Fabrication of a Hydrophobic Coating Using Acacia mearnsii De Wild Tannin Melamine Formaldehyde Microcapsules
Abstract
:1. Introduction
2. Materials and Methods
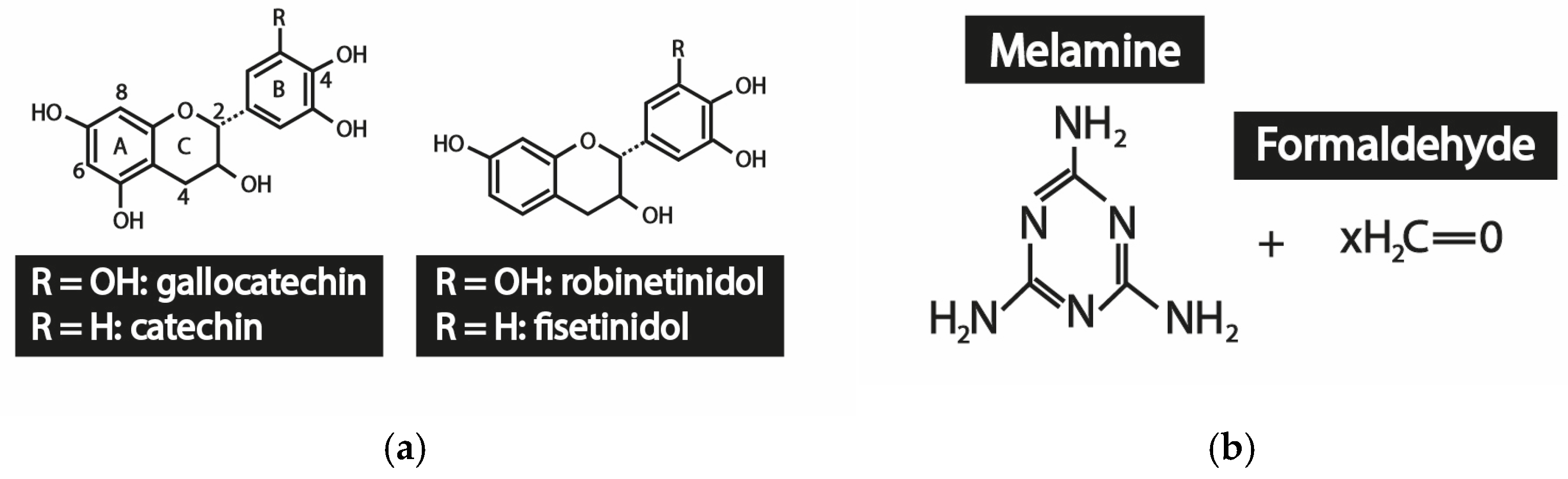
2.1. Materials
2.2. In Situ Polimerization
