Removal of Remazol Red Dyes Using Zeolites-Loaded Nanofibre Coated on Fabric Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PVA Solution and PVA/Zeolite Solution
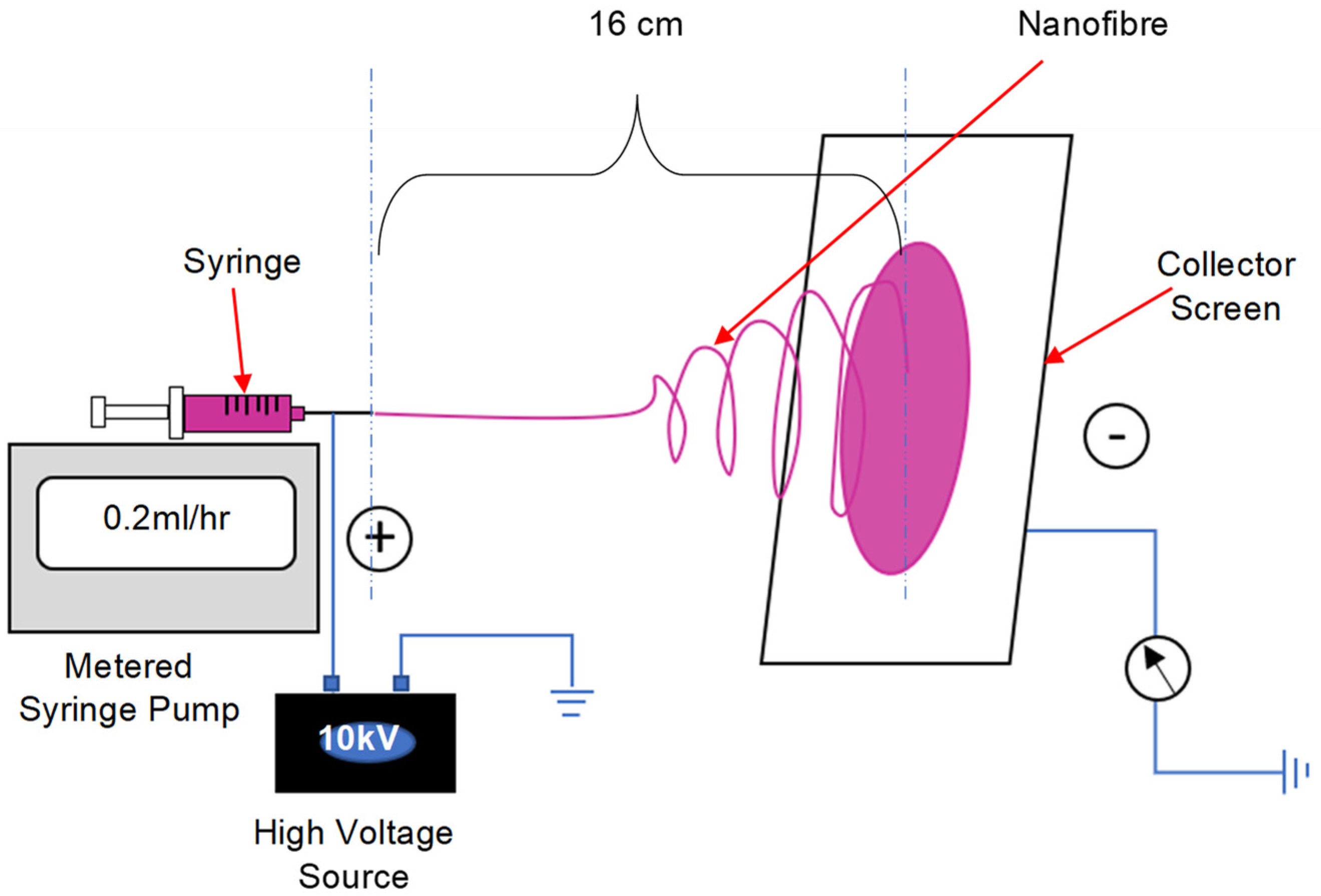
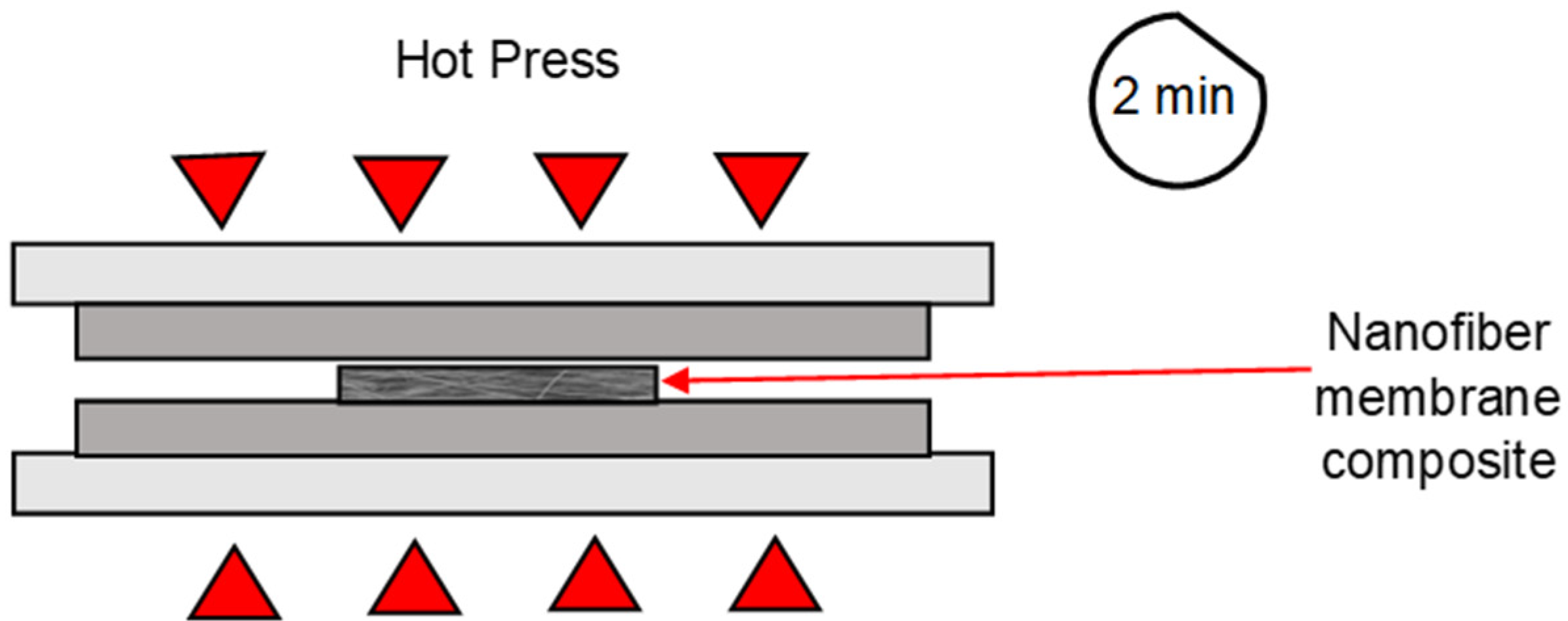
2.3. Formation of Nanofibre Composite Membranes
2.4. Membrane Characterisation Using Field Emission Scanning Electron Microscope (FESEM) and EDX Mapping
2.5. Porosity
2.6. Filtration (Water Flux)
2.7. Turbidity
2.8. Removal of Red Remazol (RR) Dye at Different Dye Concentrations and Contact Time
3. Results and Discussion
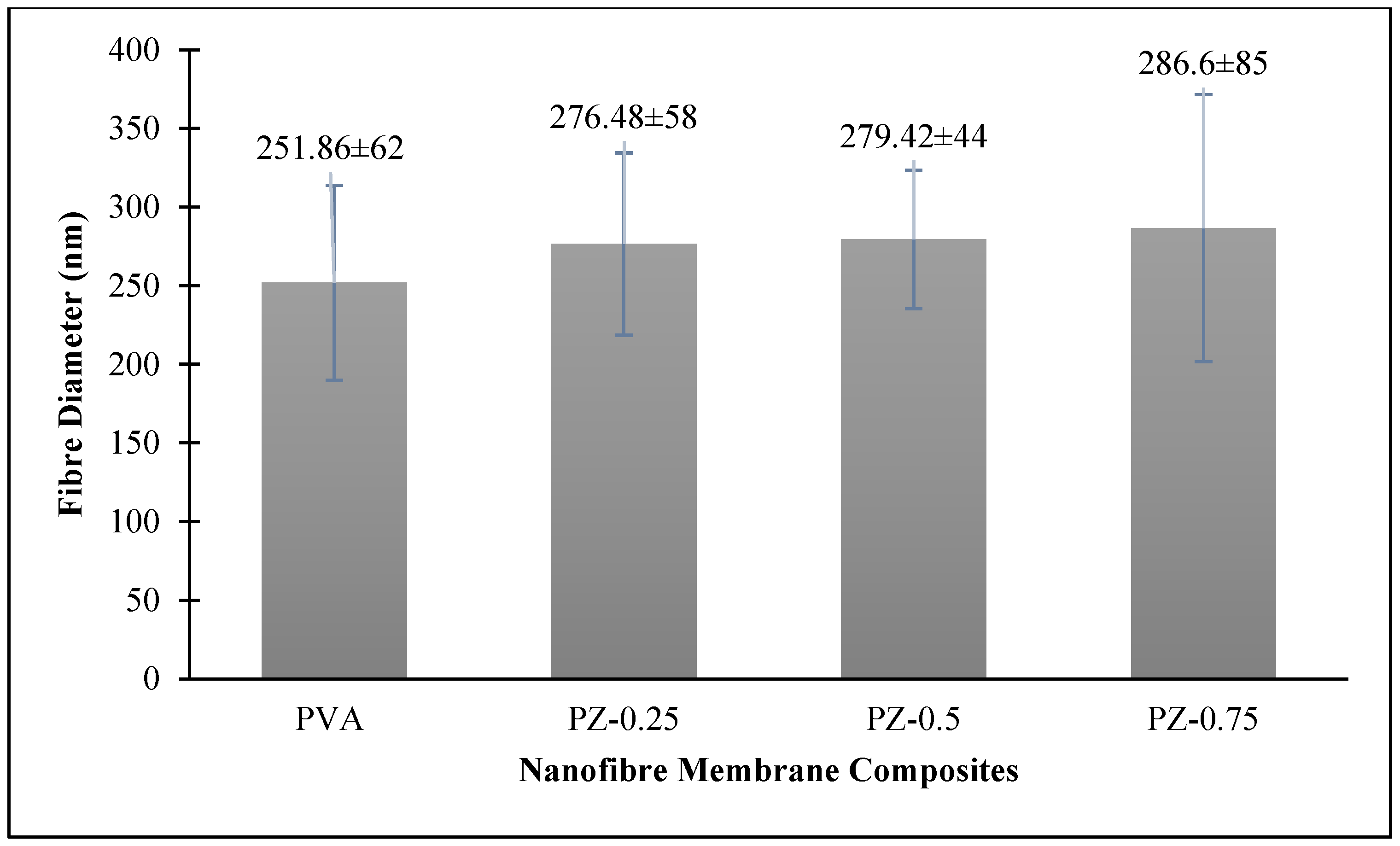
3.1. Morphology Analysis of Zeolite-Incorporated PVA Nanofibre Membranes
3.2. Pure Water Flux of Zeolite-Incorporated PVA Nanofibre Composite Membranes
3.3. Turbidity of Dye Wastewater Using Zeolite-Incorporated PVA Nanofibre Composite Membranes
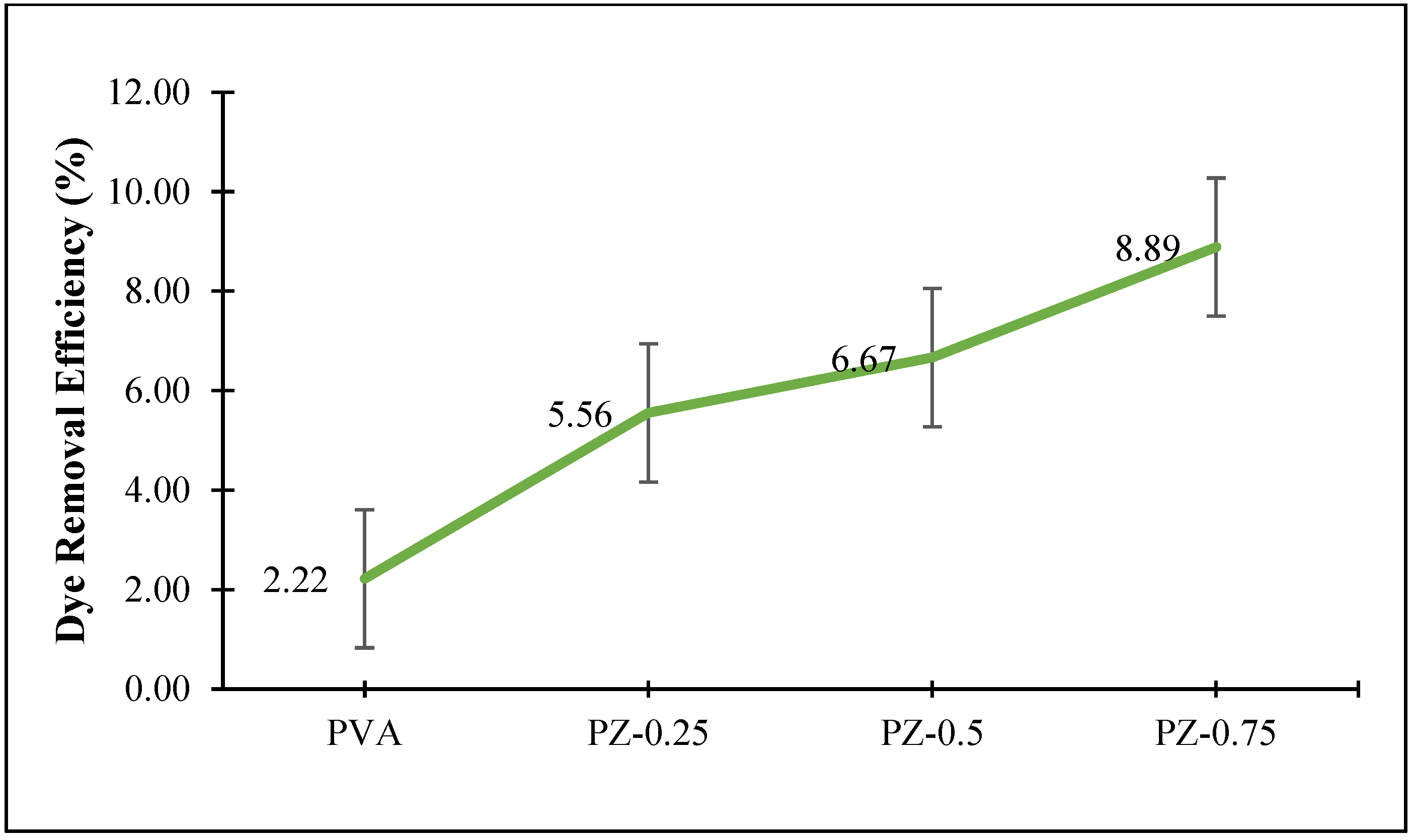
3.4. Dye Removal Using Zeolite-Incorporated PVA Nanofibre Composite Membranes
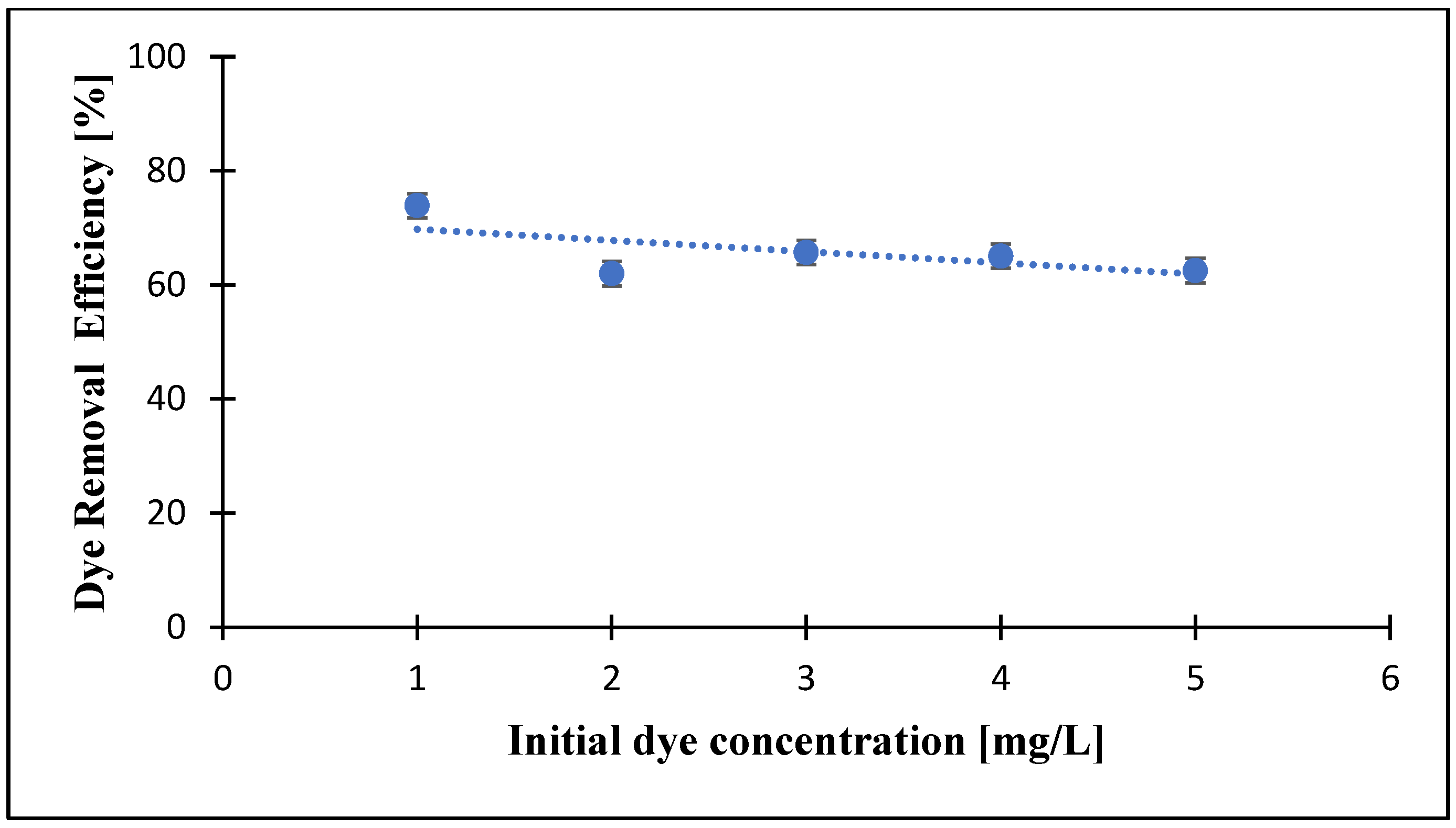
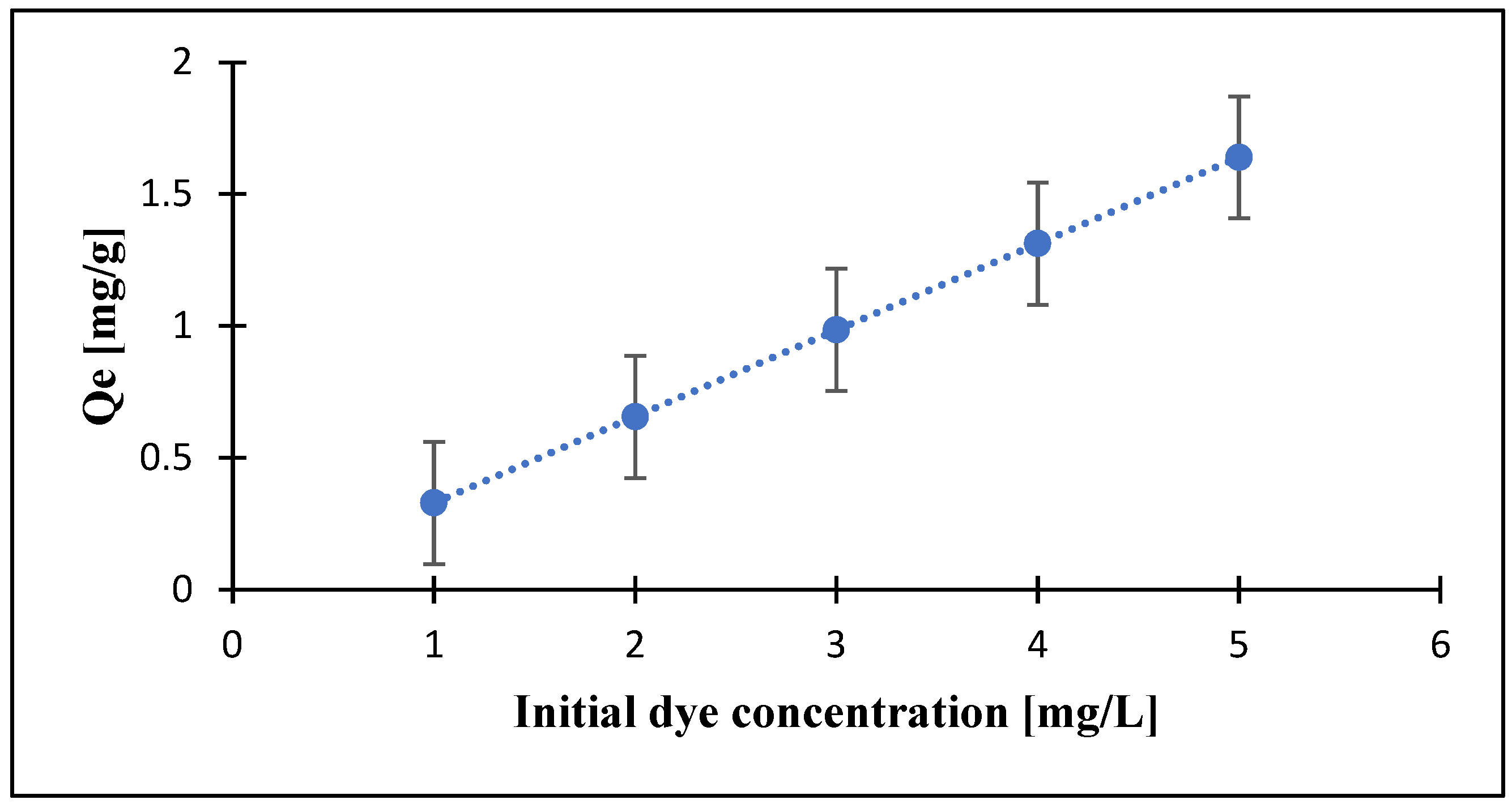
3.5. Effects of Initial Dye Concentration on the Dye Removal by PZ-0.75 Composite Membranes
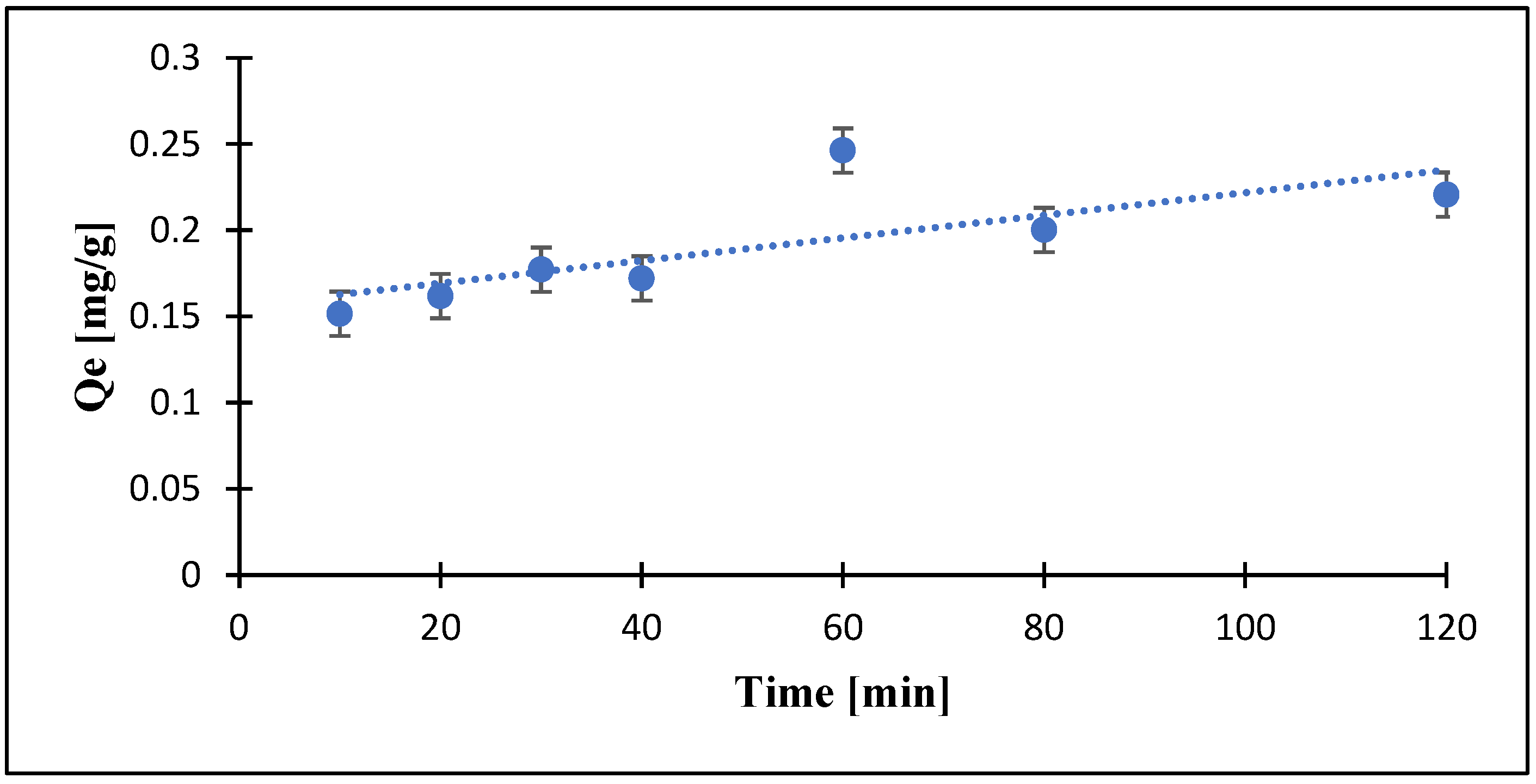
3.6. Effects of Contact Time on RR Dye Removal by PZ-0.75 Composite Membranes
3.7. Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rashidi, H.; Sulaiman, N.N.; Hashim, N. Batik industry synthetic wastewater treatment using nanofiltration membrane. Procedia Eng. 2012, 44, 2010–2012. [Google Scholar] [CrossRef]
