Abstract
This article presents the results of research on the growth kinetics, microstructure (SEM/EDS/XRD), and corrosion behavior of Zn-5Al coatings obtained using a high-temperature hot dip process on B500B reinforcing steel. The corrosion resistance of the coatings was determined using the neutral salt spray (NSS) test (EN ISO 9227). Based on chemical composition tests in micro-areas (EDS) and phase composition tests (XRD), corrosion products formed on the coating surface after exposure to a corrosive environment containing chlorides were identified. In the outer layer of the coating, areas rich in Zn and Al were found, which were solid solutions of Al in Zn (α), while the diffusion layer was formed by a layer of Fe(Al,Zn)3 intermetallics. The growth kinetics of the coatings indicate the sequential growth of the diffusion layer, controlled by diffusion in the initial phase of growth, and the formation of a periodic layered structure with a longer immersion time. The NSS test showed an improved corrosion resistance of reinforcing bars with Zn-5Al coatings compared to a conventional hot-dip-galvanized zinc coating. The increase in corrosion resistance was caused by the formation of beneficial corrosion products: layered double hydroxides (LDH) based on Zn2+ and Al3+ cations and Cl− anions and simonkolleite—Zn5(OH)8Cl2·H2O.
1. Introduction
Concrete reinforcement is an important strength element that determines the safety of a structure. A stable passive layer is formed on the surface of the concrete reinforcement, which determines the low corrosion rate of the reinforcement [1]. However, reinforced concrete structures are exposed to the surrounding external environment, and the penetration of aggressive ions through the concrete matrix to the reinforcement surface causes the destruction of the passive layer and the initiation of corrosion of reinforcing bars in concrete [2]. The presence of CO2 in the atmospheric environment causes the carbonation of concrete, which reduces its alkalinity, leading to the degradation of the passive layer and the development of uniform corrosion on the reinforcement surface [3].
The use of coatings can reduce the corrosion rate of reinforcement in aggressive atmospheric environments. To protect the concrete reinforcement, the bars are covered with various organic coating systems [4]. Paints based on alkyd resins are characterized by relatively good resistance to water, paints based on epoxy resins are relatively resistant to chemicals, while coatings based on polyurethane resins show long-term resistance to impacts, abrasion, and corrosive industrial and marine environments [5]. However, organic coatings are susceptible to aging and therefore cannot guarantee long-term corrosion resistance. Another problem with coating bars with organic coatings is that the bond strength between the bar and concrete is significantly reduced. It was found that the reduction in the bond strength of bars covered with an epoxy coating compared to uncoated bars ranges from 15 to 50% [6].
Hot-dip-galvanized coatings provide more effective protection for reinforcing steel against corrosion [7]. Galvanized reinforcing bars, unlike painted bars, have good adhesion to concrete. Zinc coatings provide barrier protection for the bars and, after penetration into the substrate, also sacrificial protection. Despite many advantages, the use of zinc coatings on reinforcing bars does not always yield the expected results. In a marine environment, the main cause of reinforcement corrosion in concrete is Cl− ions [8,9]. Cl has a smaller ion radius than CO2; therefore, it easily penetrates through defects in the structure of the concrete matrix to the reinforcement surface, causing the local corrosion of the reinforcement. Local corrosion caused by chloride ions reduces the working surface of the reinforcement and the load-bearing capacity of the reinforced concrete structure. Additionally, the stresses caused by the increase in the volume of the formed corrosion products lead to the cracking and subsequent delamination of the concrete cover [10]. Corrosion damage to reinforcements caused by chlorides is therefore much more harmful to the durability of reinforced concrete compared to the impact of CO2 ions. Therefore, in highly aggressive marine environments containing chlorides, it is reasonable to use more effective methods of protecting the reinforcement than those offered by hot-dip-galvanized coatings.
An alternative to zinc hot dip coatings are coatings obtained in Zn-5Al baths. These coatings have 2–4 times better corrosion resistance compared to conventional zinc coatings [11], and the eutectic composition of the bath is advantageous because it ensures a low melting point of the alloy—386 °C [12]. Zn-5Al coatings are used to coat sheet metal using the continuous method developed by Sendzimir [11]. The main limitation of the use of a Zn-5Al bath for the production of coatings using a batch method is the lack of a flux that would ensure adequate wetting of the steel surface by liquid zinc containing large additions of Al [13]. However, previous research [14] has shown that it is possible to produce continuous coatings in eutectic ZnAl baths while maintaining their temperature above 520 °C. However, a high bath temperature carries the risk of an excessive increase in the thickness of the coating’s diffusion layer, as well as the creation of an unfavorable periodic layered structure [15]. These features of the formation of coatings are closely related to the chemical composition and type of steel to be coated. Therefore, this article presents the test results of Zn-5Al coatings obtained on reinforcing steel in a high-temperature batch hot dip galvanizing process. The growth kinetics and microstructure of the coatings were determined in the temperature range of 540–560 °C, and their corrosion behavior was determined in an environment containing chlorides.
2. Materials and Methods
2.1. Materials and Hot Dip Procedure
Zn-5Al coatings were made on rods with a diameter of ϕ 14 mm and length of 100 mm, which were made from B500B steel with medium ductility intended for concrete reinforcement. The chemical composition of the steel was determined using a Spectro Lab M8 emission spectrometer and is presented in Table 1. Due to the Si content (0.18 wt.%), the tested steel can be classified as Sebisty steel [16].

Table 1.
Chemical compositions of B500B steel and research baths.
The surface preparation process included: acid degreasing in HydronetBase solution (SOPRIN S.r.l., Maserada Sul Piave, Italy) for 5 min, etching in 12% HCl solution (Chempur, Piekary Śląskie, Poland) for 10 min, rinsing in water, and fluxing in DomuFlux60 solution (Dipl. Ing. Herwig GmbH, Hagen, Germany) for 2 min. Before immersion in the bath, the samples were dried at 120 °C for 15 min. Then, the samples were immersed in a Zn-5Al bath at temperatures of 540 and 560 °C and immersion times of 10, 20, 40, 90, 180, and 360 s. After being removed from the bath, the samples were cooled in water. The comparative coating for corrosion tests was prepared in a conventional hot dip galvanizing bath containing a technological additive of Al at the level of 0.0062 wt.%. The temperature of the hot dip galvanizing bath was 450 °C, and the immersion time (90 s) was experimentally selected to obtain a coating thickness similar to the thickness of the Zn-5Al coating. The charge materials for the bath were super-high-grade zinc, ZnAl4 mortar, and pure aluminum granules (99.99%). After melting the components, the chemical composition of the bath was examined using an ARL 3460 emission spectrometer (Thermo ARL, Waltham, MA, USA) and is presented in Table 1.
2.2. Characterization Methods
Investigations of the microstructure and chemical composition of coating micro-areas and the morphology of corrosion products were carried out using Hitachi S-3400 N scanning electron microscopy (SEM), equipped with an energy-dispersive spectroscope (EDS) (Hitachi, Tokyo, Japan). Noran Instruments—System Six software (Thermo Fisher Scientific. Waltham, MA, USA) was used for the tests, enabling the measurement of the thickness of the coating layers.
X-ray phase analysis was performed on a Philips X’Pert 3 X-ray diffractometer (Malvern Panalytical. Malvern, UK) using a lamp with a copper anode (λCuKα = 1.54178 Ǻ) powered by a current of 30 mA at a voltage of 40 kV and a graphite monochromator. XRD tests of the phase composition were performed on the surface of the Zn-5Al coating, covering the phase composition of the outer layer, and on the oblique grinding surface, covering the phase composition over the entire cross-section of the coating. After the corrosion process, the white corrosion products were mechanically removed, and then the phase composition of the powdered white corrosion products was determined, as well as the phase composition of the coating surface after removing the white corrosion products.
The neutral salt spray (NSS) test was performed in a CORROTHERM Model 610 salt spray chamber with a volume of 400 dm3 (Erichsen, Hemer, Germany). The tests were carried out in accordance with the EN ISO 9227 standard [17] on 5 samples of the same type, maintaining the following test conditions: temperature 35 ± 1 °C, 5% aqueous NaCl solution, pH 6.8–7.2, and mist condensation rate on a flat surface of 80 cm2—1.5 ± 0.5 mL/h. The smoothness and changes on the surface of the samples were monitored every 24 h. The total exposure time in the chamber was 1000 h.
