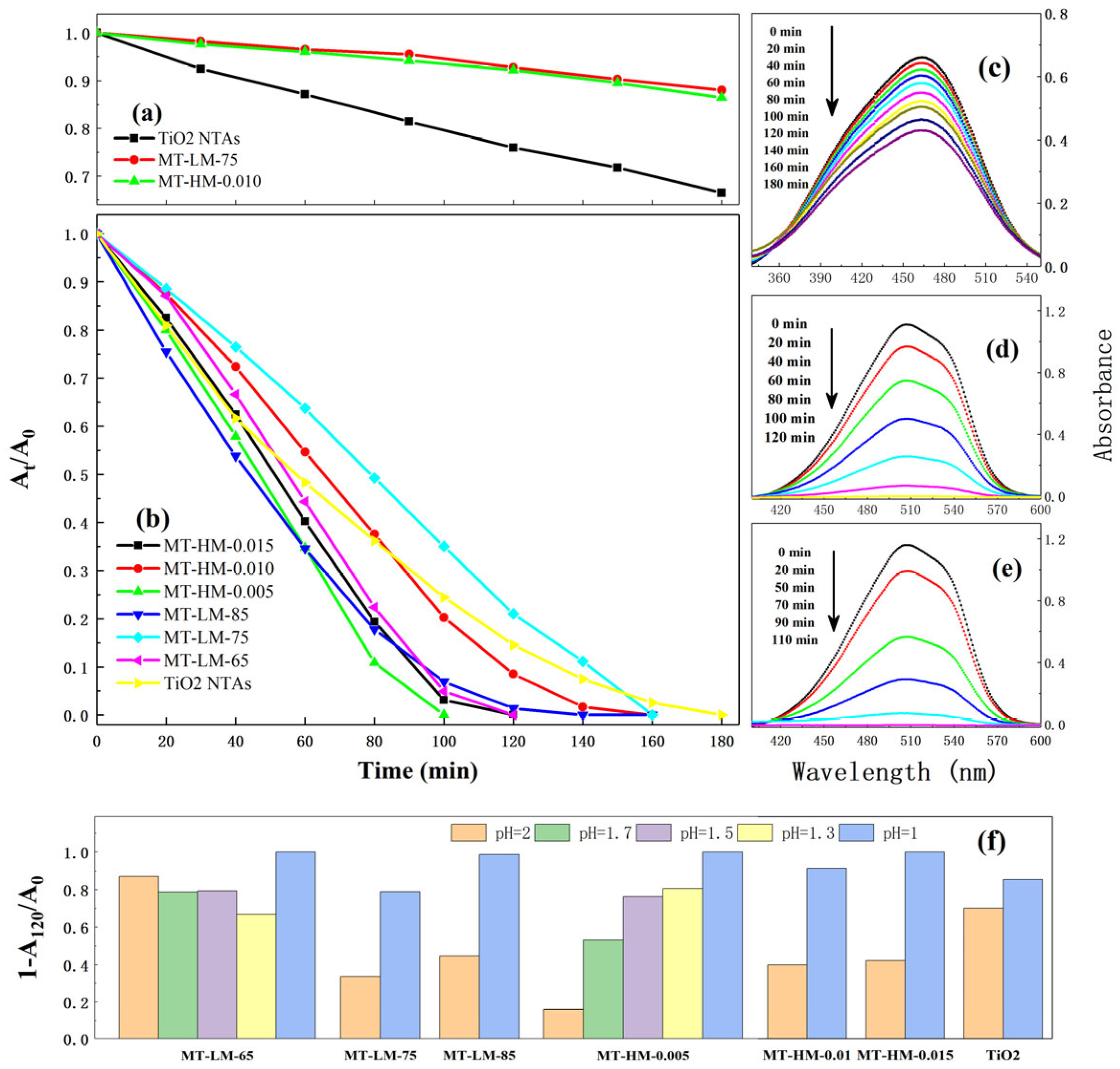
3.2.2. Photocatalytic Activity under UV Light
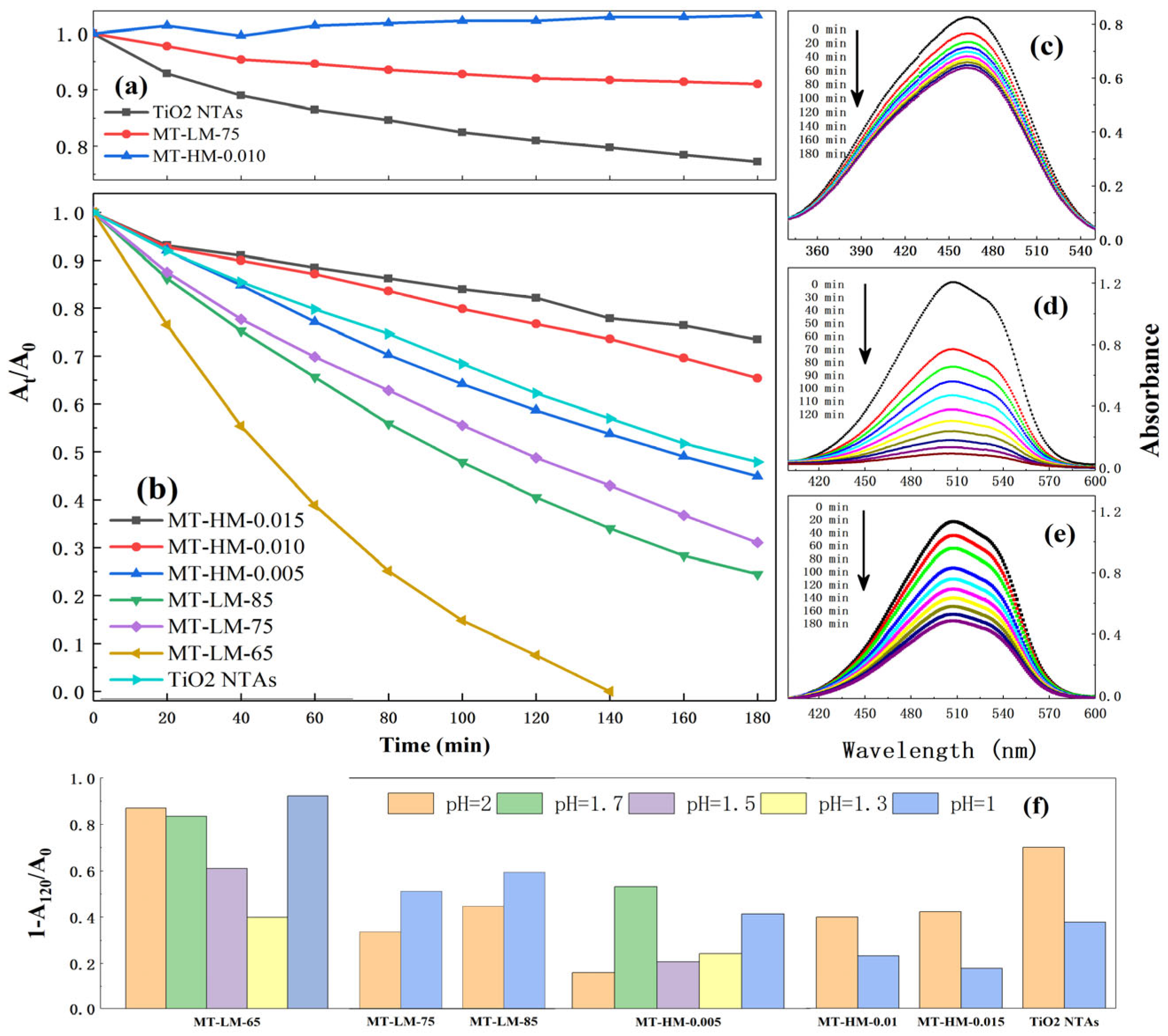
3.2.3. Photocatalytic Activity under Simulated Sunlight
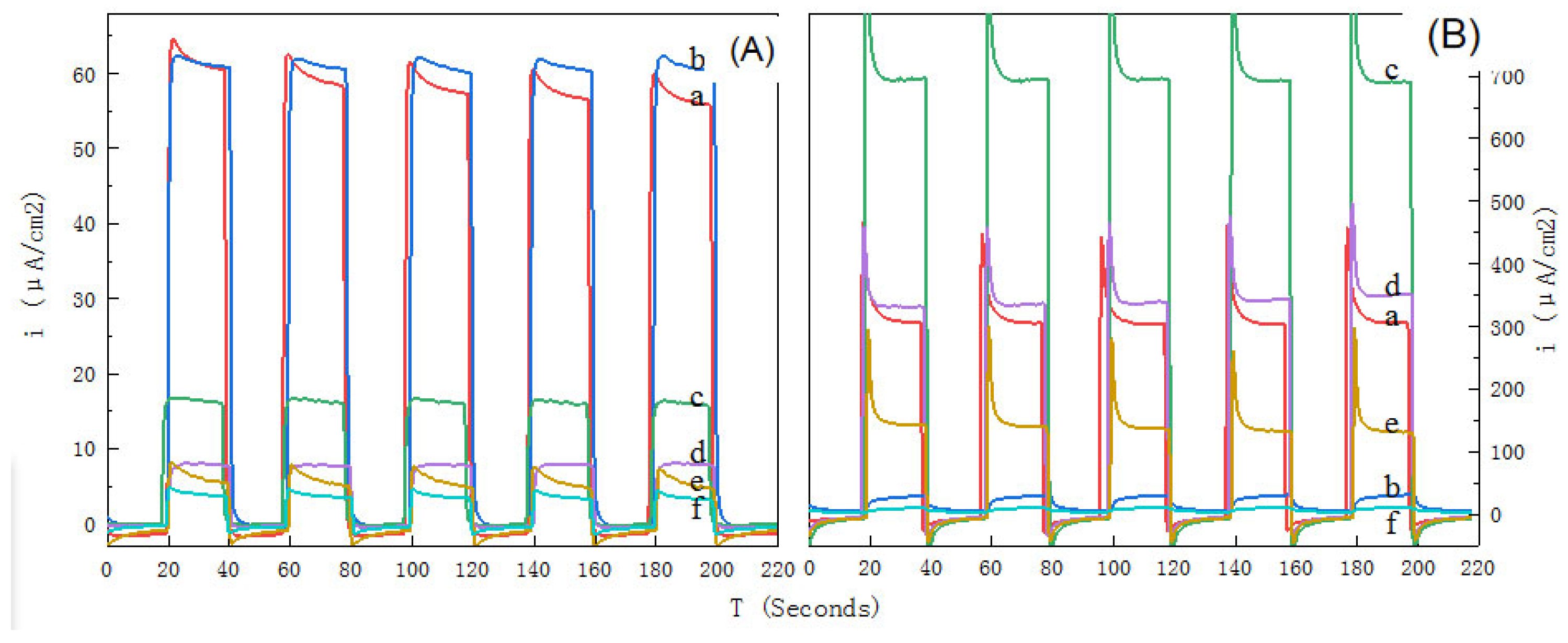
3.2.4. Photocurrent Response Capability
3.3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xue, Y.L.; Huang, J.; Lau, C.H.; Cao, B.; Li, P. Tailoring the molecular structure of crosslinked polymers for pervaporation desalination. Nat. Commun. 2020, 11, 1461. [Google Scholar] [CrossRef]
- Du Chanois, R.M.; Cooper, N.J.; Lee, B.; Patel, S.K.; Mazurowski, L.; Graedel, T.E.; Elimelech, M. Prospects of metal recovery from wastewater and brine. Nat. Water 2023, 1, 37–46. [Google Scholar] [CrossRef]
- Meng, C.; Ding, B.; Zhang, S.; Cui, L.; Ostrikov, K.K.; Huang, Z.; Yang, B.; Kim, J.-H. Angstrom-confined catalytic water purification within Co-TiOx laminar membrane nanochannels. Nat. Commun. 2022, 13, 4010. [Google Scholar] [CrossRef]
- Gholami, M.; Shirzad-Siboni, M.; Farzadkia, M.; Yang, J.K. Synthesis, characterization, and application of ZnO/TiO2, nanocomposite for photocatalysis of a herbicide. Desalination Water Treat. 2015, 57, 13632–13644. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis:design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Sato, S.; White, J.M. Photodecomposition of water over Pt/TiO2 catalysts. Chem. Phys. Lett. 1980, 72, 83–86. [Google Scholar] [CrossRef]