- Zhang, Y.; Shaad, K.; Vollmer, D.; Ma, C. Treatment of Textile Wastewater Using Advanced Oxidation Processes—A Critical Review. Water 2021, 12, 3515. [Google Scholar] [CrossRef]
- Donkadokula, N.Y.; Kola, A.K.; Naz, I.; Saroj, D. A review on advanced physico-chemical and biological textile dye wastewater treatment techniques. Rev. Environ. Sci. Biotechnol. 2020, 19, 543–560. [Google Scholar] [CrossRef]
- Affandi, N.D.; Abdul Razak, N.N. Removal of Pigment from Textile Wastewater by Electrospun Nanofibre Membrane. J. Mech. Eng. 2017, 4, 190–201. [Google Scholar]
- Siddiqui, S.I.; Ravi, R.; Rathi, G.; Tara, N.; Ul-Islam, S.; Chaudhry, S.A. Decolorization of textile wastewater using composite materials. In Nanomaterials in the Wet Processing of Textiles, 1st ed.; Islam, S.-U., Butola, B.S., Eds.; Wiley: New Jersey, NJ, USA, 2018; pp. 187–218. [Google Scholar]
- Chowdhary, P.; Bharagava, R.N.; Mishra, S.; Khan, N. Role of Industries in Water Scarcity and Its Adverse Effects on Environment and Human Health. In Environmental Concerns and Sustainable Development, 1st ed.; Shukla, V., Kumar, N., Eds.; Springer: Singapore, 2020; pp. 235–256. [Google Scholar]
- Fauzi, A.; Hapidin, D.A.; Munir, M.M.; Iskandar, F.; Khairurrijal, K. A superhydrophilic bilayer structure of a nylon 6 nanofibre/cellulose membrane and its characterization as potential water filtration media. RSC Adv. 2020, 10, 17205–17216. [Google Scholar] [CrossRef] [PubMed]
