Optimization of Preparation Process for Chitosan-Coated Pomelo Peel Flavonoid Microcapsules and Its Effect on Waterborne Paint Film Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method of Preparing Microcapsules
2.3. Preparation of Waterborne Paint Films with Different Contents of Microcapsules
2.4. Performance Test
2.4.1. The Microcapsule Yield and Coverage Rate
2.4.2. The Morphology and Chemical Composition
2.4.3. The Antibacterial Performance
2.4.4. The Optical Performance
2.4.5. The Mechanical Performance
3. Results and Discussion
3.1. The Microcapsules’ Yield and Coverage Rate
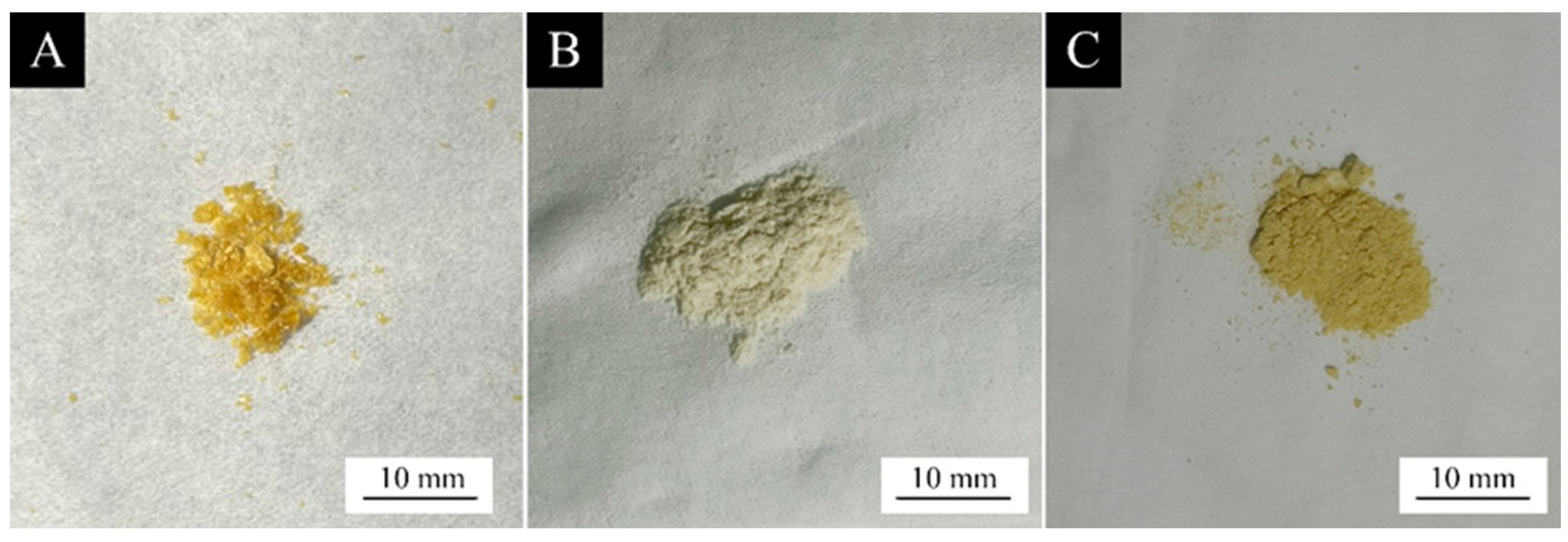
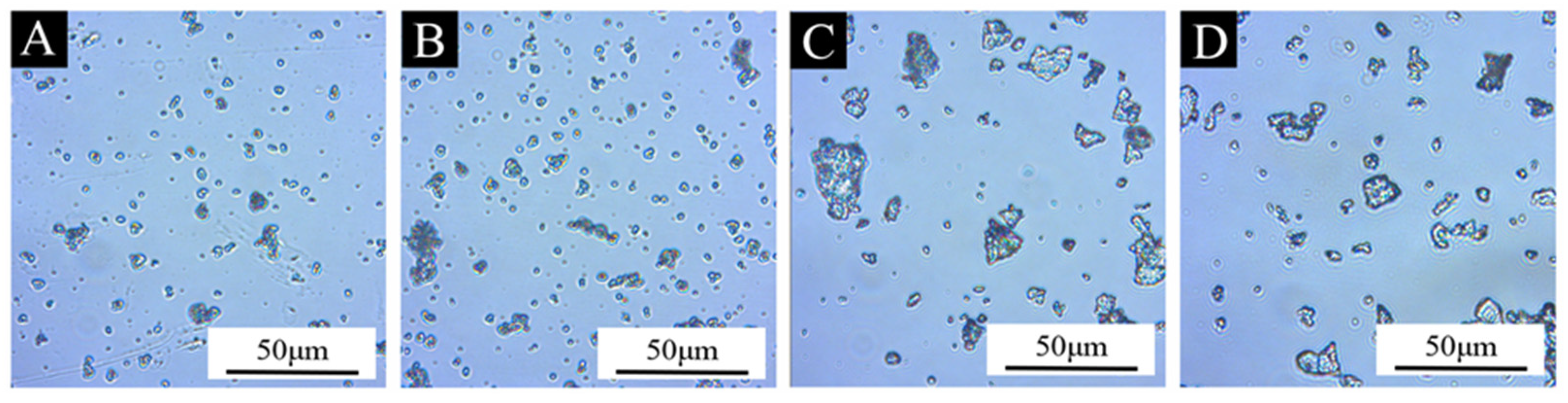
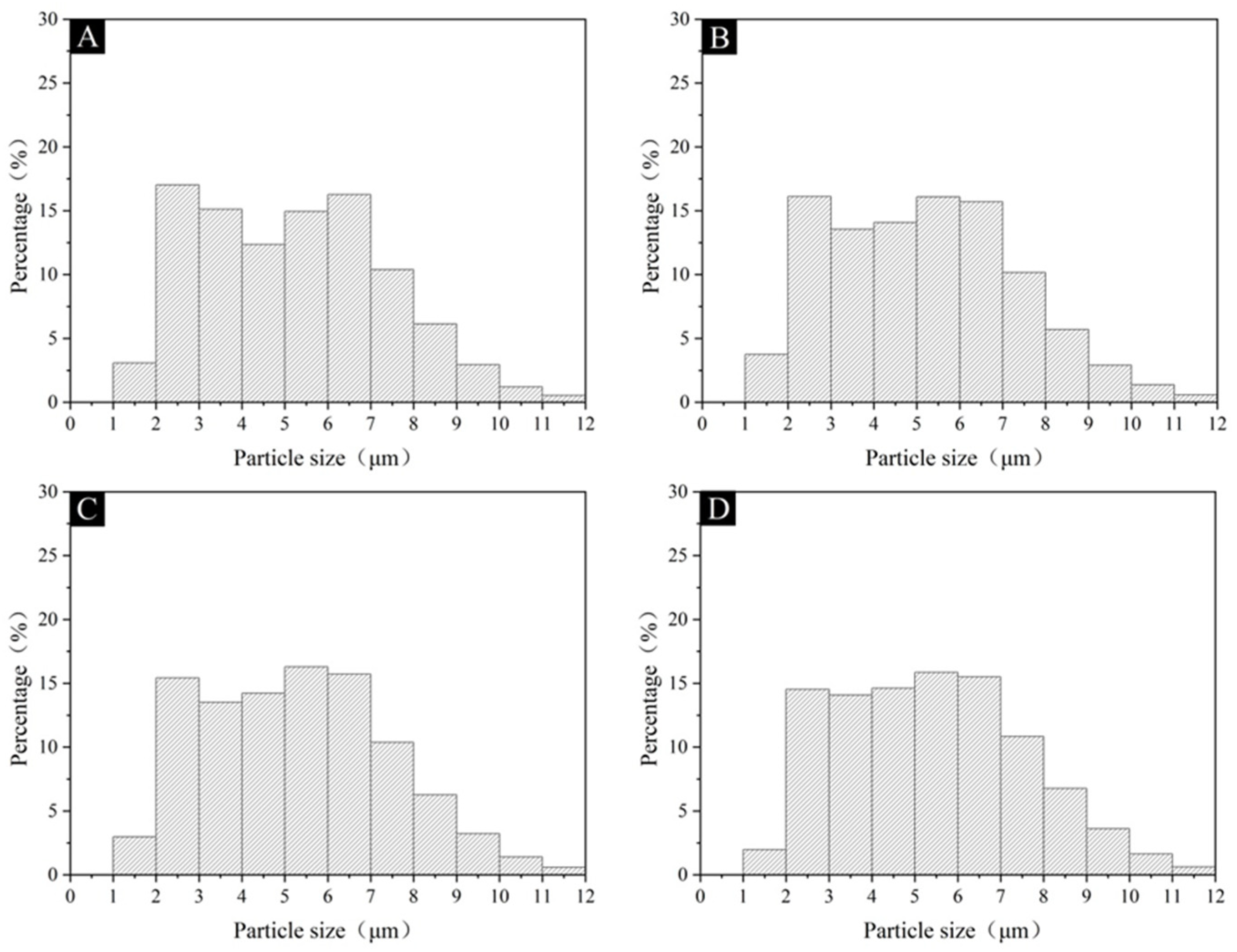
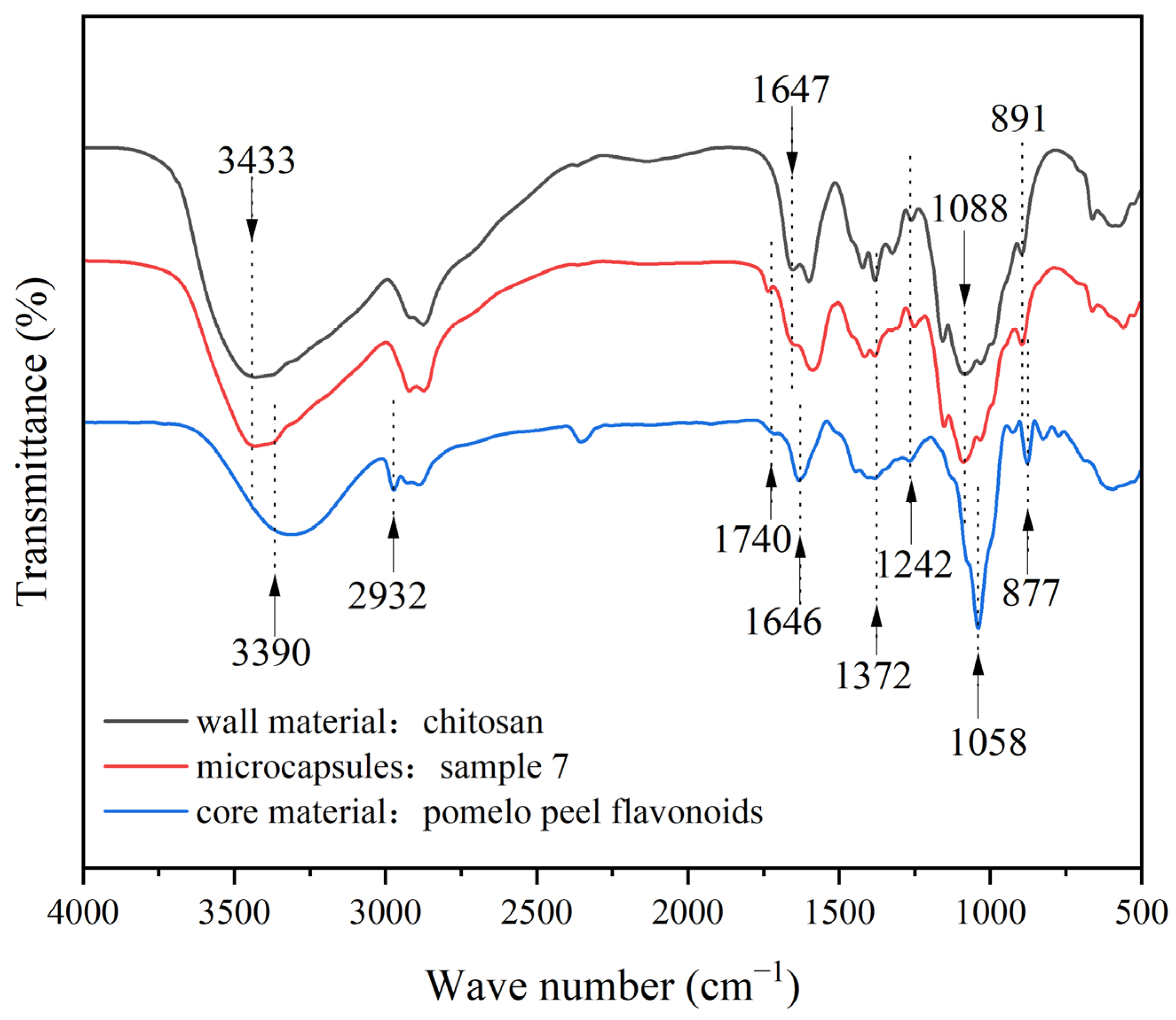
3.2. The Morphological and Chemical Composition of the Microcapsules
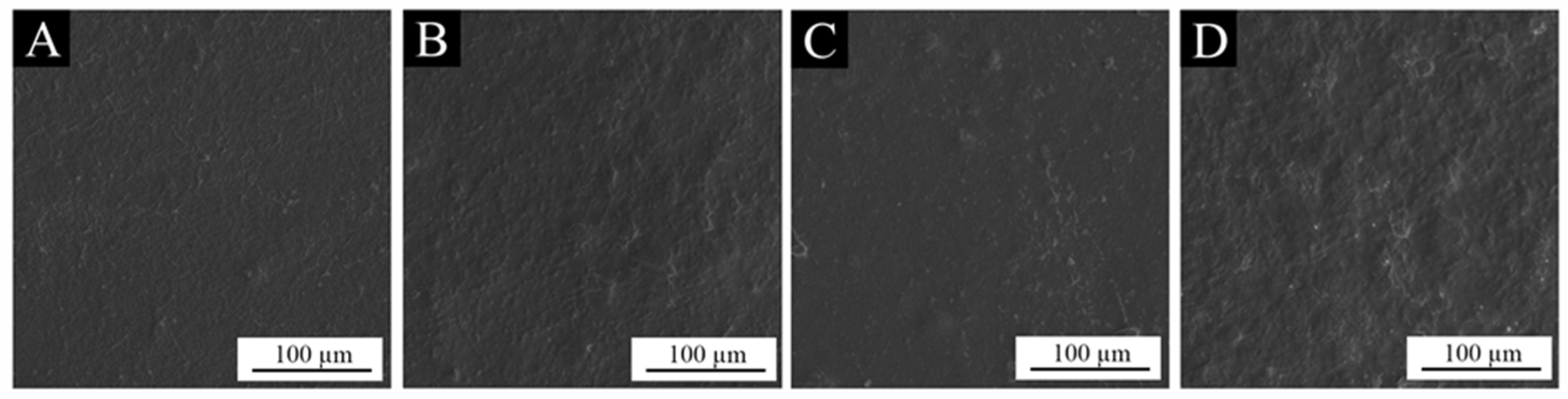
3.3. The Morphology of the Paint Film
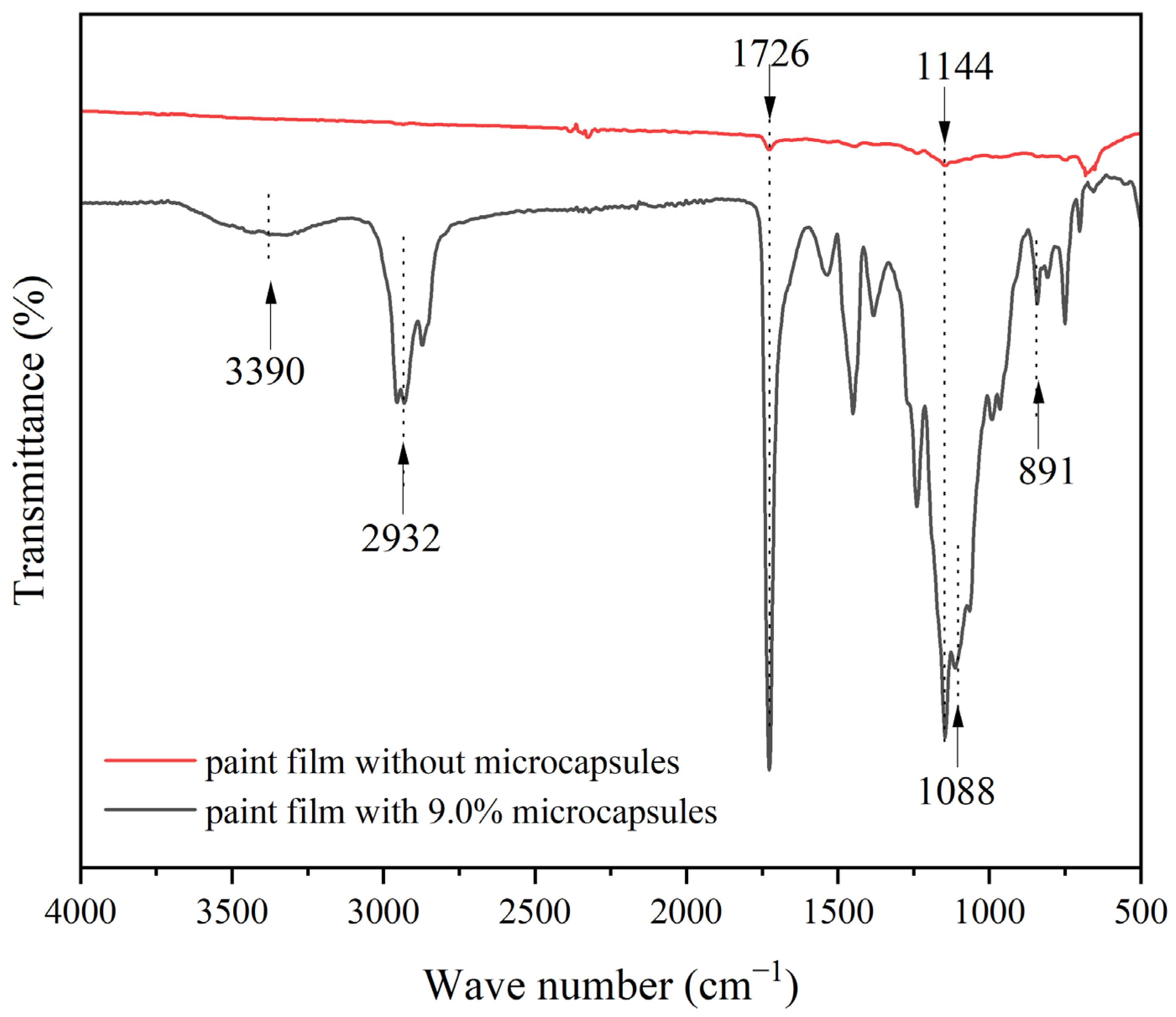
3.4. The Chemical Composition of the Paint Film
3.5. The Antibacterial Properties of the Paint Film
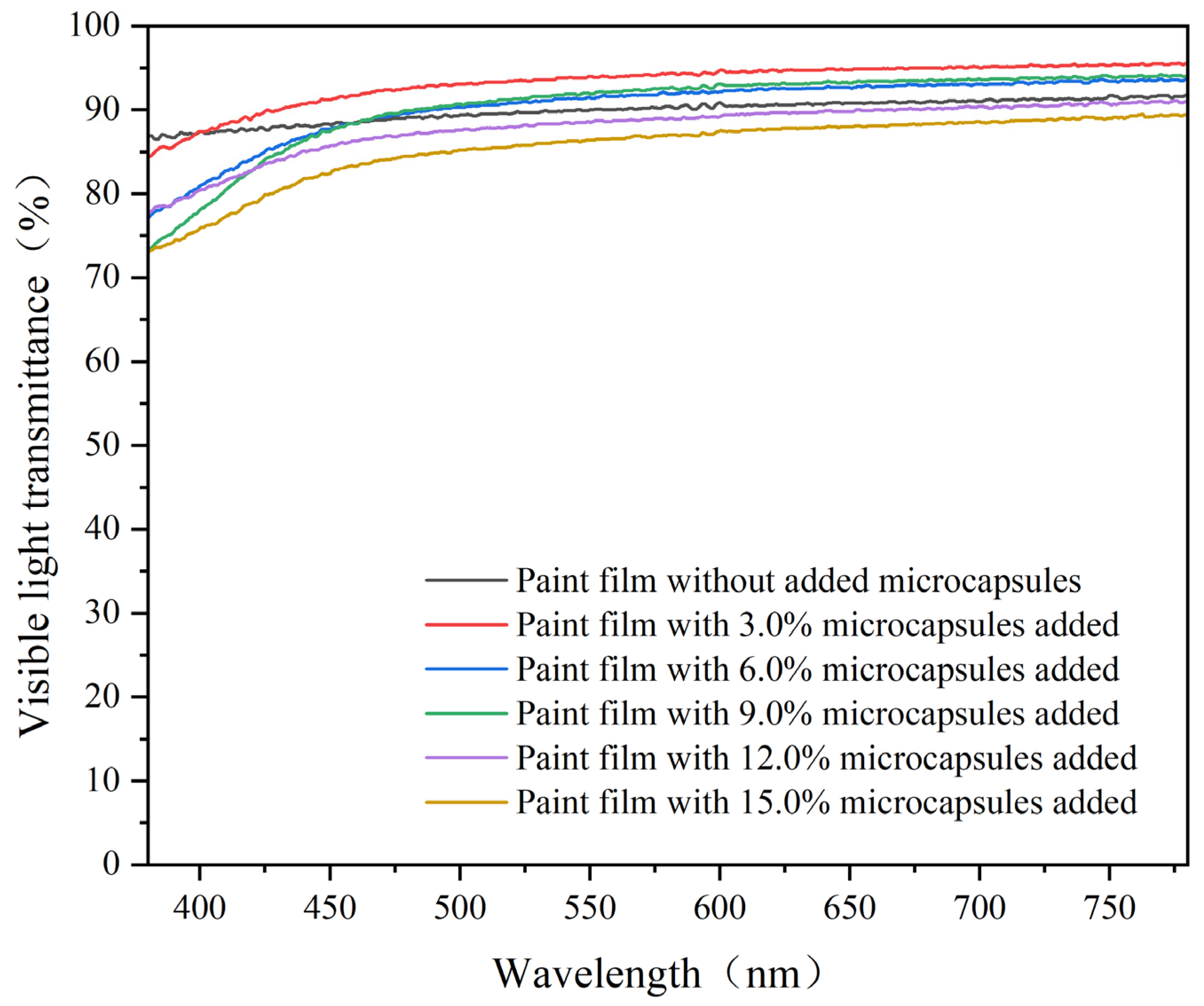
3.6. The Optical Properties of the Paint Film
- (1)
- The chromaticity value and color difference
- (2)
- Glossiness and gloss loss rate
- (3)
- Visible light transmittance
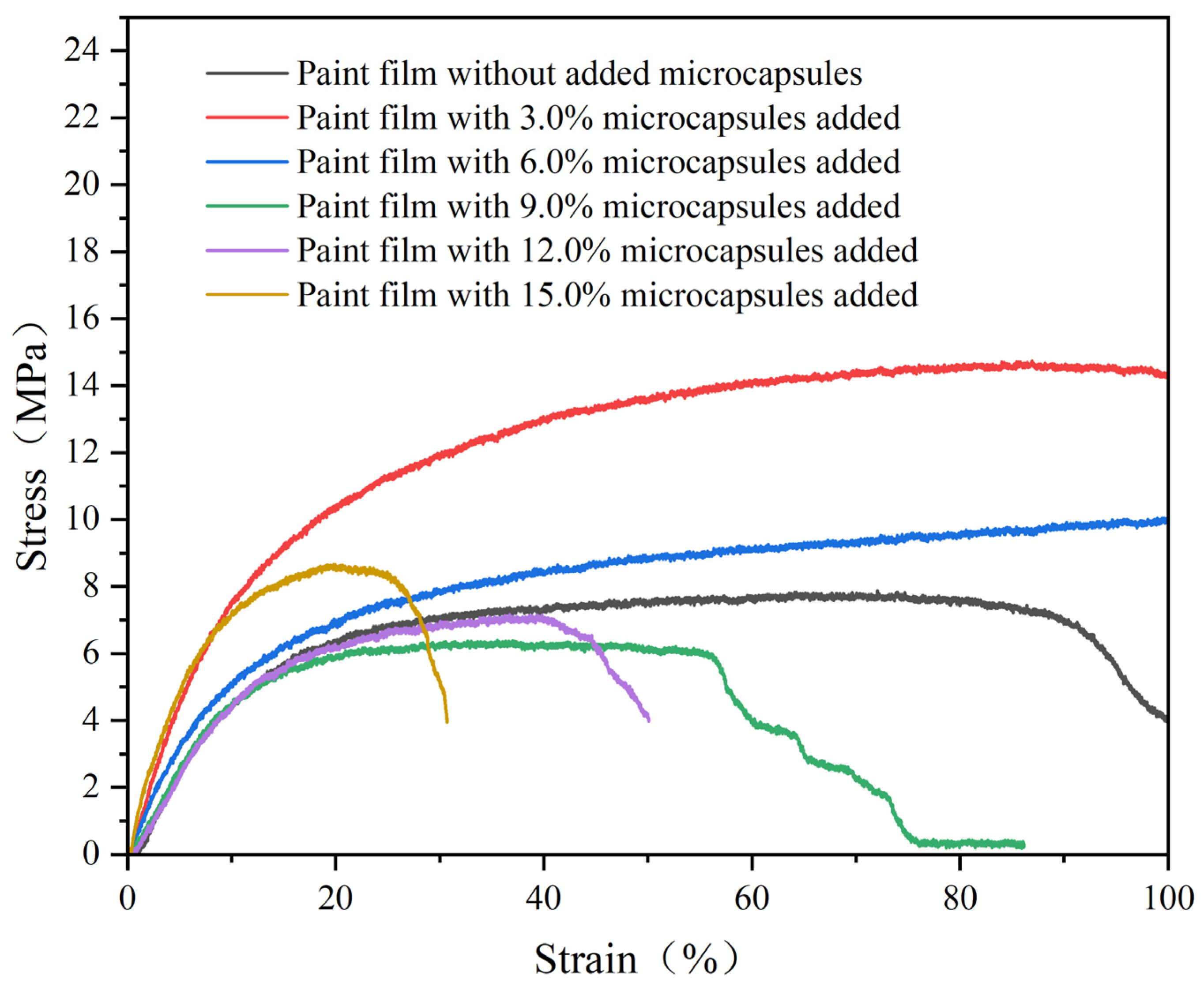
3.7. The Mechanical Properties of the Paint Film
- (1)
- Roughness
- (2)
- Elongation at break
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, J.; Liu, Y.; Xu, W. Impact of cellular structure on the thickness and light reflection properties of structural color layers on diverse wood surfaces. Wood Mater. Sci. Eng. 2024. [Google Scholar] [CrossRef]
- Wu, S.S.; Zhou, J.C.; Xu, W. A convenient approach to manufacturing lightweight and high-sound-insulation plywood using furfuryl alcohol/multilayer graphene oxide as a shielding layer. Wood Mater. Sci. Eng. 2024. [Google Scholar] [CrossRef]
