Synthesis of Eco-Friendly Narrow-Band CuAlSe2/Ga2S3/ZnS Quantum Dots for Blue Quantum Dot Light-Emitting Diodes
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Stock Solution
2.3. Synthesis of CuAlSe2/Ga2S3/ZnS QDs
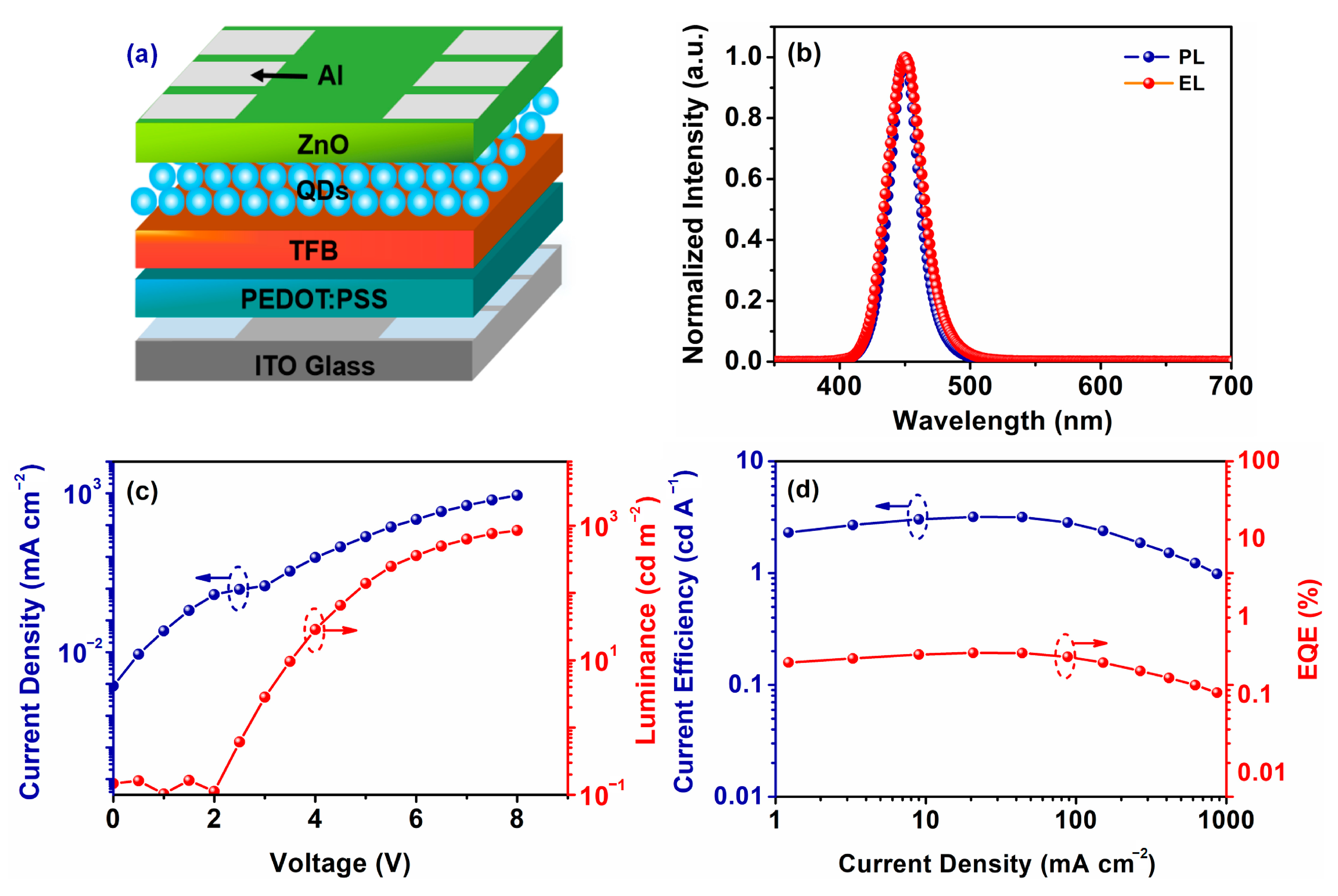
2.4. Fabrication of QLEDs
2.5. Characterization
3. Results and Discussion
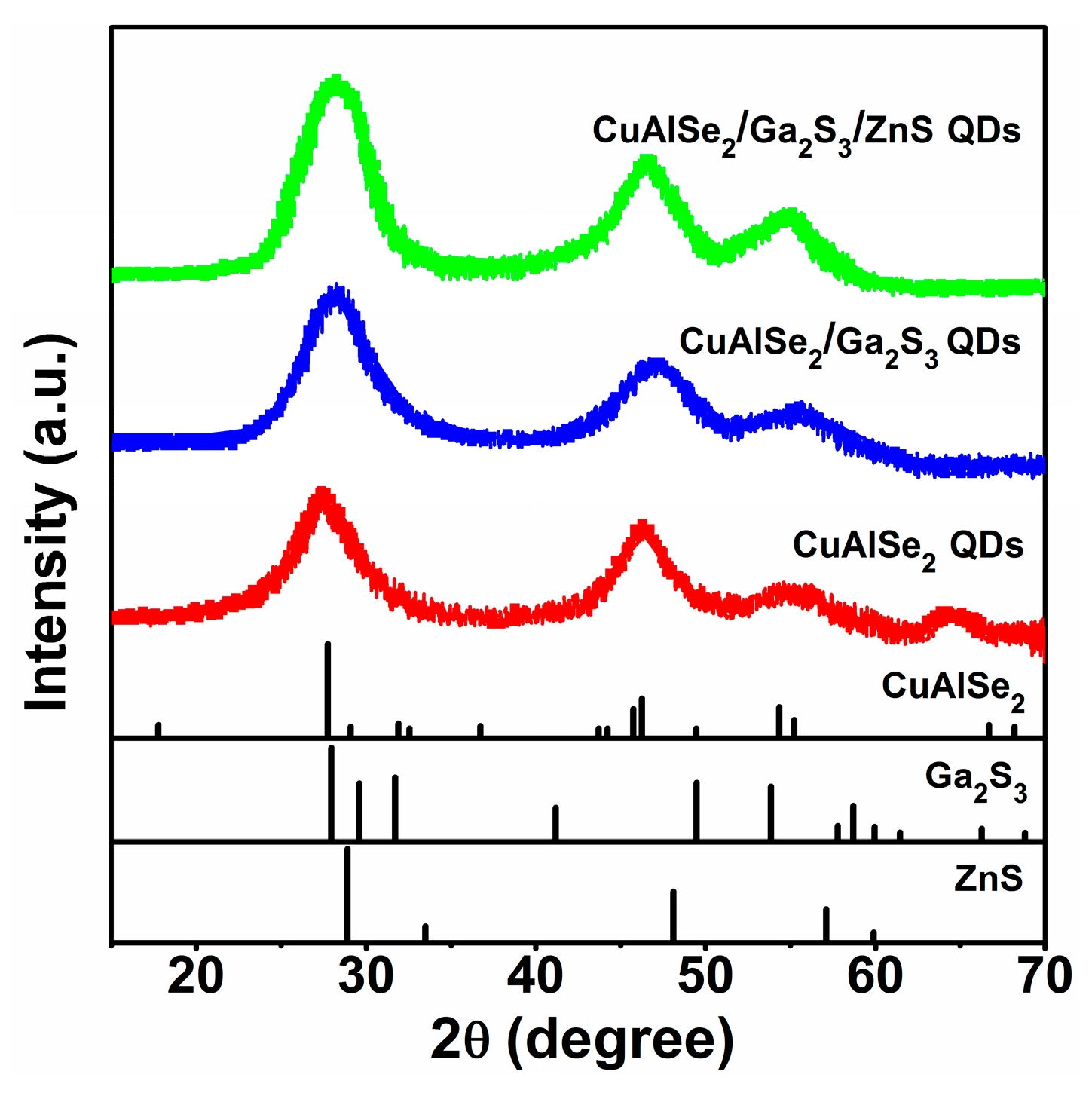
3.1. Micro-Structure Analysis
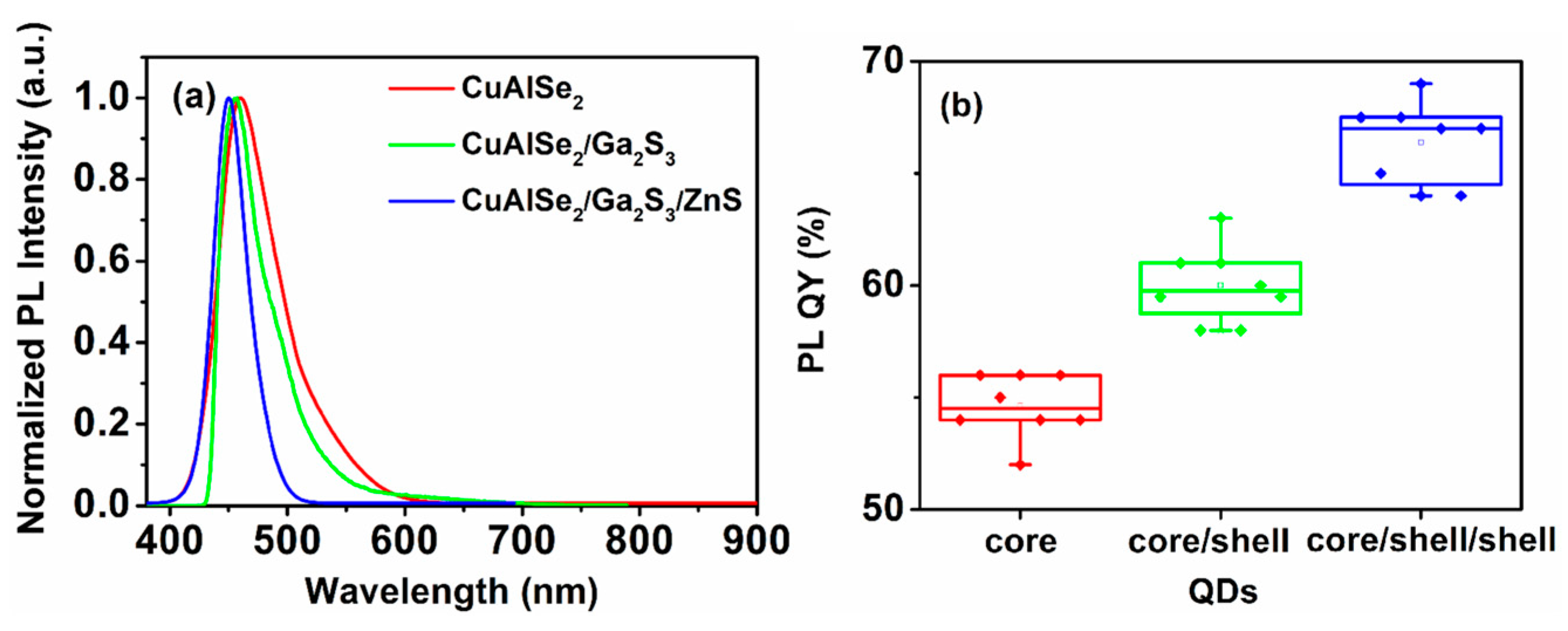
3.2. Absorption Analysis and Luminescence Properties
3.3. Fabrication of QLEDs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, Q.; Liu, K.; Han, Y.; Xia, H.; Re, Z.; Li, D.; Zhu, T.; Cheng, L.; Wang, Z.; Zhu, C.; et al. Highly stable perovskite solar cells with 0.30 voltage deficit enabled by a multi-functional asynchronous cross-linking. Nat. Commun. 2025, 16, 190. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, H.; Li, D.; Zhang, X.; Zhao, C.; Zhao, X.; Ma, W.; Yuan, J. Buried interface engineering enables efficient and refurbished CsPbI3 perovskite quantum dot solar cells. Energy Environ. Sci. 2025, 18, 972–981. [Google Scholar] [CrossRef]
- Meng, K.; Zhang, J.; Cheng, B.; Ren, X.; Xia, Z.; Xu, F.; Zhang, L. Plasmonic near-infrared-response s-scheme ZnO/CuInS2 photocatalyst for H2O2 production coupled with glycerin oxidation. Adv. Mater. 2024, 36, 2406460. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Chen, Y.; Zhang, J.; Li, W.; Zeng, F.; Liu, L.; Tian, G. Engineering of hierarchical Z-scheme ZnSe/Fe2O3 heterojunction cubic nanocages for enhanced CO2 to CO photoconversion. J. Mater. Chem. A 2025, 13, 5365–5373. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, J.; Yang, Z.; Chen, Y.; Zhang, H.; Bai, Y.; Jiang, X.; Liu, B.; Hong, J.; Chen, Y.; et al. Uncovering the performance enhancing mechanism of methanesulfonate ligands in perovskite quantum dots for light-emitting devices. ACS Appl. Mater. Interfaces 2025, 17, 6639–6647. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Dong, Z.; Wang, J.; Hu, H.; Chen, W.; Guo, T.; Li, F. Flexible ultrahigh-resolution quantum-dot light-emitting diodes. Adv. Funct. Mater. 2024, 34, 2408604. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, X.; Shi, G.; Zhang, S.; Zhou, Y.; Cui, Y.; Wang, J.; Xin, B. Green synthesis of Bio-CuS quantum dots by sulfate reducing bacteria for solid-phase photocatalytic degradation of polyethylene film. Sep. Purif. Technol. 2025, 354, 129374. [Google Scholar] [CrossRef]
- Diak, M.; Flak, D.; Jarek, M.; Przysiecka, Ł.; Nowaczyk, G. Aqueous phase transfer of near-infrared ZnCuInS2/ZnS quantum dots: Synthesis and characterization. Biomater. Adv. 2025, 166, 214083. [Google Scholar] [CrossRef]
- Matsuo, R.; Kita, M.; Tsukuda, S.; Omata, T. Design of low-lattice-mismatch type-I heterostructures of zinc chalcogenide and synthesis of ZnTeS quantum dots as key materials for realizing green and red emission. J. Mater. Chem. C 2025. [Google Scholar] [CrossRef]
- Ryu, H.; Shin, D.; Yoon, B.; Bae, W.K.; Kwak, J.; Lee, H. Direct evidence of excessive charge-carrier-induced degradation in InP quantum-dot light-emitting diodes. ACS Appl. Mater. Interfaces 2025, 17, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Luo, X.; Deng, Y.; Wei, H.; Peng, F.; Ying, L.; Huang, F.; Hu, Y.; Jin, Y. Doping bilayer hole-transport polymer strategy stabilizing solution-processed green quantum-dot light-emitting diodes. Sci. Adv. 2024, 10, eado0614. [Google Scholar] [CrossRef]
- Cai, F.; Bai, B.; Wu, Q.; Xu, R.; Xiang, C.; Yang, X.; Jia, G.; Du, Z. Blue quantum dot light-emitting diodes toward full-color displays: Materials, devices, and large-scale fabrication. Nano Lett. 2025, 25, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Yang, D.; Qin, S.; Wen, Z.; He, H.; Mei, S.; Zhang, W.; Xing, G.; Liang, C.; Guo, R. Advances, challenges, and perspectives for heavy-metal-free blue-emitting indium phosphide quantum dot light-emitting diodes. Adv. Opt. Mater. 2023, 11, 2202036. [Google Scholar] [CrossRef]