3. Results and Discussion
3.1. Growth Kinetics and Microstructure of Coatings
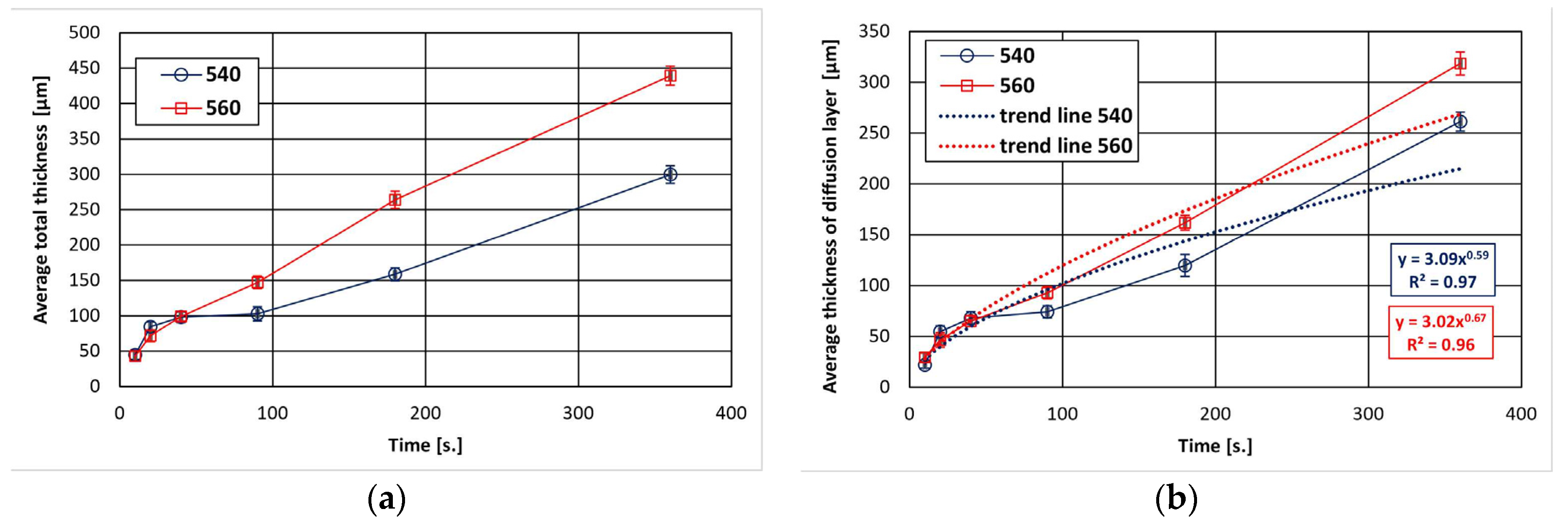
The dependence of the total thickness of the coating and the thickness of the diffusion layer in the coating on the immersion time in the bath is shown in Figure 1. The total thickness of coatings increases with the extension of the immersion time and the increase in the bath temperature. After 360 s, a coating with a thickness of 299.65 ± 12.34 µm at 540 °C and 439.38 ± 13.56 µm at 560 °C was obtained. In the initial stage, it can be seen that the increase in the total thickness of the coating is slower, but becomes more intense with longer immersion time, taking a more linear course. The growth kinetics of the diffusion layer shows that the thickness of this layer constitutes a significant part of the contribution to the formation of the total thickness of coatings (Figure 1b). After 360 s, the thickness of the diffusion layer reached 261.36 ± 11.35 µm at 540 °C and 318.56 ± 9.47 µm at 560 °C. This represents 87% and 72% of the total thickness of coatings, respectively. At the same time, the increase in the thickness of the diffusion layer with a longer immersion time is close to the parabolic relationship.
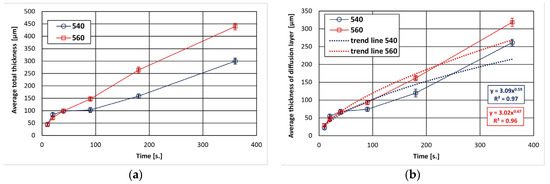
Figure 1.
Growth kinetics of ZnAl coatings obtained on reinforcement steel: (a) total thickness, (b) thickness of diffusion layer.
The growth kinetics of the intermetallic layer forming the diffusion layer of hot dip coating can be described by a power law equation:
y (thickness)—thickness of intermetallic layer [µm];
k—growth rate constant [µm/sn];
t—immersion time [s];
n—growth rate time constant.
The determined trend function for the experimental data according to the power–law relationship (Figure 1b) shows that the values of the exponent n describing the increase in diffusion layer are 0.59 at 540 °C and 0.67 at 560 °C, respectively. The growth rate time constant n determines the type of kinetics that control layer growth. The value of the exponent n is higher than 0.5, which proves that the growth of this layer is not only controlled by diffusion and is faster.
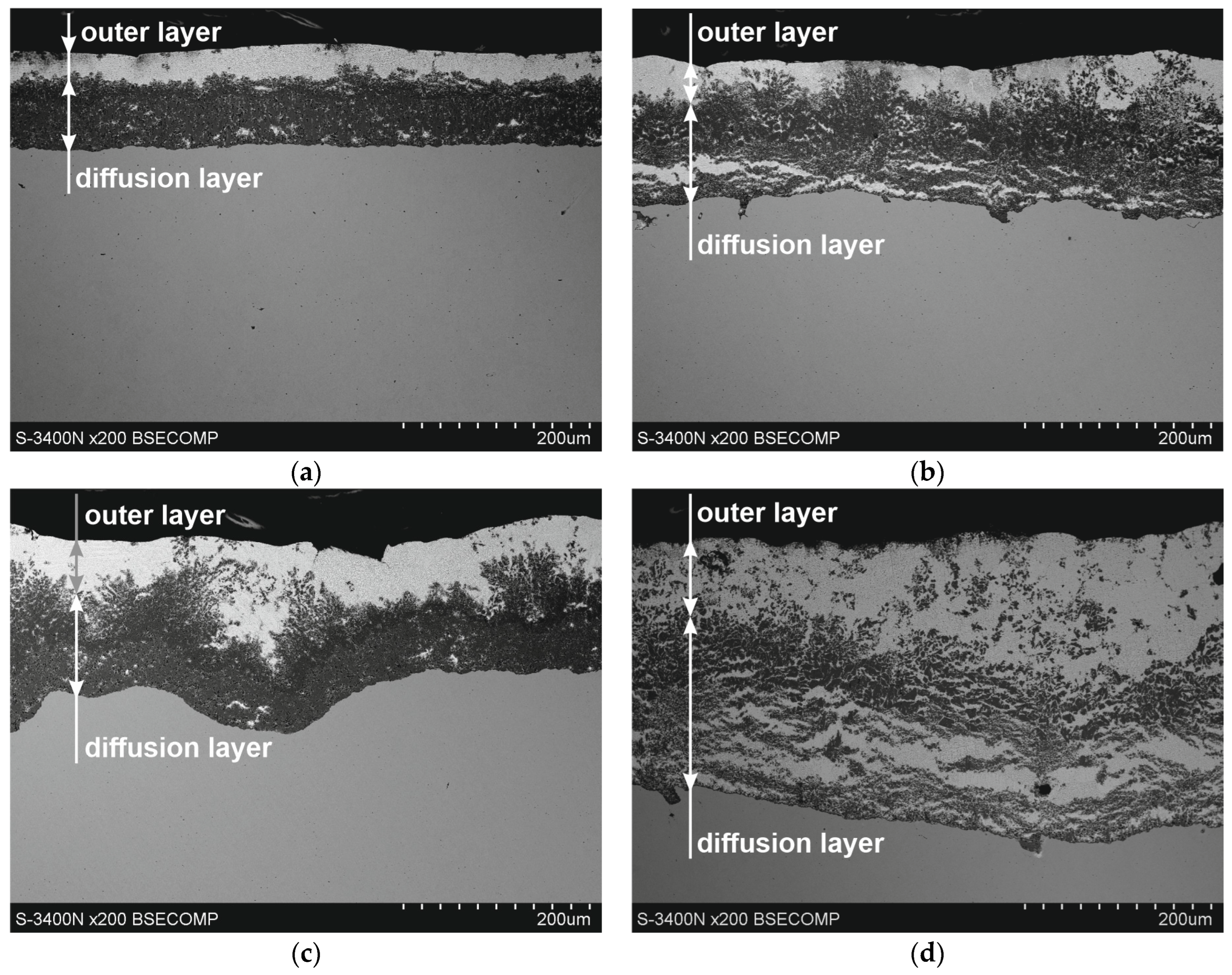
Figure 2 shows the microstructure of Zn-5Al coatings on reinforcement steel. SEM images show that the morphology of the coatings can change with increasing immersion time and temperature. The obtained coatings show a distinct two-layer structure. A diffusion layer is created on the substrate and covered with an outer layer. Over a 90 s period (Figure 2a,c), the compact structure of the diffusion layer can be clearly observed. In the coating obtained at a temperature of 540 °C after an immersion time of 180 s (Figure 1b), the diffusion layer shows structural inhomogeneities oriented parallel to the substrate. At the same time, after raising the bath temperature to 560 °C, a clear delamination of the diffusion layers and the formation of a periodic layered structure characteristic of ZnAl coatings can be observed [15].
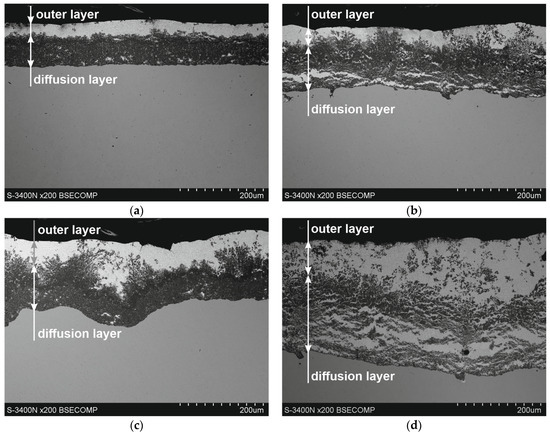
Figure 2.
Microstructure (SEM) of Zn-5Al coatings obtained on reinforcement steel; bath temperature: (a,b) 540 °C, (c,d) 560 °C; dipping time: (a,c) 90 s, (b,d) 180 s.
