A Novel Approach to Protect Brazil Nuts from Lipid Oxidation: Efficacy of Nanocellulose–Tocopherol Edible Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of the CNF
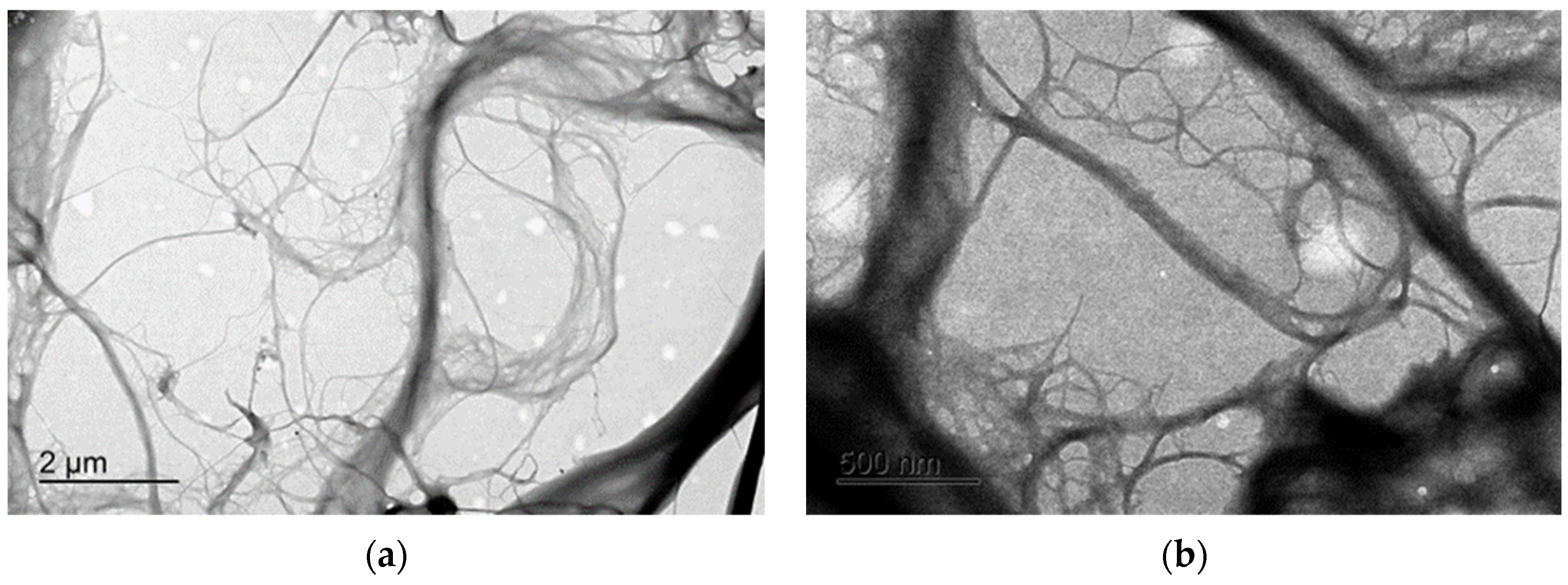
2.2.1. Transmission Electron Microscopy
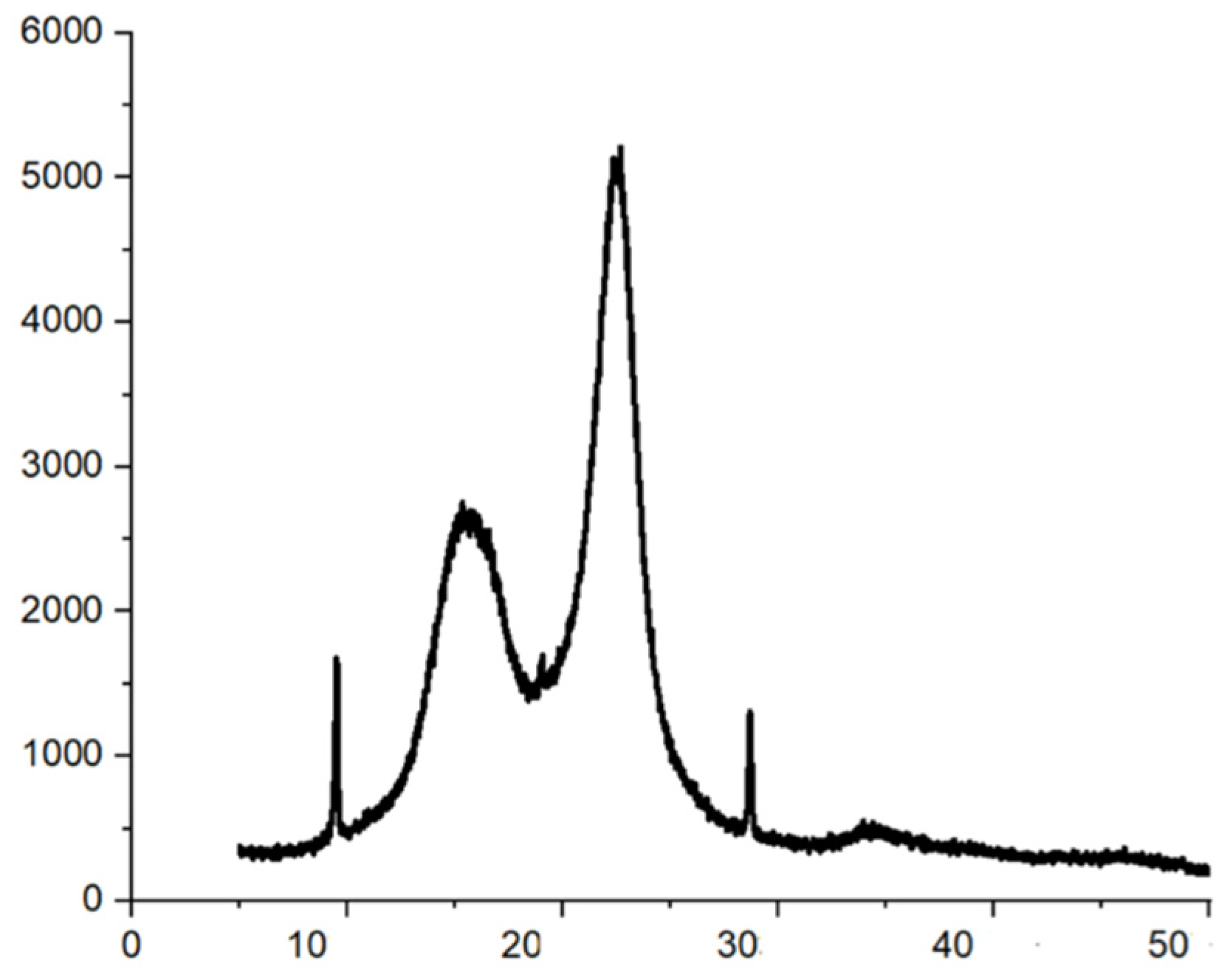
2.2.2. X-ray Diffraction
2.3. Preparation of Filmogenic Solution for Edible Films
2.4. Characterization of Film Solutions and Edible Films
2.4.1. Creaming Index
2.4.2. Water Content
2.4.3. Film Thickness
2.4.4. Color
2.4.5. Optical Properties of Light Transmission
2.4.6. Water Vapor Permeability
2.4.7. Tensile Test
2.4.8. Puncture Test
2.4.9. Biodegradability
2.5. Evaluation of the Edible Coating Applied to Unshelled Brazil Nuts
2.5.1. Edible Coating Application on Brazil Nuts
2.5.2. Accelerated Oxidation Test for Brazil Nuts Protected by Edible Coating
2.5.3. Acid Value (AV)
2.5.4. Peroxide Value (PV)
2.5.5. Conjugated Dienes (CD)
2.6. Statistical Analysis
3. Results
3.1. Fribillated Nanocellulose Characterization
TEM and X-ray Diffraction
3.2. Characterization of the Filmogenic Solution and Edible Films
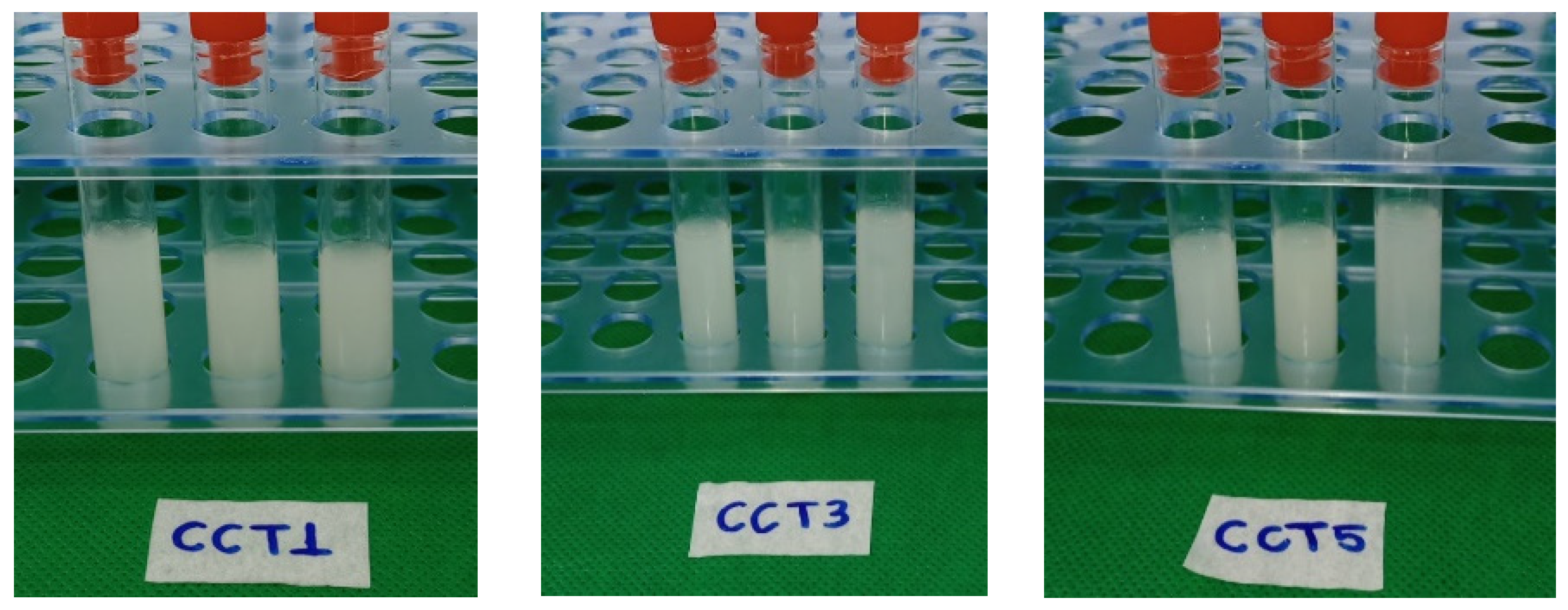
3.2.1. Creaming Index of Filmogenic Solution
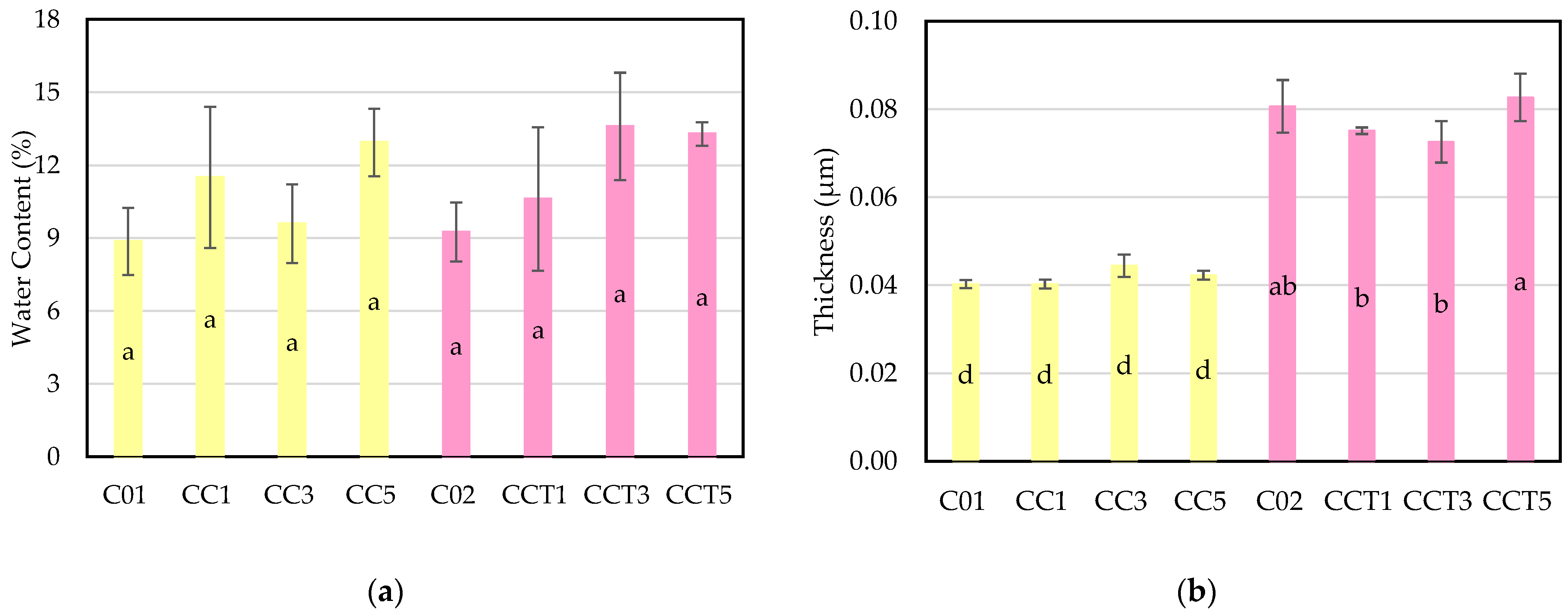
3.2.2. Water Content and Thickness of Films
3.2.3. Color Parameters
3.2.4. Optical Light Transmission Properties
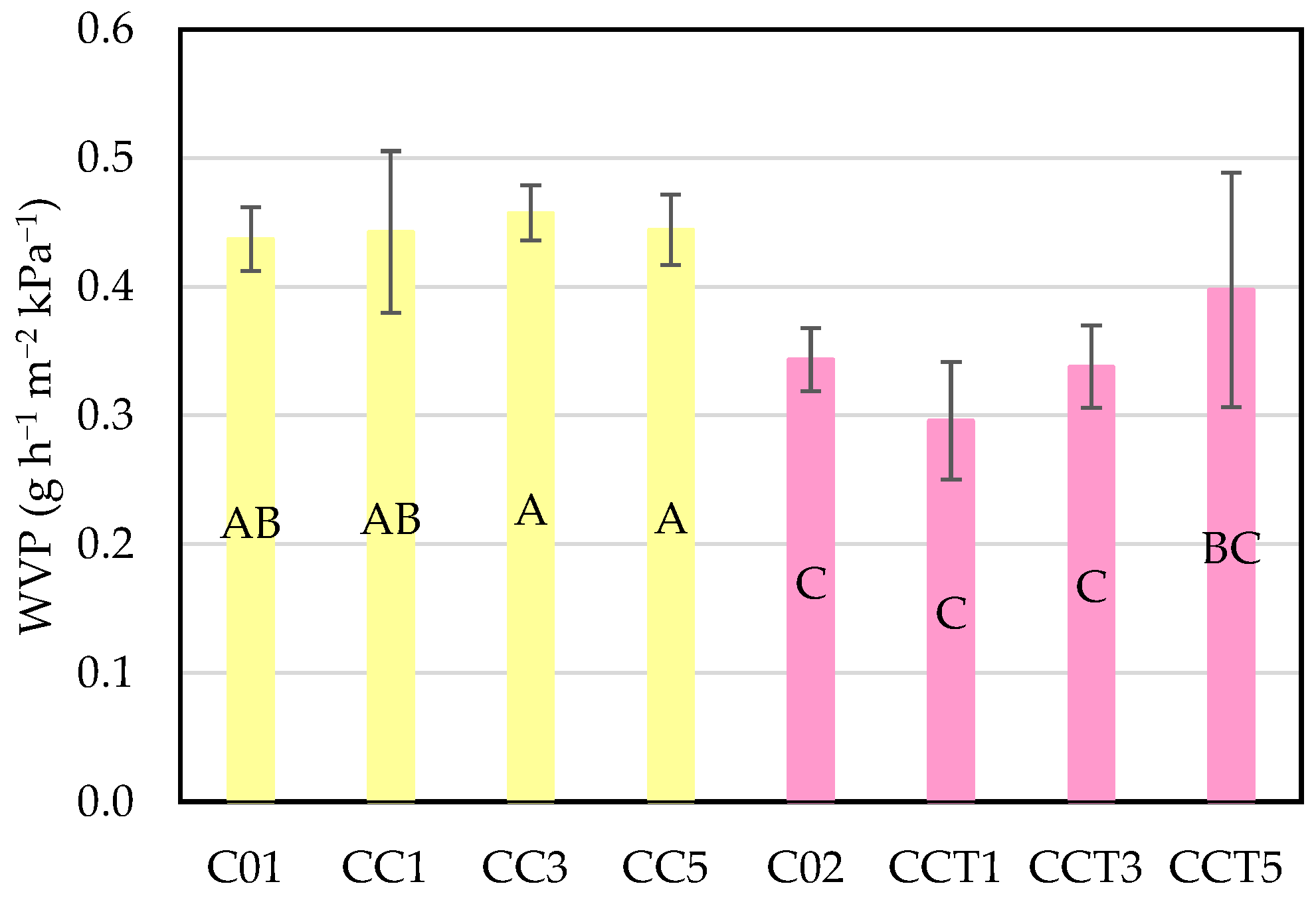
3.2.5. Water Vapor Permeability of Edible Films
3.2.6. Tensile Test of Film
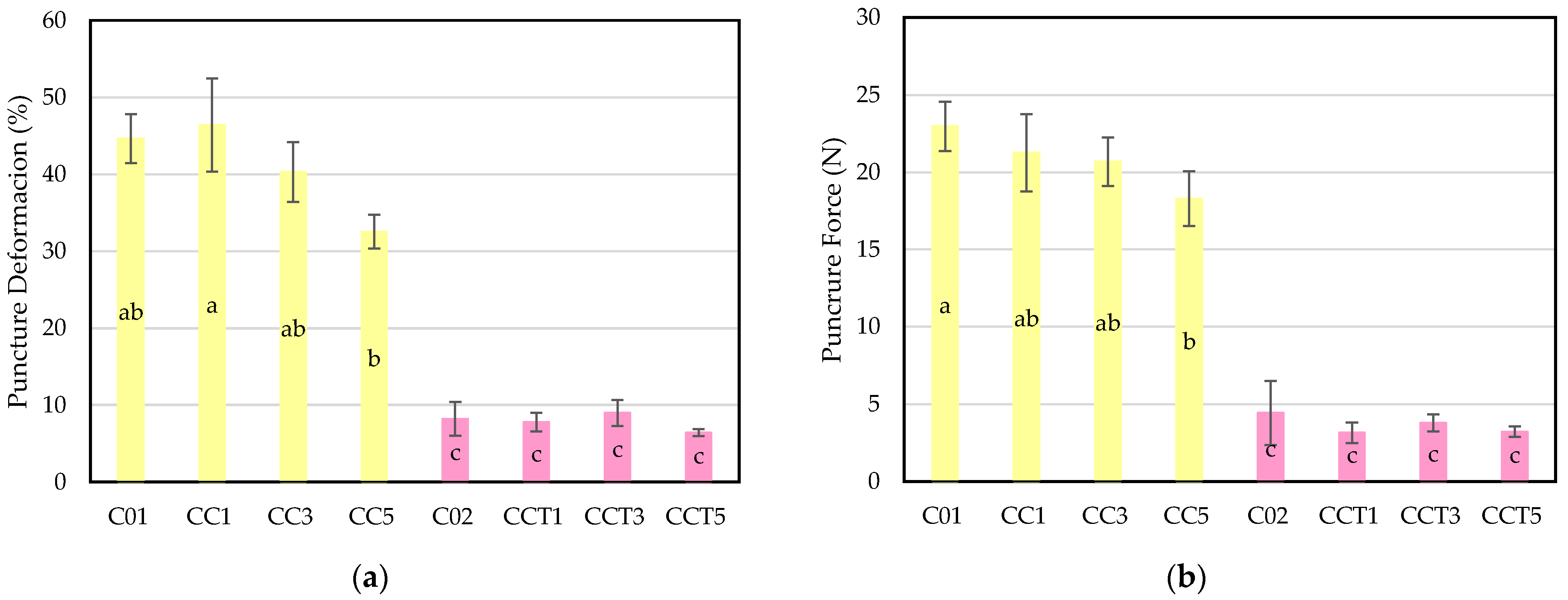
3.2.7. Puncture Test of Film
3.2.8. Biodegradability
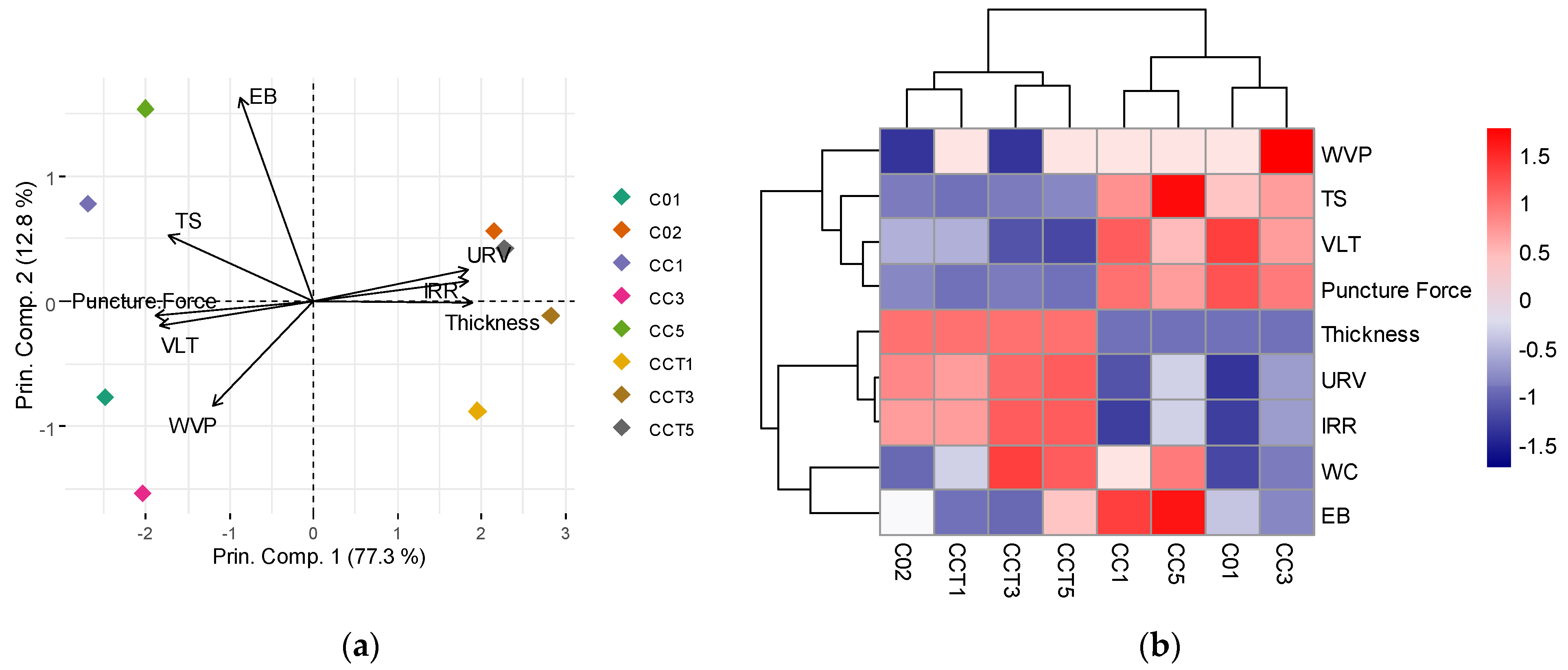
3.2.9. Principal Component Analysis and Hierarchical Clustering
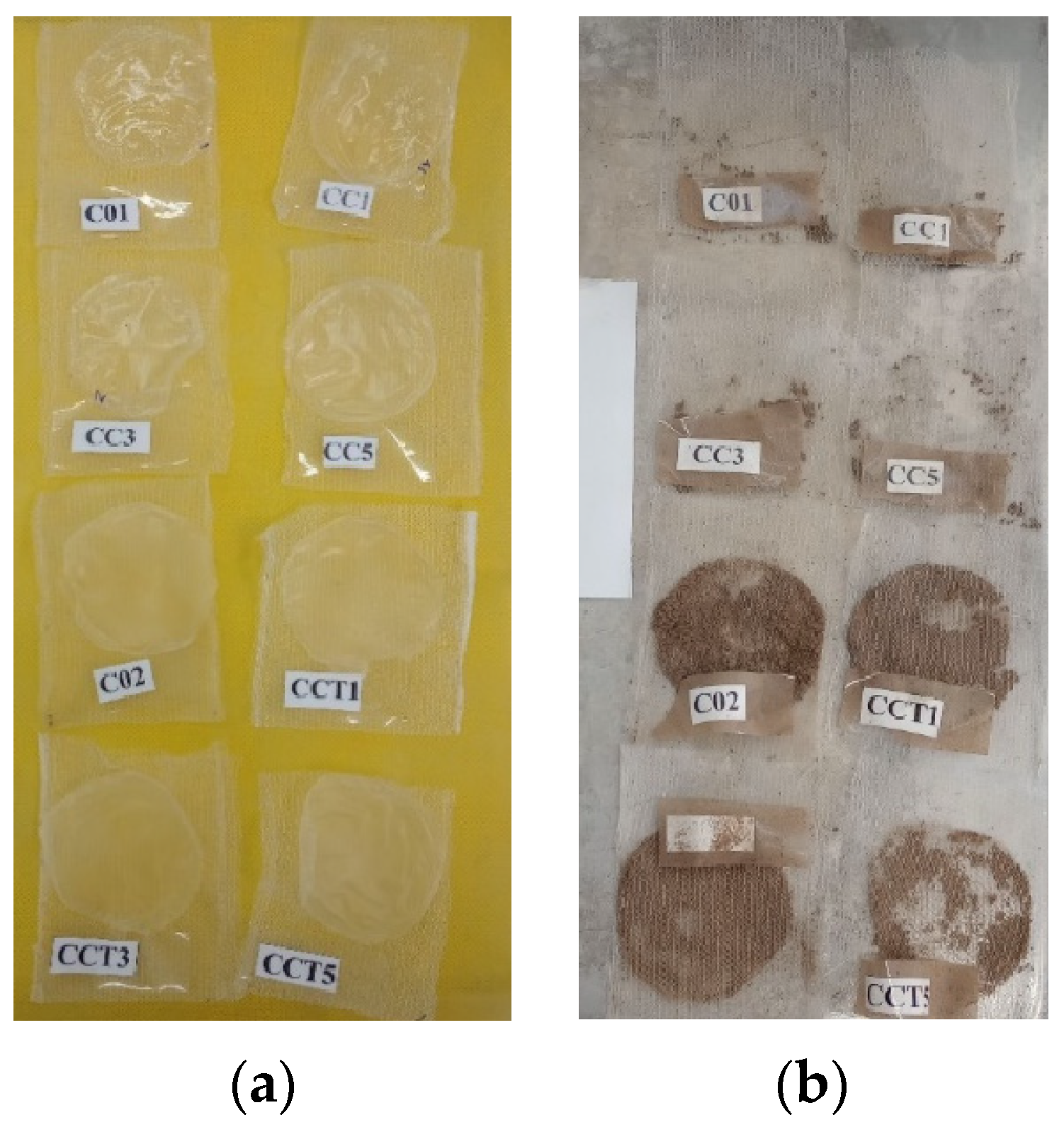
3.3. Comparative Analysis of Lipid Oxidation in Coated and Uncoated Brazil Nuts
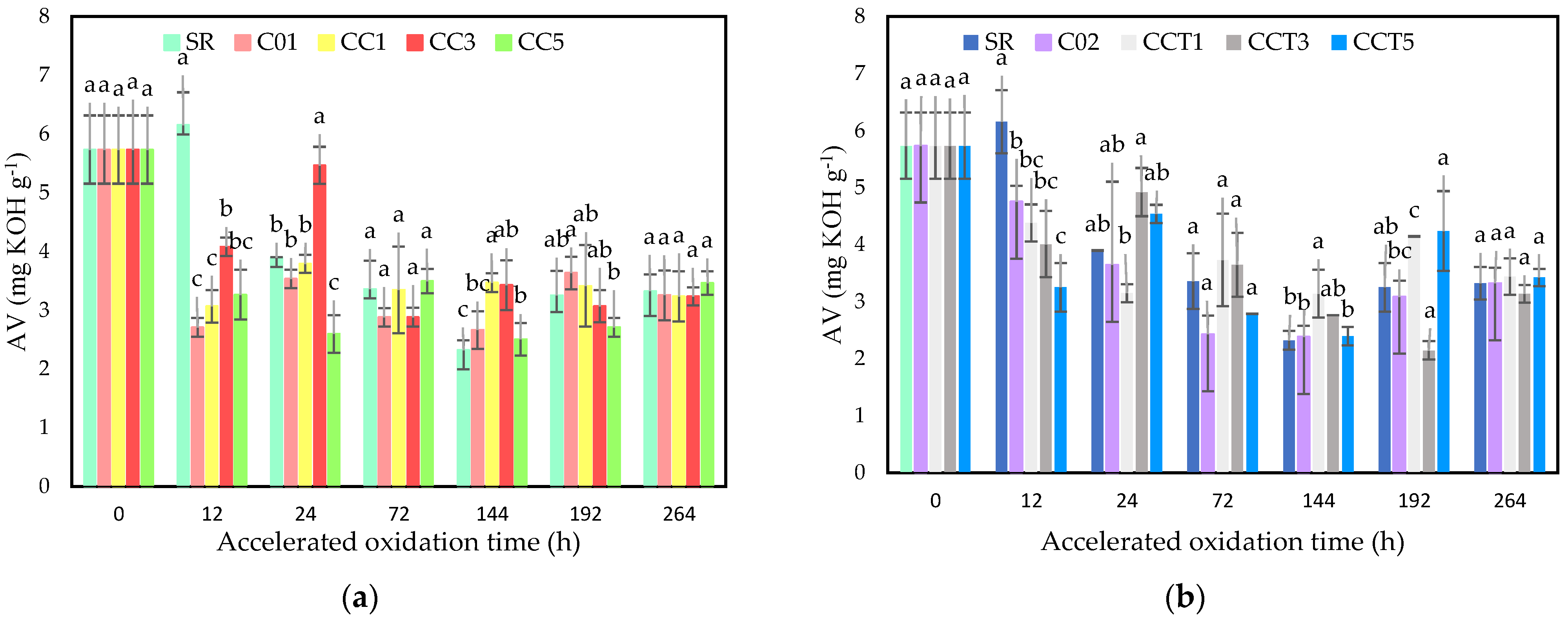
3.3.1. Acidity Index
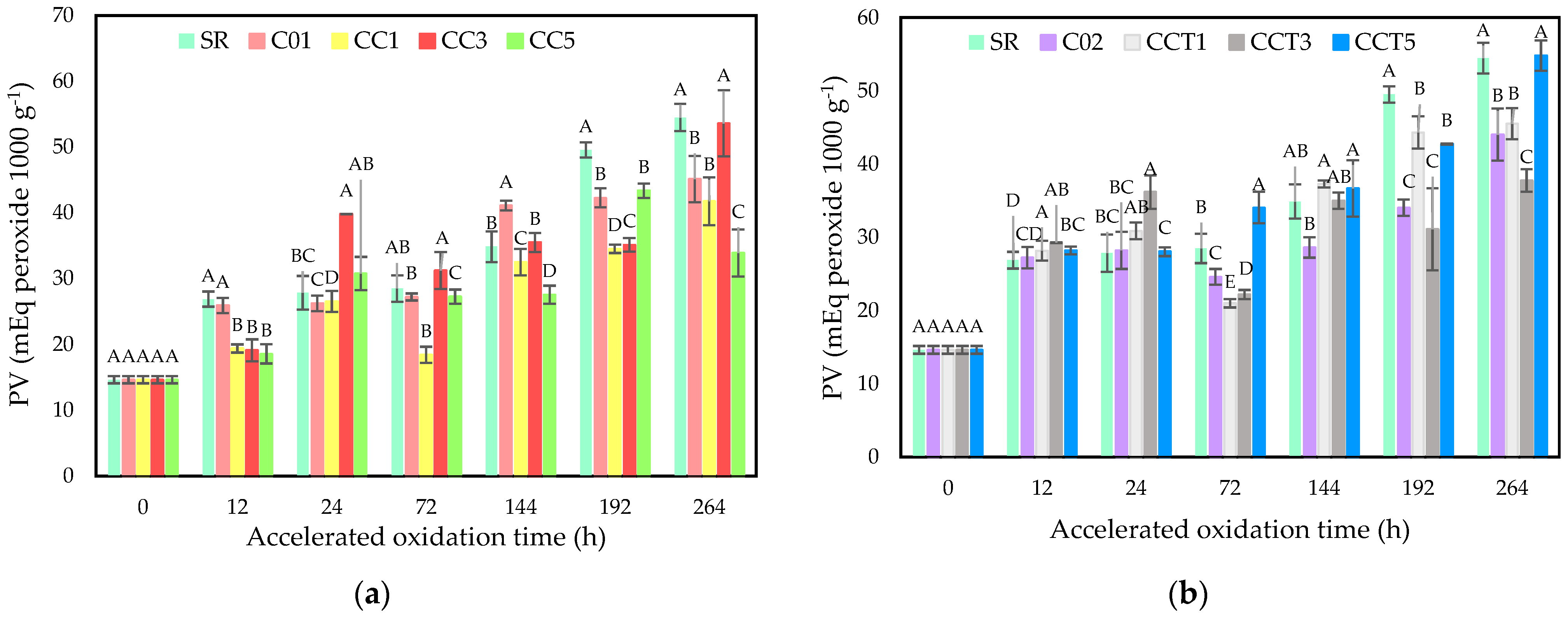
3.3.2. Peroxide Value (PV)
3.3.3. Conjugated Dienes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Declaration of Generative AI and AI-Assisted Technologies in the Writing Process
References
- Cardoso, B.R.; Duarte, G.B.S.; Reis, B.Z.; Cozzolino, S.M.F. Brazil Nuts: Nutritional Composition, Health Benefits and Safety Aspects. Food Res. Int. 2017, 100, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Alcântara, D.B.; Dionísio, A.P.; Artur, A.G.; Silveira, B.K.S.; Lopes, A.F.; Guedes, J.A.C.; Luz, L.R.; Nascimento, R.F.; Lopes, G.S.; Hermsdorff, H.H.M.; et al. Selenium in Brazil Nuts: An Overview of Agronomical Aspects, Recent Trends in Analytical Chemistry, and Health Outcomes. Food Chem. 2022, 372, 131207. [Google Scholar] [CrossRef] [PubMed]
