Effects of Precursors Ratio and Curing Treatment on the Icephobicity of Polydimethylsiloxane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Characterization
2.3. Methods
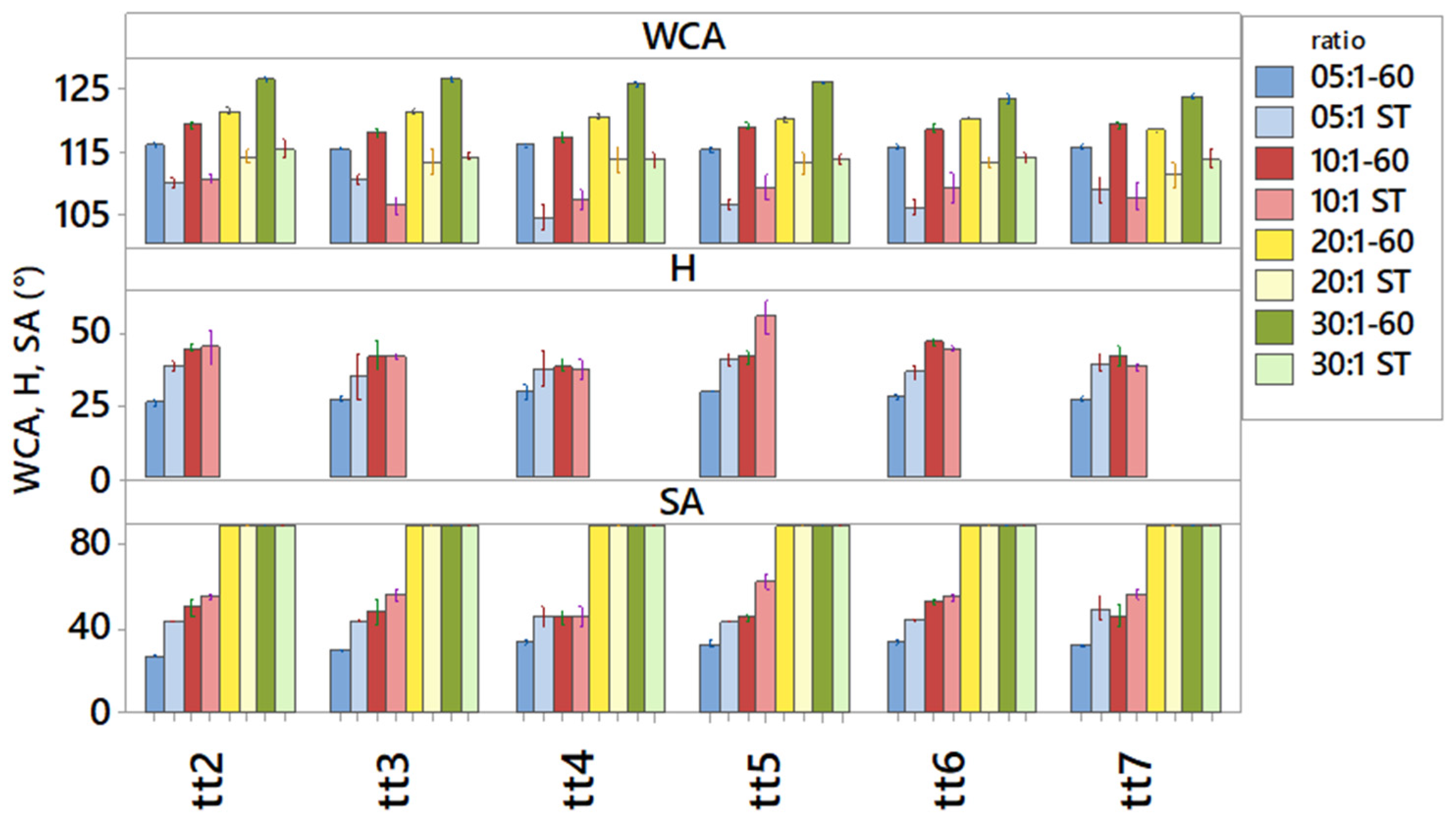
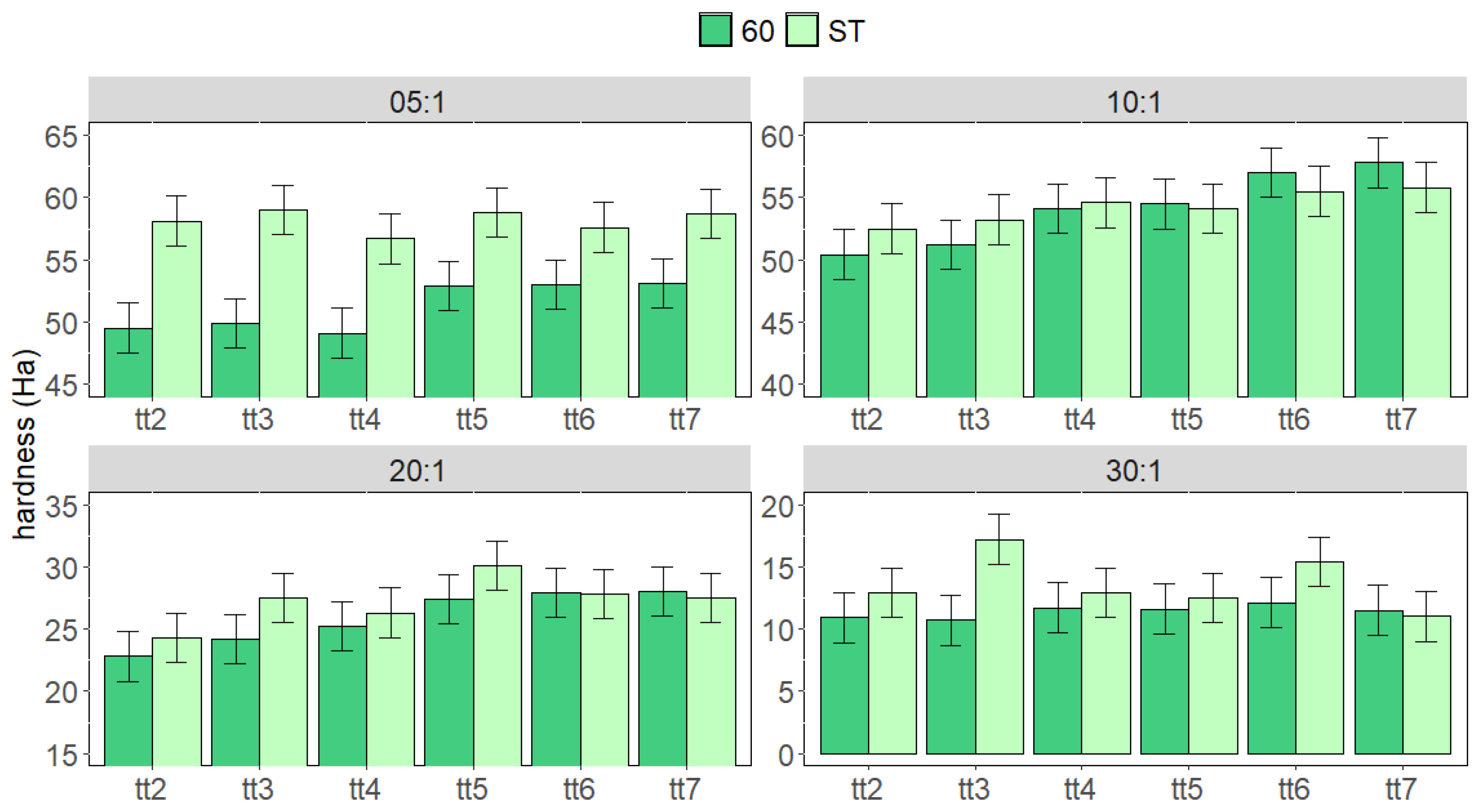
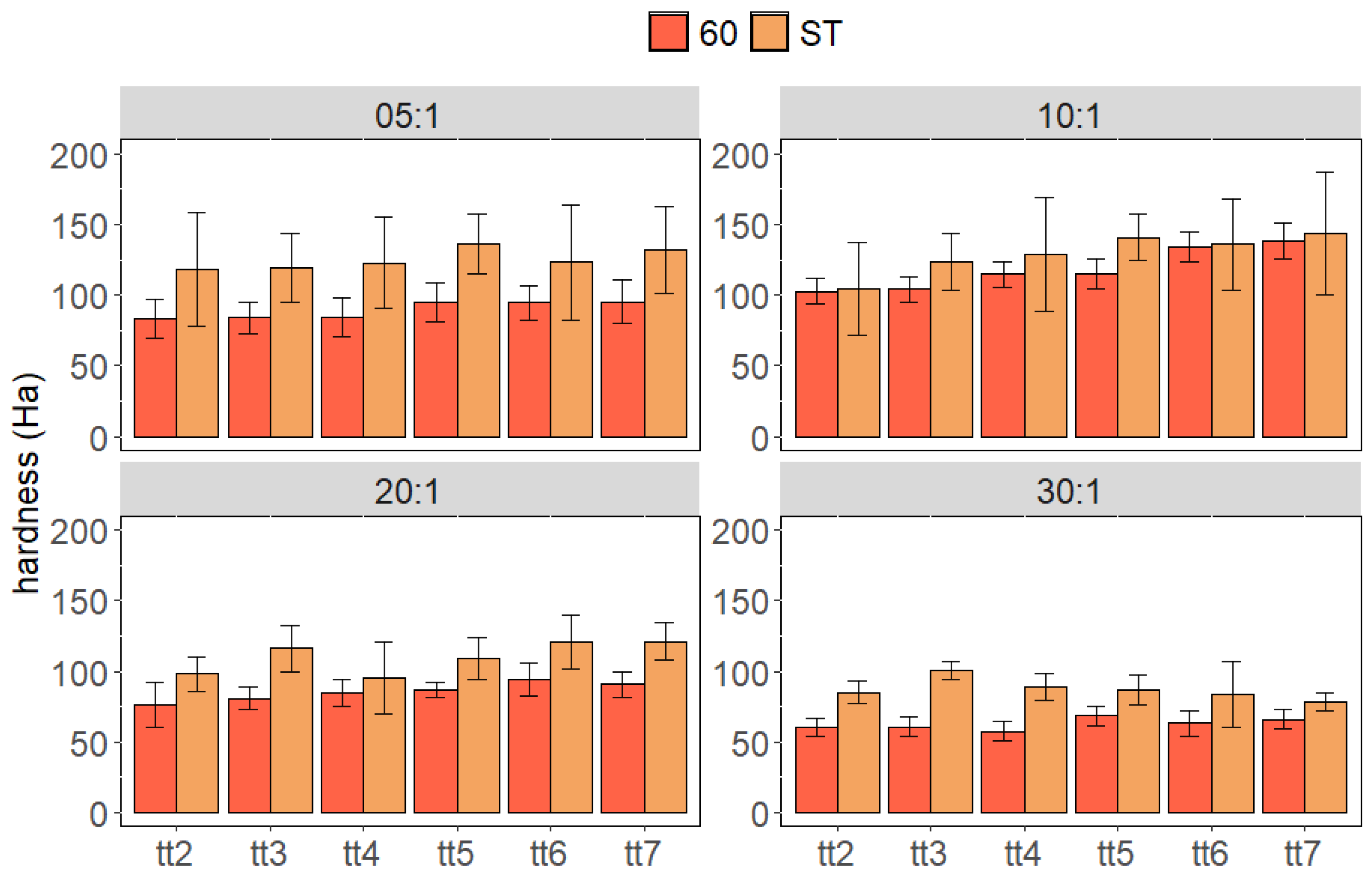
3. Results and Discussion
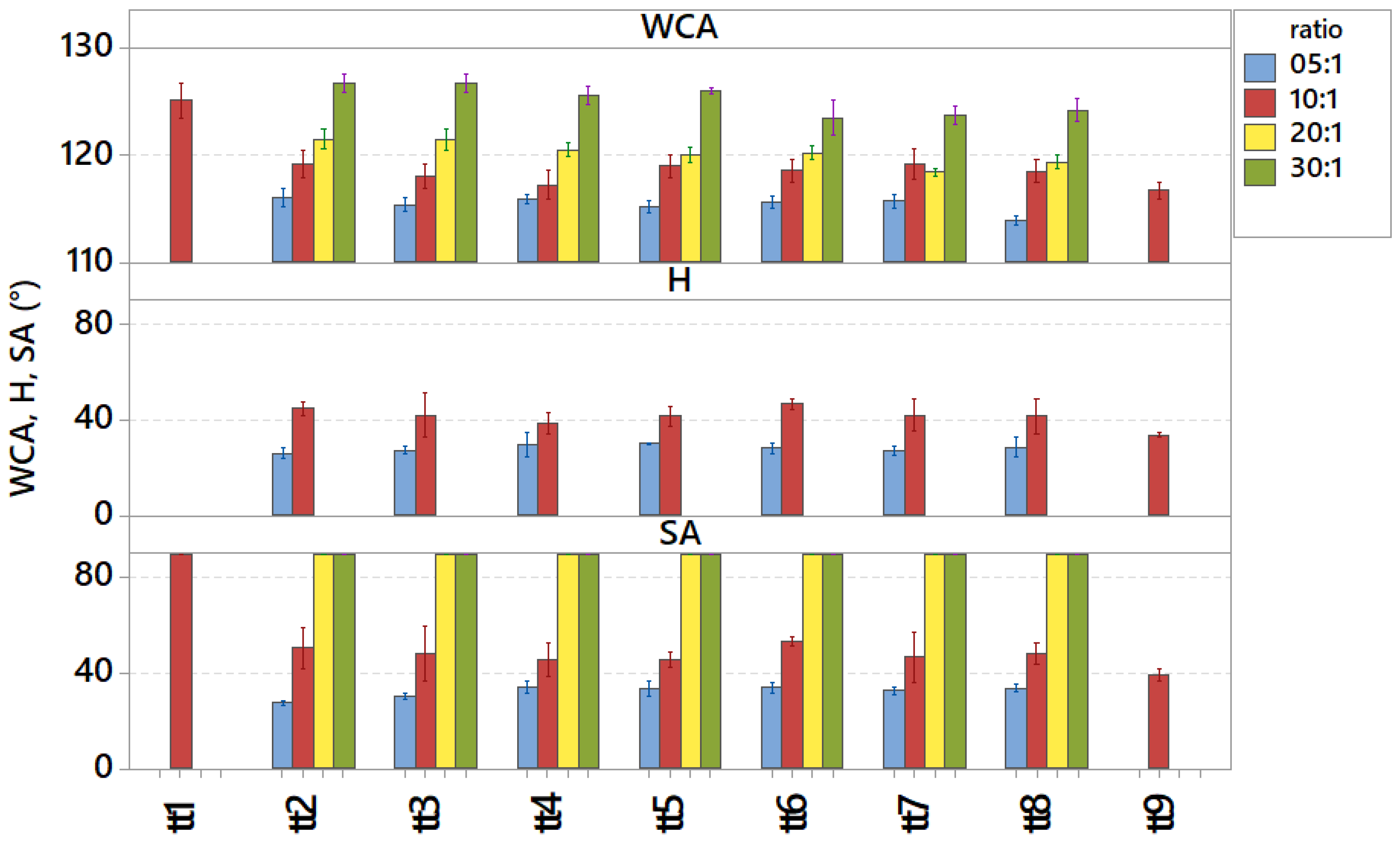
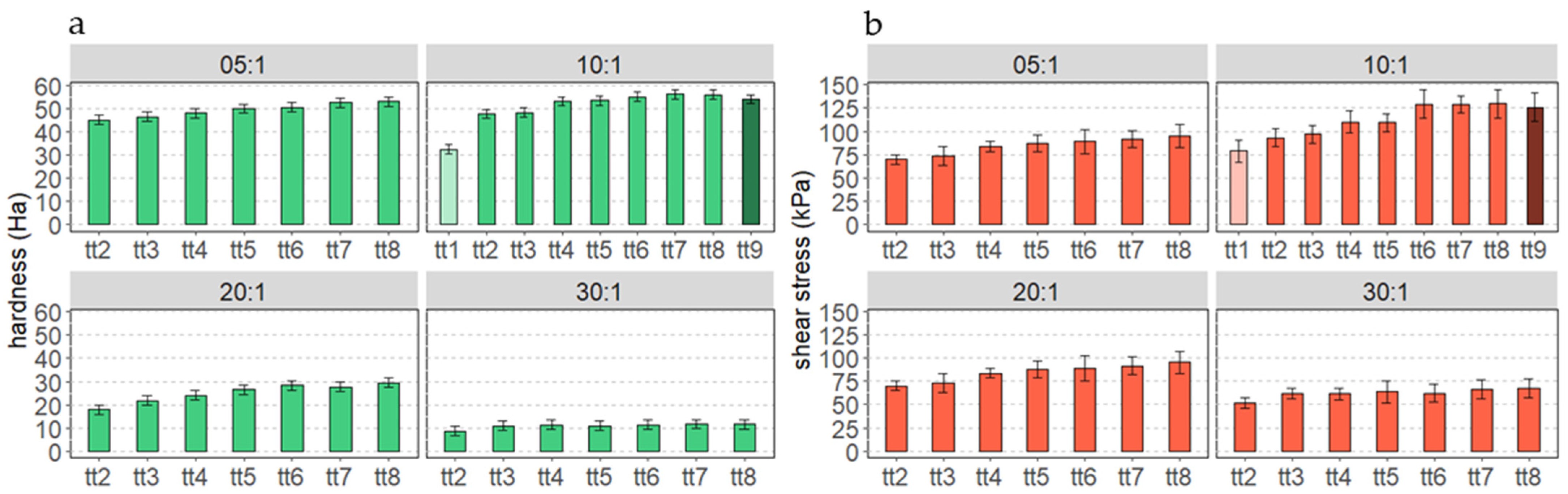
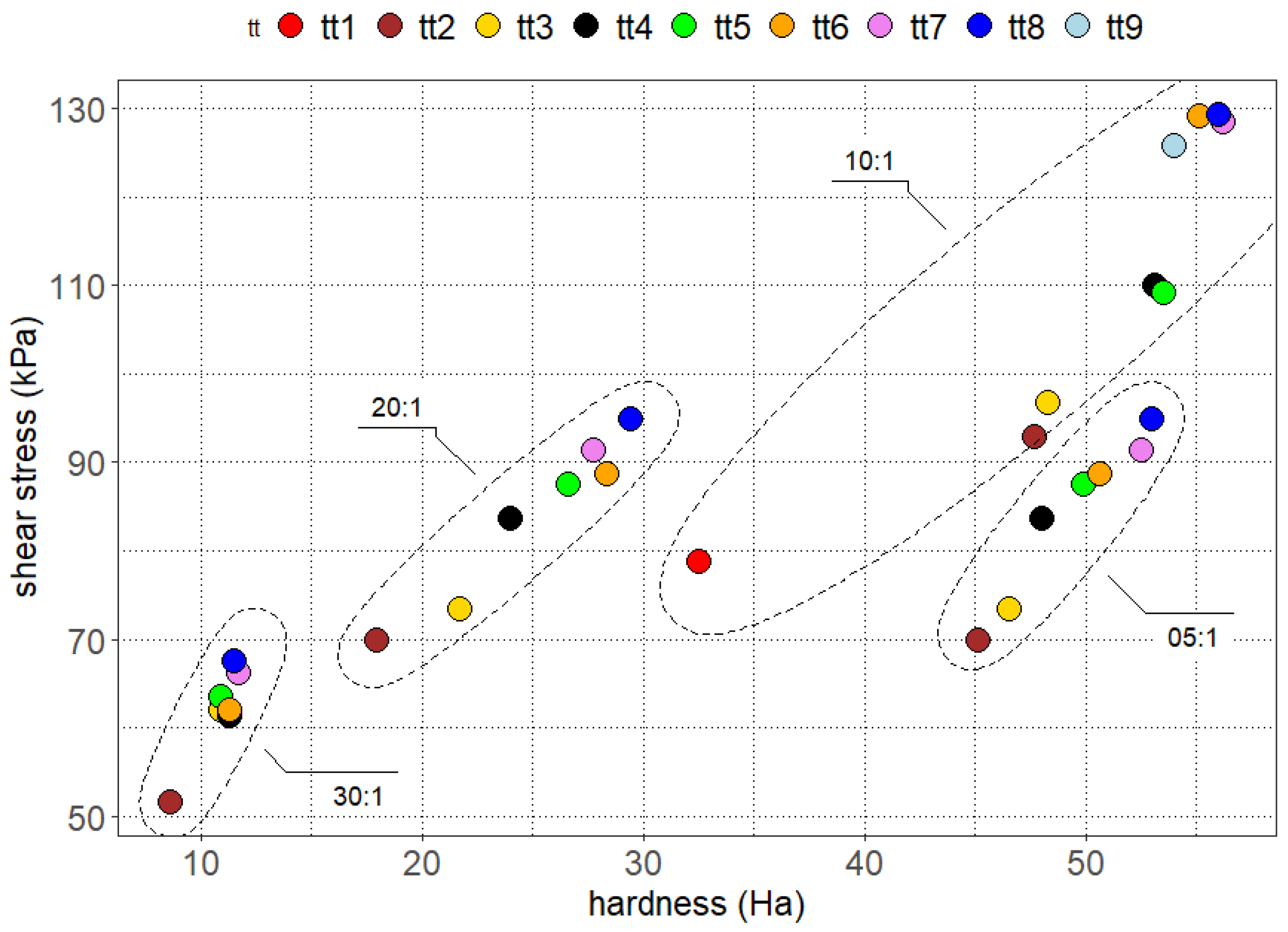