- Ding, P.; Ko, P.K.; Geng, P.; Chen, D.; Xing, Z.; Tsang, H.L.T.; Wong, K.S.; Guo, L.; Halpert, J.E. Strongly confined and spectrally tunable CsPbBr3 quantum dots for deep blue QD-LEDs. Adv. Opt. Mater. 2024, 12, 2302477. [Google Scholar] [CrossRef]
- Haque, M.A.; Lohar, A.; Jadhav, Y.; Kumar, R.; Jha, S.N.; Bhattacharyya, D.; Jadkar, S.; Sartale, S.; Mahamuni, S. Zn alloying strategy to improve the photoluminescence of CuGaS2/ZnS core/shell quantum dots. J. Mater. Chem. A 2024, 12, 10726–10736. [Google Scholar] [CrossRef]
- Shishodia, S.; Rinnert, H.; Balan, L.; Jasniewski, J.; Bruyère, S.; Medjahdi, G.; Gries, T.; Schneider, R. Microwave-assisted synthesis of highly photoluminescent core/shell CuInZnSe/ZnS quantum dots as photovoltaic absorbers. Nanoscale Adv. 2025. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Bhakat, A.; Debnath, T. Breaking AgInTe2 quantum dot chain to fabricate AgInTe2-ZnS janus nanocrystals. Inorg. Chem. 2023, 62, 20219–20227. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Kim, J.H.; Kim, K.H.; Han, C.Y.; Jo, J.H.; Jo, D.Y.; Hong, S.; Hwang, J.Y.; Do, Y.R.; Yang, H. High-efficiency blue and white electroluminescent devices based on non-Cd I-III-VI quantum dots. Nano Energy 2019, 63, 103869. [Google Scholar] [CrossRef]
- Xia, C.; Wu, W.; Yu, T.; Xie, X.; Oversteeg, C.V.; Gerritsen, H.C.; Donega, C.D.M. Size-dependent band-gap and molar absorption coefficients of colloidal CuInS2 quantum dots. ACS Nano 2018, 12, 8350–8361. [Google Scholar] [CrossRef]
- Zang, H.; Li, H.; Makarov, N.S.; Velizhanin, K.A.; Wu, K.; Park, Y.S.; Klimov, V.I. Thick-shell CuInS2/ZnS quantum dots with suppressed “blinking” and narrow single-particle emission line widths. Nano Lett. 2017, 17, 1787–1795. [Google Scholar] [CrossRef]
