Assessment of Warionia saharea Essential Oil as a Green Corrosion Inhibitor for Mild Steel in HCl: Experimental and Computational Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Corrosion Tests
2.3. Scanning Electron Microscope (SEM) Analysis
2.4. Computational Studies
2.4.1. DFT Investigations
2.4.2. Molecular Dynamics Simulation
3. Results and Discussion
3.1. WL Measurements
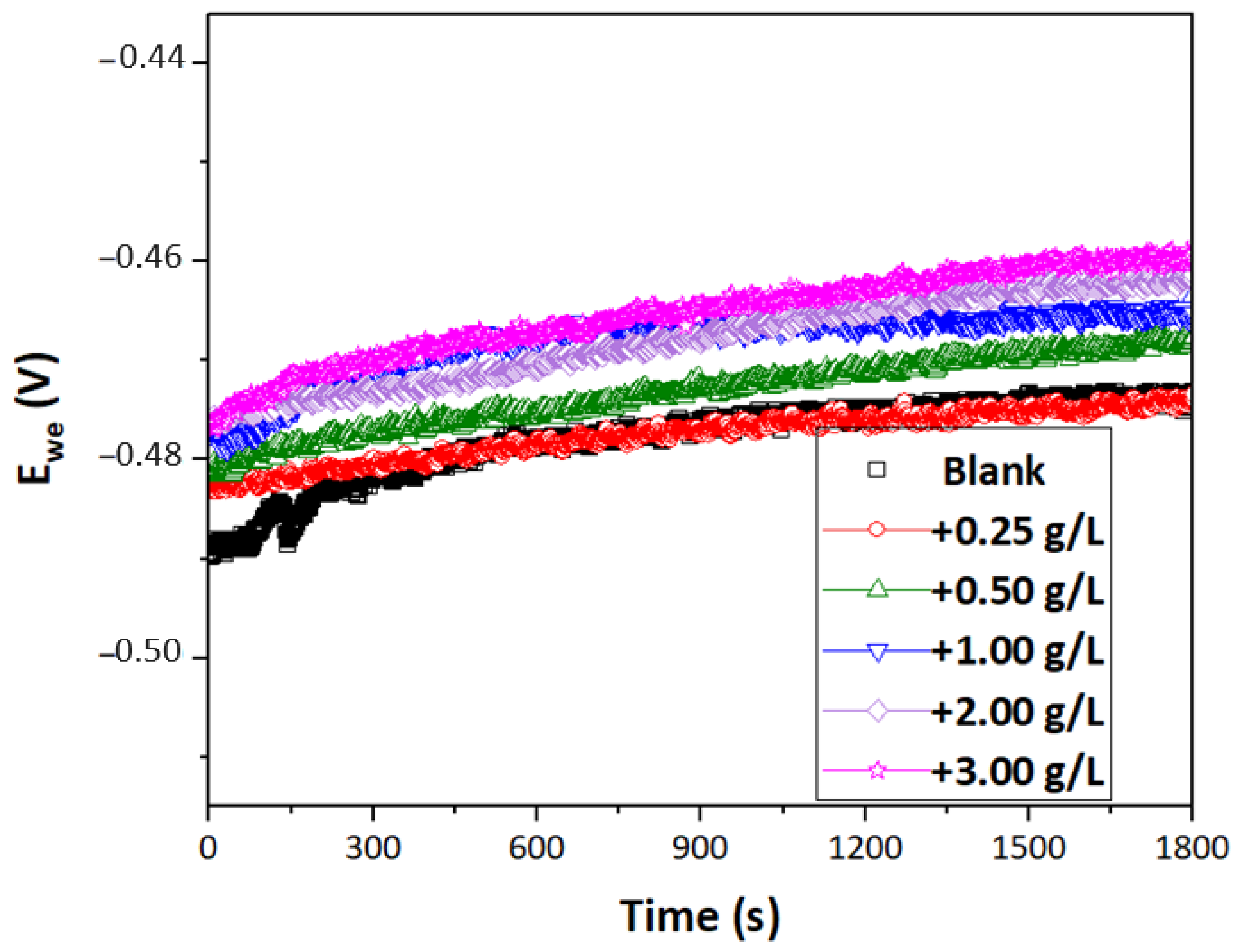
3.2. OCP Measurements
3.3. Potentiodynamic Polarization Curves
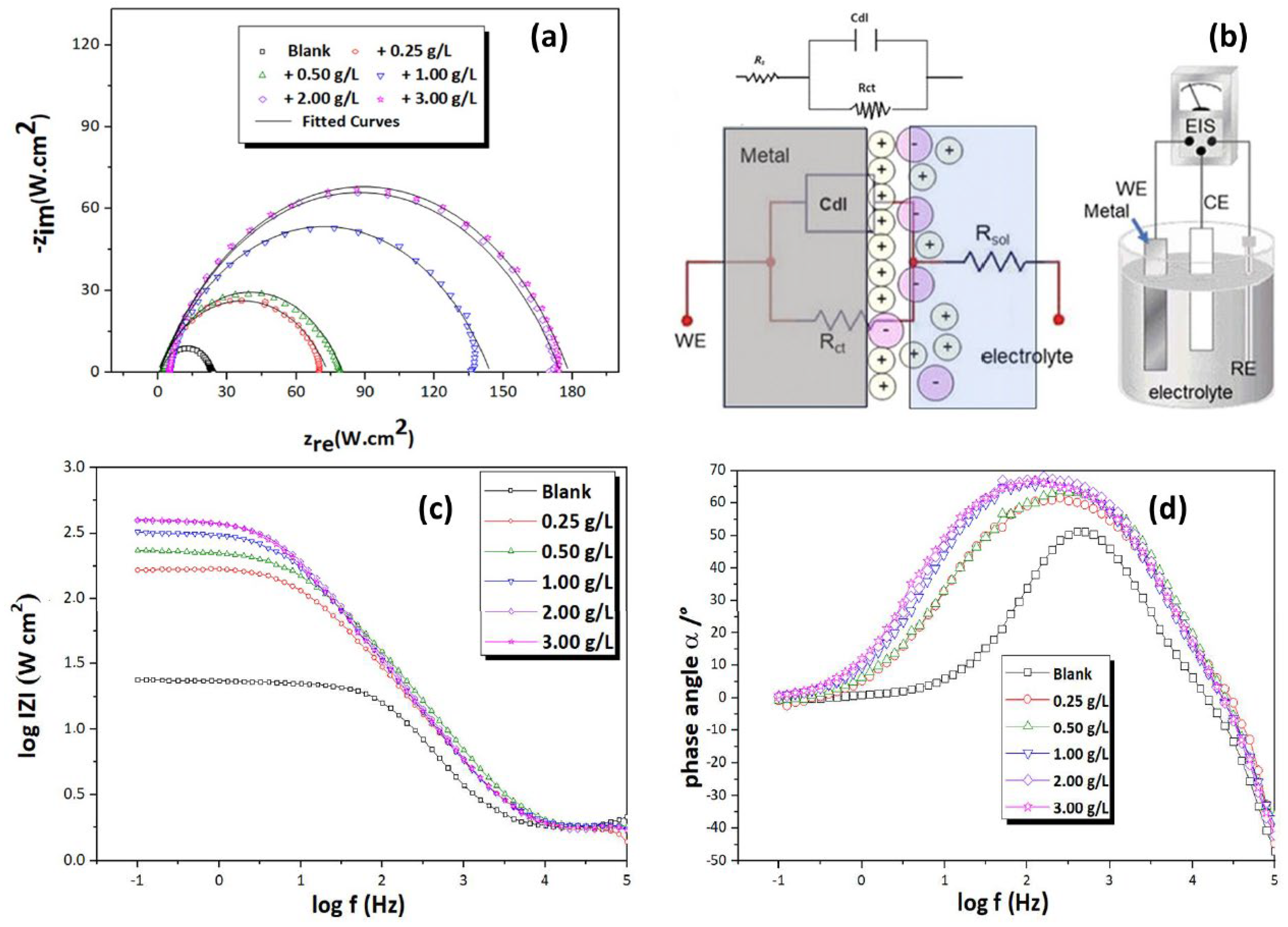
3.4. EIS Measurements
3.5. Adsorption Study
3.6. SEM Analysis