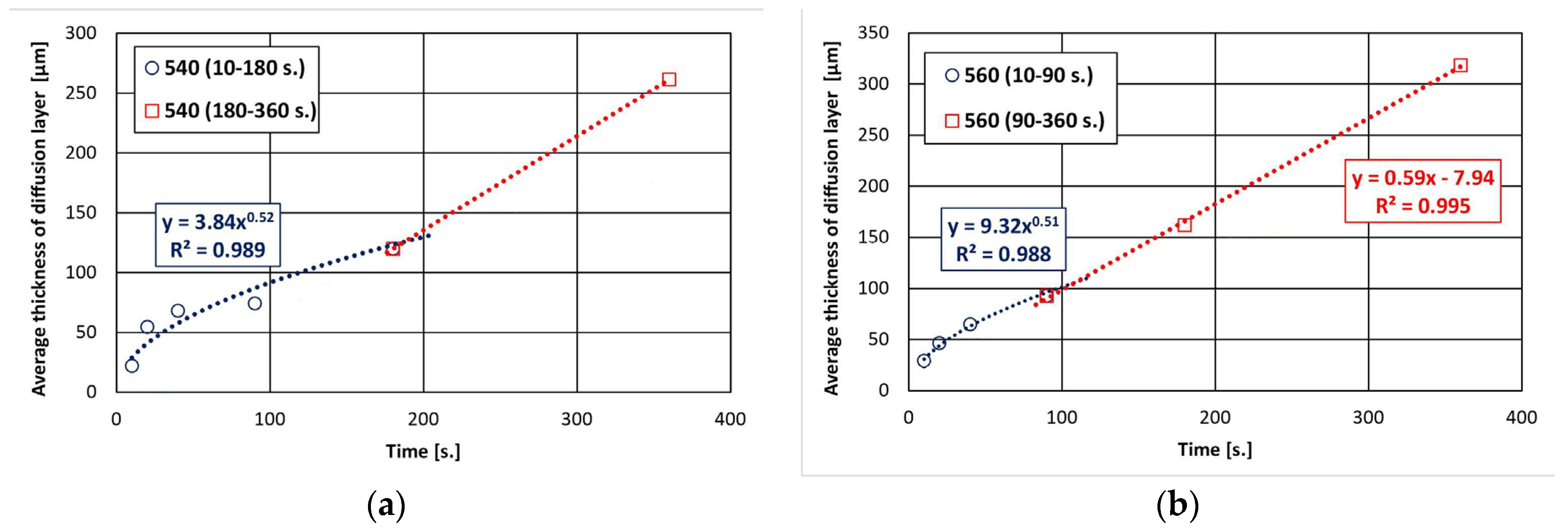
Changes in the morphology of the diffusion layer may explain the departure from the typical growth of the intermetallic layer according to the power–law relationship, and the growth of this layer may occur in two stages. In the initial stage, the intermetallic layer grows diffusively, followed by the formation of a periodic layered structure. However, the bath temperature may vary the time ranges of these stages. Within 180 s and at a temperature of 540 °C, the initiation of the formation of a periodic layered structure is visible, while at a temperature of 560 °C, it is already at an advanced stage of formation. The sequential development of the diffusion layer in the coating is confirmed by a detailed analysis of its growth kinetics, divided into two time intervals. The experimental data obtained in the time interval of 10–180 s at a temperature of 540 °C (Figure 3a) and in the time interval of 10–90 s at a temperature of 560 °C (Figure 3b) are very well described by a power trend line with a fit coefficient R2 of 0.989 and 0.988. The value of the exponent n (0.52 and 0.51, respectively) is close to 0.5, indicating that the growth of this layer in these time intervals is controlled by diffusion. At the same time, at a temperature of 560 °C, it can be observed that the further increase in the thickness of the diffusion layer in the time interval 90–360 s is more rapid and can be described by a linear relationship (fitting coefficient R2 = 0.995). This relationship cannot be clearly confirmed at a temperature of 540 °C due to the small amount of experimental data in the time interval 180–360 s. However, in this case, the change in the nature of the growth of the coating diffusion layer is also clearly visible.
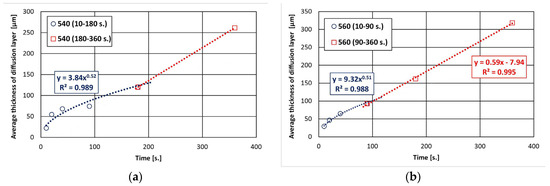
Figure 3.
Growth kinetics of diffusion layer at the following temperatures: (a) 540 °C and (b) 560 °C.
This course of coating growth kinetics requires controlling the immersion time in the bath so that the growth process of the diffusion layers takes place in the diffusion stage. In this case, it is possible to control the coating thickness and eliminate the formation of excessively thick coatings.
3.2. EDS and XRD Analysis
The microstructure of Zn-5Al coatings in the cross-section was examined using SEM, EDS, and XRD. Figure 4 shows the cross-sectional microstructure of the outer layers of the Zn-5Al coatings. Figure 1a shows that the outer layer in the coating obtained at a temperature of 540 °C is a mixture of two distinct structural components: dark precipitates (point 2) against the background of a light matrix (point 1). The dark areas vary in shape and size. Therefore, although the outer layer is formed in a bath with a eutectic composition, its morphology is not characteristic of a fine crystalline eutectic structure. EDS analysis (Table 2) shows that the light matrix (point 1) contains 99.1 at.% Zn and is therefore designated as a Zn-rich area. The dark areas contain significant amounts of Al (at point 2—6.2 at.% Al) and are therefore designated as Al-rich areas. The outer layer of the coating obtained at a temperature of 560 °C has a similar structure. However, the occurrence of distinct grains with different morphologies can be observed here (Figure 4b). The grains have a two-component structure similar to that described at 540 °C (points 3 and 4), but some of them have a more homogeneous structure (point 5). EDS analysis at point 5 indicates a much higher Al content—10.6 at.% (Table 2). The Zn-5Al bath is a eutectic composition of the Zn-Al system, in which the content of 5 wt.% corresponds to 11.3 at.% Al [12]. The Al content in the grain at point 5 is therefore very close to the eutectic content. The outer layer of Galfan coatings obtained in Zn-5Al baths is generally characterized by a eutectic structure formed by fine crystalline components of Zn in Al (β) and Al in Zn (α) solid solutions, in which the dendrites of the α solid solution are located [18]. The XRD pattern from the coating surface (Figure 5a) showed peaks coming only from Zn. Due to the high Al content in this area, it should be identified as a solid solution of Al in Zn(α). However, no solid solution of Zn in Al (β) was identified in the coating. Therefore, the morphology of the outer layer does not show a eutectic structure, and the Al contained in the bath is completely retained in the Zn solution during rapid cooling of the coating after removal from the bath.
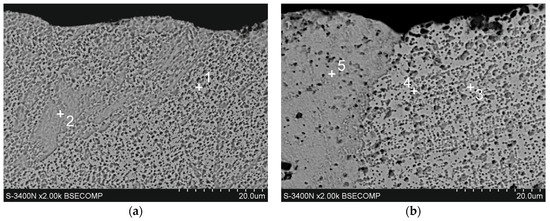
Figure 4.
The microstructure (SEM) of the outer layer of Zn-Al coatings obtained in the following conditions: (a) 540 °C, 90 s and (b) 560 °C, 90 s.
Figure 4.
The microstructure (SEM) of the outer layer of Zn-Al coatings obtained in the following conditions: (a) 540 °C, 90 s and (b) 560 °C, 90 s.
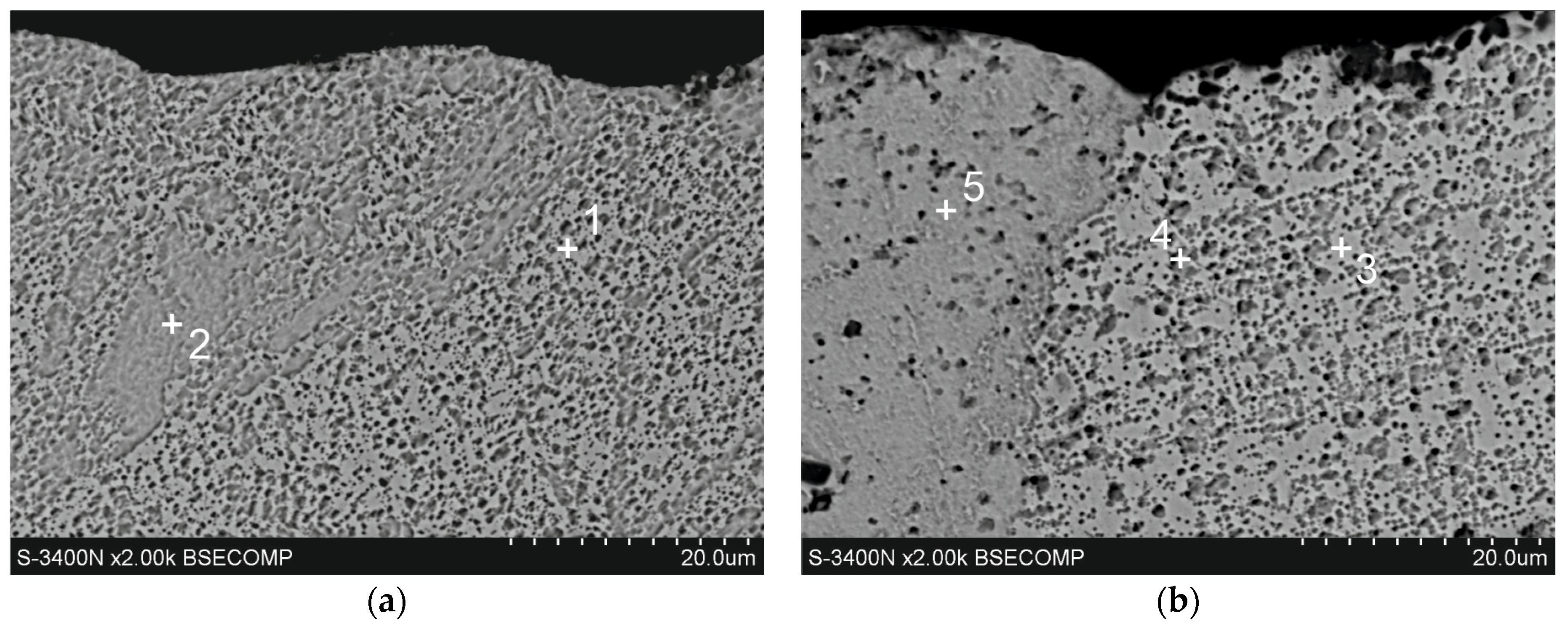

Table 2.
