Controllable Crystallization of Perovskite Films during the Blade-Coating Fabrication Process for Efficient and Stable Solar Cells
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Solution Preparation
2.3. Device Fabrication
2.4. Characterizations
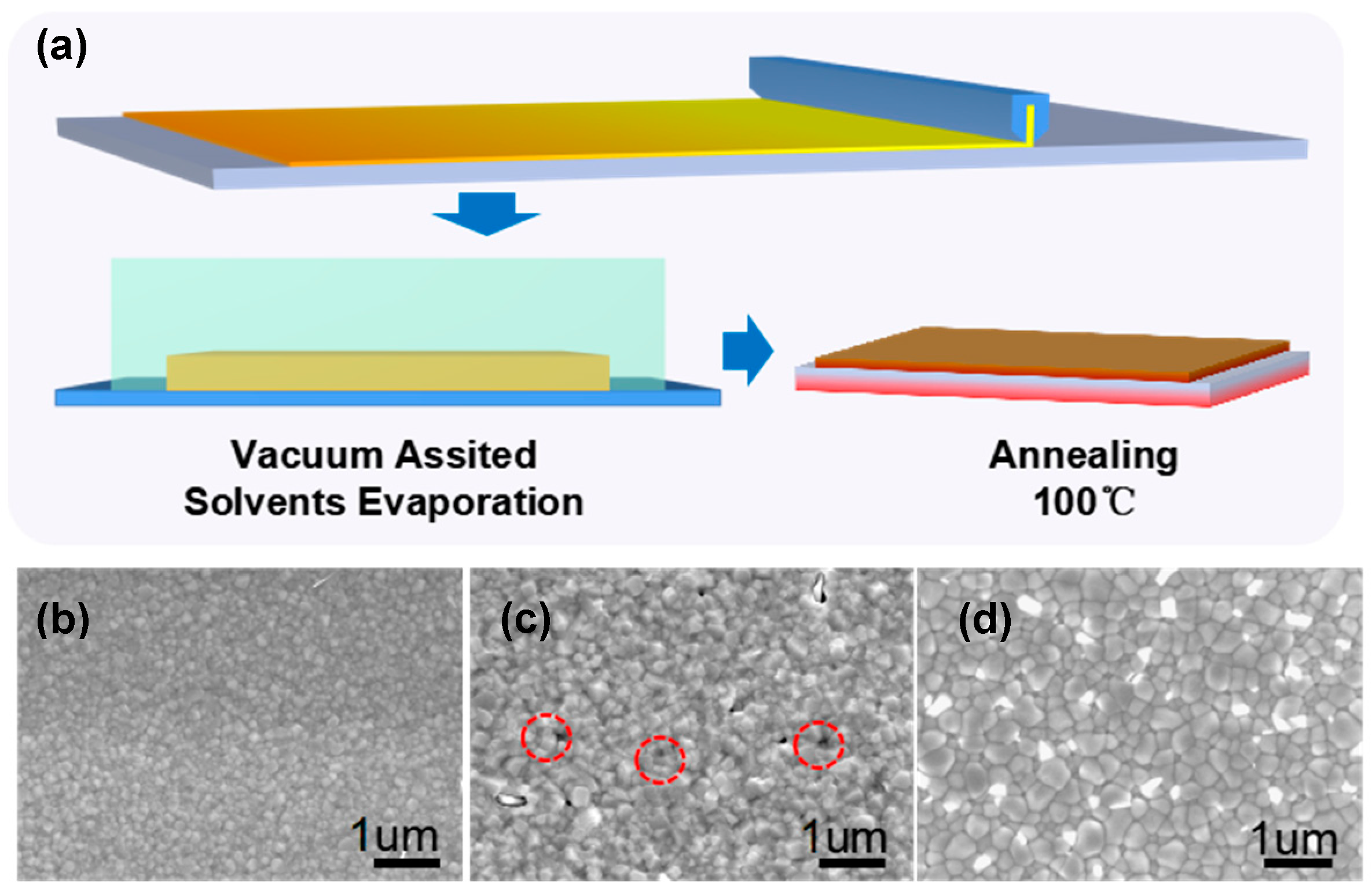
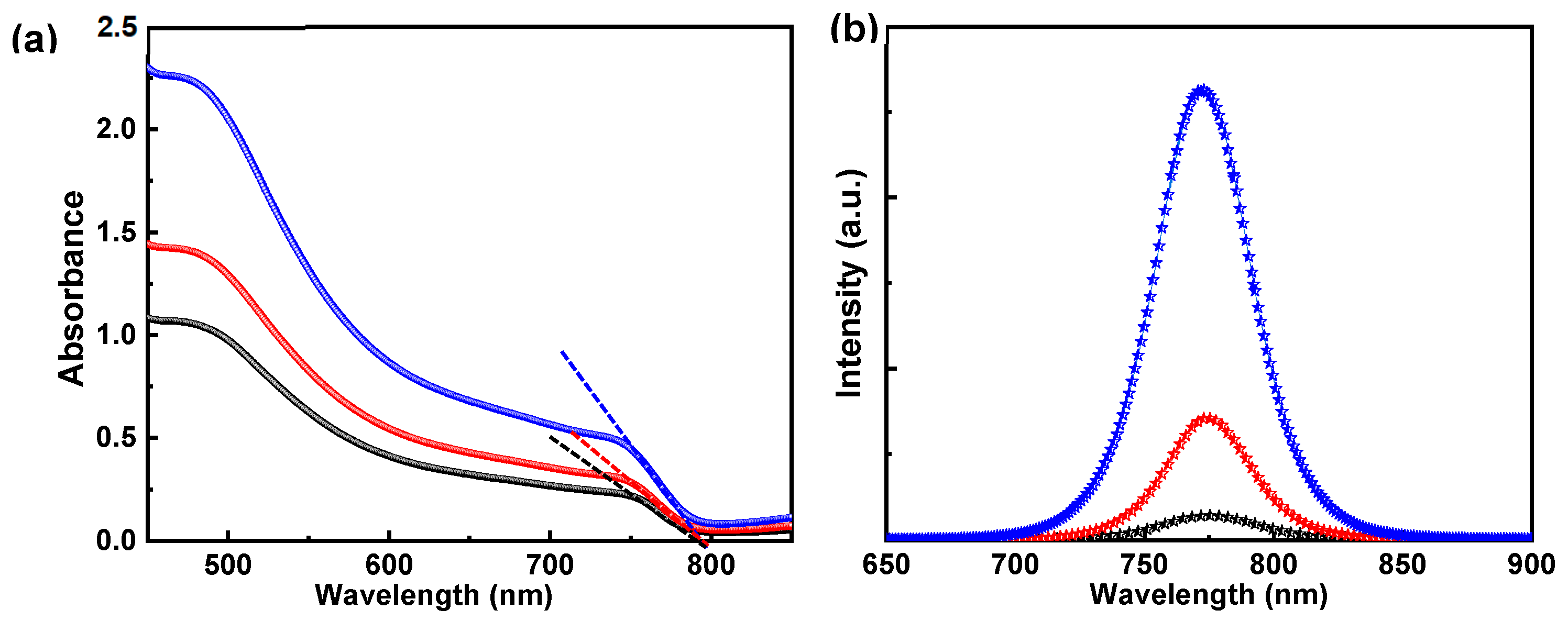
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Green, M.A. Commercial progress and challenges for photovoltaics. Nat. Energy 2016, 1, 15015. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, B.; Zhang, W.; Yang, Z.; Li, M.; Ren, F.; Imran, T.; Sun, Z.; Zhang, S.; Zhang, Y. Solvent engineering towards scalable fabrication of high-quality perovskite films for efficient solar modules. J. Energy Chem. 2023, 80, 689–710. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, W.; Guan, X.; Raza, H.; Zhang, S.; Zhang, Y.; Troshin, P.A.; Kuklin, S.A.; Liu, Z.; Chen, W. Rear electrode materials for perovskite solar cells. Adv. Funct. Mater. 2022, 32, 2200651. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, W.; Wu, S.; Zhu, H.; Liu, Z.; Liu, Z.; Jiang, Z.; Chen, R.; Zhou, J.; Lu, Q. Slot-die coating large-area formamidinium-cesium perovskite film for efficient and stable parallel solar module. Sci. Adv. 2021, 7, 3749. [Google Scholar] [CrossRef]
- Kam, M.; Zhu, Y.; Zhang, D.; Gu, L.; Chen, J.; Fan, Z. Efficient mixed-cation mixed-halide perovskite solar cells by all-vacuum sequential deposition using metal oxide electron transport layer. Sol. RRL 2019, 3, 1900050. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, S.; Chen, S.; Xiao, X.; Zhao, J.J. Huang, Defect compensation in formamidinium–caesium perovskites for highly efficient solar mini-modules with improved photostability. Nat. Energy 2021, 6, 633–641. [Google Scholar] [CrossRef]
- Bu, T.; Li, J.; Li, H.; Tian, C.; Su, J.; Tong, G.; Ono, L.K.; Wang, C.; Lin, Z.; Chai, N. Lead halide–templated crystallization of methylamine-free perovskite for efficient photovoltaic modules. Science 2021, 372, 1327–1332. [Google Scholar] [CrossRef]