- Hu, W.; Yu, R. Study on the strength mechanism of the wooden round-end mortise-and-tenon joint using the digital image correlation method. Holzforschung 2024. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.Y.; Zhu, Y. Reverse design and additive manufacturing of furniture protective foot covers. BioResources 2024, 19, 4670–4678. [Google Scholar] [CrossRef]
- Wang, C.; Yu, J.H.; Jiang, M.H.; Li, J.Y. Effect of selective enhancement on the bending performance of fused deposition methods 3D-printed PLA models. BioResources 2024, 19, 2660–2669. [Google Scholar] [CrossRef]
- Zhang, N.; Xu, W.; Tan, Y. Multi-attribute hierarchical clustering for product family division of customized wooden doors. Bioresources 2023, 18, 7889–7904. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Y.; Xu, W. Influence of cell characteristics on the construction of structural color layers on wood surfaces. Forests 2024, 15, 676. [Google Scholar] [CrossRef]
- Mai, C.; Schmitt, U.; Niemz, P. A brief overview on the development of wood research. Holzforschung 2022, 76, 102–119. [Google Scholar] [CrossRef]
- Xue, J.X.; Xu, W.; Zhou, J.C.; Mao, W.G.; Wu, S.S. Effects of High-Temperature Heat Treatment Modification by Impregnation on Physical And Mechanical Properties of Poplar. Materials 2022, 15, 7334. [Google Scholar] [CrossRef]
- Weng, M.Y.; Zhu, Y.T.; Mao, W.G.; Zhou, J.C.; Xu, W. Nano-Silica/Urea-Formaldehyde Resin-Modified Fast-Growing Lumber Performance Study. Forests 2023, 14, 1440. [Google Scholar] [CrossRef]
- Wu, S.S.; Tao, X.; Xu, W. Thermal Conductivity of Poplar Wood Veneer Impregnated with Graphene/Polyvinyl Alcohol. Forests 2021, 12, 777. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Gao, D.; Xu, W. Effect of paint process on the performance of modified poplar wood antique. Coatings 2021, 11, 1174. [Google Scholar] [CrossRef]
- Xu, W.; Chen, P.; Yang, Y.; Wang, X.; Liu, X. Effects of freezing and steam treatments on the permeability of populus tomentosa. Materialwiss. Werkst. 2021, 52, 907–915. [Google Scholar] [CrossRef]