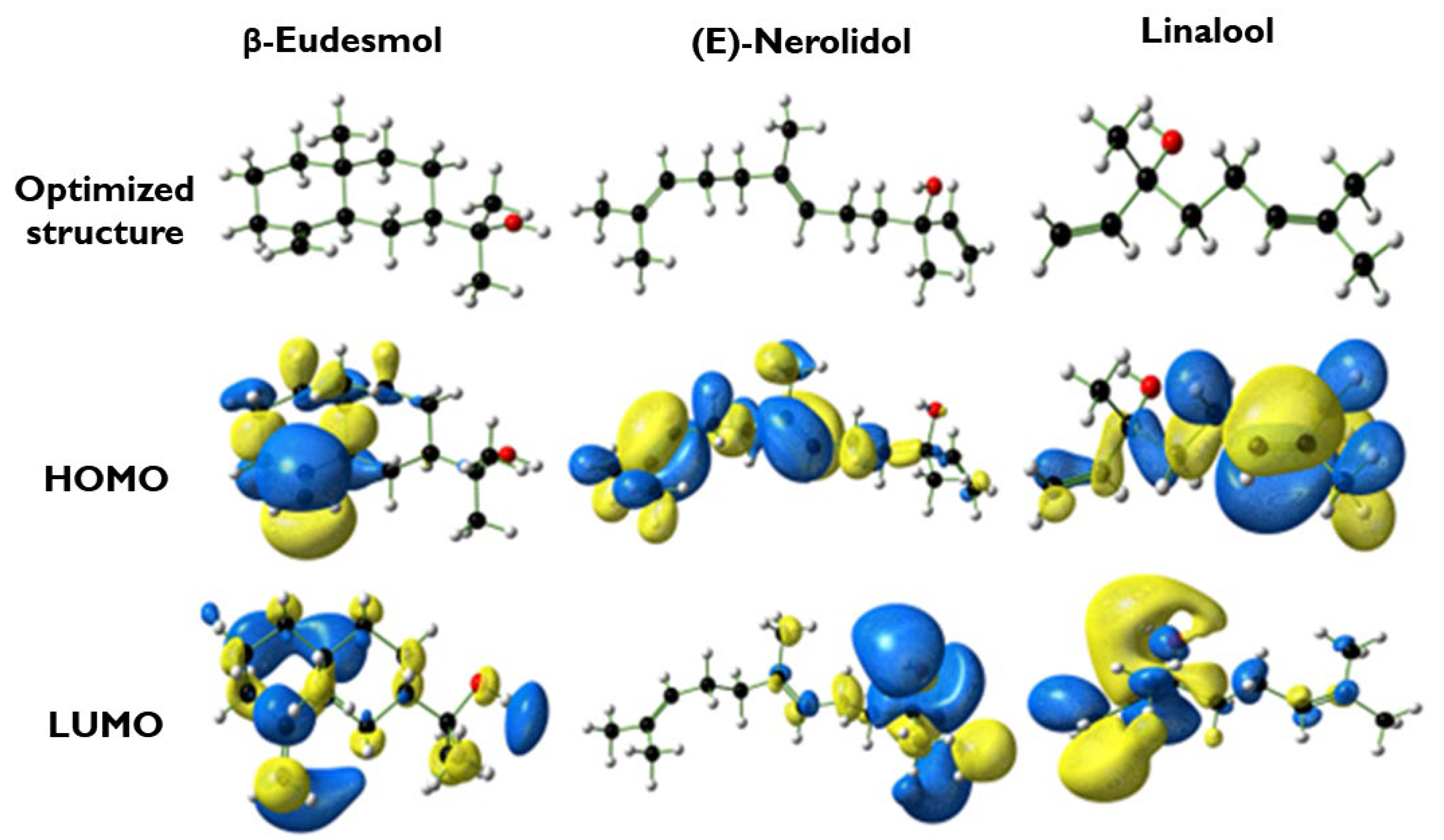
3.7. Theoretical Studies
3.7.1. DFT Calculations
3.7.2. MD Simulations
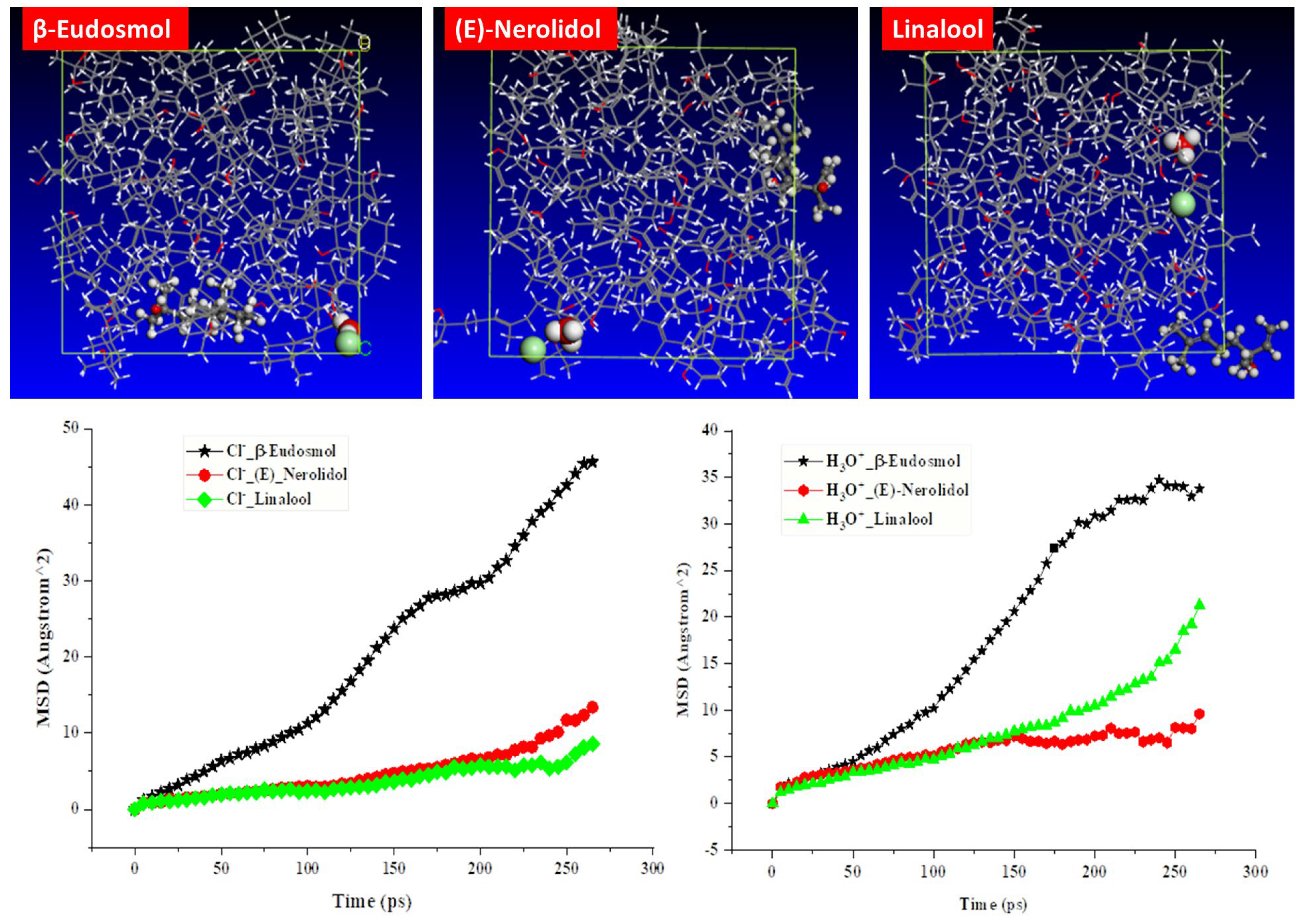
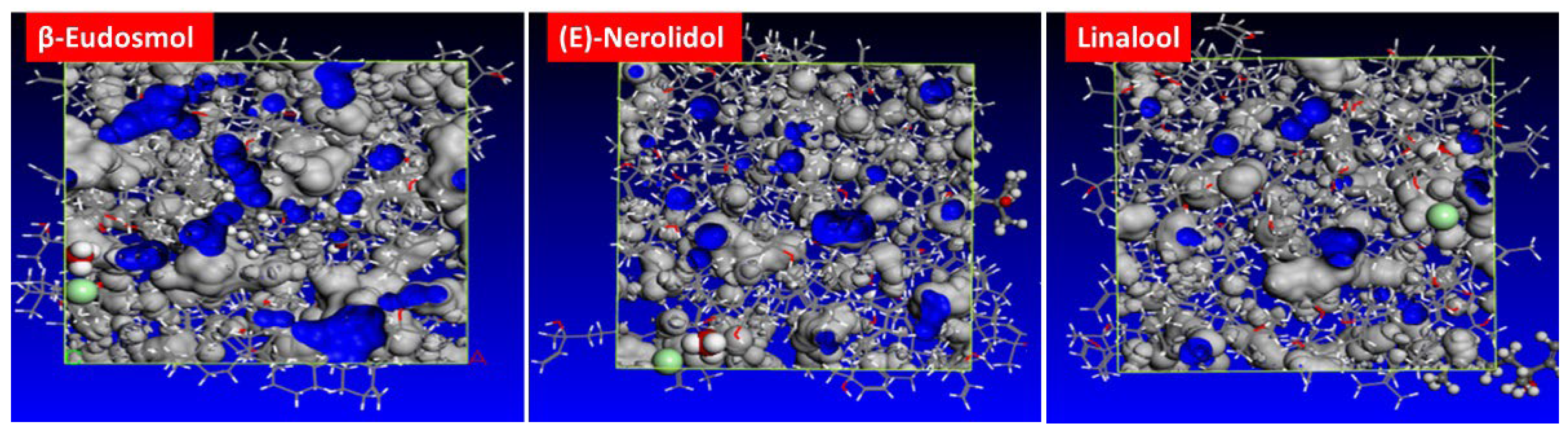
3.7.3. MSD Analysis
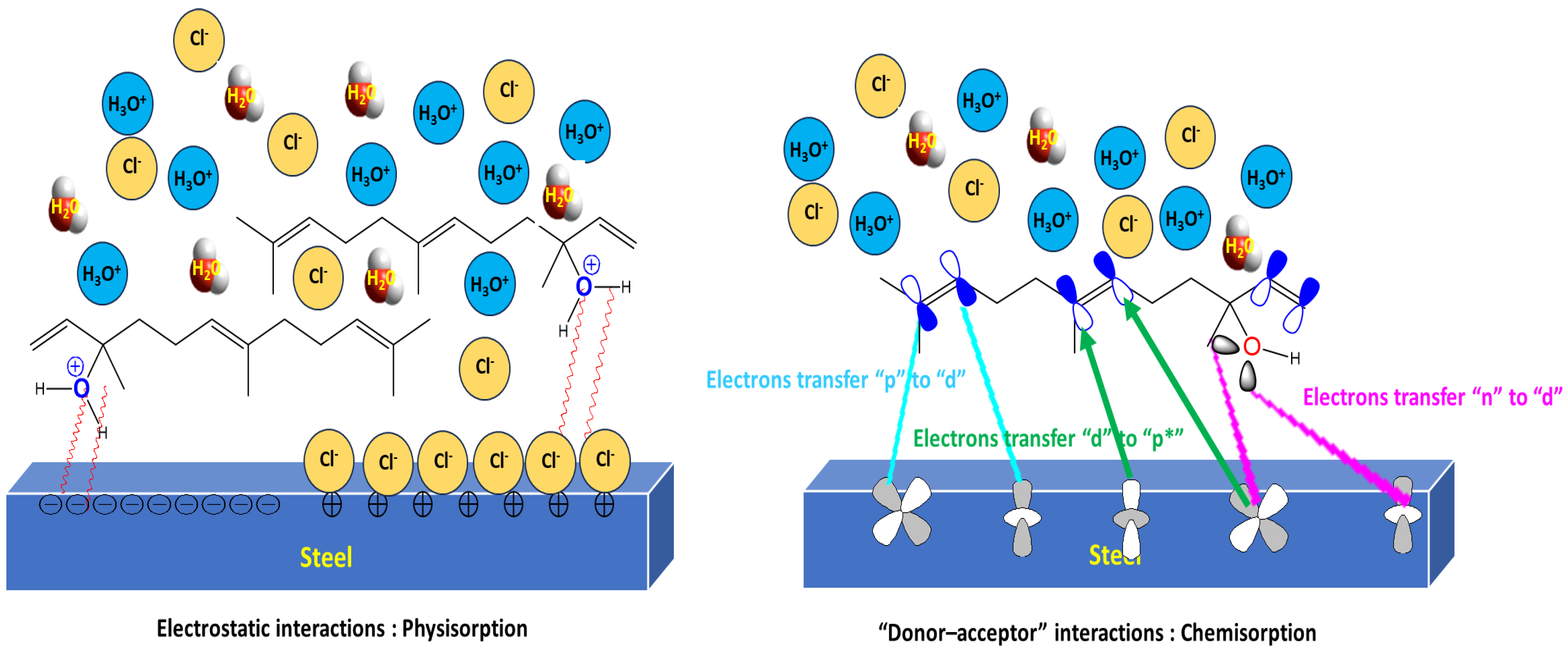
3.8. Corrosion Inhibition Mechanism of WSEO
4. Conclusions
- WSEO has been found to provide a satisfactory IE% of 86% at 2.00 g/L and 298 K, which suggests that it can be used as a good green corrosion inhibitor for MS in 1 M HCl medium.
- The PDP study demonstrated that the examined inhibitor exerts a mixed inhibition effect and with increasing WSEO concentrations, the icorr values dropped dramatically from 578.0 µA cm−2 in a blank solution to 92.4 µA cm−2 in the presence of 2.00 g/L, indicating significant corrosion inhibition potential.
- Based on the EIS data, Rct values rise with rising WSEO concentration reaching the optimal value of 165.8 Ω cm2 at 2.00 g/L, indicating the adsorption of inhibitor and the development of a protective layer at the MS/solution interface.
- The thermodynamic study revealed that the adsorption of WSEO on the MS surface aligns with the Langmuir isotherm model involving both chemical and physical adsorption.
- DFT calculations provided a detailed explanation of the relationship between the inhibitory efficacy of WSEO and the electronic properties of its main constituents.
- Monte Carlo (MC) simulations indicated that the primary compounds preferentially adsorb onto the Fe (110) surface in a flat, parallel orientation.
- The MSD calculations reveal that the main component of the studied inhibitor creates a barrier film on the MS surface and limits the migration of corrosive species. SEM analysis confirms the existence of an inhibitor protective film on the MS steel surface.