- Ohtani, B.; Osaki, H.; Nishimoto, S.; Kagiya, T. A novel photocatalytic process of amine N-alkylation by platinized semiconductor particles suspended in alcohols. J. Am. Chem. Soc. 1986, 108, 308–310. [Google Scholar] [CrossRef]
- Chatterjee, D.; Mahata, A. Demineralization of organic pollutants on the dye modified TiO2 semiconductor particulate system using visible light. Appl. Catal. B Environ. 2001, 33, 119–125. [Google Scholar] [CrossRef]
- Diak, M.; Grabowska, E.; Zaleska, A. Synthesis, characterization and photocatalytic activity of noble metal-modified TiO2 nanosheets with exposed {001} facets. Appl. Surf. Sci. 2015, 347, 275–285. [Google Scholar] [CrossRef]
- Bu, Y.; Chen, Z.; Li, W.; Yu, J. High-Efficiency Photoelectrochemical Properties by a Highly Crystalline CdS-Sensitized ZnO Nanorod Array. ACS Appl. Mater. Interfaces 2013, 5, 5097–5104. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasure. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Toupin, M.; Brousse, T.; Bélanger, D. Charge Storage Mechanism of MnO2 Electrode Used in Aqueous Electrochemical Capacitor. Chem. Mater. 2004, 16, 3184–3190. [Google Scholar] [CrossRef]