- Tao, M.X.; Liu, X.; Xu, W. Effect of the vacuum impregnation process on water absorption and nail-holding power of silica sol-modified Chinese Fir. Forests 2024, 15, 261. [Google Scholar] [CrossRef]
- Singh, A.P.; Kim, Y.S.; Chavan, R.R. Relationship of wood cell wall ultrastructure to bacterial degradation of wood. IAWA J. 2019, 40, 845–870. [Google Scholar] [CrossRef]
- Xu, W.; Fang, X.Y.; Han, J.T.; Wu, Z.H. Effect of Coating Thickness on Sound Absorption Property of Four Wood Species Commonly Used for Piano Soundboards. Wood Fiber Sci. 2020, 52, 28–43. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Feng, X.H.; Wu, Y.; Wu, Z.H. Factors influencing the properties of UV-cured self-matting film. Prog. Org. Coat. 2024, 189, 108241. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Feng, X.H.; Wu, Y.; Wu, Z.H. Self-matting waterborne polyurethane acrylate wood coating by 222 nm far-UVC irradiation in ambient air. Prog. Org. Coat. 2024, 189, 108305. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Gao, D.; Xu, W. Effect of sanding processes on the surface properties of modified poplar coated by primer compared with mahogany. Coatings 2020, 10, 856. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Gao, D.; Xu, W. Effect of polyurethane non-transparent coating process on paint film performance applied on modified poplar. Coatings 2022, 12, 39. [Google Scholar] [CrossRef]
- Jiang, G.F.; Li, X.F.; Che, Y.L.; Lv, Y.; Liu, F.; Wang, Y.Q.; Zhao, C.C.; Wang, X.J. Antibacterial and anticorrosive properties of CuZnO@RGO waterborne polyurethane coating in circulating cooling water. Environ. Sci. Pollut. R. 2019, 26, 9027–9040. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, T.; Hao, R.D.; Wang, Y.M. Construction strategy and mechanism of a novel wood preservative with excellent antifungal effects. Molecules 2024, 29, 1313. [Google Scholar] [CrossRef] [PubMed]