- Manea, L.R.; Bertea, A.; Bertea, A.P. Electrospun nanofibre membranes for textile wastewater treatment. IOP Conf. Ser. Mater. Sci. Eng. 2019, 572, 012077. [Google Scholar] [CrossRef]
- Hami, S.S.B.M.; Affandi, N.D.N.; Indrie, L.; Harun, A.M.; Ahmad, M.R. Enhancing Mechanical Properties and Flux of Nanofibre Membranes for Water Filtration. Polymers 2023, 15, 3281. [Google Scholar] [CrossRef]
- Makarov, I.S.; Shambilova, G.K.; Vinogradov, M.I.; Anokhina, T.S.; Bukanova, A.S.; Kairliyeva, F.B.; Bukanova, S.K.; Levin, I.S. Membranes Based on Cellulose and Copolymers of Acrylonitrile Prepared from Joint Solutions. Membranes 2023, 13, 667. [Google Scholar] [CrossRef]
- Mansor, E.S.; Ali, H.; Abdel-Karim, A. Efficient and reusable polyethylene oxide/polyaniline composite membrane for dye adsorption and filtration. Colloids Inter. Sci. Comm. 2020, 39, 100314. [Google Scholar] [CrossRef]
- Rafieian, F.; Jonoobi, M.; Yu, Q. A Novel Nanocomposite Membrane Containing Modified Cellulose Nanocrystals for Copper Ion Removal and Dye Adsorption from Water. Cellulose 2019, 26, 3359–3373. [Google Scholar] [CrossRef]
- Huang, L.; Manickam, S.S.; McCutcheon, J.R. Increasing strength of electrospun nanofibre membranes for water filtration using solvent vapor. J. Membr. Sci. 2013, 423, 213–220. [Google Scholar] [CrossRef]