- Izgorodin, A.; Hocking, R.; Winther-Jensen, O.; Hilder, M.; Winther-Jensen, B.; MacFarlane, D.R. Phosphorylated manganese oxide electrodeposited from ionic liquid as a stable, high efficiency water oxidation catalyst. Catal. Today 2013, 200, 36–40. [Google Scholar] [CrossRef]
- Li, S.; Ma, Z.; Wang, L.; Liu, J. Influence of MnO2 on the photocatalytic activity of P-25 TiO2 in the degradation of methyl orange. Sci. China 2008, 51, 179–185. [Google Scholar] [CrossRef]
- Ghaemi, M.; Khosravi-Fard, L.; Neshati, J. Improved performance of rechargeable alkaline batteries via surfactant-mediated electrosynthesis of MnO2. J. Power Sources 2004, 141, 340–350. [Google Scholar] [CrossRef]
- Sakai, N.; Ebina, Y.; Takada, K.; Sasaki, T. Photocurrent generation from semiconducting manganese oxide nanosheets in response to visible light. J. Phys. Chem. B 2005, 109, 9651–9655. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, X.; Li, X.; Yang, F.; Jin, B.; Xu, T.; Li, G.; Li, M. Electrodeposition synthesis of MnO2/TiO2 nanotube arrays nanocomposites and their visible light photocatalytic activity. Mater. Res. Bull. 2014, 59, 32–36. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M.; Zhang, Y.; Yu, C.; Qi, W.; Cui, J.; Wang, Y.; Qin, Y.; Liu, J.; Shu, X.; et al. Controlled synthesis of MnO2@TiO2 hybrid nanotube arrays with enhanced oxygen evolution reaction performance. Int. J. Hydrogen Energy 2018, 43, 14369–14378. [Google Scholar] [CrossRef]