- Vainio-Kaila, T.; Harju, A.; Rohumaa, A.; Paajanen, O.; Venäläinen, M.; Seppä, J.; Veijalainen, A.M.; Pasanen, P. The effect of surface treatment on the antibacterial properties of wood and the possibility to detect the antibacteriality with fluorescence method. Forests 2023, 14, 23. [Google Scholar] [CrossRef]
- Chang, Y.J.; Wu, Z.H. Synthesized high performance UV-cured wood wax oil using Irgacure 2959 modified thistle oil and linseed oil. Ind. Crop. Prod. 2024, 218, 118952. [Google Scholar] [CrossRef]
- Veiko, A.G.; Olchowik-Grabarek, E.; Sekowski, S.; Roszkowska, A.; Lapshina, E.A.; Dobrzynska, I.; Zamaraeva, M.; Zavodnik, I.B. Antimicrobial activity of quercetin, naringenin and catechin: Flavonoids inhibit staphylococcus aureus-induced hemolysis and modify membranes of bacteria and erythrocytes. Molecules 2023, 28, 1252. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, W.J.; Li, X.Y.; Liu, J.C.; Chen, Y. Antimicrobial activity and mechanisms of walnut green husk extract. Molecules 2023, 28, 7981. [Google Scholar] [CrossRef]
- Tran, N.D.N.; Bui, T.H.; Nguyen, A.P.; Nguyen, T.T.; Nguyen, V.M.; Duong, N.L.; Nguyen, T. The ability of silver-biochar green-synthesized from Citrus maxima peel to adsorb pollutant organic compounds and antibacterial activity. Green Chem. Lett. Rev. 2022, 15, 16–25. [Google Scholar] [CrossRef]
- Irkni, R.; Esmer, O.K. Novel food packaging systems with natural antimicrobial agents. J. Food Sci. Tech. Mys. 2015, 52, 6095–6111. [Google Scholar] [CrossRef]
- Van, C.K.; Nguyen, P.T.N.; Nguyen, T.T.T.; Bach, L.G. Microencapsulation of Citrus latifolia peel essential oil by spray-drying using maltodextrin: Characterization, antimicrobial activities, and release profile. Lwt-Food Sci. Technol. 2024, 197, 115825. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Yang, A.; Tan, W.H.; Yang, W.C. Development, Physicochemical Properties, and Antibacterial Activity of Propolis Microcapsules. Foods 2023, 12, 3191. [Google Scholar] [CrossRef]