- Au, A.; Mojadadi, A.; Shao, J.Y.; Ahmad, G.; Witting, P.K. Physiological Benefits of Novel Selenium Delivery via Nanoparticles. Int. J. Mol. Sci. 2023, 24, 6068. [Google Scholar] [CrossRef]
- López-De-Dicastillo, C.; Pezo, D.; Nerín, C.; López-Carballo, G.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Reducing Oxidation of Foods through Antioxidant Active Packaging Based on Ethyl Vinyl Alcohol and Natural Flavonoids. Packag. Technol. Sci. 2012, 25, 457–466. [Google Scholar] [CrossRef]
- Machado, M.; Rodriguez-Alcalá, L.M.; Gomes, A.M.; Pintado, M. Vegetable Oils Oxidation: Mechanisms, Consequences and Protective Strategies. Food Rev. Int. 2023, 39, 4180–4197. [Google Scholar] [CrossRef]
- Medina-Meza, I.G.; Barnaba, C.; Barbosa-Cánovas, G.V. Effects of High Pressure Processing on Lipid Oxidation: A Review. Innov. Food Sci. Emerg. 2014, 22, 1–10. [Google Scholar] [CrossRef]
- Albertos, I.; Martin-Diana, A.B.; Burón, M.; Rico, D. Development of Functional Bio-Based Seaweed (Himanthalia elongata and Palmaria palmata) Edible Films for Extending the Shelflife of Fresh Fish Burgers. Food Packag. Shelf Life 2019, 22, 100382. [Google Scholar] [CrossRef]
- Riveros, C.G.; Nepote, V.; Grosso, N.R. Thyme and Basil Essential Oils Included in Edible Coatings as a Natural Preserving Method of Oilseed Kernels. J. Sci. Food Agric. 2016, 96, 183–191. [Google Scholar] [CrossRef]
- Riveros, C.G.; Martin, M.P.; Aguirre, A.; Grosso, N.R. Film Preparation with High Protein Defatted Peanut Flour: Characterisation and Potential Use as Food Packaging. Int. J. Food Sci. Technol. 2018, 53, 969–975. [Google Scholar] [CrossRef]
- Gniewosz, M.; Pobiega, K.; Kraśniewska, K.; Synowiec, A.; Chaberek, M.; Galus, S. Characterization and Antifungal Activity of Pullulan Edible Films Enriched with Propolis Extract for Active Packaging. Foods 2022, 15, 2319. [Google Scholar] [CrossRef]
- Kumar, N.; Pratibha, P.; Neeraj, N. Functional Properties of Pomegranate Peel in Edible Coating/Film: A Review. Int. J. Postharvest Technol. Innov. 2020, 7, 205–216. [Google Scholar] [CrossRef]