- Wu, H.; Hu, Z.; Geng, Q.; Chen, Z.; Song, Y.; Chu, J.; Ning, X.; Dong, S.; Yuan, D. Facile preparation of CuMOF-modified multifunctional nanofibre membrane for high-efficient filtration/separation in complex environments. Colloids Surf. Phyicochem. Eng. Asp. 2022, 651, 129656. [Google Scholar] [CrossRef]
- Hamid, S.A.; Azha, S.F.; Sellaoui, L.; Bonilla-Petriciolet, A.; Ismail, S. Adsorption of Copper (II) Cation on Polysulfone/Zeolite Blend Sheet Membrane: Synthesis, Characterization, Experiments and Adsorption Modelling. Colloids Surf. Phyicochem. Eng. Asp. 2020, 601, 124980. [Google Scholar] [CrossRef]
- Habiba, U.; Siddique, T.A.; Lee, J.J.L.; Joo, T.C.; Ang, B.C.; Afifi, A.M. Adsorption Study of Methyl Orange by Chitosan/Polyvinyl Alcohol/Zeolite Electrospun Composite Nanofibrous Membrane. Carbohydr. Polym. 2018, 191, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Habiba, U.; Afifi, A.M.; Salleh, A.; Ang, B.C. Chitosan/(Polyvinyl Alcohol)/Zeolite Electrospun Composite Nanofibrous Membrane for Adsorption of Cr6+, Fe3+ and Ni2+. J. Hazard. Mater. 2017, 322, 182–194. [Google Scholar] [CrossRef]
- Radoor, S.; Karayil, J.; Jayakumar, A.; Parameswaranpillai, J.; Lee, J.; Shivanna, J.M.; Nithya, R.; Siengchin, S. Adsorption of Anionic Dye Onto ZSM-5 Zeolite-based Bio Membrane: Characterizations, Kinetics and Adsorption Isotherm. J. Polym. Environ. 2022, 30, 3279–3292. [Google Scholar] [CrossRef]