- Feng, Q.; Huang, X.; Tang, Z.; Hou, Y.; Chang, Q.; Nie, S.; Cao, F.; Niu, X.; Yin, J.; Li, J. Governing PbI6 octahedral frameworks for high-stability perovskite solar modules. Energy Environ. Sci. 2022, 15, 4404–4413. [Google Scholar] [CrossRef]
- Si, H.; Liao, Q.; Kang, Z.; Ou, Y.; Meng, J.; Liu, Y.; Zhang, Z.; Zhang, Y. Deciphering the NH4PbI3 Intermediate Phase for Simultaneous Improvement on Nucleation and Crystal Growth of Perovskite. Adv. Funct. Mater. 2017, 17, 1701804. [Google Scholar] [CrossRef]
- Deng, Y.; Zheng, X.; Bai, Y.; Wang, Q.; Zhao, J.; Huang, J. Surfactant-controlled ink drying enables high-speed deposition of perovskite films for efficient photovoltaic modules. Nat. Energy 2018, 3, 560–566. [Google Scholar] [CrossRef]
- Guo, F.; Qiu, S.; Hu, J.; Wang, H.; Cai, B.; Li, J.; Yuan, X.; Liu, X.; Forberich, K.; BrabecC, J. A Generalized Crystallization Protocol for Scalable Deposition of High-Quality Perovskite Thin Films for Photovoltaic Applications. Adv. Sci. 2019, 6, 1901067. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Van Brackle, C.H.; Dai, X.; Zhao, J.; Chen, B.; Huang, J. Tailoring solvent coordination for high-speed, room-temperature blading of perovskite photovoltaic films. Sci. Adv. 2019, 5, 7537. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, Y.; Lu, H.; Chen, C.; Liu, Y.; Bai, X.; Yang, L.; Yu, W.W.; Dai, Q.; Zhang, Y. CsPbBr3 perovskite nanoparticles as additive for environmentally stable perovskite solar cells with 20.46% efficiency. Nano Energy 2019, 59, 517–526. [Google Scholar] [CrossRef]
- Lee, J.W.; Bae, S.H.; Marco, N.D.; Hsieh, Y.T.; Dai, Z.; Yang, Y. The role of grain boundaries in perovskite solar cells. Mater. Today Energy 2018, 7, 149–160. [Google Scholar] [CrossRef]
- Wang, B.; Wong, K.Y.; Yang, S.; Chen, T. Crystallinity and defect state engineering in organo-lead halide perovskite for high-efficiency solar cells. J. Mater. Chem. A 2016, 4, 3806–3812. [Google Scholar] [CrossRef]