- Huang, N.; Yan, X.X. Preparation of aloe-emodin microcapsules and its effect on antibacterial and optical properties of water-based coating. Polymers 2023, 15, 1728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, S.S.; Su, R.R.; Xiong, D.; Feng, W.; Chen, J.P. Controlled release mechanism and antibacterial effect of layer-by-layer self-assembly thyme oil microcapsule. J. Food Sci. 2019, 84, 1427–1438. [Google Scholar] [CrossRef] [PubMed]
- Shilpa, V.S.; Shams, R.; Dash, K.K.; Pandey, V.K.; Dar, A.H.; Mukarram, S.A.; Harsányi, E.; Kovács, B. Phytochemical properties, extraction, and pharmacological benefits of naringin: A review. Molecules 2023, 28, 5623. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, X.J.; Cheng, Y.; Gao, H.S.; Chen, X.H. A review of classification, biosynthesis, biological activities and potential applications of flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, Y.C.; Ni, J.; Jiang, L.L.; Hu, Y.; He, C.B.; Ma, Y.; Wu, G.H.; Xiong, H.J. Response surface methodology-optimized extraction of flavonoids from pomelo peels and isolation of naringin with antioxidant activities by Sephadex LH20 gel chromatography. Cur. Res. Food Sci. 2023, 7, 100610. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.A.A.; Rodrigues, J.B.D.; Magnani, M.; de Souza, E.L.; de Siqueira, J.P. Inhibitory effects of flavonoids on biofilm formation by Staphylococcus aureus that overexpresses efflux protein genes. Microb. Pathog. 2017, 107, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Pan, Y.R.; Li, X.S.; Jie, J.X.; Zeng, M.Y. Chemical composition, antimicrobial and anti-quorum sensing activities of pummelo peel flavonoid extract. Ind. Crop. Prod. 2017, 109, 862–868. [Google Scholar] [CrossRef]
- Valle, J.A.B.; Valle, R.D.S.C.; Bierhalz, A.C.K.; Bezerra, F.M.; Hernandez, A.L.; Arias, M.J.L. Chitosan microcapsules: Methods of the production and use in the textile finishing. J. Appl. Polym. Sci. 2021, 138, e50482. [Google Scholar] [CrossRef]