- Chen, Y.S.; Ooi, C.W.; Show, P.L.; Hoe, B.C.; Chai, W.S.; Chiu, C.Y.; Wang, S.S.S.; Chang, Y.K. Removal of Ionic Dyes by Nanofiber Membrane Functionalized with Chitosan and Egg White Proteins: Membrane Preparation and Adsorption Efficiency. Membranes 2022, 12, 63. [Google Scholar] [CrossRef]
- Wangi, G.M.; Olupot, P.W.; Byaruhanga, J.; Kulabako, R. Characterization of natural zeolite and determination of its ion-exchange potential for selected metal ions in water. Environ. Process. 2023, 10, 2023. [Google Scholar] [CrossRef]
- Takai, R.; Kurimoto, R.; Nakagawa, Y.; Kotsuchibashi, Y.; Namekawa, K.; Ebara, M. Towards a Rational Design of Zeolite-Polymer Composite Nanofibres for Efficient Adsorption of Creatinine. J. Nanomater. 2016, 2016, 5638905. [Google Scholar] [CrossRef]
- Rad, L.R.; Momeni, A.; Ghazani, B.F.; Irani, M.; Mahmoudi, M.; Noghreh, B. Removal of Ni2+ and Cd2+ Ions from Aqueous Solutions Using Electrospun PVA/Zeolite Nanofibrous Adsorbent. Chem. Eng. J. 2014, 256, 119–127. [Google Scholar] [CrossRef]
- Radoor, S.; Karayil, J.; Parameswaranpillai, J.; Siengchin, S. Removal of Anionic Dye Congo Red from Aqueous Environment Using Polyvinyl Alcohol/Sodium Alginate/ZSM-5 Zeolite Membrane. Sci. Rep. 2020, 10, 15452. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, Z.; Gong, W.; Xu, F.; Song, X.; He, X.; Fan, M. Electrospun carbon nanofibres and their applications in several areas. ACS Omega 2023, 8, 22316–22330. [Google Scholar] [CrossRef]