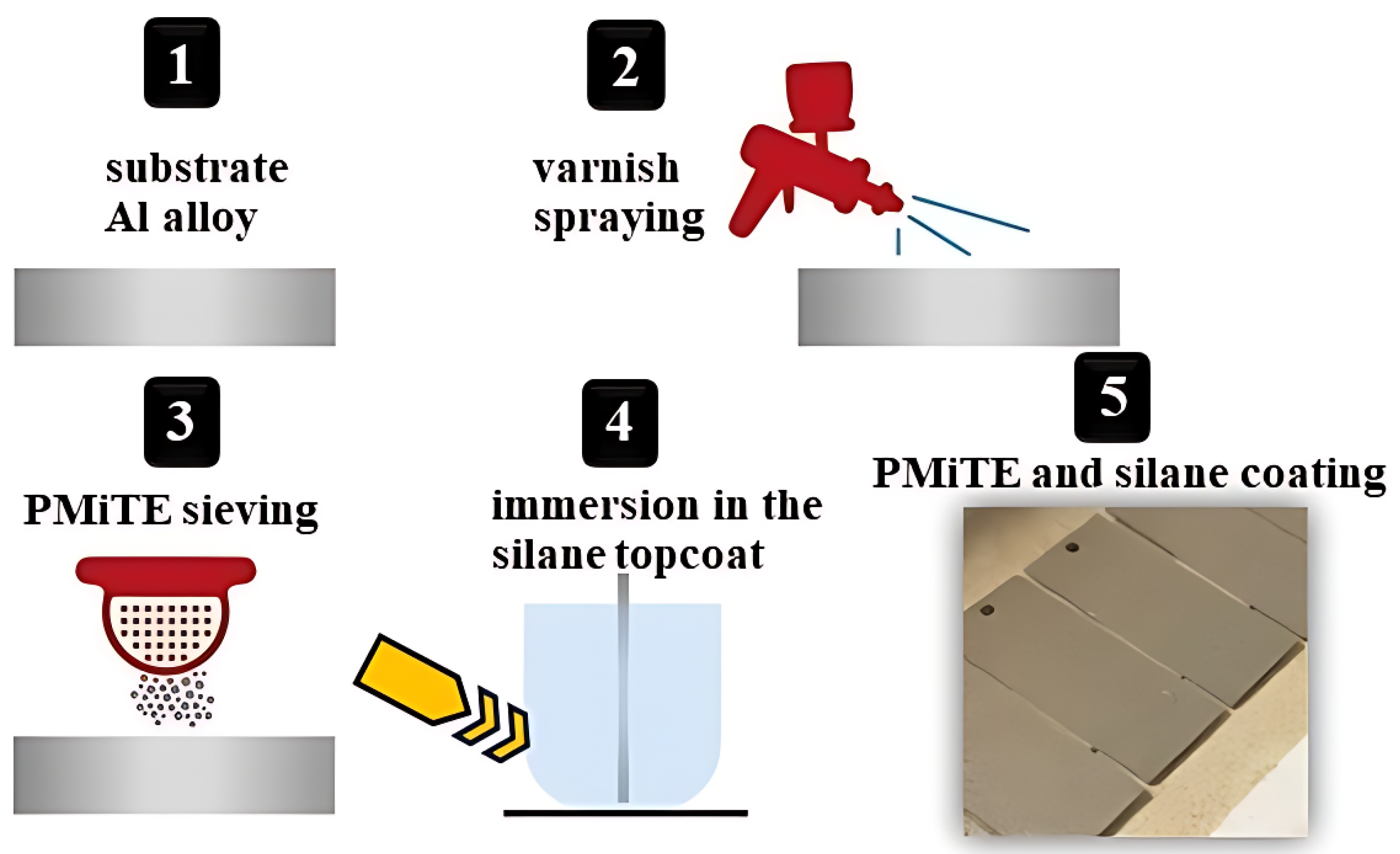
2.3. Coating Application
2.4. Characterization
3. Results and Discussion
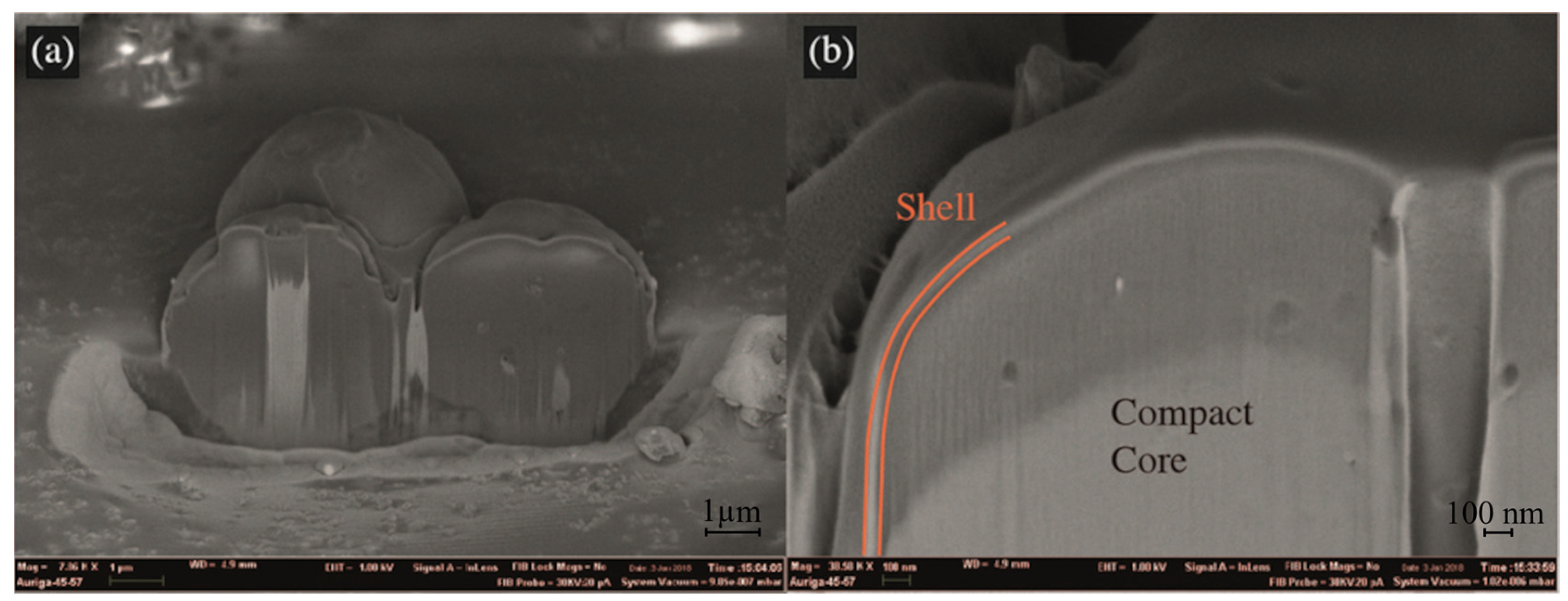