3.1. Cured Samples Characterization
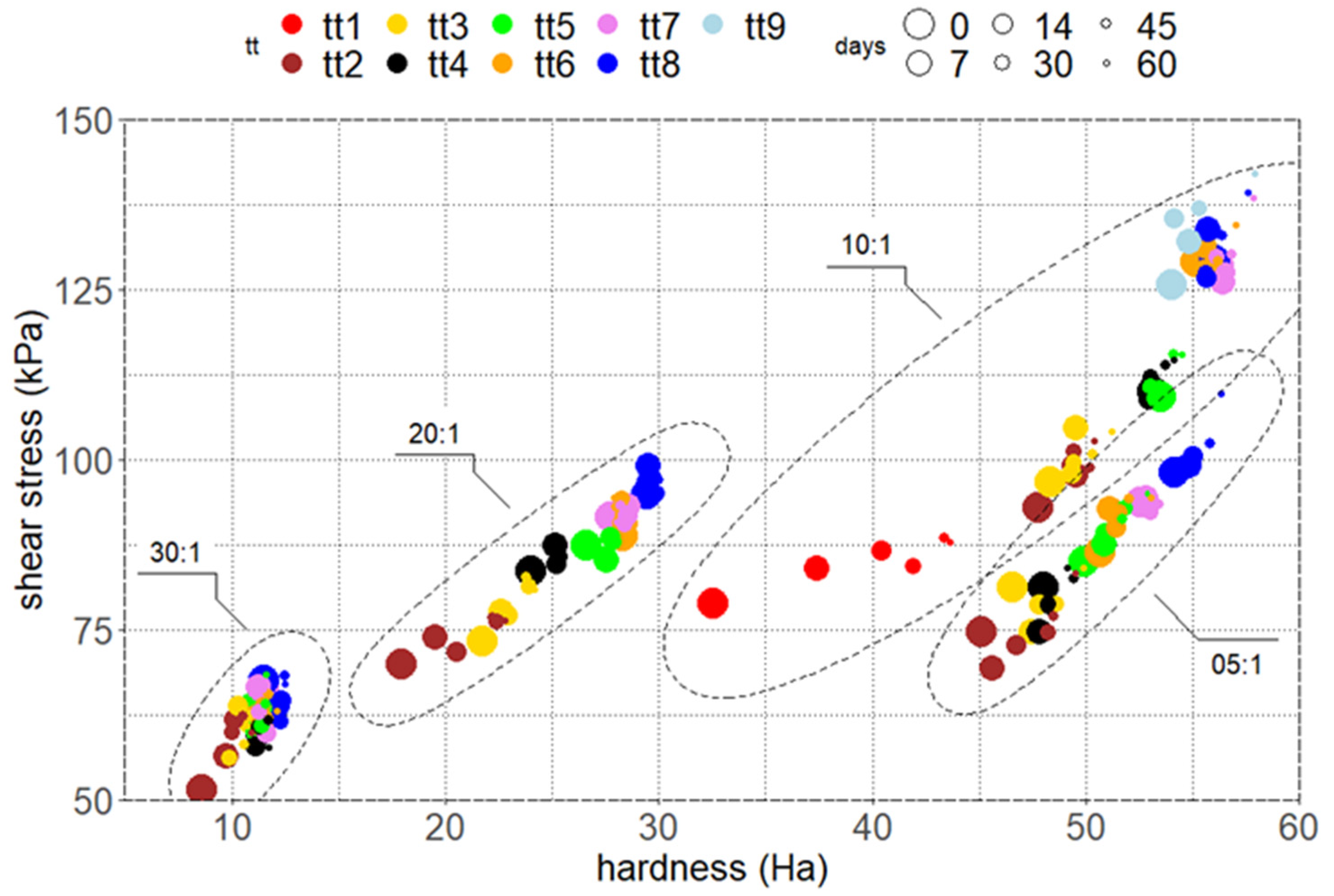
3.2. Aged Samples Characterization
3.3. Stressed Samples Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Golovin, K.; Kobaku, S.P.R.; Lee, D.H.; DiLoreto, E.T.; Mabry, J.M.; Tuteja, A. Designing Durable Icephobic Surfaces. Sci. Adv. 2016, 2, e1501496. [Google Scholar] [CrossRef] [PubMed]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.S.; Lima, R.; Minas, G. Properties