- Dong, X.; Li, X.; Yin, S.; Li, Z.; Li, L.; Li, J. Regulation of photophysical and electronic properties of I-III-VI quantum dots for light-emitting diodes. Sci. China Mater. 2024, 67, 2734–2748. [Google Scholar] [CrossRef]
- Niu, W.; Xie, X.; Chen, Z.; Sun, R.; Li, Y.; Wang, S.; Zhang, Y.; Yang, C. Realization of narrow-bandwidth Cu-Ga-S-based quantum dots with controllable luminescence. Adv. Opt. Mater. 2024, 12, 2400762. [Google Scholar] [CrossRef]
- Tozawa, M.; Ofuji, S.; Tanaka, M.; Akiyoshi, K.; Kameyama, T.; Yamamoto, T.; Motomura, G.; Fujisaki, Y.; Uematsu, T.; Kuwabata, S.; et al. Spectrally narrow blue-light emission from nonstoichiometric AgGaS2 quantum dots for application to light-emitting diodes. ACS Appl. Mater. Interfaces 2024, 16, 68169–68180. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Li, X.; Yin, S.; Li, J.; Zhang, P. Synthesis of blue-emitting CuAlSe2 quantum dots and their luminescent properties. Coatings 2024, 14, 1291. [Google Scholar] [CrossRef]
- Bai, T.; Wang, X.; Dong, Y.; Xing, S.; Shi, Z.; Feng, S. One-pot synthesis of high-quality AgGaS2/ZnS-based photoluminescent nanocrystals with widely tunable band gap. Inorg. Chem. 2020, 59, 5975–5982. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, J.; Lin, O.; Yin, Z.; Li, X.; Zhang, Y.; Tang, A. Narrow-bandwidth blue-emitting Ag-Ga-Zn-S semiconductor nanocrystals for quantum-dot light-emitting diodes. J. Phys. Chem. Lett. 2022, 13, 11857–11863. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, B.Y.; Jang, E.P.; Yoon, S.Y.; Kim, K.H.; Do, Y.R.; Yang, H. Synthesis of widely emission-tunable Ag-Ga-S and its quaternary derivative quantum dots. Chem. Eng. J. 2018, 347, 791–797. [Google Scholar] [CrossRef]
- Chen, B.; Zhong, H.; Zhang, W.; Tan, Z.; Li, Y.; Yu, C.; Zhai, T.; Bando, Y.; Yang, S.; Zou, B. Highly emissive and color-tunable CuInS2-based colloidal semiconductor nanocrystals: Off-stoichiometry effects and improved electroluminescence performance. Adv. Funct. Mater. 2012, 22, 2081–2088. [Google Scholar] [CrossRef]