Results for the EDS analysis of the Zn-5Al coatings and corresponding phase analysis points (as shown in Figure 4 and Figure 6).
| Point No. | Al (at.%) | Fe (at.%) | Zn (at.%) | Phase |
|---|---|---|---|---|
| 1 | 0.9 | - | 99.1 | Zn-rich phase |
| 2 | 6.2 | - | 93.8 | Al-rich phase |
| 3 | 0.7 | - | 99.3 | Zn-rich phase |
| 4 | 3.9 | - | 96.1 | Al-rich phase |
| 5 | 10.6 | - | 89.4 | Al-rich phase |
| 6 | 68.9 | 24.7 | 6.4 | Fe(Al,Zn)3 |
| 7 | 0.2 | - | 99.8 | Zn-rich phase |
| 8 | 0.3 | - | 99.7 | Zn-rich phase |
| 9 | 69.5 | 25.2 | 5.3 | Fe(Al,Zn)3 |
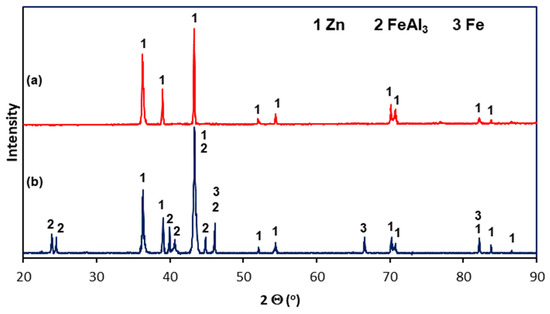
Figure 5.
XRD spectra of the Zn-Al coating obtained in reinforcement steel at 540 °C in 90 s: (a) from the flat ground surface of the outer layer of the coating; (b) from the bevel cut surface on the cross-section of the coating.
Figure 5.
XRD spectra of the Zn-Al coating obtained in reinforcement steel at 540 °C in 90 s: (a) from the flat ground surface of the outer layer of the coating; (b) from the bevel cut surface on the cross-section of the coating.
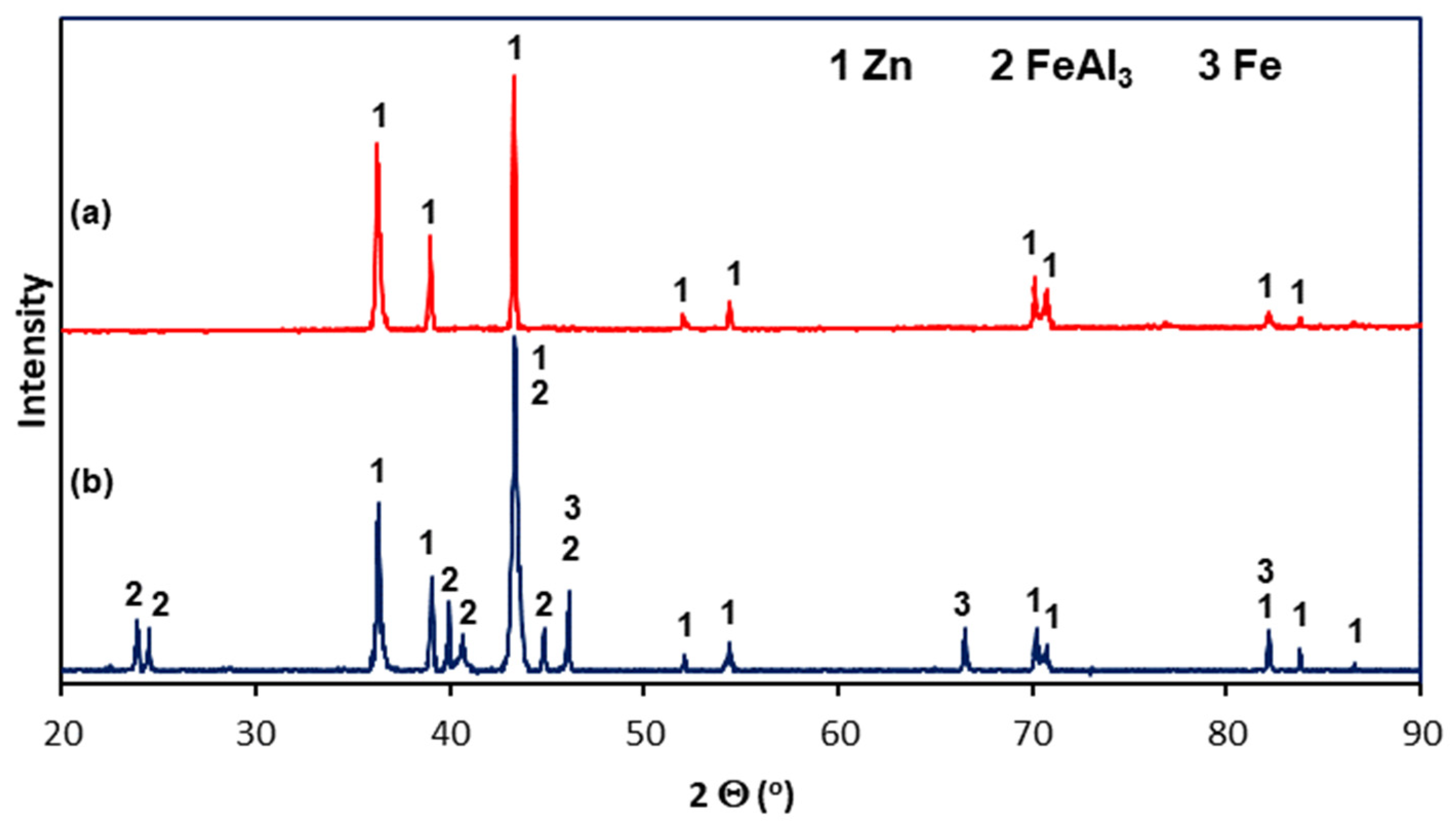
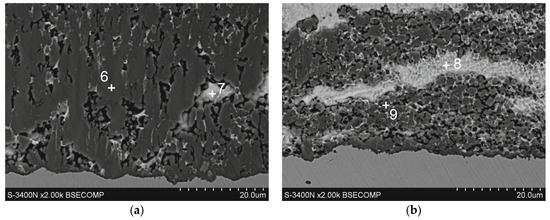
Figure 6.
Microstructure (SEM) of diffusion layer of Zn-Al coatings obtained in the following conditions: (a) 540 °C, 90 s and (b) 560 °C, 180 s.
Figure 6.
Microstructure (SEM) of diffusion layer of Zn-Al coatings obtained in the following conditions: (a) 540 °C, 90 s and (b) 560 °C, 180 s.
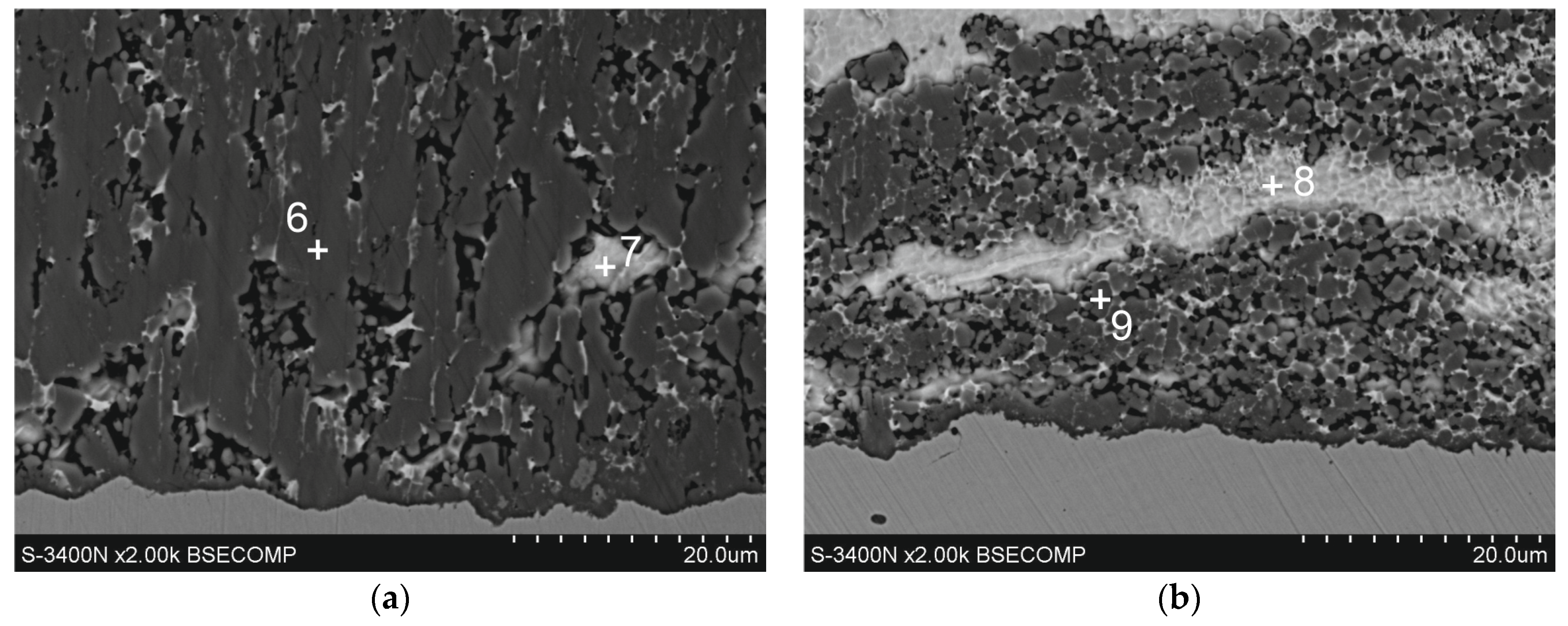
The diffusion layer has different morphologies depending on the temperature and immersion time. Figure 6a shows SEM images of the diffusion layer in the coating obtained at a temperature of 540 °C and an immersion time of 90 s, when it forms a compact layer. The morphology of this layer indicates some heterogeneity. EDS analysis at point 7 confirms the presence of a Zn-rich area (Table 2). For the most part, the diffusion layer exhibits a closely packed crystal structure. EDS analysis at point 6 shows a content of 68.9 at.% Al and 24.7 at.% Fe and 6.4 at.% Zn (Table 2). A similar chemical composition occurs in the diffusion layer in the coating obtained at a temperature of 560 °C (Table 2, point 9—69.5 at.% Al and 25.2 at.% Fe and 5.3 at.% Zn). However, with an immersion time of 180 s, a clear periodic layered structure is formed (Figure 6b). The XRD pattern from the oblique cut surface (Figure 5b) confirms the presence of FeAl3 intermetallics in the cross-section of the coating. Fe-Al intermetallics show high concentrations of vacancies [19], which control the diffusion processes and determine the growth of the diffusion layer of coatings. At the same time, Zn has high solubility in the solid state in the crystallographic lattice of Fe-Al intermetallics [20], which may be facilitated by the presence of structural defects. The ideal stoichiometry of the crystallographic lattice of FeAl3 intermetallic contains 25 at.% Fe and 75 at.% Al. In this study, the Al/Fe atomic ratio is 2.78 at point 6, 2.75 at point 9, and always less than 3.0. At the same time, the total content (Al + Zn) at these points is close to 75 at.%, which gives an atomic ratio (Al + Zn)/Fe close to 3.0 (3.04 in point 6 and 2.96 in point 9, respectively). This may suggest that Zn atoms replace Al atoms in the FeAl3 phase lattice, which is clearly identified in the XRD pattern (Figure 5b). Assuming that Al atoms are replaced by Zn atoms as proposed by Qian et al. [21] model, the phase forming the diffusion layer of the coating can be written as Fe(Al,Zn)3.