- Zhong, Y.; Cavender, G.; Zhao, Y. Investigation of Different Coating Application Methods on the Performance of Edible Coatings on Mozzarella Cheese. Food Sci. Technol. 2014, 56, 1–8. [Google Scholar] [CrossRef]
- Nascimento, D.R.; Guerra, I.C.; Mesquita, J.D.A.; Hernande, T.; Takeuchi, K.P. Importância dos revestimentos comestíveis ativos como estratégia para proteção das oleaginosas contra processos de oxidação lipídica encontrados na literatura. Res. Soc. Dev. 2022, 11, 21911629080. [Google Scholar] [CrossRef]
- do Val Siqueira, L.; Arias, C.I.L.F.; Maniglia, B.C.; Tadini, C.C. Starch-Based Biodegradable Plastics: Methods of Production, Challenges and Future Perspectives. Curr. Opin. Food Sci. 2021, 38, 122–130. [Google Scholar] [CrossRef]
- Chacon, X.R.; Contretas-Esquivel, J.C.; Carbo, F.A.; Vega, M.d.l.L.R.; Rodriguez, R.D.P.; Brambila, G.S. Water Vapor Permeability, Mechanical, Optical, and Sensorial Properties of Plasticized Guar Gum Edible Films. Res. Methodol. Food Sci. 2018, 1, 21–37. [Google Scholar]
- Jakubowska, E.; Gierszewska, M.; Szydłowska-Czerniak, A.; Nowaczyk, J.; Olewnik-Kruszkowska, E. Development and Characterization of Active Packaging Films Based on Chitosan, Plasticizer, and Quercetin for Repassed Oil Storage. Food Chem. 2023, 399, 133934. [Google Scholar] [CrossRef]
- Balasubramaniam, S.P.L.; Patel, A.S.; Nayak, B. Surface Modification of Cellulose Nanofiber Film with Fatty Acids for Developing Renewable Hydrophobic Food Packaging. Food Packag. Shelf Life 2020, 26, 100587. [Google Scholar] [CrossRef]
- Hasnida Raja Hashim, R.; Anas Nagoor Gunny, A.; Sung Ting, S.; Helya Iman Kamaludim, N.; Gopinath, S.C.B. The Effect of Nanofillers on the Functional Properties of PLA and Chitosan Based Film. MJAS 2023, 27, 63–73. [Google Scholar]
- Pereda, M.; Dufresne, A.; Aranguren, M.I.; Marcovich, N.E. Polyelectrolyte Films Based on Chitosan/Olive Oil and Reinforced with Cellulose Nanocrystals. Carbohydr. Polym. 2014, 101, 1018–1026. [Google Scholar] [CrossRef]
- Lengowski, E.C.; Franco, T.S.; Viana, L.C.; Bonfatti Júnior, E.A.; de Muñiz, G.I.B. Micro and Nanoengineered Structures and Compounds: Nanocellulose. Cellulose 2023, 30, 10595–10632. [Google Scholar] [CrossRef]