- Wang, J.; Long, Y.; Zhang, Y.; Zhong, X.; Zhu, L. Preparation of highly luminescent CdTe/CdS core/shell quantum dots. ChemPhysChem 2009, 10, 680–685. [Google Scholar] [CrossRef]
- Hergert, F.; Jost, S.; Hock, R.; Purwins, M. Prediction of solid-state reactions for the formation of the chalcopyrites CuInS2, CuGaS2, CuAlS2 and CuAlSe2 starting from binary compounds. Phys. Status Solidi A 2006, 203, 2598–2602. [Google Scholar] [CrossRef]
- Gariano, G.; Lesnyak, V.; Brescia, R.; Bertoni, G.; Dang, Z.; Gaspari, R.; Trizio, L.D.; Manna, L. Role of the crystal structure in cation exchange reactions involving colloidal Cu2Se nanocrystals. J. Am. Chem. Soc. 2017, 139, 9583–9590. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.S.; Shojaei, F.; Park, K.; Oh, J.Y.; Im, H.S.; Jang, D.M.; Park, J.; Kang, H.S. Red-to-ultraviolet emission tuning of two-dimensional gallium sulfide/selenide. ACS Nano 2015, 9, 9585–9593. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Liu, Y.; Xu, W.; Li, Y. Ultracompact temperature-compensated all-fiber ultraviolet sensor based on CdZnSe/ZnSe/ZnS quantum dots sealed in liquid cavity. IEEE Sens. J. 2024, 24, 34417–34423. [Google Scholar] [CrossRef]
- Dénoue, K.; Cheviré, F.; Calers, C.; Verger, L.; Coq, D.L.; Calvez, L. Mechanochemical synthesis and structural characterization of gallium sulfide Ga2S3. J. Solid State Chem. 2020, 292, 121743. [Google Scholar] [CrossRef]
- Lee, S.H.; Song, S.W.; Yoon, S.Y.; Jo, D.Y.; Kim, S.K.; Kim, H.M.; Kim, Y.; Park, S.M.; Yang, H. Heterostructural tailoring of blue ZnSeTe quantum dots toward high-color purity and high-efficiency electroluminescence. Chem. Eng. J. 2022, 429, 132464. [Google Scholar] [CrossRef]