At this stage of research, it is not possible to explain the mechanism for the formation of a periodic layered structure at high temperatures. Selverian et al. [22] indicate the presence of two layers of the FeAl3 phase and the Fe2Al5 phase in ZnAl coatings. The presence of Fe2Al5 intermetallics causes an increase in volume. This leads to stresses at the interface and, as a result, cracks and delaminations. Although the mechanism seems probable, it cannot be confirmed in this study because no intermetallics were found in the Fe2Al5 coating. However, the presence of Fe2Al5 intermetallics and the ongoing transformation of Fe2Al5 intermetallics into FeAl3 intermetallics cannot be clearly ruled out. However, this requires detailed studies of the sequence of formation of intermetallics in the diffusion layer of the coating. However, this study shows that the formation of Zn-5Al coatings on reinforcement steel is very complex and requires controlling the process parameters.
3.3. Corrosion Resistance and Corrosion Products
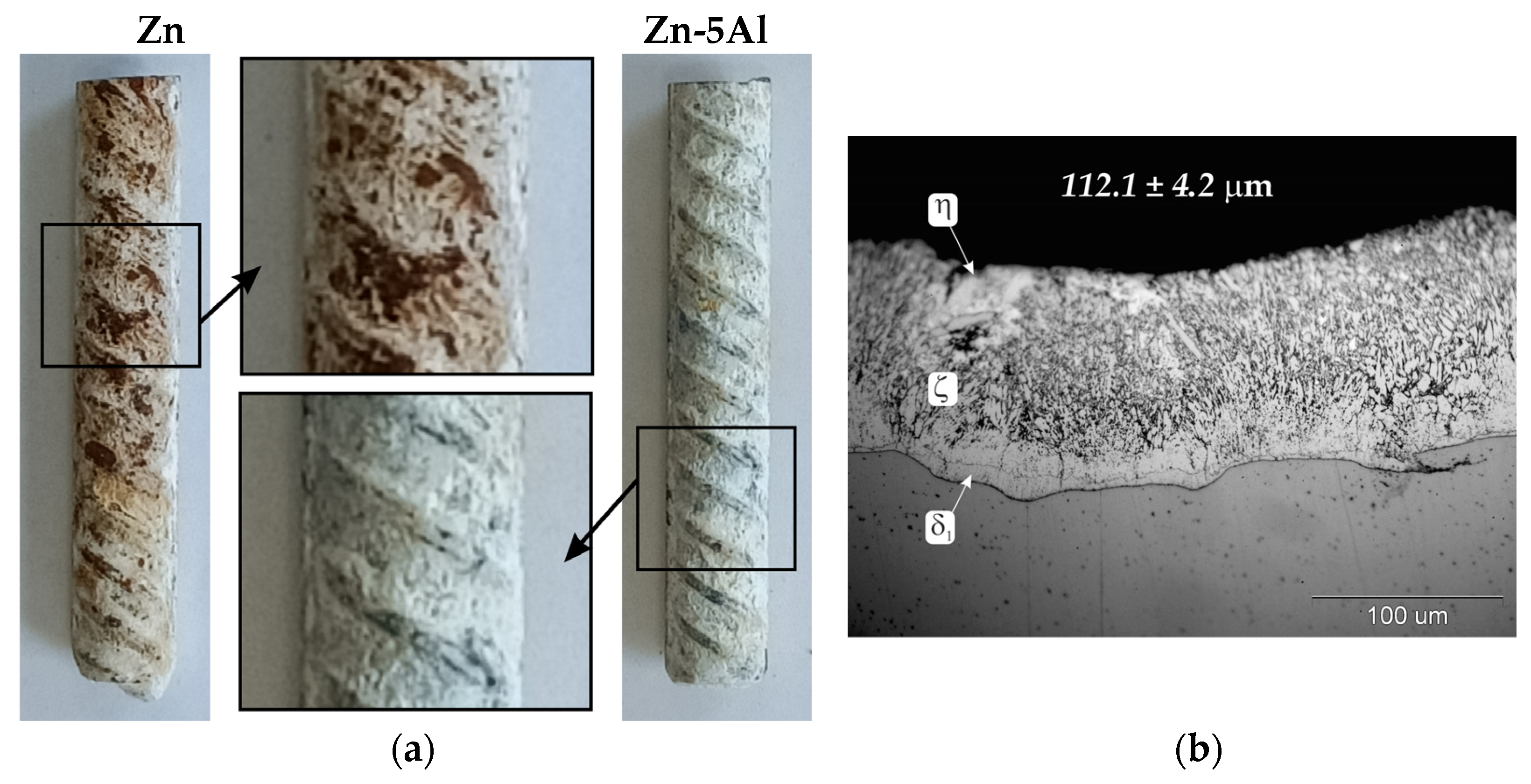
The corrosion resistance of Zn-5Al coatings was compared with the corrosion resistance of conventional hot-dip-galvanized coatings in a neutral salt spray test. The surface appearance of reinforcement bars with coatings after 1000 h salt spray exposure is shown in Figure 7a. After completing the corrosion test, Zn-5Al coatings were covered with white corrosion products and did not penetrate the substrate. Comparative Zn coating, however, shows local penetration into the substrate and the presence of red corrosion products on the surface, which indicates a loss of protective properties. The thickness of the coatings measured before starting the corrosion test was 102.91 ± 7.1 µm, respectively, for Zn-5Al coating and 112.1 ± 4.2 µm for Zn coating. Despite the similar thickness of the tested coatings, Zn coating showed lower protection effectiveness. This behavior of coatings under corrosion test conditions can be explained by their structure. The morphology of the structural components of the Zn coating (Figure 7b) indicates the typical structure of the phases of the Fe-Zn system: outer layer of η phase (solid solution of Fe in Zn) and diffusion layer of δ1, ζ intermetallics. The δ1 intermetallics layer has an uneven thickness, while the ζ intermetallics shows significant growth towards the coating surface and is only locally covered by the layer of η phase [23]. This coating structure can be considered typical for steels from the Sebisty range. The Zn-5Al coating, whose diffusion layer is formed by FeAl3 intermetallics, and the outer layer—a solution of Al in Zn (α)—therefore shows better anti-corrosion protection properties in this corrosion test.
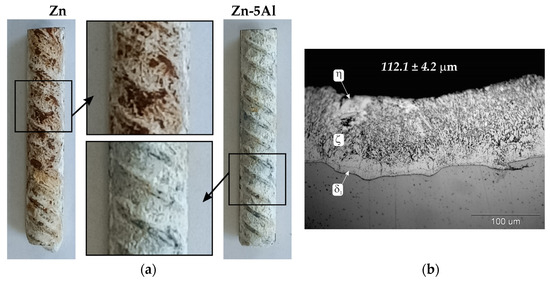
Figure 7.
Results of neutral salt spray test: (a) surface appearance of coatings after 1000 h exposure; (b) structure of comparative hot-dip-galvanized coating.
Figure 8a shows the SEM images of the corroded Zn-5Al coating surface that was exposed to the neutral salt spray test. EDS analysis (Figure 8b) indicates that the average chemical composition of the white corrosion products in area A includes Zn, Cl and O.

Figure 8.
SEM images (a) and corresponding EDS patterns (b) of the corroded Zn-5Al coating surface after 1000 h exposure in NSS test.