- Huang, F.; Dkhissi, Y.; Huang, W.; Xiao, M.; Benesperi, L.; Rubanov, S.; Rubanov, S.; Zhu, Y.; Lin, X.; Jiang, L.; et al. Gas-assisted preparation of lead iodide perovskite films consisting of a monolayer of single crystalline grains for high efficiency planar solar cells. Nano Energy 2014, 10, 10–18. [Google Scholar] [CrossRef]
- Wang, M.; Cao, F.; Deng, K.; Li, L. Adduct phases induced controlled crystallization for mixed-cation perovskite solar cells with efficiency over 21%. Nano Energy 2019, 63, 103867. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Y.L.; Lu, C.Q.; Zhang, R.; Wang, X.J.; Zhang, W.J.; Jiang, Z.Y. MACl-induced controlled crystallization in sequentially deposited perovskites for high-efficiency and stable perovskite solar cells. Coatings 2023, 13, 1885. [Google Scholar] [CrossRef]
- Docampo, P.; HanuschF, C.; Stranks, S.D.; Doblinger, M.; Feckl, J.M.; Ehrensperger, M.; Minar, N.K.; Johnston, M.B.; Snaith, H.J.; Bein, T. Solution Deposition-Conversion for Planar Heterojunction Mixed Halide Perovskite Solar Cells. Adv. Energy Mater. 2014, 4, 1400355. [Google Scholar] [CrossRef]
- Chen, J.; Zuo, L.; Zhang, Y.; Lian, X.; Fu, W.; Yan, J.; Li, J.; Wu, G.; Li, C.Z.; Chen, H. High-Performance Thickness Insensitive Perovskite Solar Cells with Enhanced Moisture Stability. Adv. Energy Mater. 2018, 8, 1800438. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Z.; Zhang, W.; Yan, L.; Lu, C.; Ni, C. Pre-crystallization applied in two-step spin-coating method for high-quality perovskite films in photovoltaic devices. J. Alloys Compd. 2020, 832, 153616. [Google Scholar] [CrossRef]
- Zhao, Y.; Duan, J.; Wang, Y.; Yang, X.; Tang, Q. Precise stress control of inorganic perovskite films for carbon-based solar cells with an ultrahigh voltage of 1.622 V. Nano Energy 2020, 67, 104286. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, L.; Xiang, S.; Wei, Y.; Xie, M.; Liu, H.; Li, W.; Chen, H. Growing high-quality CsPbBr3 by using porous CsPb2Br5 as an intermediate: A promising light absorber in carbon-based perovskite solar cells. Sustain. Energy Fuels 2019, 3, 184–194. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Z.; Ye, H.; Tu, Y.; Sun, B.; Tan, X.; Shi, T.; Tang, Z.; Liao, G. Novel efficient C60-based inverted perovskite solar cells with negligible hysteresis. Electrochim. Acta 2018, 288, 115–125. [Google Scholar] [CrossRef]
- Teng, P.; Han, X.; Li, J.; Xu, Y.; Kang, L.; Wang, Y.; Yang, Y.; Yu, T. Elegant Face-Down Liquid-Space-Restricted Deposition of CsPbBr3 Films for Efficient Carbon-Based All-Inorganic Planar Perovskite Solar Cells. ACS Appl. Mater. Inter. 2018, 10, 9541. [Google Scholar] [CrossRef]
- Luo, J.; Jia, C.; Wan, Z.; Han, F.; Zhao, B.; Wang, R. The novel dopant for hole-transporting material opens a new processing route to efficiently reduce hysteresis and improve stability of planar perovskite solar cells. J. Power Sources 2017, 342, 886–895. [Google Scholar] [CrossRef]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef]
- Xiao, Z.; Dong, Q.; Bi, C.; Shao, Y.; Yuan, Y.; Huang, J. Solvent Annealing of Perovskite-Induced Crystal Growth for Photovoltaic-Device Efficiency Enhancement. Adv. Mater. 2014, 26, 6503–6509. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Liu, Z.; Chen, Q.; Wang, X.; Zhou, H. The additive coordination effect on hybrids perovskite crystallization and high-performance solar cell. Adv. Mater. 2016, 28, 9862–9868. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Etgar, L. Current density mismatch in perovskite solar cells. ACS Energy Lett. 2020, 5, 2886–2888. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Jiang, Z.Y.; Li, J.C.; Meng, G.X.; Guo, J.J.; Zhang, W.J. Preparation of efficiency and stability all-inorganic CsPbBr3 perovskite solar cells by pre-crystallization multi-step spin coating method. Coatings 2024, 14, 918. [Google Scholar] [CrossRef]