- Zhong, H.; Zhou, Y.; Ye, M.; He, Y.; Ye, J.; He, C.; Yang, C.; Li, Y. Controlled synthesis and optical properties of colloidal ternary chalcogenide CuInS2 nanocrystals. Chem. Mater. 2008, 20, 6434–6443. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, Q.; Lin, G.; Zhou, X.; Wu, W.; Yang, X.; Zhang, J.; Li, W. All-solution processed inverted green quantum dot light-emitting diodes with concurrent high efficiency and long lifetime. Mater. Horiz. 2019, 6, 2009–2015. [Google Scholar] [CrossRef]




| Element | CuAlSe2/Ga2S3 | CuAlSe2/Ga2S3/ZnS |
|---|---|---|
| Atomic Percentage (%) | ||
| Cu | 13.3 | 9.62 |
| Al | 21.53 | 16.5 |
| Se | 27.7 | 10.19 |
| Ga | 17.35 | 12.02 |
| S | 20.12 | 35.87 |
| Zn | — | 15.8 |
| Device | QDs | EL Peak (nm) | EL FWHM (nm) | Lmax (cd m−2) | CE (cd A−1) | EQEmax (%) |
|---|---|---|---|---|---|---|
| A | CuAlSe2 | 463 | 60 | 1.6 | 0.03 | 0.02 |
| B | CuAlSe2/Ga2S3 | 460 | 45 | 53.7 | 1.93 | 0.21 |
| C | CuAlSe2/Ga2S3/ZnS | 453 | 39 | 138.9 | 3.16 | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, S.; Liu, L.; Dong, X.; Li, X.; Yin, S.; Li, J. Synthesis of Eco-Friendly Narrow-Band CuAlSe2/Ga2S3/ZnS Quantum Dots for Blue Quantum Dot Light-Emitting Diodes. Coatings 2025, 15, 245. https://doi.org/10.3390/coatings15020245
Yuan S, Liu L, Dong X, Li X, Yin S, Li J. Synthesis of Eco-Friendly Narrow-Band CuAlSe2/Ga2S3/ZnS Quantum Dots for Blue Quantum Dot Light-Emitting Diodes. Coatings. 2025; 15(2):245. https://doi.org/10.3390/coatings15020245
Chicago/Turabian StyleYuan, Shenghua, Liyuan Liu, Xiaofei Dong, Xianggao Li, Shougen Yin, and Jingling Li. 2025. "Synthesis of Eco-Friendly Narrow-Band CuAlSe2/Ga2S3/ZnS Quantum Dots for Blue Quantum Dot Light-Emitting Diodes" Coatings 15, no. 2: 245. https://doi.org/10.3390/coatings15020245
APA StyleYuan, S., Liu, L., Dong, X., Li, X., Yin, S., & Li, J. (2025). Synthesis of Eco-Friendly Narrow-Band CuAlSe2/Ga2S3/ZnS Quantum Dots for Blue Quantum Dot Light-Emitting Diodes. Coatings, 15(2), 245. https://doi.org/10.3390/coatings15020245