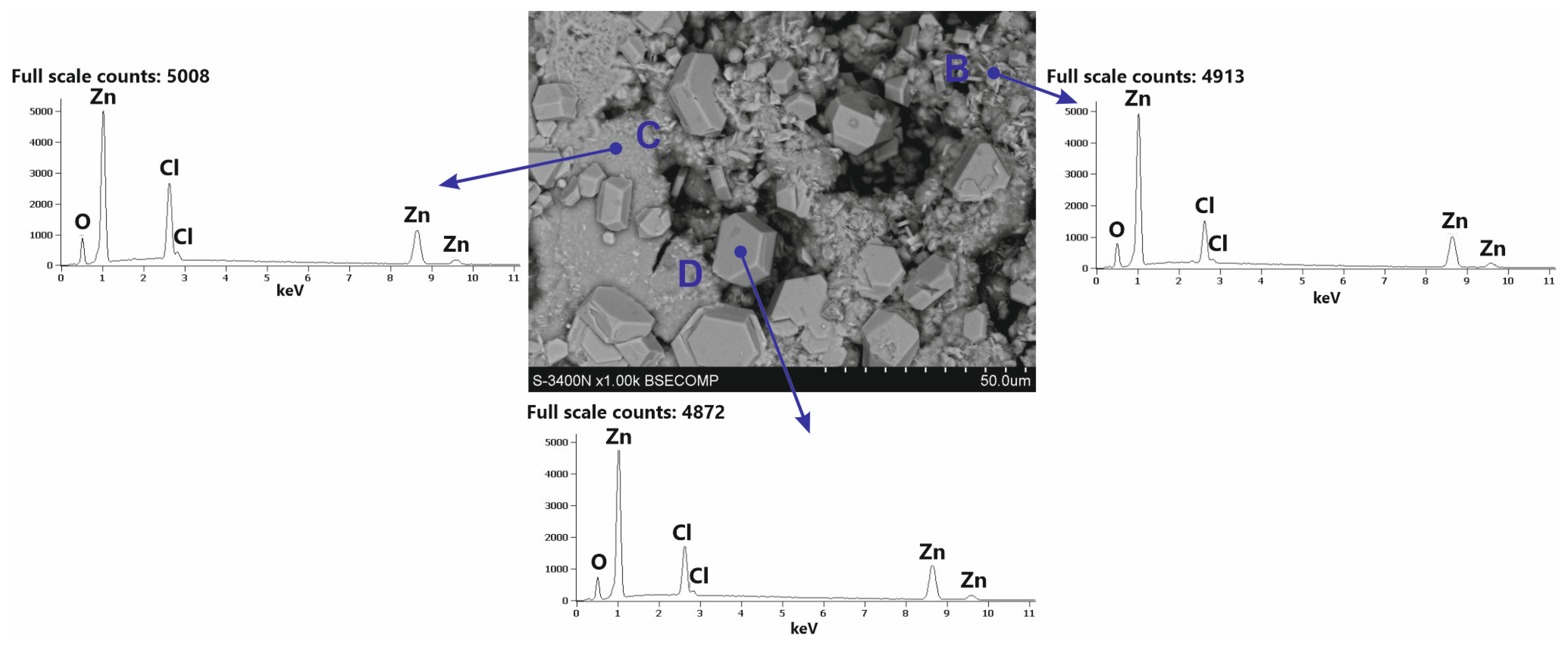
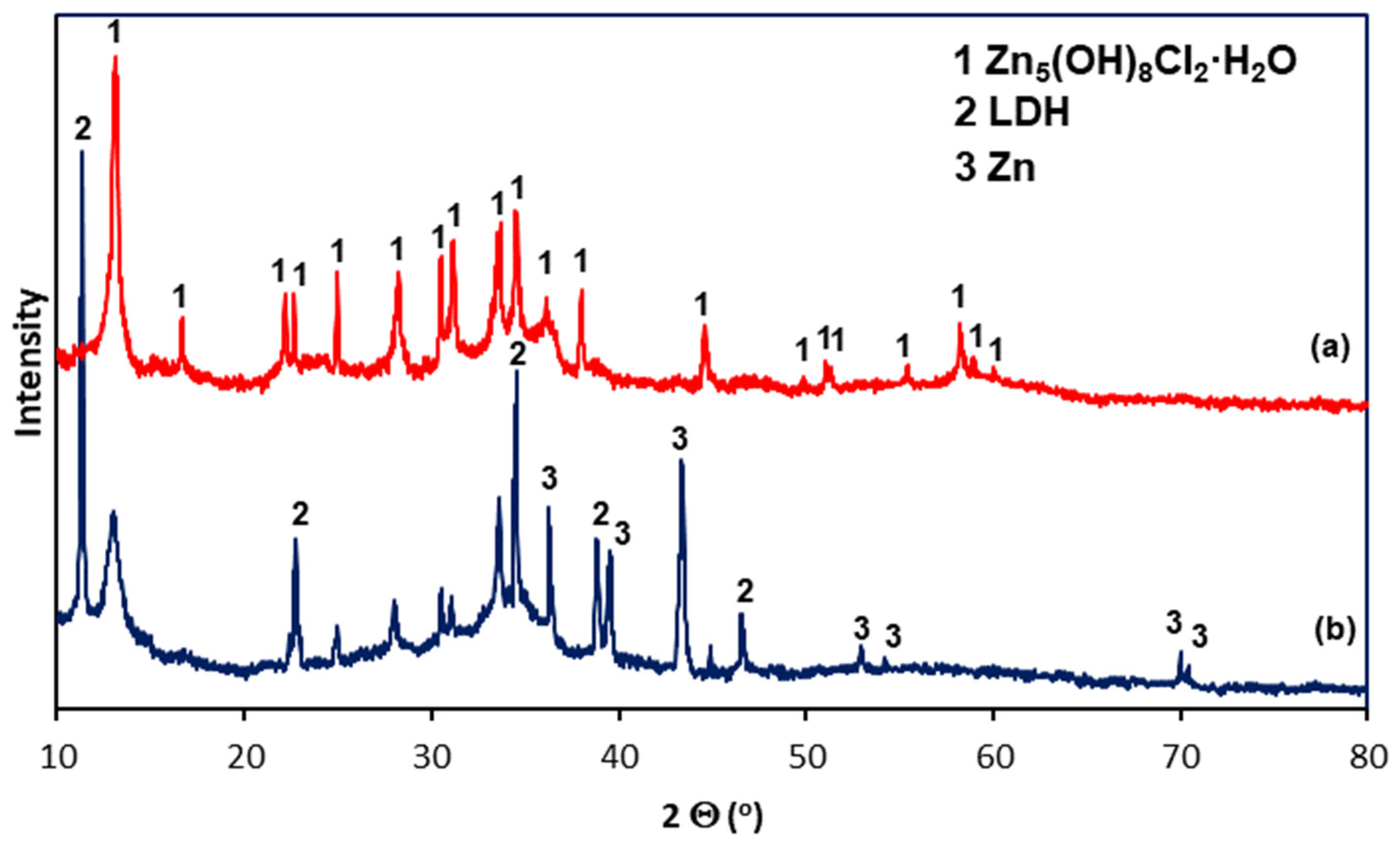
White corrosion products have a very complex morphology. SEM images of white corrosion products (Figure 9) indicate the presence of structural components with a compact structure at point C, transforming into a heterogeneous structure at point B, and locally distributed crystals with regular shapes. However, EDS analysis in separate structural components of white corrosion products does not show significant differences in the chemical composition at points A, B and C, indicating the presence of similar amounts of Zn, Cl, and O. This may suggest that all structural components of the products white corrosion are composed of the same phase. This is confirmed by XRD studies of the phase composition in powdered white corrosion products (Figure 10a), where one phase was clearly identified: simonkolleite—Zn5(OH)8Cl2·H2O.
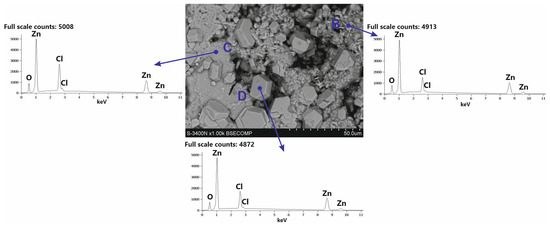
Figure 9.
Microstructure (SEM) and EDS patterns of structural components of the white corrosion products on the surface of the Zn-5Al coating.
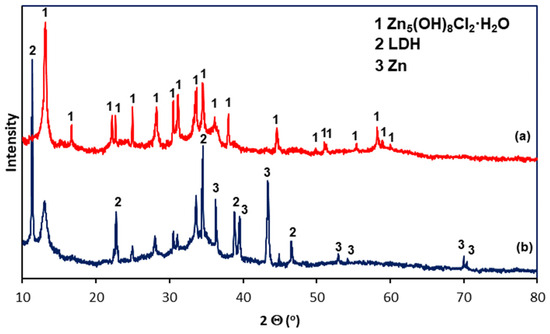
Figure 10.
XRD spectra of corrosion products removed (a) from the coating surface and (b) from the coating surface after the removal of white corrosion products.
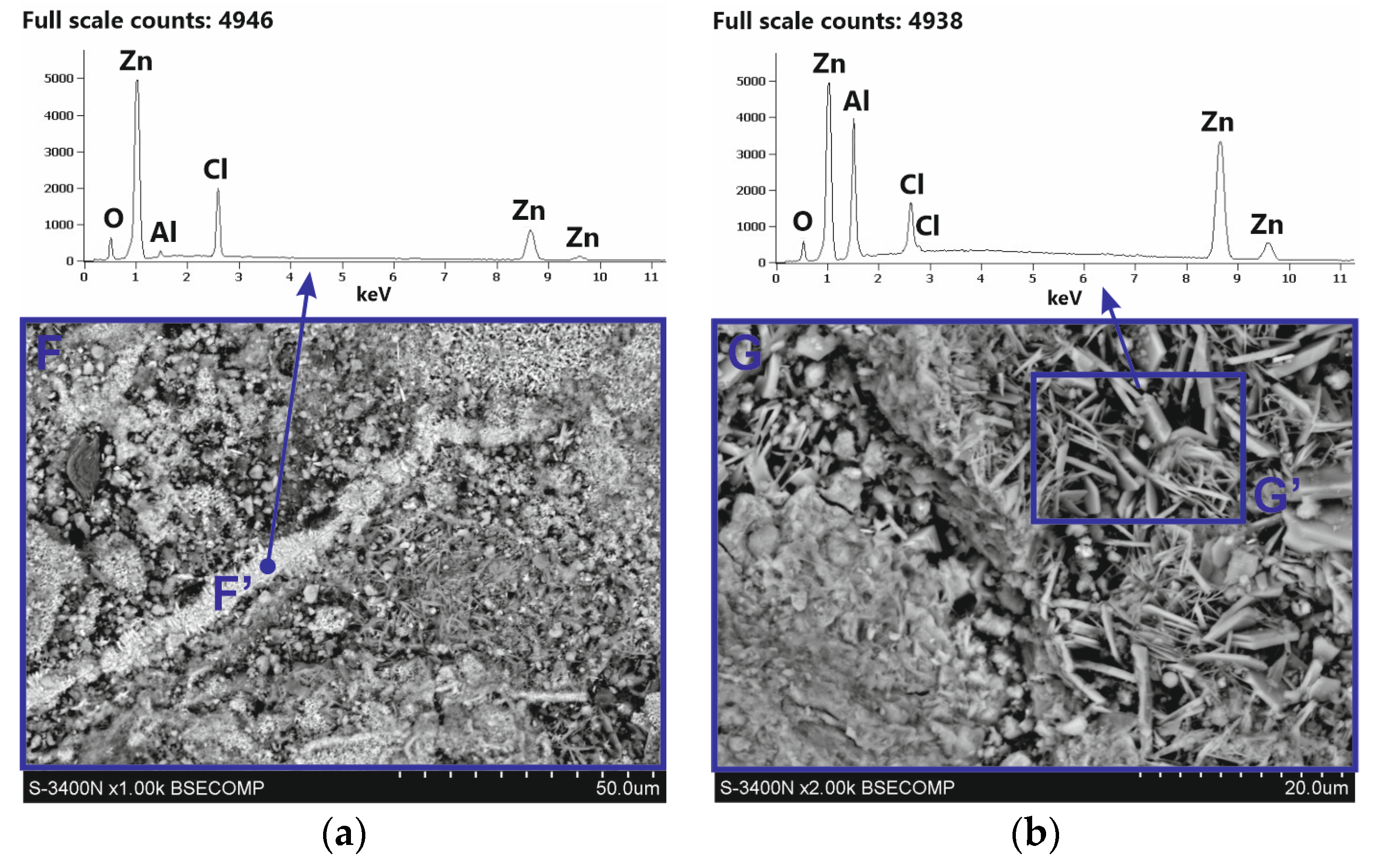
After removing white corrosion products from the coating surface, two characteristic areas with different morphologhies can be observed: heterogeneous (F) and compact (G) (Figure 11a). EDS analysis also indicates differences in the chemical composition in these areas. The heterogeneous area F is characterized by high contents of Zn, Cl, and O, while the compact area G also shows a high content of Al, also with high contents of Zn, Cl, and O (Figure 11b). It can therefore be argued that the differences in appearance are caused by the course of corrosion in other structural components of the coating and the exposure to other types of corrosion products. This is confirmed by the analysis of the phase composition from the coating surface after removing the white corrosion products (Figure 10b), where the XRD pattern clearly identified the presence of layered double hydroxides (LDH) in the corrosion products, as well as simonkolleite—Zn5(OH)8Cl2·H2O. However, the simonkolleite peaks observed after the removal of white corrosion products are much weaker. The dominant corrosion product on the coating surface after removing the white corrosion products is therefore LDH. A detailed EDS point analysis performed in the inhomogeneous area F and the morphology of the corrosion products occurring there (Figure 12a) show a high content of Zn, Cl, and O. Area F can therefore be identified as a component of the Zn-rich coating, and at the same time, the corrosion products formed there are identified as simonkolleite. The morphology of the G area, as well as the EDS analysis in this area (Figure 12b), allows us to identify this area as a component of the Al-rich coating, and the formed corrosion products as LDH.