- Li, N.; Xia, W.Y.; Wang, J.; Liu, Z.L.; Li, Q.Y.; Chen, S.Z.; Xu, C.W.; Lu, X.H. Manganese oxide supported on hydrogenated TiO2 nanowire array catalysts for the electrochemical oxygen evolution reaction in water electrolysis. J. Mater. Chem. A 2015, 3, 21308–21313. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, H.; Zhang, H.; Cheng, X.; Xie, M.; Cheng, Q. Fabrication of MnO2/TiO2 nano-tube arrays photoelectrode and its enhanced visible light photoelectrocatalytic performance and mechanism. Sep. Purif. Technol. 2017, 189, 193–203. [Google Scholar] [CrossRef]
- Nevárez-Martínez, M.C.; Kobylański, M.P.; Mazierski, P.; Wółkiewicz, J.; Trykowski, G.; Malankowska, A.; Kozak, M.; Espinoza-Montero, P.J.; Zaleska-Medynska, A. Self-Organized TiO2-MnO2 Nanotube Arrays for Efficient Photocatalytic Degradation of Toluene. Molecules 2017, 22, 564. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.; Li, X.; Zhu, J.; Li, H. Mesoporous yolk-shell SnS2-TiO2 visible photocatalysts with enhanced activity and durability in Cr(VI) reduction. Nanoscale 2013, 5, 1876–1881. [Google Scholar] [CrossRef] [PubMed]