- Daraei, A.; Pieters, M.; Baker, S.R.; De Lange-Loots, Z.; Siniarski, A.; Litvinov, R.I.; Veen, C.S.B.; de Maat, M.P.M.; Weisel, J.W.; Ariens, R.A.S.; et al. Automated Fiber Diameter and Porosity Measurements of Plasma Clots in Scanning Electron Microscopy Images. Biomolecules 2021, 11, 1536. [Google Scholar] [CrossRef] [PubMed]
- Thoola, M.I. Performance Characteristics of Bio-Ultrafiltration on Local Surface Waters. Ph.D. Thesis, Durban University of Technology, KwaZulu-Natal, South Africa, 2014. [Google Scholar]
- Kastali, M.; Mouhir, L.; Chatoui, M.; Souabi, S.; Anouzla, A. Removal of turbidity and sludge production from industrial process wastewater treatment by a rejection of steel rich in FeCl3 (SIWW). Biointer. Res. Appl. Chem. 2021, 11, 13359–13376. [Google Scholar]
- Cardoso, N.F.; Pinto, R.A.; Lima, E.C.; Calvete, T.; Amavisca, C.V.; Royer, B.; Cunha, M.; Freire, M.; Pinto, I.S. Removal of remazol black B textile dye from aqueous solution by adsorption. Desalination 2011, 269, 92–103. [Google Scholar] [CrossRef]
- Lu, L.; Samarasekera, C.; Yeow, J.T.W. Creatinine adsorption capacity of electrospun polyacrylonitrile (PAN)-zeolite nanofibre membranes for potential artificial kidney applications. J. Appl. Polym. Sci. 2015, 132, 42418. [Google Scholar] [CrossRef]
- Khmiri, Y.; Attia, A.; Aloulou, H.; Dammak, L.; Baklouti, L.; Amar, R.B. Preparation and characterization of new and low-cost ceramic flat membranes based on zeolite-clay for the removal of indigo blue dye molecules. Membranes 2023, 13, 865. [Google Scholar] [CrossRef]