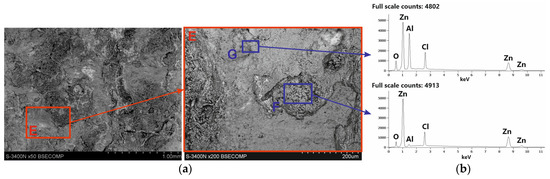
Figure 11.
SEM images (a) and corresponding EDS patterns (b) of corroded Zn-5Al coating after removal of white corrosion products.
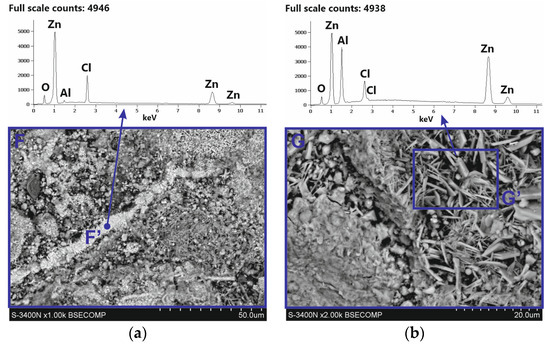
Figure 12.
Microstructure (SEM) and EDS patterns of corroded Zn-Al coatings in the following areas: (a) Zn-rich and (b) Al-rich.
LDH are hydroxides of two metallic elements with the general formula
[M1−x2+ Mx3+ +·(OH)2]x+(An−)x/n·mH2O
- -
- M2+—divalent metal cation;
- -
- M3+—trivalent metal cation;
- -
- An−—interlayer anion [24].
EDS analysis in area G (Figure 11b) shows high Zn and Al content. The Al-rich coating component may therefore be a source of divalent Zn2+ and trivalent Al3+ cations. Under neutral salt spray test conditions, the aggressiveness of the corrosive environment is determined by the presence of a high chloride concentration. The most probable anion is Cl− [25]. The morphology of corrosion products in the G′ area (Figure 12b) indicates the location of LDH there. EDS analysis in the G′ micro-area, which includes high concentrations of Zn and Al, may confirm the presence of divalent cations Zn2+ and trivalent Al3+. However, the high Cl concentration and O content indicate that the Cl− anion is incorporated into the LDH structure. The results of SEM/EDS tests in correlation with the XRD results confirm that the corrosion products formed on the coating surface in Al-rich areas are most likely Zn–Al layered double hydroxides intercalated with chloride, which can be written with the general formula ZnAl LDH-Cl−. In Zn-rich areas, however, the formation of simonkolleite is preferential, covering the entire surface of the rod as well as the ZnAl LDH-Cl− products formed beneath it.
The increased corrosion resistance of reinforcement bars coated with Zn-5Al can be explained by the formation of a favorable configuration of corrosion products. Simonkolleite has a very compact structure [26] and forms an adherent and insoluble layer [27]. Therefore, it can act as a barrier coating to prevent further corrosion. However, the formation of simonkolleite is considered to be preferential in the initial stages of corrosion [28]. The presence of carbonate ions in the atmospheric environment caused by the dissolution of CO2 leads to the easy transformation of simonkolleite into hydrozincite—Zn5(OH)6(CO3)2 [29]. Hydrozincite has a porous structure and poor adhesion to the coating surface [30], which significantly reduces its anti-corrosion protection effectiveness. Although simonkolleite was stable in this study and no hydrozincite was found, the transformation of corrosion products under long-term exposure to the atmospheric environment cannot be ruled out. However, the use of reinforcement bars in concrete significantly limits the access of CO2 to the surface of the bar. Therefore, it should be expected that simonkolleite will effectively protect the surface of the coating in concrete in the initial stage of concrete corrosion, and only its carbonation will promote the transformation of simonkolleite into hydrozincite.
Research has shown that, under the simonkolleite layer, a layer of zinc aluminum layered double hydroxides—ZnAl LDH-Cl−—is formed on the surface of the coating. It is most likely formed on the surface of the Al-rich areas. Yang et al. [31] showed that, in Galvalume coatings (Zn-55Al-1.6Si), the ZnAl layered double hydroxide layer supported by CO32− anions has a compact structure and is very stable, which is the reason for the high corrosion resistance of the coatings. LDH compounds easily exchange cations and anions and show excellent anion adsorption capacity [24]. Therefore, the formation of the ZnAl LDH-Cl− compound on the surface of the tested coatings may limit the diffusion of chlorides to the reinforcement surface, leading to a delay in the initiation of reinforcement corrosion and extending the service life of the reinforced concrete. The corrosion tests carried out are of a model nature, where in a corrosive environment the concentration and diffusion rate of Cl− are much higher compared to other ions (e.g., CO32− and SO42−), which are commonly occurring aggressive components of the atmospheric environment [32,33]. The priority of ion exchange depends on the bond strength between the anion and cation layers. Costa et al. [34] showed that the binding strength of CO32− anions with cations in ZnAl LDH is higher than that of Cl− anions, while a similar relationship of the binding strength of SO42− > Cl− anions was presented by Zhao et.al. [35] for MgAl LDH compounds. As shown by studies of ZnAlMg coatings [36,37,38], in various atmospheric environments, the most stable were LDH compounds based on CO32− anions, while studies in a model environment containing SO2 [39] showed that the protective properties of ZnAlMg coatings are provided by LDH compounds based on SO42− anions. Therefore, it should be assumed that Zn-5Al coatings will provide high corrosion resistance of reinforcement bars in various corrosive environments due to the formation of a beneficial corrosion product on the surface in the form of ZnAl LDH, which is capable of absorbing various aggressive anions in the environment. Also, due to its unique ion exchange properties, the presence of LDH may also contribute to the protection of the concrete matrix itself, acting as an inhibitor of mainly carbonate, sulfate, and chloride corrosion [40].
4. Conclusions
The growth kinetics, microstructure, and corrosion resistance of high-temperature ZnAl-galvanized coatings obtained on reinforcement steel were investigated. The conclusions are as follows:
- The growth of high-temperature ZnAl-galvanized coatings takes place in two stages. In the initial stage, a compact diffusion layer of the coating is formed, and its growth is controlled by diffusion. In the second stage, the periodic layered structure is formed, the growth of which is close to linear. The time sequences of coating growth depend on the bath temperature, which, when raised, accelerates the sequence of formation of the periodic layered structure.
- High-temperature ZnAl-galvanized coatings exhibit a two-layer microstructure. A diffusion layer of Fe(Al,Zn)3 intermetallics is formed near the substrate, which is covered with an outer layer composed of Zn-rich and Al-rich areas.
- Zn-5Al coatings showed better corrosion resistance than conventional hot-dip-galvanized coatings due to the formation of favorable corrosion products. The NSS test showed that a layer of simonkolleite—Zn5(OH)8Cl2·H2O—and a layered double hydroxide (LDH) layer based on divalent cations of Zn2+, trivalent cations, and Al3+ and Cl− anions are formed on the coating surface. This configuration of corrosion products and the ease of exchange of cations and anions in LDH structures constitute an effective protective barrier, delaying the corrosion process in various corrosive environments.