- Lengowski, E.C.; Bonfatti Júnior, E.A.; Simon, L.; de Muñiz, G.I.B.; de Andrade, A.S.; Nisgoski, S.; Klock, U. Different Degree of Fibrillation: Strategy to Reduce Permeability in Nanocellulose-Starch Films. Cellulose 2020, 27, 10855–10872. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Ling, J.K.U.; Tiong, M.; Barhoum, A.; Chan, Y.S.; Acquah, C.; Danquah, M.K. Nanocelluloses: Sources, Types, Unique Properties, Market, and Regulations. In Handbook of Nanocelluloses: Classification, Properties, Fabrication, and Emerging Applications; Barhoum, A., Ed.; Springer: Dublin, Ireland, 2022; pp. 3–34. [Google Scholar]
- Jung, S.; Cui, Y.; Barnes, M.; Satam, C.; Zhang, S.; Chowdhury, R.A.; Adumbumkulath, A.; Sahin, O.; Miller, C.; Sajadi, S.M.; et al. Multifunctional Bio-Nanocomposite Coatings for Perishable Fruits. Adv. Mater. 2020, 32, 1908291. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.S.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A. Preparation and Characterization of PVA/Nanocellulose/Ag Nanocomposite Films for Antimicrobial Food Packaging. Carbohydr. Polym. 2018, 184, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Afonso, C.; Bandarra, N.M.; Nunes, L.; Cardoso, C. Tocopherols in Seafood and Aquaculture Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 128–140. [Google Scholar] [CrossRef]
- Kamal-Eldin, A. Effect of Fatty Acids and Tocopherols on the Oxidative Stability of Vegetable Oils. Eur. J. Lipid Sci. Technol. 2006, 108, 1051–1061. [Google Scholar] [CrossRef]
- Ferreira, L.F.; Figueiredo, L.P.; Martins, M.A.; Luvizaro, L.B.; bLara, B.R.B.D.; Oliveira, C.R.D.; Júnior, M.G.; Tonoli, G.H.D.; Dias, M.V. Active Coatings of Thermoplastic Starch and Chitosan with Alpha-Tocopherol/Bentonite for Special Green Coffee Beans. Int. J. Biol. Macromol. 2021, 170, 810–819. [Google Scholar] [CrossRef]
- Nakazone, P.H. Development of Multifunctional Liquid Crystalline Formulations Containing Titanium Dioxide Nanoparticles and Alpha-Tocopherol; Final Course Work (Pharmacy-Biochemistry); Universidade Estadual Paulista: Araraquara, Brazil, 2012. [Google Scholar]
- List, G.R. Soybean Lecithin: Food, Industrial Uses, and Other Applications. Polar Lip 2015, 1–33. [Google Scholar] [CrossRef]
- Bonilla, J.; Poloni, T.; Sobral, P.J.A. Active Edible Coatings with Boldo Extract Added and Their Application on Nut Products: Reducing the Oxidative Rancidity Rate. Int. J. Food Sci. Technol. 2018, 53, 700–708. [Google Scholar] [CrossRef]