- Salmankhani, A.; Khadem, S.S.M.; Seidi, F.; Mashhadzadeh, A.H.; Zarrintaj, P.; Habibzadeh, S.; Mohaddespour, A.; Rabiee, N.; Lima, E.C.; Shokouhimehr, M.; et al. Adsorption onto Zeolites: Molecular Perspective. Chem. Pap. 2021, 75, 6217–6239. [Google Scholar] [CrossRef]
- Aljerf, L. High-efficiency extraction of bromocresol purple dye and heavy metals as chromium from industrial effluent by adsorption onto a modified surface of zeolite: Kinetics and equilibrium study. J. Environ. Manag. 2018, 225, 120–132. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Alipour, M.R.; Mahvi, A.H. Textile wastewater treatment by application of combined chemical coagulation, electrocoagulation, and adsorption processes. Desalin. Water Treat. 2016, 57, 9203–9215. [Google Scholar] [CrossRef]
- Hammood, Z.A.; Chyad, T.F.; Al-Saedi, R. Adsorption Performance of Dyes Over Zeolite for Textile Wastewater Treatment. Ecol. Chem. Eng. 2021, 28, 329–337. [Google Scholar] [CrossRef]
- Zhu, M.-X.; Lee, L.; Wang, H.-H.; Wang, Z. Removal of an anionic dye by adsorption/precipitation processes using alkaline white mud. J. Hazard. Mater. 2007, 149, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Chikri, R.; Elhadiri, N.; Benchanaa, M.; El Maguana, Y. Efficiency of Sawdust as Low-Cost Adsorbent for Dyes Removal. J. Chem. 2020, 17, 8813420. [Google Scholar] [CrossRef]
- Maimulyanti, A.; Nurhidayati, I.; Widarsih, R.W. Adsorption Isotherm Models and Kinetics for Phosphate Adsorption in Sediment. Rasayan J. Chem. 2022, 15, 2626–2631. [Google Scholar] [CrossRef]