Author Contributions
Conceptualization, A.M. and P.P.; methodology, A.M. and V.S.; validation, H.K.; investigation, A.M., P.P. and V.S.; writing—original draft preparation, A.M.; writing—review and editing, H.K.; Formal analysis and visualization, F.B., supervision, H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
Author Franciszek Berger was employed by the company Rockwell Automation sp.z o.o. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Gao, D.Y.; Tai, Y.Y.; Yang, L.; Zhang, Z.Q.; Liu, G.J.; You, P.B. Corrosion of steel fibers in chloride-contaminated simulated concrete pore solutions. J. Mater. Civ. Eng. 2023, 35, 04022429. [Google Scholar] [CrossRef]
- Yang, L.; Liu, G.J.; Gao, D.Y.; Zhang, C.X. Experimental study on water absorption of unsaturated concrete: W/c ratio, coarse aggregate and saturation degree. Construct. Build. Mater. 2021, 272, 121945. [Google Scholar] [CrossRef]
- Huet, B.; L’Hostis, V.; Miserque, F.; Idrissi, H. Electrochemical behavior of mild steel in concrete: Influence of pH and carbonate content of concrete pore solution. Electrochim. Acta 2005, 51, 172–180. [Google Scholar] [CrossRef]
- ISO 12944-5:2019; Paints and Varnishes—Corrosion Protection of Steel Structures by Protective Paint Systems—Part 5: Protective Paint Systems. International Organization for Standardization: Geneva, Switzerland, 2019.
- Schweitzer, P.A. Paint and Coatings: Applications and Corrosion Resistance; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: New York, NY, USA; Consultant York: Pennsylvania, PA, USA, 2005; pp. 34–114. [Google Scholar]
- Assaad, J.J.; Issa, C.A. Bond strength of epoxy-coated bars in underwater concrete. Construct. Build. Mater. 2012, 30, 667–674. [Google Scholar] [CrossRef]
- Yeomans, S.R. Use of galvanized rebars in RC structures. In A Presentation to a Seminar on Durability of Reinforced Concrete Structures; Standing Committee on Concrete Technology Civil Engineering and Development Hong Kong Government: Hong Kong, China, 2005. [Google Scholar]
- Cao, Y.; Gehlen, C.; Angst, U.; Wang, L.; Wang, Z.D.; Yao, Y. Critical chloride content in reinforced concrete—An updated review considering Chinese experience. Cement Concr. Res. 2019, 117, 58–68. [Google Scholar] [CrossRef]
- Liu, G.J.; Zhang, Y.S.; Ni, Z.W.; Huang, R. Corrosion behavior of steel submitted to chloride and sulphate ions in simulated concrete pore solution. Construct. Build. Mater. 2016, 115, 1–5. [Google Scholar] [CrossRef]
- Liu, Y.P.; Weyers, R.E. Modeling the time-to-corrosion cracking in chloride contaminated reinforced concrete structures. ACI Mater. J. 1998, 95, 675–681. [Google Scholar]
- Goodwin, F.E. An Update on Galfan. In Proceedings of the 14th International Galvanizing Conference, Munich, Germany, 9–14 June 1985. [Google Scholar]
- Massalski, T.B.; Okamoto, H. Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Materials Park, OH, USA, 1990. [Google Scholar]
- Kim, K.-Y.; Grandhi, S.; Oh, M.-S. Improving the Coatability of Zn–Mg–Al Alloy on Steel Substrate by the Surface Pretreatment of SnCl2-Added Zinc Ammonium Chloride. Appl. Sci. 2023, 13, 950. [Google Scholar] [CrossRef]
- Kania, H. Structure Shaping and Corrosion Resistance of Zn-Al Coatings Obtained in Hot Dip Metalization; Silesian University of Technology: Gliwice, Poland, 2017. [Google Scholar]
- Gao, L.; Li, Z.; Kuang, X.; Yin, F.; Ji, H. Formation of periodic layered structure during hot-dip galvanizing in Al-Zn-Mg bath. Surf. Coat. Technol. 2016, 304, 306–315. [Google Scholar] [CrossRef]
- Schulz, W.D.; Thiele, M. General Hot-Dip Galvanizing; Leuze Verlag: Bad Saulgau, Germany, 2012. [Google Scholar]
- ISO 9227:2017; Corrosion Tests in Artificial Atmospheres—Salt Spray Tests. Polish Version PN EN ISO 9227:2017; Polish Committee for Standardization: Warszawa, Poland, 2017.
- Marder, A.R. The metallurgy of zinc-coated steel. Prog Mater Sci. 2000, 45, 191–271. [Google Scholar] [CrossRef]
- Zamanzadea, M.; Hasemannb, G.; Motza, C.; Krügerb, M.; Barnoushac, A. Vacancy effects on the mechanical behaviour of B2-FeAl intermetallics. Mater. Sci. Eng. A 2018, 712, 88–96. [Google Scholar] [CrossRef]
- Zamanzade, M.; Barnoush, A.; Motz, C. A Review on the Properties of Iron Aluminide Intermetallics. Crystals 2016, 6, 10. [Google Scholar] [CrossRef]
- Qian, W.J.; Gu, W.G. Inhibitory action of Si on growth of interfacial compound layer during hot-dip aluminizing. Acta Metall. Sin. 1994, 30, 404–408. [Google Scholar]
- Selverian, J.H.; Marder, A.R.; Notis, M.R. The effects of silicon on the reaction between solid iron and liquid 55 wt pct Al-Zn baths. Met. Trans. A 1989, 20, 543–555. [Google Scholar] [CrossRef]
- Kania, H.; Mendala, J.; Kozuba, J.; Saternus, M. Development of bath chemical composition for batch hot-dip galvanizing—A Review. Materials 2020, 13, 4168. [Google Scholar] [CrossRef]
- Evans, D.G.; Slade, R.C.T. Structural Aspects of Layered Double Hydroxides. In Layered Double Hydroxides. Structure and Bonding; Duan, X., Evans, D.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 119. [Google Scholar] [CrossRef]
- LeBozec, N.; Thierry, D.; Persson, D.; Riener, C.K.; Luckeneder, G. Influence of microstructure of zinc-aluminium-magnesium alloy coated steel on the corrosion behavior in outdoor marine atmosphere. Surf. Coat. Technol. 2019, 374, 897–909. [Google Scholar] [CrossRef]
- Volovitch, P.; Allely, C.; Ogle, K. Understanding corrosion via corrosion product characterization: I. Case study of the role of Mg alloying in Zn–Mg coating on steel. Corros. Sci. 2009, 51, 1251–1262. [Google Scholar] [CrossRef]
- Eaves, D.R. A Study of Novel Filiform Corrosion Phenomena on Hot Dip Organically Coated Zn-Al-Mg Steel. PhD Thesis, Swansea University, Swansea, UK, 2013. [Google Scholar]
- Prosek, T.; Thierry, D.; Taxen, C.; Maixner, J. Effect of cations on corrosion of zincand carbon steel covered with chloride deposits under atmospheric conditions. Corros. Sci. 2007, 49, 2676–2693. [Google Scholar] [CrossRef]
- Hosking, N.C.; Ström, M.A.; Shipway, P.H.; Rudd, C.D. Corrosion resistance of zinc–magnesium coated steel. Corros. Sci. 2007, 49, 3669–3695. [Google Scholar] [CrossRef]
- Komatsu, A.; Izutani, H.; Tsujimura, T.; Andoh, A.; Kittaka, T. Corrosion resistanceand protection mechanism of hot-dip Zn–Al–Mg alloy coated steel sheet under accelerated corrosion environment. Tetsu-to-Hagane 2000, 86, 534–541. [Google Scholar] [CrossRef]
- Yang, D.; Chen, J.; Han, Q.; Liu, K.R. Effects of lanthanum addition on corrosion resistance of hot-dipped galvalume coating. J. Rare Earths 2009, 27, 114–118. [Google Scholar] [CrossRef]
- Chen, Y.; Shui, Z.; Chen, W.; Chen, G. Chloride binding of synthetic Ca–Al–NO3 LDHs in hardened cement paste. Construct. Build. Mater. 2015, 93, 1051–1058. [Google Scholar] [CrossRef]
- Li, K.F.; Zhang, D.D.; Li, Q.W.; Fan, Z.H. Durability for concrete structures in marine environments of HZM project: Design, assessment and beyond. Cement Concr. Res. 2019, 115, 545–558. [Google Scholar] [CrossRef]
- Costa, D.G.; Rocha, A.B.; Souza, W.F.; Chiaro, S.S.X.; Leitao, A.A. Comparative Structural, thermodynamic and electronic analyses of ZnAlAn−hydrotalcite-like (An− = Cl−, F−, Br−, OH−, CO32− or NO3−): An ab initio study. Appl. Clay Sci. 2012, 56, 16–22. [Google Scholar] [CrossRef]
- Zhao, X.J.; Zhu, Y.Q.; Xu, S.M.; Liu, H.M.; Yin, P.; Feng, Y.L.; Yan, H. Anion exchange behavior of M(II)Al layered double hydroxides: A molecular dynamics and DFT study. Phys. Chem. Chem. Phys. 2020, 22, 19758–19768. [Google Scholar] [CrossRef] [PubMed]
- LeBozec, N.; Thierry, D.; Rohwerder, M.; Persson, D.; Luckeneder, G.; Luxem, L. Effect of carbon dioxide on the atmospheric corrosion of Zn–Mg–Al coated steel. Corros. Sci. 2013, 74, 379–386. [Google Scholar] [CrossRef]
- Persson, D.; Thierry, D.; LeBozec, N.; Prosek, T. In situ infrared reflection spectroscopy studies of the initial atmospheric corrosion of Zn–Al–Mg coated steel. Corros. Sci. 2013, 72, 54–63. [Google Scholar] [CrossRef]
- Prosek, T.; Persson, D.; Stoulil, J.; Thierry, D. Composition of corrosion products formed on Zn–Mg, Zn–Al and Zn–Al–Mg coatings in model atmospheric conditions. Corros. Sci. 2014, 86, 231–238. [Google Scholar] [CrossRef]
- Kania, H.; Marek, A. Corrosion behavior of Zn-Al-Mg-Si coatings in sulfur dioxide-containing environment. Appl. Sci. 2024, 14, 2120. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Y.; Zhang, Z.; Wang, Y.; Hu, J.; Ma, Y.; Zhang, Z.; Huang, H.; Wei, J.; Yu, Q.; et al. A review on the research progress of LDHs as corrosion inhibitors for reinforced concrete. J. Build. Eng. 2023, 70, 106303. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).