- González, M.G.; Chaparro, A.M.; Salvador, P. Photoelectrochemical study of the TiO2 Cr system. Observation of strong (001) rutile photoetching in the presence of Cr(VI). J. Photochem. Photobiol. Chem. 1993, 73, 221–231. [Google Scholar] [CrossRef]
- Raja, K.S.; Gandhi, T.; Misra, M. Effect of water content of ethylene glycol as electrolyte for synthesis of ordered titania nanotubes. Electrochem. Commun. 2007, 9, 1069–1076. [Google Scholar] [CrossRef]
- Di, J.; Fu, X.; Zheng, H.; Jia, Y. H-TiO2/C/MnO2 nanocomposite materials for high-performance supercapacitors. J. Nanoparticle Res. 2015, 17, 255. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y. Enhanced electrochemical performance of manganese dioxide spheres deposited on a titanium dioxide nanotube arrays substrate. J. Power Sources 2014, 272, 866–879. [Google Scholar] [CrossRef]
- Hao, X.; Zhao, J.; Zhao, Y.; Ma, D.; Lu, Y.; Guo, J.; Zeng, Q. Mild aqueous synthesis of urchin-like MnOx hollow nanostructures and their properties for RhB degradation. Chem. Eng. J. 2013, 229, 134–143. [Google Scholar] [CrossRef]
- Ronca, E.; Marotta, G.; Pastore, M.; De Angelis, F. Effect of Sensitizer Structure and TiO2 Protonation on Charge Generation in Dye-Sensitized Solar Cells. J. Phys. Chem. C 2014, 87, 405–418. [Google Scholar] [CrossRef]