| Woven Polyester Fabric | Nonwoven Interfacing | ||
|---|---|---|---|
| Thickness (mm) | 1.83 | 1.75 | |
| Weight (g/m2) | 110.07 | 34.54 | |
| Density | Warp/cm | Weft/cm | - |
| ≈55 | ≈37 | - | |
| Atomic % | |||
|---|---|---|---|
| Membrane | Na | Si | Total |
| PZ-0.25 | 68.91 | 31.09 | 100 |
| PZ-0.5 | 45.8 | 54.2 | 100 |
| PZ-0.75 | 27.63 | 72.37 | 100 |
| Membrane | Porosity (%) |
|---|---|
| PVA | 12.51 |
| PZ-0.25 | 16.47 |
| PZ -0.5 | 25.41 |
| PZ-0.75 | 39.07 |
| Langmuir Constant | Freundlich Constant | ||||
|---|---|---|---|---|---|
| KL | qmax | R2 | Kf | R2 | |
| 0.454 | 3.052 | 0.979 | 0.949 | 0.828 | 0.983 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hami, S.S.b.M.; Affandi, N.D.N.; Indrie, L.; Harun, A.M. Removal of Remazol Red Dyes Using Zeolites-Loaded Nanofibre Coated on Fabric Substrates. Coatings 2024, 14, 1155. https://doi.org/10.3390/coatings14091155
Hami SSbM, Affandi NDN, Indrie L, Harun AM. Removal of Remazol Red Dyes Using Zeolites-Loaded Nanofibre Coated on Fabric Substrates. Coatings. 2024; 14(9):1155. https://doi.org/10.3390/coatings14091155
Chicago/Turabian StyleHami, Siddratul Sarah binti Mohd, Nor Dalila Nor Affandi, Liliana Indrie, and Ahmad Mukifza Harun. 2024. "Removal of Remazol Red Dyes Using Zeolites-Loaded Nanofibre Coated on Fabric Substrates" Coatings 14, no. 9: 1155. https://doi.org/10.3390/coatings14091155