- Leme, C.M.M.; de Carvalho, A.S.; de Carvalho Rodrigues, V.; dos Santos, A.R.; Tanamati, A.A.C.; Gonçalves, O.H.; Valderrama, P.; Leimann, F.V. Active Packaging to Prevent Lipid Oxidation on Brazil Nuts (Bertholletia Excelsa HBK) Stored under Varying Temperatures. Packag. Technol. Sci. 2023, 36, 985–993. [Google Scholar] [CrossRef]
- Creely, J.J.; Segal, L.; Loeb, L. An X-ray Study of New Cellulose Complexes with Diamines Containing Three, Five, Six, Seven, and Eight Carbon Atoms. J. Polym. Sci. 1959, 36, 205–214. [Google Scholar] [CrossRef]
- da Silva, T.G.; Guerra, I.C.; Mesquita, J.d.A.; Hernandes, T.; Takeuchi, K.P. Development of Film-Forming Solutions for the Production of Biodegradable, Edible Films with Antioxidant Activity: Systematic Review. Res. Soc. Dev. 2022, 11, e59511730139. [Google Scholar]
- Ruggeri, E.; Kim, D.; Cao, Y.; Farè, S.; De Nardo, L.; Marelli, B. A Multilayered Edible Coating to Extend Produce Shelf Life. ACS Sustain. Chem. Eng. 2020, 8, 14312–14321. [Google Scholar] [CrossRef]
- Sepeidnameh, M.; Fazlara, A.; Hosseini, S.M.H.; Pourmahdi Borujeni, M. Encapsulation of Grape Seed Oil in Oil-in-Water Emulsion Using Multilayer Technology: Investigation of Physical Stability, Physicochemical and Oxidative Properties of Emulsions under the Influence of the Number of Layers. Curr. Res. Food Sci. 2024, 8, 100771. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; Nasri, R.; Li, S.; Nasri, M. Bioactive Composite Films with Chitosan and Carotenoproteins Extract from Blue Crab Shells: Biological Potential and Structural, Thermal, and Mechanical Characterization. Food Hydrocoll. 2019, 89, 802–812. [Google Scholar] [CrossRef]
- Andrade, R.M.S.; Ferreira, M.S.L.; Gonçalves, É.C.B.A. Development and Characterization of Edible Films Based on Fruit and Vegetable Residues. J. Food Sci. 2016, 81, E412–E418. [Google Scholar] [CrossRef]
- Ju, A.; Song, K. Bin Active Biodegradable Films Based on Water Soluble Polysaccharides from White Jelly Mushroom (Tremella fuciformis) Containing Roasted Peanut Skin Extract. LWT 2020, 126, 109293. [Google Scholar] [CrossRef]
- Khater, E.S.; Bahnasawy, A.; Gabal, B.A.; Abbas, W.; Morsy, O. Effect of Adding Nano-Materials on the Properties of Hydroxypropyl Methylcellulose (HPMC) Edible Films. Sci. Rep. 2023, 13, 5063. [Google Scholar] [CrossRef]
- ASTM E96/E96M-22ae1; Standard Test Methods for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 2010.