- Zhou, J.C.; Xu, W. An aesthetic transparent wood resistant to Escherichia coli based on interface optimization. Eur. J. Wood Wood Prod. 2023, 81, 1569–1579. [Google Scholar] [CrossRef]
- Chen, K.L.; Ni, Y.Z.; Shi, X.; Jia, Z.H.; Qiu, H.; Portale, G. Green fabrication of chitosan microcapsules via double emulsion-simple coacervation and their application in fabrics. Cellulose 2023, 30, 11861–11873. [Google Scholar] [CrossRef]
- Utami, P.D.; Setianingsih, H.; Tirto Sari, D.R. Microencapsulation, Physicochemical Characterization, and Antioxidant, Antibacterial, and Antiplasmodial Activities of Holothuria atra Microcapsule. Scientifica 2024, 2024, 5559133. [Google Scholar] [CrossRef] [PubMed]
- Comini, S.; Mandras, N.; Iannantuoni, M.R.; Menotti, F.; Musumeci, A.G.; Piersigilli, G.; Allizond, V.; Banche, G.; Cuffini, A.M. Positive and Negative Ions Potently Inhibit the Viability of Airborne Gram-Positive and Gram-Negative Bacteria. Microbiol. Spectr. 2021, 9, e00651. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.; Cardoso, J.; Guerra, N.; Ribeiro, E.; Viegas, C.; Verde, S.C.; Sousa-Uva, A. Exposure and Health Effects of Bacteria in Healthcare Units: An Overview. Appl. Sci.-Basel 2022, 12, 1958. [Google Scholar] [CrossRef]
- Tehrani, E.; Amiri, S. Synthesis and characterization PVA electro-spun nanofibers containing encapsulated vitamin C in chitosan microspheres. J. Text. I. 2022, 113, 212–223. [Google Scholar] [CrossRef]
- Deng, J.Z.; Ding, T.T.; Yan, X.X. Effect of Two Pomelo Peel Flavonoid Microcapsules on the Performance of Waterborne Coatings on the Surface of Poplar Boards. Coatings 2024, 14, 937. [Google Scholar] [CrossRef]
- GB/T 21866-2008; Test Method and Effect for Antibacterial Capability of Paints Film. Standardization Administration of the People’s Republic of China: Beijing, China, 2008.