- The computational results also revealed that (E)-Nerolidol may play an important role in the overall inhibitory action of WSEO in 1 M HCl.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lavanya, D.; Priya, F.V.; Vijaya, D. Green Approach to Corrosion Inhibition of Mild Steel in Hydrochloric Acid by 1-[Morpholin-4-yl (thiophen-2-yl) methyl] thiourea. J. Fail. Anal. Prev. 2020, 20, 494–502. [Google Scholar] [CrossRef]
- Obot, I.; Meroufel, A.; Onyeachu, I.B.; Alenazi, A.; Sorour, A.A. Corrosion inhibitors for acid cleaning of desalination heat exchangers: Progress, challenges and future perspectives. J. Mol. Liq. 2019, 296, 111760. [Google Scholar] [CrossRef]
- Mzioud, K.; Habsaoui, A.; Ouakki, M.; Galai, M.; El Fartah, S.; Ebn Touhami, M. Inhibition of copper corrosion by the essential oil of Allium sativum in 0.5 M H2SO4 solutions. SN Appl. Sci. 2020, 2, 1611. [Google Scholar] [CrossRef]
- Znini, M. Application of Essential Oils as green corrosion inhibitors for metals and alloys in different aggressive mediums. Arab. J. Med. Aromat. Plants 2019, 5, 1–34. [Google Scholar]
- Sultana, N.; Hossain, S.; Mohammed, M.E.; Irfan, M.; Haq, B.; Faruque, M.; Razzak, S.; Hossain, M. Experimental study and parameters optimization of microalgae based heavy metals removal process using a hybrid response surface methodology-crow search algorithm. Sci. Rep. 2020, 10, 15068. [Google Scholar] [CrossRef]
- Mellak, N.; Ghali, N.; Messaoudi, N.; Benhelima, A.; Ferhat, M.; Addou, A. Study of corrosion inhibition properties of Schinus molle essential oil on carbon steel in HCl. Mater. Corros. 2021, 72, 1270–1278. [Google Scholar] [CrossRef]
- Manssouri, M.; Znini, M.; El Ouadi, Y.; Ansari, A.; Costa, J.; Majidi, L. Essential Oil of Aaronsohnia Pubescens Subsp. Pubescens as Novel Eco-Friendly Inhibitor for Mild Steel in 1.0 M HCl. Anal. Bioanal. Electrochem. 2020, 12, 841–856. [Google Scholar]
- Bui, H.T.; Dang, T.-D.; Le, H.T.; Hoang, T.T. Comparative study on corrosion inhibition of vietnam orange peel essential oil with urotropine and insight of corrosion inhibition mechanism for mild steel in hydrochloric solution. J. Electrochem. Sci. Technol. 2019, 10, 69–81. [Google Scholar]
- Znini, M.; Ansari, A.; Costa, J.; Senhaji, O.; Paolini, J.; Majidi, L. Experimental, quantum chemical and molecular dynamic simulations studies on the corrosion inhibition of c38 steel in 1M HCl by Anethum graveolens essential oil. Anal. Bioanal. Electrochem. 2019, 11, 1426–1451. [Google Scholar]
- Saha, S.K.; Murmu, M.; Murmu, N.C.; Obot, I.; Banerjee, P. Molecular level insights for the corrosion inhibition effectiveness of three amine derivatives on the carbon steel surface in the adverse medium: A combined density functional theory and molecular dynamics simulation study. Surf. Interfaces 2018, 10, 65–73. [Google Scholar] [CrossRef]
- Znini, M.; Majidi, L.; Laghchimi, A.; Paolini, J.; Hammouti, B.; Costa, J.; Bouyanzer, A.; Al-Deyab, S. Chemical composition and anticorrosive activity of Warionia saharea essential oil against the corrosion of mild steel in 0.5 M H2SO4. Int. J. Electrochem. Sci. 2011, 6, 5940–5955. [Google Scholar] [CrossRef]
- Frisch, M. Gaussian. 2009. Available online: http://www.gaussian.com/ (accessed on 21 July 2023).
- Umar, B.A.; Uzairu, A. In-silico approach to understand the inhibition of corrosion by some potent triazole derivatives of pyrimidine for steel. SN Appl. Sci. 2019, 1, 1413. [Google Scholar] [CrossRef]
- El Ibrahimi, B.; Jmiai, A.; El Mouaden, K.; Baddouh, A.; El Issami, S.; Bazzi, L.; Hilali, M. Effect of solution’s pH and molecular structure of three linear α-amino acids on the corrosion of tin in salt solution: A combined experimental and theoretical approach. J. Mol. Struct. 2019, 1196, 105–118. [Google Scholar] [CrossRef]
- Murmu, M.; Saha, S.K.; Murmu, N.C.; Banerjee, P. Effect of stereochemical conformation into the corrosion inhibitive behaviour of double azomethine based Schiff bases on mild steel surface in 1 mol L−1 HCl medium: An experimental, density functional theory and molecular dynamics simulation study. Corros. Sci. 2019, 146, 134–151. [Google Scholar] [CrossRef]