- ASTM D88-12; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2010.
- ASTM F1342-05; Standard Test Method for Test Method for Protective Clothing Material Resistance to Puncture. ASTM International: West Conshohocken, PA, USA, 2010.
- ASTM G160; Standard Practice for Evaluating Microbial Susceptibility of Nonmetallic Materials By Laboratory Soil Burial. ASTM International: West Conshohocken, PA, USA, 2010.
- Khoshnoudi-Nia, S.; Sedaghat, N. Effect of Active Edible Coating and Temperature on Quality Properties of Roasted Pistachio Nuts during Storage. J. Food Process Preserv. 2019, 43, e14121. [Google Scholar] [CrossRef]
- Miraliakbari, H.; Shahidi, F. Oxidative Stability of Tree Nut Oils. J. Agric. Food Chem. 2008, 56, 4751–4759. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- AOCS Official Method Cd3d-63. Acid value of fats and oils. In Official Methods and Recommended Practices of the AOCS; AOCS Press: Urbana, IL, USA, 2017. [Google Scholar]
- AOCS Official Method Cd 8b-90. Peroxide value, acetic acid, isooctane method. In Official Methods and Recommended Practices of the AOCS; AOCS Press: Urbana, IL, USA, 2017. [Google Scholar]
- AOCS Oficial Method Ti 1a-64. Spectrophotometric Determination of Conjugated Dienoic Acid in Dehydrated Castor Oils and Acids. In Official Methods and Recommended Practices of the AOCS; AOCS Press: Champaign, IL, USA, 1989. [Google Scholar]
- Sokal, R.R.; Michener, C.D. A Statistical Method for Evaluating Systematic Relationships. Univ. Kans. 1958, 38, 1409–1438. [Google Scholar]
- Wairegi, L.; van Asten, P. Norms for Multivariate Diagnosis of Nutrient Imbalance in the East African Highland Bananas (Musa spp. AAA). J. Plant Nutr. 2011, 34, 1453–1472. [Google Scholar] [CrossRef]
- Zimmermann, T.; Pöhler, E.; Geiger, T. Cellulose Fibrils for Polymer Reinforcement. Adv. Eng. Mater. 2004, 6, 754–761. [Google Scholar] [CrossRef]
- Pääkko, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic Hydrolysis Combined with Mechanical Shearing and High-Pressure Homogenization for Nanoscale Cellulose Fibrils and Strong Gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Siaueira, G.; Bras, J.; Dufresne, A. Cellulose Whiskers versus Microfibrils: Influence of the Nature of the Nanoparticle and Its Surface Functionalization on the Thermal and Mechanical Properties of Nanocomposites. Biomacromolecules 2009, 10, 425–432. [Google Scholar]
- Calderari, T.O.; Iamanaka, B.T.; Frisvad, J.C.; Pitt, J.I.; Sartori, D.; Pereira, J.L.; Fungaro, M.H.P.; Taniwaki, M.H. The Biodiversity of Aspergillus Section Flavi in Brazil Nuts: From Rainforest to Consumer. Int. J. Food Microbiol. 2013, 160, 267–272. [Google Scholar] [CrossRef]
- Moura Eça Felix, A.; Regina de Ávila Oliveira, C.; Rodrigo Guerreiro, J. Contaminação de Castanha Do Brasil Por Aflatoxinas: Uma Revisão Do Panorama Atual Contamination of Brazil Nuts by Aflatoxin: A Review of the Current Picture. J. Health Sci. Inst. 2018, 36, 205–210. [Google Scholar]
- Shahidi, F.; Hossain, A. Preservation of Aquatic Food Using Edible Films and Coatings Containing Essential Oils: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 66–105. [Google Scholar] [CrossRef]
- Monteiro, L.d.M.; Nogueira, R.M.; Pires, E.M. A Valid Method for Determining the Water Content of the Brazil Nut (Bertholletia excelsa). Biosci. J. 2016, 32, 952–959. [Google Scholar] [CrossRef]
- Matloob, A.; Ayub, H.; Mohsin, M.; Ambreen, S.; Khan, F.A.; Oranab, S.; Rahim, M.A.; Khalid, W.; Nayik, G.A.; Ramniwas, S.; et al. A Review on Edible Coatings and Films: Advances, Composition, Production Methods, and Safety Concerns. ACS Omega 2023, 8, 28932–28944. [Google Scholar] [CrossRef]
- Gumus, T.; Kaynarca, G.B.; Kamer, D.D.A. Optimization of an Edible Film Formulation by Incorporating Carrageenan and Red Wine Lees into Fish Gelatin Film Matrix. Int. J. Biol. Macromol. 2024, 258, 128854. [Google Scholar] [CrossRef] [PubMed]
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film Formation and Deposition Methods of Edible Coating on Food Products: A Review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Fernández, X.; Vega-Ahuatzin, A.; Ochoa-Velasco, C.E.; Cid-Pérez, S.; Hernández-Carranza, P.; Ávila-Sosa, R. Physical and Antioxidant Characterization of Edible Films Added with Red Prickly Pear (Opuntia ficus-Indica L.) Cv. San Martín Peel and/or Its Aqueous Extracts. Food Bioprocess Technol. 2018, 11, 368–379. [Google Scholar] [CrossRef]
- Atef, M.; Rezaei, M.; Behrooz, R. Preparation and Characterization Agar-Based Nanocomposite Film Reinforced by Nanocrystalline Cellulose. Int. J. Biol. Macromol. 2014, 70, 537–544. [Google Scholar] [CrossRef]
- Chu, M.; Feng, N.; An, H.; You, G.; Mo, C.; Zhong, H.; Pan, L.; Hu, D. Design and Validation of Antibacterial and PH Response of Cationic Guar Gum Film by Combining Hydroxyethyl Cellulose and Red Cabbage Pigment. Int. J. Biol. Macromol. 2020, 162, 1311–1322. [Google Scholar] [CrossRef]
- Espinosa, E.; Rincón, E.; Morcillo-Martín, R.; Rabasco-Vílchez, L.; Rodríguez, A. Orange Peel Waste Biorefinery in Multi-Component Cascade Approach: Polyphenolic Compounds and Nanocellulose for Food Packaging. Ind. Crop. Prod. 2022, 187, 11541. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; Chiralt, A. Starch-Polyester Bilayer Films with Phenolic Acids for Pork Meat Preservation. Food Chem. 2022, 385, 132650. [Google Scholar] [CrossRef]
- Nayak, P.K.; Dash, U.; Rayaguru, K.; Krishnan, K.R. Physio-Chemical Changes During Repeated Frying of Cooked Oil: A Review. J. Food Biochem. 2016, 40, 371–390. [Google Scholar] [CrossRef]
- Krishnamurthy, K.; Khurana, H.K.; Soojin, J.; Irudayaraj, J.; Demirci, A. Infrared Heating in Food Processing: An Overview. Compr. Rev. Food Sci. Food Saf. 2008, 7, 2–13. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Sharma, L.; Kaur, M.; Kaur, R. Physical, Structural and Thermal Properties of Composite Edible Films Prepared from Pearl Millet Starch and Carrageenan Gum: Process Optimization Using Response Surface Methodology. Int. J. Biol. Macromol. 2020, 143, 704–713. [Google Scholar] [CrossRef]
- Yıldırım-Yalçın, M.; Sadıkoğlu, H.; Şeker, M. Characterization of Edible Film Based on Grape Juice and Cross-Linked Maize Starch and Its Effects on the Storage Quality of Chicken Breast Fillets. LWT 2021, 142, 111012. [Google Scholar] [CrossRef]
- Ameur, A.; Bensid, A.; Ozogul, F.; Ucar, Y.; Durmus, M.; Kulawik, P.; Boudjenah-Haroun, S. Application of Oil-in-Water Nanoemulsions Based on Grape and Cinnamon Essential Oils for Shelf-Life Extension of Chilled Flathead Mullet Fillets. J. Sci. Food Agric. 2022, 102, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Cortés, N.M.; Lorenzo, G.; Califano, A.N. Food Grade Microemulsion Systems: Sunflower Oil/Castor Oil Derivative-Ethanol/Water. Rheological and Physicochemical Analysis. Food Res. Int. 2018, 107, 41–47. [Google Scholar] [CrossRef]
- Panigrahi, J.; Gheewala, B.; Patel, M.; Patel, N.; Gantait, S. Gibberellic Acid Coating: A Novel Approach to Expand the Shelf-Life in Green Chilli (Capsicum annuum L.). Sci. Hortic. 2017, 225, 581–588. [Google Scholar] [CrossRef]
- Fabra, M.J.; Hambleton, A.; Talens, P.; Debeaufort, F.; Chiralt, A. Effect of Ferulic Acid and α-Tocopherol Antioxidants on Properties of Sodium Caseinate Edible Films. Food Hydrocoll. 2011, 25, 1441–1447. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food Applications of Emulsion-Based Edible Films and Coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Mikus, M.; Galus, S.; Ciurzyńska, A.; Janowicz, M. Development and Characterization of Novel Composite Films Based on Soy Protein Isolate and Oilseed Flours. Molecules 2021, 26, 3738. [Google Scholar] [CrossRef]
- Amjadi, S.; Nazari, M.; Alizadeh, S.A.; Hamishehkar, H. Multifunctional Betanin Nanoliposomes-Incorporated Gelatin/Chitosan Nanofiber/ZnO Nanoparticles Nanocomposite Film for Fresh Beef Preservation. Meat Sci. 2020, 167, 108161. [Google Scholar] [CrossRef]