- Motomizu, S.; Oshima, M.; Hosoi, Y. Spectrophotometric determination of cationic and anionic surfactants with anionic dyes in the presence of nonionic surfactants, part I: A general aspect. Microchim. Acta 1992, 106, 57–66. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]

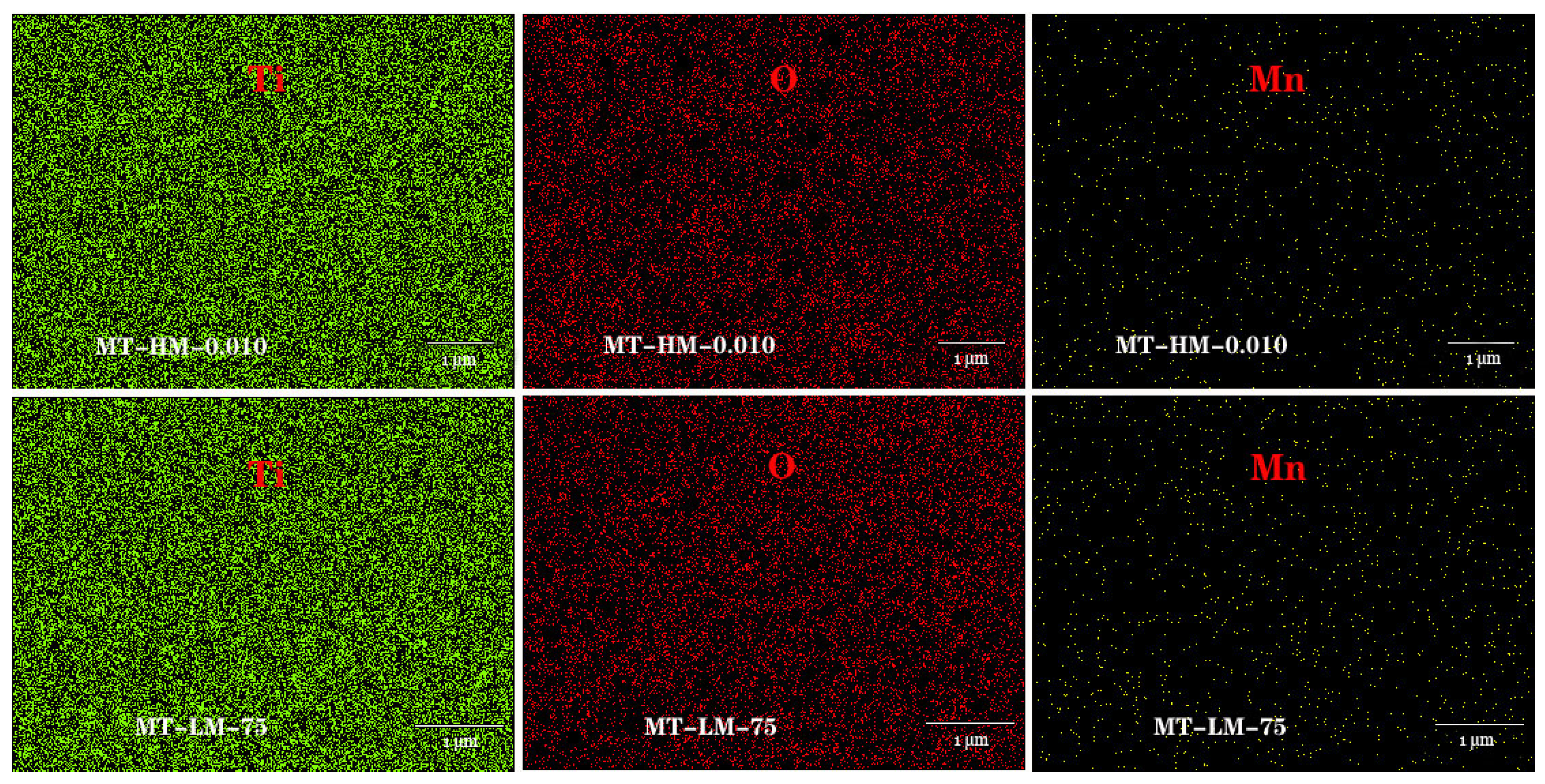



| Element | Mn (at%) | Ti (at%) | O (at%) | |
|---|---|---|---|---|
| TiO2 NTAs | 0 | 47.64 | 52.36 | |
| MnO2/TiO2 NTAs-HM | 0.005 mol/L | 0.56 | 49.69 | 49.75 |
| 0.010 mol/L | 0.77 | 42.32 | 56.91 | |
| 0.015 mol/L | 1.34 | 41.98 | 56.68 | |
| MnO2/TiO2 NTAs-LM | 65 °C | 0.52 | 41.93 | 57.55 |
| 75 °C | 0.73 | 42.93 | 56.35 | |
| 85 °C | 0.93 | 40.4 | 58.67 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, F.; Xiang, Y.; Yu, Y.; Zou, C. Bamboo-like MnO2/TiO2 Nanotube Arrays with Enhanced Photocatalytic Degradation. Coatings 2024, 14, 894. https://doi.org/10.3390/coatings14070894
Liang F, Xiang Y, Yu Y, Zou C. Bamboo-like MnO2/TiO2 Nanotube Arrays with Enhanced Photocatalytic Degradation. Coatings. 2024; 14(7):894. https://doi.org/10.3390/coatings14070894
Chicago/Turabian StyleLiang, Feng, Yanxiong Xiang, Yunjiang Yu, and Changwei Zou. 2024. "Bamboo-like MnO2/TiO2 Nanotube Arrays with Enhanced Photocatalytic Degradation" Coatings 14, no. 7: 894. https://doi.org/10.3390/coatings14070894