- Errahmany, N.; Rbaa, M.; Abousalem, A.S.; Tazouti, A.; Galai, M.; Touhami, M.E.; Lakhrissi, B.; Touir, R. Experimental, DFT calculations and MC simulations concept of novel quinazolinone derivatives as corrosion inhibitor for mild steel in 1.0 M HCl medium. J. Mol. Liq. 2020, 312, 113413. [Google Scholar] [CrossRef]
- Haldhar, R.; Kim, S.-C.; Prasad, D.; Bedair, M.; Bahadur, I.; Kaya, S.; Dagdag, O.; Guo, L. Papaver somniferum as an efficient corrosion inhibitor for iron alloy in acidic condition: DFT, MC simulation, LCMS and electrochemical studies. J. Mol. Struct. 2021, 1242, 130822. [Google Scholar] [CrossRef]
- Ouakki, M.; Galai, M.; Aribou, Z.; Benzekri, Z.; Dahmani, K.; Ech-chihbi, E.; Abousalem, A.S.; Boukhris, S.; Cherkaoui, M. Detailed experimental and computational explorations of pyran derivatives as corrosion inhibitors for mild steel in 1.0 M HCl: Electrochemical/surface studies, DFT modeling, and MC simulation. J. Mol. Struct. 2022, 1261, 132784. [Google Scholar] [CrossRef]
- Berisha, A. Ab inito exploration of nanocars as potential corrosion inhibitors. Comput. Theor. Chem. 2021, 1201, 113258. [Google Scholar] [CrossRef]
- Kalajahi, S.T.; Mofradnia, S.R.; Yazdian, F.; Rasekh, B.; Neshati, J.; Taghavi, L.; Pourmadadi, M.; Haghirosadat, B.F. Inhibition performances of graphene oxide/silver nanostructure for the microbial corrosion: Molecular dynamic simulation study. Environ. Sci. Pollut. Res. 2022, 29, 49884–49897. [Google Scholar] [CrossRef]
- Benhiba, F.; Hsissou, R.; Abderrahim, K.; Serrar, H.; Rouifi, Z.; Boukhris, S.; Kaichouh, G.; Bellaouchou, A.; Guenbour, A.; Oudda, H. Development of New pyrimidine derivative inhibitor for mild steel corrosion in acid medium. J. Bio-Tribo-Corros. 2022, 8, 36. [Google Scholar] [CrossRef]
- Benhiba, F.; Hsissou, R.; Benzekri, Z.; Echihi, S.; El-Blilak, J.; Boukhris, S.; Bellaouchou, A.; Guenbour, A.; Oudda, H.; Warad, I. DFT/electronic scale, MD simulation and evaluation of 6-methyl-2-(p-tolyl)-1,4-dihydroquinoxaline as a potential corrosion inhibition. J. Mol. Liq. 2021, 335, 116539. [Google Scholar] [CrossRef]
- Deng, S.; Li, X. Inhibition by Ginkgo leaves extract of the corrosion of steel in HCl and H2SO4 solutions. Corros. Sci. 2012, 55, 407–415. [Google Scholar] [CrossRef]
- Ngouné, B.; Pengou, M.; Nouteza, A.M.; Nanseu-Njiki, C.P.; Ngameni, E. Performances of alkaloid extract from Rauvolfia macrophylla Stapf toward corrosion inhibition of C38 steel in acidic media. ACS Omega 2019, 4, 9081–9091. [Google Scholar] [CrossRef] [PubMed]
- Dhouibi, I.; Masmoudi, F.; Bouaziz, M.; Masmoud, M. A study of the anti-corrosive effects of essential oils of rosemary and myrtle for copper corrosion in chloride media. Arab. J. Chem. 2021, 14, 102961. [Google Scholar] [CrossRef]
- Sathiya Priya, A.; Muralidharan, V.; Subramania, A. Development of novel acidizing inhibitors for carbon steel corrosion in 15% boiling hydrochloric acid. Corrosion 2008, 64, 541–552. [Google Scholar] [CrossRef]
- Zarrouk, A.; Zarrok, H.; Ramli, Y.; Bouachrine, M.; Hammouti, B.; Sahibed-Dine, A.; Bentiss, F. Inhibitive properties, adsorption and theoretical study of 3,7-dimethyl-1-(prop-2-yn-1-yl) quinoxalin-2 (1H)-one as efficient corrosion inhibitor for carbon steel in hydrochloric acid solution. J. Mol. Liq. 2016, 222, 239–252. [Google Scholar] [CrossRef]
- Pavithra, M.; Venkatesha, T.; Kumar, M.P.; Tondan, H. Inhibition of mild steel corrosion by Rabeprazole sulfide. Corros. Sci. 2012, 60, 104–111. [Google Scholar] [CrossRef]
- Bouklah, M.; Elmsellem, H.; Krim, O.; Serdaroğlu, G.; Hammouti, B.; Elidrissi, A.; Kaya, S.; Warad, I. Effect of substitution on corrosion inhibition properties of three Imidazole derivatives on mild steel in 1 M HCl. Arab. J. Chem. Environ. Res. 2020, 7, 126–143. [Google Scholar]