- Li, M.; Li, B.; Cao, G.; Tian, J. Monolithic MAPbI3 films for high-efficiency solar cells via coordination and a heat assisted process. J. Mater. Chem. A 2017, 40, 21313–21319. [Google Scholar] [CrossRef]
- Qiu, J.J.; Yang, T.T.; Li, Y.F.; Qian, W.H.; Liu, X.Y. Au@Ag@Pt core–shell nanorods regulating Ag release behavior endow titanium antibacterial activity and biocompatibility. Rare Met. 2022, 41, 630–638. [Google Scholar] [CrossRef]
- Aristidou, N.; Eames, C.; Sanchez-Molina, I.; Bu, X.; Kosco, J.; Islam, M.S.; Haque, S.A. Fast oxygen diffusion and iodide defects mediate oxygen-induced degradation of perovskite solar cells. Nat. Commun. 2017, 8, 15218. [Google Scholar] [CrossRef]


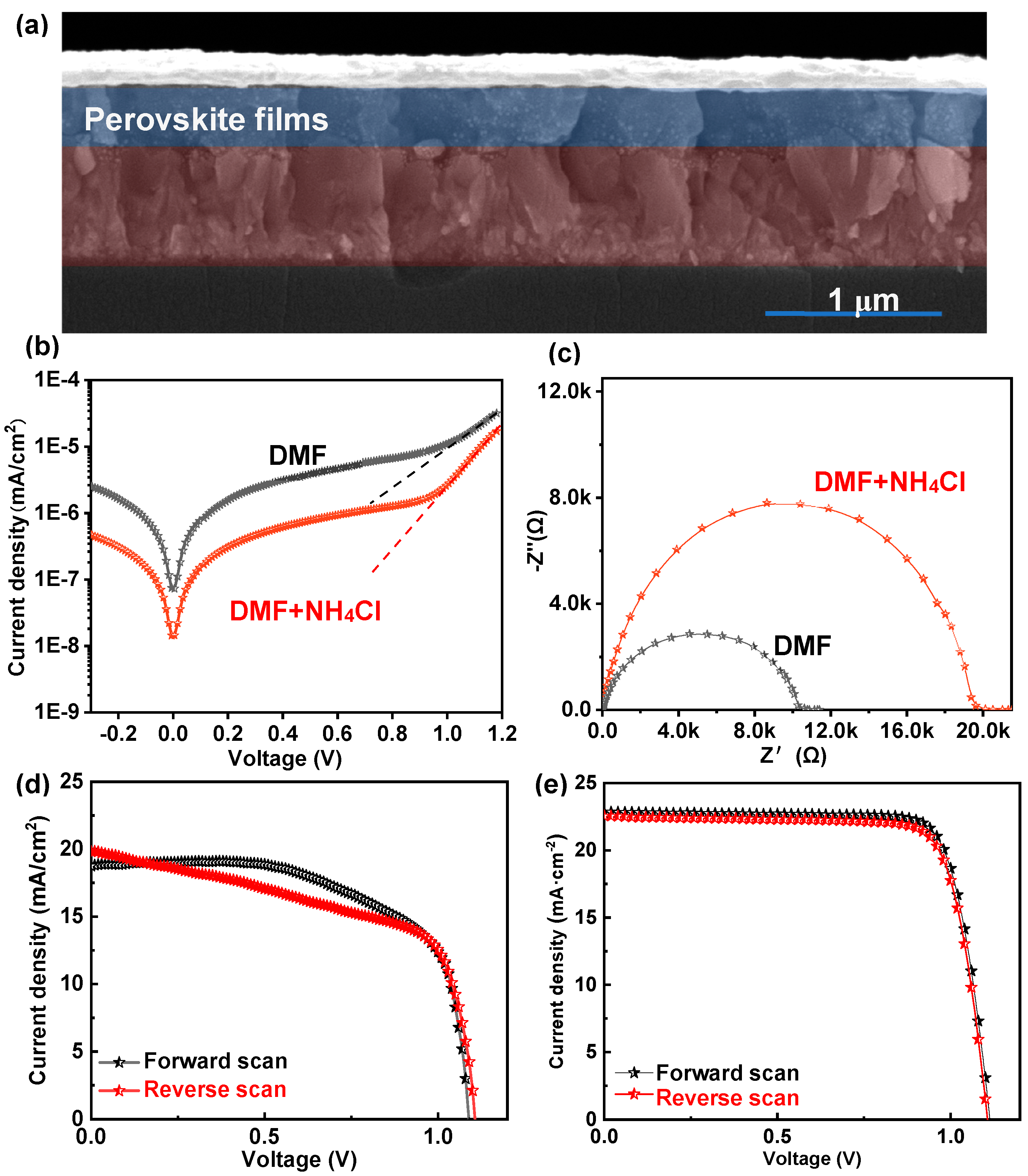

| JSC (mA/cm2) | VOC (V) | FF (%) | PCE (%) | ||
|---|---|---|---|---|---|
| DMF | Forward scan | 18.76 | 1.09 | 64.8% | 13.25% |
| Reverse scan | 19.90 | 1.10 | 59.6% | 13.05% | |
| DMF + NH4Cl | Forward scan | 22.86 | 1.11 | 79.3% | 20.12% |
| Reverse scan | 22.46 | 1.10 | 79.9% | 19.74% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Li, J.; Li, K.; Zhang, R.; Qin, Z.; Zhang, Y.; Wu, B.; Ma, Q.; Zhang, Y.; Zhang, W. Controllable Crystallization of Perovskite Films during the Blade-Coating Fabrication Process for Efficient and Stable Solar Cells. Coatings 2024, 14, 1113. https://doi.org/10.3390/coatings14091113
Jiang Z, Li J, Li K, Zhang R, Qin Z, Zhang Y, Wu B, Ma Q, Zhang Y, Zhang W. Controllable Crystallization of Perovskite Films during the Blade-Coating Fabrication Process for Efficient and Stable Solar Cells. Coatings. 2024; 14(9):1113. https://doi.org/10.3390/coatings14091113
Chicago/Turabian StyleJiang, Zhaoyi, Jiaqi Li, Kun Li, Rui Zhang, Zhenxing Qin, Yufei Zhang, Boheng Wu, Qiang Ma, Yulong Zhang, and Weijia Zhang. 2024. "Controllable Crystallization of Perovskite Films during the Blade-Coating Fabrication Process for Efficient and Stable Solar Cells" Coatings 14, no. 9: 1113. https://doi.org/10.3390/coatings14091113