and Applications of PDMS for Biomedical Engineering: A Review. JFB 2021, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Borók, A.; Laboda, K.; Bonyár, A. PDMS Bonding Technologies for Microfluidic Applications: A Review. Biosensors 2021, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Sekitani, T.; Nakajima, H.; Maeda, H.; Fukushima, T.; Aida, T.; Hata, K.; Someya, T. Stretchable Active-Matrix Organic Light-Emitting Diode Display Using Printable Elastic Conductors. Nat. Mater. 2009, 8, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Mark, J. Physical Properties of Polymers Handbook; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Vaicekauskaite, J.; Mazurek, P.; Vudayagiri, S.; Ladegaard Skov, A. Silicone Elastomer Map: Design the Ideal Elastomer. In Electroactive Polymer Actuators and Devices (EAPAD) XXI; Bar-Cohen, Y., Anderson, I.A., Eds.; SPIE: Denver, CO, USA, 2019; p. 61. [Google Scholar]
- Yu, Y.; Sanchez, D.; Lu, N. Work of Adhesion/Separation between Soft Elastomers of Different Mixing Ratios. J. Mater. Res. 2015, 30, 2702–2712. [Google Scholar] [CrossRef]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with Designer Functionalities—Properties, Modifications Strategies, and Applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Placet, V.; Delobelle, P. Mechanical Properties of Bulk Polydimethylsiloxane for Microfluidics over a Large Range of Frequencies and Aging Times. J. Micromech. Microeng. 2015, 25, 035009. [Google Scholar] [CrossRef]
- Sales, F.C.; Ariati, R.M.; Noronha, V.T.; Ribeiro, J.E. Mechanical Characterization of PDMS with Different Mixing Ratios. Procedia Struct. Integr. 2022, 37, 383–388. [Google Scholar] [CrossRef]
- Liu, M.; Sun, J.; Chen, Q. Influences of Heating Temperature on Mechanical Properties of Polydimethylsiloxane. Sens. Actuators A Phys. 2009, 15, 42–45. [Google Scholar] [CrossRef]
- Seghir, R.; Arscott, S. Extended PDMS Stiffness Range for Flexible Systems. Sens. Actuators A Phys. 2015, 230, 33–39. [Google Scholar] [CrossRef]
- ASTM-D2240; Test Method for Rubber Property-Durometer Hardness. ASTM International: West Conshohocken, PA, USA, 2015.