- Bockris, J.M.; Green, M.; Swinkels, D. Adsorption of naphthalene on solid metal electrodes. J. Electrochem. Soc. 1964, 111, 743. [Google Scholar] [CrossRef]
- Wang, P.; Xiong, L.; He, Z.; Xu, X.; Hu, J.; Chen, Q.; Zhang, R.; Pu, J.; Guo, L. Synergistic Effect of Imidazoline Derivative and Benzimidazole as Corrosion Inhibitors for Q235 Steel: An Electrochemical, XPS, FT-IR and MD Study. Arab. J. Sci. Eng. 2022, 47, 7123–7134. [Google Scholar] [CrossRef]
- Kowsari, E.; Payami, M.; Amini, R.; Ramezanzadeh, B.; Javanbakht, M. Task-specific ionic liquid as a new green inhibitor of mild steel corrosion. Appl. Surf. Sci. 2014, 289, 478–486. [Google Scholar] [CrossRef]
- Zehra, B.F.; Said, A.; Eddine, H.M.; Hamid, E.; Najat, H.; Rachid, N.; Toumert, L.I. Crataegus oxyacantha leaves extract for carbon steel protection against corrosion in 1M HCl: Characterization, electrochemical, theoretical research, and surface analysis. J. Mol. Struct. 2022, 1259, 132737. [Google Scholar] [CrossRef]
- Adewuyi, A.; Oluwaseyifunmi, A.; Kaki, S.S.; Oderinde, R.A. Synthesis of fatty phenylthiosemicarbazide from underutilized Sesamum indicum seed oil: A promising corrosion inhibitor of carbon steel in developing country. SN Appl. Sci. 2019, 1, 637. [Google Scholar] [CrossRef]
- Verma, C.; Obot, I.; Bahadur, I.; Sherif, E.-S.M.; Ebenso, E.E. Choline based ionic liquids as sustainable corrosion inhibitors on mild steel surface in acidic medium: Gravimetric, electrochemical, surface morphology, DFT and Monte Carlo simulation studies. Appl. Surf. Sci. 2018, 457, 134–149. [Google Scholar] [CrossRef]
- Salman, T.A.; Zinad, D.S.; Jaber, S.H.; Al-Ghezi, M.; Mahal, A.; Takriff, M.S.; Al-Amiery, A.A. Effect of 1,3,4-thiadiazole scaffold on the corrosion inhibition of mild steel in acidic medium: An experimental and computational study. J. Bio-Tribo-Corros. 2019, 5, 48. [Google Scholar] [CrossRef]
- Zhang, X.; Kang, Q.; Wang, Y. Theoretical study of N-thiazolyl-2-cyanoacetamide derivatives as corrosion inhibitor for aluminum in alkaline environments. Comput. Theor. Chem. 2018, 1131, 25–32. [Google Scholar] [CrossRef]
- Hu, S.-Q.; Guo, A.-L.; Yan, Y.-G.; Jia, X.-L.; Geng, Y.-F.; Guo, W.-Y. Computer simulation of diffusion of corrosive particle in corrosion inhibitor membrane. Comput. Theor. Chem. 2011, 964, 176–181. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.; Chauhan, D.S.; Quraishi, M.; Lgaz, H.; Chung, I.-M. Comprehensive investigation of steel corrosion inhibition at macro/micro level by ecofriendly green corrosion inhibitor in 15% HCl medium. J. Colloid Interface Sci. 2020, 560, 225–236. [Google Scholar] [CrossRef]
- Hsissou, R.; Benhiba, F.; Dagdag, O.; El Bouchti, M.; Nouneh, K.; Assouag, M.; Briche, S.; Zarrouk, A.; Elharfi, A. Development and potential performance of prepolymer in corrosion inhibition for carbon steel in 1.0 M HCl: Outlooks from experimental and computational investigations. J. Colloid Interface Sci. 2020, 574, 43–60. [Google Scholar] [CrossRef]
- Fernine, Y.; Arrousse, N.; Haldhar, R.; Raorane, C.J.; Kim, S.-C.; El Hajjaji, F.; Touhami, M.E.; Beniken, M.; Haboubi, K.; Taleb, M. Synthesis and characterization of phenolphthalein derivatives, detailed theoretical DFT computation/molecular simulation, and prevention of AA2024-T3 corrosion in medium 3.5% NaCl. J. Taiwan Inst. Chem. Eng. 2022, 140, 104556. [Google Scholar] [CrossRef]




| CWSEO (g/L) | 0.00 | 0.25 | 0.50 | 1.00 | 2.00 | 3.00 |
| Wcorr (mg·cm−2h−1) | 0.381 | 0.098 | 0.090 | 0.076 | 0.065 | 0.063 |
| IEWL (%) | - | 74.15 | 76.36 | 79.96 | 82.93 | 83.34 |
| Surface coverage (θ) | - | 0.7415 | 0.7636 | 0.7996 | 0.8293 | 0.8334 |
| CWSEO (g/L) | −Ecorr (mV/SCE) | icorr (µA cm−2) | −βc (mV dec−1) | βa (mV dec−1) | IEPDP (%) | θ |
|---|---|---|---|---|---|---|
| 0.00 | 490 | 578.0 | 147.7 | 74.8 | - | - |
| 0.25 | 490 | 152.5 | 157.3 | 56.7 | 73.62 | 0.7362 |
| 0.50 | 490 | 130.6 | 149.8 | 53.6 | 77.40 | 0.7740 |
| 1.00 | 480 | 101.6 | 144.1 | 53.2 | 82.42 | 0.8242 |
| 2.00 | 480 | 92.4 | 140.8 | 49.9 | 84.01 | 0.8401 |