- GB/T 4789.2-2022; National Food Safety Standard Food Microbiological Examination: Aerobic Plate Count. Standardization Administration of the People’s Republic of China: Beijing, China, 2022.
- GB/T 11186.3-1989; Methods for Measuring the Colour of Paint Films. Part III: Calculation of Colour Differences. Standardization Administration of the People’s Republic of China: Beijing, China, 1990.
- GB/T 4893.6-2013; Test of Surface Coatings of Furniture. Standardization Administration of the People’s Republic of China: Beijing, China, 2013.
- Zhang, H.; Zhou, L.M.; Shehzad, H.; Farooqi, Z.H.; Sharif, A.; Ahmed, E.; Habiba, U.; Qaisar, F.; Fatima, N.-E.; Begum, R.; et al. Innovative free radical induced synthesis of WO3-doped diethyl malonate grafted chitosan encapsulated with phosphorylated alginate matrix for UO22+ adsorption: Parameters optimisation through response surface methodology. Sep. Purif. Technol. 2024, 353, 128455. [Google Scholar] [CrossRef]
- Ding, T.T.; Yan, X.X. Preparation process optimization for melamine resin-covered pomelo peel flavonoid antibacterial microcapsules and their effect on waterborne paint film performance. Coatings 2024, 14, 654. [Google Scholar] [CrossRef]



| Material | Molecular Formula | CAS No. |
|---|---|---|
| Chitosan | (C6H11NO4)n | 9012-76-4 |
| Acetic acid | CH3COOH | 64-19-7 |
| Tween-80 | C24H44O6 | 9005-65-6 |
| Sodium tripolyphosphate | Na5P3O10 | 7758-29-4 |
| Anhydrous ethanol | C2H6O | 64-17-5 |
| Staphylococcus aureus | - | - |
| Escherichia coli | - | - |
| Nutrient agar medium | - | - |
| Nutritious broth | - | - |
| Equipment | Model | Manufacturing |
|---|---|---|
| Optical microscope (OM) | AX10 | Carl Zeiss AG. Oberkochen, Germany |
| Scanning electron microscope (SEM) | Quanta-200 | Thermo Fisher Scientific, Waltham, MA, USA |
| Magnetic stirrer | DF-101Z | Nanbei Scientific Instrument Technology Co., Ltd., Beijing, China |
| Fourier Transform Infrared (FTIR) spectrometer | VERTEX 80V | Bruker Corporation, Karlsruhe, Germany |
| Humidity chamber | HWS-50 | Shanghai Shangyi Instrument Equipment Co., Ltd., Shanghai, China |
| Freeze-dryer | YTLG-10A | Shanghai Yetuo Technology Co., Ltd., Shanghai, China |
| Colony counter | XK97-A | Hangzhou Qiwei Instrument Co., Ltd., Hangzhou, China |
| Portable colorimeter | SC-10 | Zhuhai Tianchuang Instrument Co., Ltd., Zhuhai, China |
| Gloss meter | HG268 | Shenzhen ThreeNH Technology Co., Ltd., Shenzhen, China |
| Ultraviolet spectrophotometer | U-3900 | Hitachi Scientific Instruments (Suzhou) Co., Ltd., Suzhou, China |
| Universal mechanical testing machine | AGS-X | Shimadzu Manufacturing Co., Ltd., Kyoto, Japan |
| Fine roughness tester | JB-4C | Suliang Instrument Technology Co., Ltd., Suzhou, China |
| Level | Factor A pH Value | Factor B m(Core Material):m(Wall Material) | Factor C Concentration of Emulsifier (%) |
|---|---|---|---|
| 1 | 6 | 1.0:1 | 1 |
| 2 | 8 | 1.2:1 | 3 |
| Sample | pH | m(Core Material):m(Wall Material) | Concentration of Emulsifier (%) |
|---|---|---|---|
| 1 | 6 | 1.0:1 | 1 |
| 2 | 6 | 1.2:1 | 3 |
| 3 | 8 | 1.0:1 | 3 |
| 4 | 8 | 1.2:1 | 1 |
| Test | Sample | Chitosan (g) | 1% Acetic Acid Solution (g) | Pomelo Peel Flavonoids (g) | Anhydrous Ethanol (g) | Emulsifier (g) | Deionized Water (mL) | STPP (g) |
|---|---|---|---|---|---|---|---|---|
| Orthogonal test | 1 | 0.80 | 39.20 | 0.80 | 7.20 | 0.72 | 71.28 | 0.80 |
| 2 | 0.80 | 39.20 | 0.96 | 8.64 | 2.11 | 68.29 | 0.80 | |
| 3 | 0.80 | 39.20 | 0.80 | 7.20 | 2.10 | 69.90 | 0.80 | |
| 4 | 0.80 | 39.20 | 0.96 | 8.64 | 0.70 | 69.70 | 0.80 | |
| One-factor test | 5 | 0.80 | 39.20 | 0.80 | 7.20 | 0.72 | 71.28 | 0.80 |
| 6 | 0.80 | 39.20 | 0.80 | 7.20 | 0.72 | 71.28 | 0.80 | |
| 7 | 0.80 | 39.20 | 0.80 | 7.20 | 0.72 | 71.28 | 0.80 | |
| 8 | 0.80 | 39.20 | 0.80 | 7.20 | 0.72 | 71.28 | 0.80 | |
| 9 | 0.80 | 39.20 | 0.80 | 7.20 | 0.72 | 71.28 | 0.80 |
| Content of Microcapsules (%) | Mass of Microcapsules (g) | Mass of Waterborne Paint (g) |
|---|---|---|
| 0 | 0 | 1.00 |
| 3.0 | 0.03 | 0.97 |
| 6.0 | 0.06 | 0.94 |
| 9.0 | 0.09 | 0.91 |
| 12.0 | 0.12 | 0.88 |
| 15.0 | 0.15 | 0.85 |
| Category | Sample | Factor A pH Value | Factor B m(Core Material):m(Wall Material) | Factor C Concentration of Emulsifier (%) | Yield (%) |
|---|---|---|---|---|---|
| Range | 1 | 6 | 1.0:1 | 1 | 20 |
| 2 | 6 | 1.2:1 | 3 | 20 | |
| 3 | 8 | 1.0:1 | 3 | 22 | |
| 4 | 8 | 1.2:1 | 1 | 21 | |