- Balordi, M.; Pini, F.; Santucci de Magistris, G. Superhydrophobic Ice-Phobic Zinc Surfaces. Surf. Interfaces 2022, 30, 101855. [Google Scholar] [CrossRef]
- Lamberti, A.; Quaglio, M.; Sacco, A.; Cocuzza, M.; Pirri, C. Surface Energy Tailoring of Glass by Contact Printed PDMS. Appl. Surf. Sci. 2012, 258, 9427–9431. [Google Scholar] [CrossRef]
- Viola, I.; Zacheo, A.; Arima, V.; Aricò, A.S.; Cortese, B.; Manca, M.; Zocco, A.; Taurino, A.; Rinaldi, R.; Gigli, G. The Influence of Polydimethylsiloxane Curing Ratio on Capillary Pressure in Microfluidic Devices. Appl. Surf. Sci. 2012, 258, 8032–8039. [Google Scholar] [CrossRef]
- Ibáñez-Ibáñez, P.F.; Montes Ruiz-Cabello, F.J.; Cabrerizo-Vílchez, M.A.; Rodríguez-Valverde, M.A. Ice Adhesion of PDMS Surfaces with Balanced Elastic and Water-Repellent Properties. J. Colloid Interface Sci. 2022, 608, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Chen, N.; Tirrell, M.; Israelachvili, J.N. Adhesion and Friction Mechanisms of Polymer-on-Polymer Surfaces. Science 2002, 297, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Khanafer, K.; Duprey, A.; Schlicht, M.; Berguer, R. Effects of Strain Rate, Mixing Ratio, and Stress–Strain Definition on the Mechanical Behavior of the Polydimethylsiloxane (PDMS) Material as Related to Its Biological Applications. Biomed. Microdevices 2009, 11, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Johnston, I.D.; McCluskey, D.K.; Tan, C.K.; Tracey, M.C. Mechanical Characterization of Bulk Sylgard 184 for Microfluidics and Microengineering. J. Micromech. Microeng. 2014, 24, 035017. [Google Scholar] [CrossRef]
- Chaudhury, M.K.; Kim, K.H. Shear-Induced Adhesive Failure of a Rigid Slabin Contact with a Thin Confined Film. Eur. Phys. J. E 2007, 23, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Mark, J.E. Polymer Data Handbook; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Bolis, F.M. Studio di Rivestimenti per Strutture Morphing con Impiego di Materiali Elastomerici e Compositi; PolitTesi: Milano, Italy, 2011. [Google Scholar]



| Thermal Treatment | Temperature | Time |
|---|---|---|
| tt1 | 25 °C | 48 h |
| tt2 | 100 °C | 35 m |
| tt3 | 100 °C | 1 h |
| tt4 | 100 °C | 3 h |
| tt5 | 100 °C | 6 h |
| tt6 | 100 °C | 9 h |
| tt7 | 100 °C | 12 h |
| tt8 | 100 °C | 24 h |
| tt9 | 200 °C | 18 m |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balordi, M.; Casali, A.; Gadia, P.; Pelagatti, P.; Pini, F.; Santucci de Magistris, G. Effects of Precursors Ratio and Curing Treatment on the Icephobicity of Polydimethylsiloxane. Coatings 2024, 14, 901. https://doi.org/10.3390/coatings14070901
Balordi M, Casali A, Gadia P, Pelagatti P, Pini F, Santucci de Magistris G. Effects of Precursors Ratio and Curing Treatment on the Icephobicity of Polydimethylsiloxane. Coatings. 2024; 14(7):901. https://doi.org/10.3390/coatings14070901
Chicago/Turabian StyleBalordi, Marcella, Alessandro Casali, Paolo Gadia, Paolo Pelagatti, Francesco Pini, and Giorgio Santucci de Magistris. 2024. "Effects of Precursors Ratio and Curing Treatment on the Icephobicity of Polydimethylsiloxane" Coatings 14, no. 7: 901. https://doi.org/10.3390/coatings14070901