- Chauhan, P.; Sharma, M.; Sharma, R.; Kumar, D. Nanocomposites-Based Biodegradable Polymers. In Nanomaterials in Clinical Therapeutics: Synthesis and Applications; Mukhopadhyay, M., Kuila, A., Eds.; Wiley: Hoboken, NJ, USA, 2022; pp. 285–316. [Google Scholar]
- Shen, Z.; Kamdem, D.P. Development and Characterization of Biodegradable Chitosan Films Containing Two Essential Oils. Int. J. Biol. Macromol. 2015, 74, 289–296. [Google Scholar] [CrossRef]
- Song, X.; Zuo, G.; Chen, F. Effect of Essential Oil and Surfactant on the Physical and Antimicrobial Properties of Corn and Wheat Starch Films. Int. J. Biol. Macromol. 2018, 107, 1302–1309. [Google Scholar] [CrossRef]
- Tian, D.; He, C.H.; He, J.H. Macromolecule Orientation in Nanofibers. Nanomaterials 2018, 8, 918. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.; Salmieri, S.; Dussault, D.; Uribe-Calderon, J.; Kamal, M.R.; Safrany, A.; Lacroix, M. Production and Properties of Nanocellulose-Reinforced Methylcellulose-Based Biodegradable Films. J. Agric. Food Chem. 2010, 58, 7878–7885. [Google Scholar] [CrossRef]
- García-Betanzos, C.I.; Hernández-Sánchez, H.; Quintanar-Guerrero, D.; Del Real, L.A.; de la Luz Zambrano-Zaragoza, M. The Evaluation of Mechanical, Thermal, Optical and Microstructural Properties of Edible Films with Solid Lipid Nanoparticles-Xanthan Gum Stored at Different Temperatures and Relative Humidities. Food Bioprocess Technol. 2016, 9, 1756–1768. [Google Scholar] [CrossRef]
- Shen, G.; Yu, G.; Wu, H.; Li, S.; Hou, X.; Li, M.; Li, Q.; Liu, X.; Zhou, M.; Chen, A.; et al. Incorporation of Lipids into Wheat Bran Cellulose/Wheat Gluten Composite Film Improves Its Water Resistance Properties. Membranes 2021, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Chambi, H.; Grosso, C. Effect of Surfactants on the Functional Properties of Gelatin-Polysaccharide-Based Films. Eur. Food Res. Technol. 2011, 232, 63–69. [Google Scholar] [CrossRef]
- Liu, L.; Kerry, J.F.; Kerry, J.P. Effect of Food Ingredients and Selected Lipids on the Physical Properties of Extruded Edible Films/Casings. Int. J. Food Sci. Technol. 2006, 41, 295–302. [Google Scholar] [CrossRef]
- De Oliveira, A.F.; Assmann, V.; Soldi, V. Influência de plastificantes e umidade relativa em filmes de derivados de celulose: Carboximetilcelulose e hidroxipropil-metilcelulose. In Proceedings of the 9th Brazilian Polymer Congress, Campina Grande, Brazil, 7 October 2007; pp. 1–7. [Google Scholar]
- Tyagi, V.; Thakur, A. Carboxymethyl Cellulose-Polyvinyl Alcohol Based Materials: A Review. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Yaradoddi, J.S.; Banapurmath, N.R.; Ganachari, S.V.; Soudagar, M.E.M.; Mubarak, N.M.; Hallad, S.; Hugar, S.; Fayaz, H. Biodegradable Carboxymethyl Cellulose Based Material for Sustainable Packaging Application. Sci. Rep. 2020, 10, 21960. [Google Scholar] [CrossRef]
- European Commission Regulation (EU) No 1169/2011. Available online: http://data.europa.eu/eli/reg/2011/1169/oj (accessed on 7 September 2024).
- European Commission Regulation EC 1333/2008: Food. Additives. Available online: https://food.ec.europa.eu/safety/food-improvement-agents/additives/eurules_en#:~:text=Regulation%20EC%201333%2F2008%20sets%20the%20rules%20on%20food,additives%3A%20definitions%2C%20conditions%20of%20use%2C%20labelling%20and%20procedures (accessed on 7 September 2024).
- Berry, R. Improving Barrier Properties with Cellulose Nanocrystal. Available online: https://www.tappi.org/globalassets/documents/events/europlace_proceedings/04_6_richard-berry_improving-barrier-properties-with-cellulose-nanocrystals_tappi-place-porto_2019.pdf (accessed on 7 September 2024).
- US Food and Drug Administration. Inventory of Effective Food Contact Substance (FCS) Notifications. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=FCN (accessed on 7 September 2024).
- Ministério da Saúde; Agência Nacional de Vigilância Sanitária. INSTRUÇÃO NORMATIVA—IN N° 87, DE 15 DE MARÇO DE 2021. In Regulamento Técnico Para Óleos Vegetais, Gorduras Vegetais e Creme Vegetal; Ministério da Saúde: Brasília, Brasil, 2020; pp. 1–13. [Google Scholar]
- Kowalczyk, D. Original Article Effect of Carboxymethyl Cellulose/Candelilla Wax Coating Containing Ascorbic Acid on Quality of Walnut (Juglans regia L.) Kernels. Int. J. Food Sci. Technol. 2017, 52, 1425–1431. [Google Scholar] [CrossRef]
- Razavi, R.; Maghsoudlou, Y.; Aalami, M.; Ghorbani, M. Impact of Carboxymethyl Cellulose Coating Enriched with Thymus vulgaris L. Extract on Physicochemical, Microbial, and Sensorial Properties of Fresh Hazelnut (Corylus avellana L.) during Storage. J. Food Process Preserv. 2021, 45, e15313. [Google Scholar] [CrossRef]
- López-Uriarte, P.; Bulló, M.; Casas-Agustench, P.; Babio, N.; Salas-Salvadó, J. Nuts and Oxidation: A Systematic Review. Nutr. Rev. 2009, 67, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Gahruie, H.H.; Hosseini, S.M.H.; Taghavifard, M.H.; Eskandari, M.H.; Golmakani, M.-T.; Shad, E. Lipid Oxidation, Color Changes, and Microbiological Quality of Frozen Beef Burgers Incorporated with Shirazi Thyme, Cinnamon, and Rosemary Extracts. J. Food Qual. 2017, 2017, 6350156. [Google Scholar]
- Pei, J.; Mei, J.; Wu, G.; Yu, H.; Xie, J. Gum Tragacanth-Sodium Alginate Active Coatings Containing Epigallocatechin Gallate Reduce Hydrogen Peroxide Content and Inhibit Lipid and Protein Oxidations of Large Yellow Croaker (Larimichthys crocea) during Superchilling Storage. Food Chem. 2022, 397, 133792. [Google Scholar] [CrossRef]
- Wu, Y.; Liang, Y.; Mei, C.; Cai, L.; Nadda, A.; Van Le, Q.; Peng, Y.; Lam, S.S.; Sonne, C.; Xia, C. Advanced Nanocellulose-Based Gas Barrier Materials: Present Status and Prospects. Chemosphere 2022, 286, 131891. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Santos, J.; Valls, C.; Cusola, O.; Roncero, M.B. Improving Filmogenic and Barrier Properties of Nanocellulose Films by Addition of Biodegradable Plasticizers. ACS Sustain. Chem. Eng. 2021, 9, 9647–9660. [Google Scholar] [CrossRef]
- Pirozzi, A.; Ferrari, G.; Donsì, F. The Use of Nanocellulose in Edible Coatings for the Preservation of Perishable Fruits and Vegetables. Coatings 2021, 11, 990. [Google Scholar] [CrossRef]
- Fang, H.; Chen, X.; Wang, S.; Cheng, S.; Ding, Y. Enhanced Mechanical and Oxygen Barrier Performance in Biodegradable Polyurethanes by Incorporating Cellulose Nanocrystals with Interfacial Polylactide Stereocomplexation. Cellulose 2019, 26, 9751–9764. [Google Scholar] [CrossRef]
- Hill, R.; Phipps, J.; Greenwood, R.; Skuse, D.; Zhang, Z.J. The Effect of Pre-Treatment and Process Conditions on the Gas Barrier Properties of Fibrillated Cellulose Films and Coatings: A Review. Carbohydr. Polym. 2024, 337, 122085. [Google Scholar] [CrossRef]
- Tardy, B.L.; Yokota, S.; Ago, M.; Xiang, W.; Kondo, T.; Bordes, R.; Rojas, O.J. Nanocellulose–Surfactant Interactions. Curr. Opin. Colloid. Interface Sci. 2017, 29, 57–67. [Google Scholar] [CrossRef]
- Joshi, M.; Adak, B.; Butola, B.S. Polyurethane Nanocomposite Based Gas Barrier Films, Membranes and Coatings: A Review on Synthesis, Characterization and Potential Applications. Prog. Mater. Sci. 2018, 97, 230–282. [Google Scholar] [CrossRef]
- Introzzi, L.; Blomfeldt, T.O.J.; Trabattoni, S.; Tavazzi, S.; Santo, N.; Schiraldi, A.; Piergiovanni, L.; Farris, S. Ultrasound-Assisted Pullulan/Montmorillonite Bionanocomposite Coating with High Oxygen Barrier Properties. Langmuir 2012, 28, 11206–11214. [Google Scholar] [CrossRef] [PubMed]
- Popovici, V.; Sturza, R.; Ghendov-Mosanu, A. Technological and Environmental Factors Impact on the Antioxidation Mechanism of Oil Lipids. In Environmental and Technological Aspects of Redox Processes; IGI Global: Hershey, PA, USA, 2023; pp. 212–237. Available online: https://services.igi-global.com/resolvedoi/resolve.aspx?doi=10.4018/979-8-3693-0512-6.ch012 (accessed on 7 May 2024).