| 3.00 | 480 | 91.8 | 138.9 | 48.6 | 84.11 | 0.8411 |
| CWSEO (g/L) | Rct (Ω cm2) | Cdl (µF cm2) | CPE | IEEIS (%) | θ | χ2 (10−3) | |
|---|---|---|---|---|---|---|---|
| Q (Ω−1Sncm−2) | n | ||||||
| 0.00 | 21.71 | 73.37 | 314.51 | 0.877 | - | - | 2.25 |
| 0.25 | 69.1 | 52.52 | 219.23 | 0.865 | 68.58 | 0.6858 | 3.52 |
| 0.50 | 80.2 | 48.08 | 118.04 | 0.866 | 72.93 | 0.7293 | 5.40 |
| 1.00 | 134.8 | 42.78 | 96.70 | 0.864 | 83.90 | 0.8390 | 3.25 |
| 2.00 | 165.8 | 38.42 | 82.21 | 0.836 | 86.91 | 0.8691 | 5.25 |
| 3.00 | 166.1 | 37.64 | 82.11 | 0.834 | 86.92 | 0.8792 | 5.41 |
| EO Inhibitor | Medium and Substrate | Concentration | IE (%) | Ref |
|---|---|---|---|---|
| Schinus mole | CS in 1 M HCl | 2.00 g/L | 70% | [6] |
| Vietnam orange peel | MS in 1 M HCl | 4.00 g/L | about 90% | [7] |
| A. pubescens subsp. pubescens | MS in 1 M HCl | 3.00 g/L | 89.88% | [8] |
| Myrtle | Copper in 3% NaCl | 10.00 g/L | 91.88% | [27] |
| Rosemary | 92.54% | |||
| Asteriscus graveolens | MS in 0.5 M H2SO4 | 3.00 g/L | 82.89% | [28] |
| Warionia saharea | MS in 0.5 M H2SO4 | 3.00 g/L | 74% | [11] |
| Warionia saharea | MS in 1 M HCl | 2.00 g/L | 84%–87% | In this work |
| Adsorption | Method | R2 | Kads (L/g) | Isotherm Property | ΔG°ads (KJ·mol−1) |
|---|---|---|---|---|---|
| Langmuir | WL | 1 | 0.79 | RL = 0.39 | −16.55 |
| PDP | 0.9997 | 0.81 | RL = 0.38 | −16.60 | |
| EIS | 0.9998 | 0.80 | RL = 0.38 | −16.58 | |
| El-Awady | WL | 0.9831 | 459.43 | 1/y = 4.54 | −32.33 |
| PDP | 0.9415 | 192.48 | 1/y = 3.65 | −30.18 | |
| EIS | 0.9343 | 18.72 | 1/y = 2.00 | −24.40 | |
| Freundlich | WL | 0.9831 | 0.79 | z = 0.047 | −16.55 |
| PDP | 0.926 | 0.80 | z = 0.056 | −16.58 | |
| EIS | 0.9194 | 0.80 | z = 0.104 | −16.58 | |
| Temkin | WL | 0.9838 | 250 × 106 | a = −13.66 | −65.08 |
| PDP | 0.9303 | 78.6 × 106 | a = −11.28 | −62.22 | |
| EIS | 0.9228 | 19.7 × 103 | A = −6.15 | −41.65 |
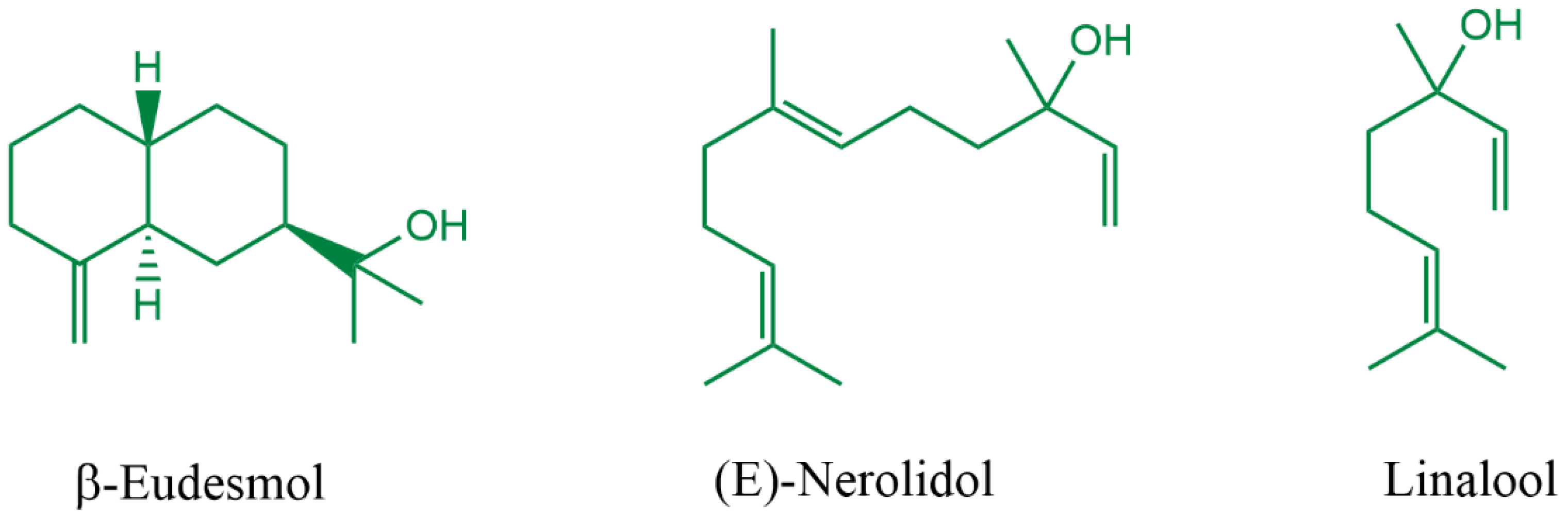
| Molecule | EHOMO (eV) | ELUMO (eV) | ∆Egap (eV) | ∆N | η |
|---|---|---|---|---|---|
| β-Eudesmol | −6.35 | 0.86 | 7.21 | 0.29 | 3.61 |
| (E)-Nerolidol | −6.01 | 0.29 | 6.30 | 0.31 | 3.15 |
| Linalool | −6.36 | 0.56 | 6.92 | 0.28 | 3.46 |
| Molecule | Eads |
|---|---|
| β-Eudesmol | −215.18 |
| (E)-Nerolidol | −258.15 |
| Linalool | −140.60 |
| Molecule | Dion (H3O+) (m2 s−1) | Dion (Cl−) (m2 s−1) | FFV (%) |
|---|---|---|---|
| (E)-Nerolidol | 1.926 × 10−3 | 1.062 × 10−2 | 6.50 |
| Linalool | 1.57 × 10−2 | 5.114 × 10−3 | 6.94 |
| β-Eudesmol | 2.144 × 10−2 | 3.153 × 10−2 | 13.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari, A.; Youssefi, Y.; Tanghourte, M.; Ouassou, N.; Asoufar, N.; Znini, M.; Lgaz, H.; Mabrouk, E.H.; Azrour, M.; Lee, H.-S.; et al. Assessment of Warionia saharea Essential Oil as a Green Corrosion Inhibitor for Mild Steel in HCl: Experimental and Computational Studies. Coatings 2024, 14, 1164. https://doi.org/10.3390/coatings14091164
Ansari A, Youssefi Y, Tanghourte M, Ouassou N, Asoufar N, Znini M, Lgaz H, Mabrouk EH, Azrour M, Lee H-S, et al. Assessment of Warionia saharea Essential Oil as a Green Corrosion Inhibitor for Mild Steel in HCl: Experimental and Computational Studies. Coatings. 2024; 14(9):1164. https://doi.org/10.3390/coatings14091164
Chicago/Turabian StyleAnsari, Abdeslam, Youssef Youssefi, Mohamed Tanghourte, Nazih Ouassou, Nazih Asoufar, Mohamed Znini, Hassane Lgaz, El Houssine Mabrouk, Mohamed Azrour, Han-Seung Lee, and et al. 2024. "Assessment of Warionia saharea Essential Oil as a Green Corrosion Inhibitor for Mild Steel in HCl: Experimental and Computational Studies" Coatings 14, no. 9: 1164. https://doi.org/10.3390/coatings14091164
APA StyleAnsari, A., Youssefi, Y., Tanghourte, M., Ouassou, N., Asoufar, N., Znini, M., Lgaz, H., Mabrouk, E. H., Azrour, M., Lee, H.-S., & Hammouti, B. (2024). Assessment of Warionia saharea Essential Oil as a Green Corrosion Inhibitor for Mild Steel in HCl: Experimental and Computational Studies. Coatings, 14(9), 1164. https://doi.org/10.3390/coatings14091164