| Mean value 1 | 20.000 | 21.000 | 20.500 | ||
| Mean value 2 | 21.500 | 20.500 | 21.000 | ||
| R | 1.500 | 0.500 | 0.500 | ||
| Factor primary and secondary levels | A > B = C | ||||
| Optimal level | A2 | B1 | C2 | ||
| Optimal solution | A2 B1 C2 | ||||
| Variance | Sum of squared deviations | 2.250 | 0.250 | 0.250 | |
| Degree of freedom | 1 | 1 | 1 | ||
| Fratio | 2.455 | 0.273 | 0.273 | ||
| Fcritical value | 10.100 | 10.100 | 10.100 | ||
| Significance | |||||
| Category | Sample | Factor A pH Value | Factor B m(Core Material):m(Wall Material) | Factor C Concentration of Emulsifier (%) | Coverage Rate (%) |
|---|---|---|---|---|---|
| Range | 1 | 6 | 1.0:1 | 1 | 45 |
| 2 | 6 | 1.2:1 | 3 | 31 | |
| 3 | 8 | 1.0:1 | 3 | 53 | |
| 4 | 8 | 1.2:1 | 1 | 51 | |
| Mean value 1 | 38.000 | 49.000 | 48.000 | ||
| Mean value 2 | 52.000 | 41.000 | 42.000 | ||
| R | 14.000 | 8.000 | 6.000 | ||
| Factor primary and secondary levels | A > B > C | ||||
| Optimal level | A2 | B1 | C1 | ||
| Optimal solution | A2 B1 C1 | ||||
| Variance | Sum of squared deviations | 196.000 | 64.000 | 36.000 | |
| Degree of freedom | 1 | 1 | 1 | ||
| Fratio | 1.986 | 0.649 | 0.365 | ||
| Fcritical value | 10.100 | 10.100 | 10.100 | ||
| Significance | |||||
| Sample | pH Value | Yield Rate (%) | Coverage Rate (%) |
|---|---|---|---|
| 5 | 5.5 | 24 | 38 |
| 6 | 6.5 | 20 | 41 |
| 7 | 7.5 | 22 | 50 |
| 8 | 8.5 | 22 | 47 |
| 9 | 9.5 | 23 | 44 |
| Content of Microcapsules (%) | Average Number of Recovered Escherichia coli (CFU·Piece−1) | Antibacterial Rate against Escherichia coli (%) | Average Number of Recovered Staphylococcus aureus (CFU·Piece−1) | Antibacterial Rate against Staphylococcus aureus (%) |
|---|---|---|---|---|
| 0 | 190 | - | 432 | - |
| 3.0 | 134 | 29.5 ± 0.6 | 289 | 33.1 ± 0.8 |
| 6.0 | 102 | 46.3 ± 1.6 | 187 | 56.7 ± 1.5 |
| 9.0 | 73 | 61.6 ± 0.9 | 126 | 70.8 ± 1.2 |
| 12.0 | 51 | 73.2 ± 1.4 | 74 | 82.9 ± 2.7 |
| 15.0 | 32 | 83.2 ± 1.1 | 36 | 91.7 ± 1.7 |
| Item | SS | df | MS | F | p-Value | F Crit |
|---|---|---|---|---|---|---|
| Content of microcapsules | 10,537.29 | 5 | 2107.457 | 243.8058 | 0.00 | 5.050329 |
| Antibacterial species | 142.83 | 1 | 142.83 | 16.5236 | 0.009682 | 6.607891 |
| Error | 43.22 | 5 | 8.644 | |||
| Total | 10,723.34 | 11 |
| Content of Microcapsules (%) | L | a | b | ΔE |
|---|---|---|---|---|
| 0 | 81.91 | 1.73 | −2.27 | - |
| 3.0 | 44.93 | 0.97 | 0.50 | 37.09 |
| 6.0 | 43.50 | 0.97 | 1.30 | 38.58 |
| 9.0 | 43.00 | 0.90 | 1.57 | 39.11 |
| 12.0 | 42.20 | 0.87 | 1.60 | 39.91 |
| 15.0 | 41.40 | 0.63 | 1.87 | 40.74 |
| Content of Microcapsules (%) | 20° (%) | 60° (%) | 85° (%) | Gloss Loss Rate (%) |
|---|---|---|---|---|
| 0 | 6.10 | 17.45 | 31.17 | - |
| 3.0 | 4.47 | 14.13 | 14.93 | 19.1 |
| 6.0 | 2.40 | 10.30 | 7.27 | 41.0 |
| 9.0 | 2.13 | 10.17 | 4.10 | 41.7 |
| 12.0 | 2.10 | 10.17 | 3.40 | 41.7 |
| 15.0 | 1.77 | 8.10 | 3.27 | 53.6 |
| Content of Microcapsules (%) | Visible Light Transmittance (%) |
|---|---|
| 0 | 89.89 |
| 3.0 | 93.25 |
| 6.0 | 90.40 |
| 9.0 | 90.47 |
| 12.0 | 87.94 |
| 15.0 | 85.54 |
| Content of Microcapsules (%) | Roughness (%) |
|---|---|
| 0 | 0.27 |
| 3.0 | 0.46 |
| 6.0 | 1.46 |
| 9.0 | 2.60 |
| 12.0 | 2.65 |
| 15.0 | 2.70 |
| Content of Microcapsules (%) | Elongation at Break (%) |
|---|---|
| 0 | 18.9 |
| 3.0 | 22.3 |
| 6.0 | 21.5 |
| 9.0 | 16.1 |
| 12.0 | 10.5 |
| 15.0 | 7.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.; Ding, T.; Yan, X. Optimization of Preparation Process for Chitosan-Coated Pomelo Peel Flavonoid Microcapsules and Its Effect on Waterborne Paint Film Properties. Coatings 2024, 14, 1003. https://doi.org/10.3390/coatings14081003
Deng J, Ding T, Yan X. Optimization of Preparation Process for Chitosan-Coated Pomelo Peel Flavonoid Microcapsules and Its Effect on Waterborne Paint Film Properties. Coatings. 2024; 14(8):1003. https://doi.org/10.3390/coatings14081003
Chicago/Turabian StyleDeng, Jinzhe, Tingting Ding, and Xiaoxing Yan. 2024. "Optimization of Preparation Process for Chitosan-Coated Pomelo Peel Flavonoid Microcapsules and Its Effect on Waterborne Paint Film Properties" Coatings 14, no. 8: 1003. https://doi.org/10.3390/coatings14081003
APA StyleDeng, J., Ding, T., & Yan, X. (2024). Optimization of Preparation Process for Chitosan-Coated Pomelo Peel Flavonoid Microcapsules and Its Effect on Waterborne Paint Film Properties. Coatings, 14(8), 1003. https://doi.org/10.3390/coatings14081003