- Kyselka, J.; Cihelková, K.; Lopes-Lutz, D.; Chudoba, J.; Váchalová, T.; Alishevich, K.; Hrádková, I.; Berčíková, M.; Mikolášková, M.; Filip, V. Mechanism Controlling High-Temperature Degradation of Sunflower Oil Triacylglycerols in the Absence of Oxygen. Eur. J. Lipid Sci. Technol. 2021, 123, 2000228. [Google Scholar] [CrossRef]
- Sartoria, A.G.d.O.; D’Arce, M.A.B.R.; Skibsted, L.H.; Bastos, D.H.M. Mild storage conditions affect tendency of lipis formation and volatiles in Brazil Nuts (Bertholletia excelsa). In Chemical Changes in Brazil Nusts and Co-Products: Characterization and Stratefies of Control and Monitoring; USP: Piracicaba, Brasil, 2017; pp. 6–9. [Google Scholar]

| Samples | L | a* | b* | C* | °Hue |
|---|---|---|---|---|---|
| C01 | 89.7 ± 0.2 A | 1.6 ± 0.2 A | −2.8 ± 0.1 D | 3.2 ± 0.2 CD | 300.0 ± 2.5 B |
| CC1 | 89.6 ± 0.2 A | 1.5 ± 0.1 A | −2.6 ± 0.1 D | 3.0 ± 0.1 D | 301.2 ± 2.2 AB |
| CC3 | 89.4 ± 0.2 A | 1.6 ± 0.1 A | −2.6 ± 0.1 D | 3.1 ± 0.1 D | 301.9 ± 1.8 AB |
| CC5 | 89.4 ± 0.1 A | 1.5 ± 0.1 A | −2.2 ± 0.1 D | 2.7 ± 0.1 E | 304.7 ± 3.0 A |
| C02 | 87.5 ± 0.3 B | 1.5 ± 0.2 A | 3.6 ± 0.2 B | 3.9 ± 0.3 B | 67.1 ± 2.1 C |
| CCT1 | 87.3 ± 0.25 B | 1.5 ± 0.1 A | 3.0± 0.1 C | 3.4 ± 0.1 C | 62.0 ± 3.3 E |
| CCT3 | 87.4 ± 0.09 BC | 1.6 ± 0.1 A | 4.0 ±0.1 A | 4.3 ± 0.3 A | 67.3 ± 0.6 CD |
| CCT5 | 87.0 ± 0.13 C | 1.7 ± 0.1 A | 3.5 ± 0.3 B | 3.9 ± 0.3 AB | 63.6 ± 0.6 DE |
| Samples | VLT (%) | UVR (%) | IRR (%) |
|---|---|---|---|
| C01 | 88.5 ± 1.8 a | 15.5 ± 0.7 e | 11.1 ± 0.4 e |
| CC1 | 86.0 ± 0.9 ab | 20.1 ± 1.3 e | 11.1 ± 0.4 e |
| CC3 | 79.6 ± 3.5 bc | 29.1 ± 1.0 d | 21.3 ± 2.4 cd |
| CC5 | 76.4 ± 3.5 c | 36.4 ± 5.9 c | 26.4 ± 0.8 c |
| C02 | 62.5 ± 2.8 d | 58.7 ± 3.6 ab | 42.7 ± 3.1 b |
| CCT1 | 62.8 ± 4.0 d | 56.0 ± 3.1 b | 42.7 ±4.1 b |
| CCT3 | 54.1 ± 6.0 e | 63.1 ± 1.9 a | 49.5 ± 6.3 a |
| CCT5 | 52.9 ± 4.0 e | 65.3 ± 2.0 a | 50.3 ± 2.6 a |
| Samples | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| C01 | 20.57 ± 3.83 B | 11.14 ± 7.44 BC |
| CC1 | 24.02 ± 4.79 B | 16.33 ± 2.64 A |
| CC3 | 22.99 ± 6.70 B | 15.92 ± 4.08 A |
| CC5 | 33.43 ± 9.07 A | 17.20 ± 4.54 A |
| C02 | 6.58 ± 1.44 CD | 9.87 ± 3.94 AB |
| CCT1 | 6.16 ± 2.00 C | 9.44 ± 6.03 C |
| CCT3 | 7.47 ± 1.38 C | 9.10 ± 5.56 C |
| CCT5 | 8.05 ± 1.93 C | 13.36 ± 2.17 A |
| Samples | Conjugated Dienes (g L−1) | |||||
|---|---|---|---|---|---|---|
| 12 h | 24 h | 72 h | 144 h | 192 h | 264 h | |
| SR | 0.34 | NA | 0.41 | 0.36 | 0.44 | 0.50 |
| C01 | 0.34 | 0.40 | 0.25 | 0.48 | 0.40 | 0.34 |
| CC1 | 0.45 | 0.40 | 0.30 | 0.34 | 0.40 | 0.40 |
| CC3 | 0.15 | 0.15 | 0.10 | 0.14 | 0.05 | 0.12 |
| CC5 | NA | 0.42 | NA | 0.44 | 0.28 | 0.45 |
| C02 | 0.30 | 0.30 | 0.26 | 0.23 | 0.21 | 0.52 |
| CCT1 | 0.39 | 0.39 | 0.20 | 0.29 | 0.42 | 0.56 |
| CCT3 | 0.21 | 0.31 | 0.11 | 0.42 | 0.39 | 0.45 |
| CCT5 | 0.36 | 0.47 | 0.30 | 0.36 | 0.49 | 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, D.R.; Mesquita, J.; da Silva, T.; Hernandes, T.; Lengowski, E.C.; Takeuchi, K. A Novel Approach to Protect Brazil Nuts from Lipid Oxidation: Efficacy of Nanocellulose–Tocopherol Edible Coatings. Coatings 2024, 14, 1182. https://doi.org/10.3390/coatings14091182
Nascimento DR, Mesquita J, da Silva T, Hernandes T, Lengowski EC, Takeuchi K. A Novel Approach to Protect Brazil Nuts from Lipid Oxidation: Efficacy of Nanocellulose–Tocopherol Edible Coatings. Coatings. 2024; 14(9):1182. https://doi.org/10.3390/coatings14091182
Chicago/Turabian StyleNascimento, Debora Ribeiro, Juliana Mesquita, Thayanne da Silva, Thais Hernandes, Elaine Cristina Lengowski, and Katiuchia Takeuchi. 2024. "A Novel Approach to Protect Brazil Nuts from Lipid Oxidation: Efficacy of Nanocellulose–Tocopherol Edible Coatings" Coatings 14, no. 9: 1182. https://doi.org/10.3390/coatings14091182