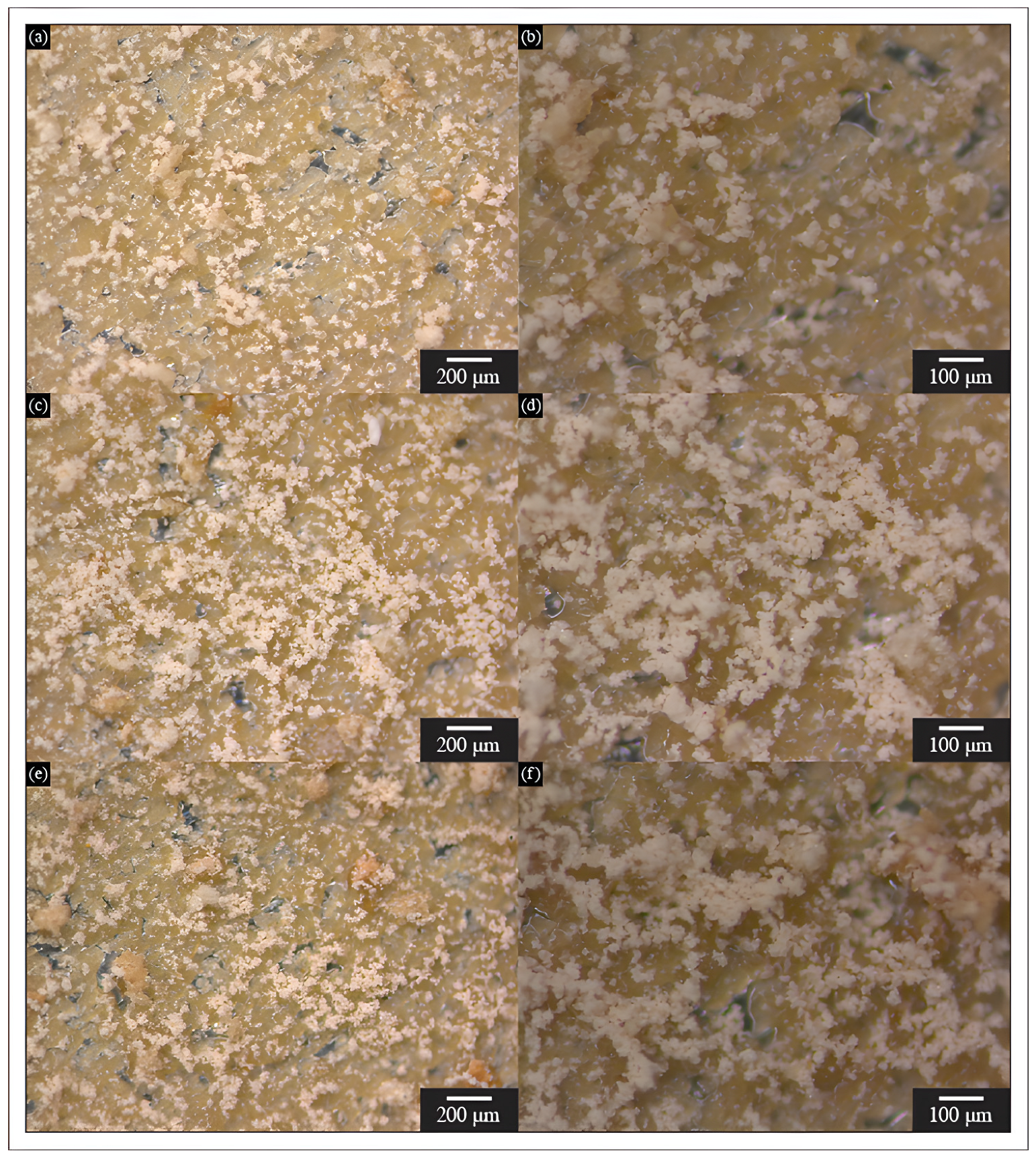
3.1. Microcapsules Morphology
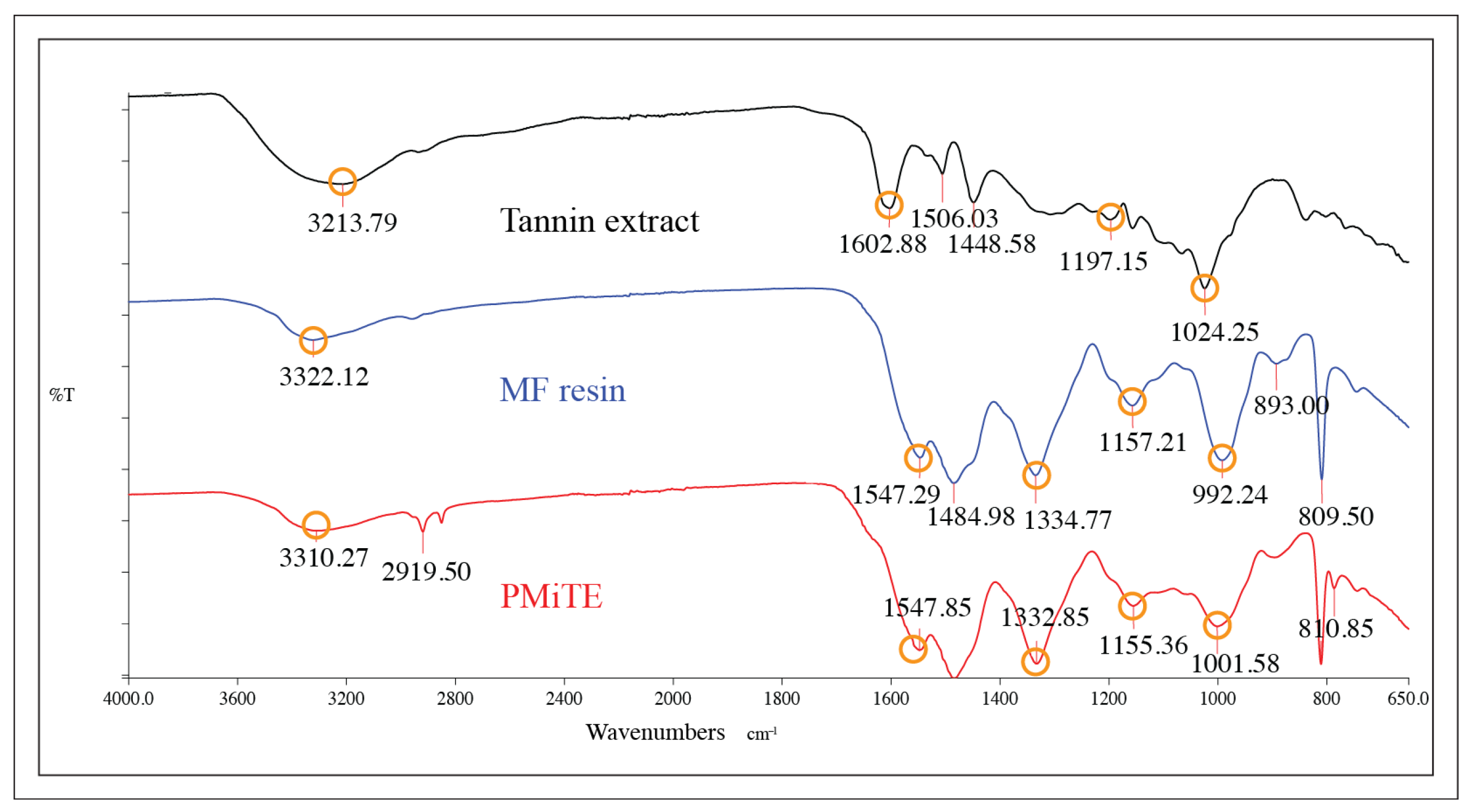
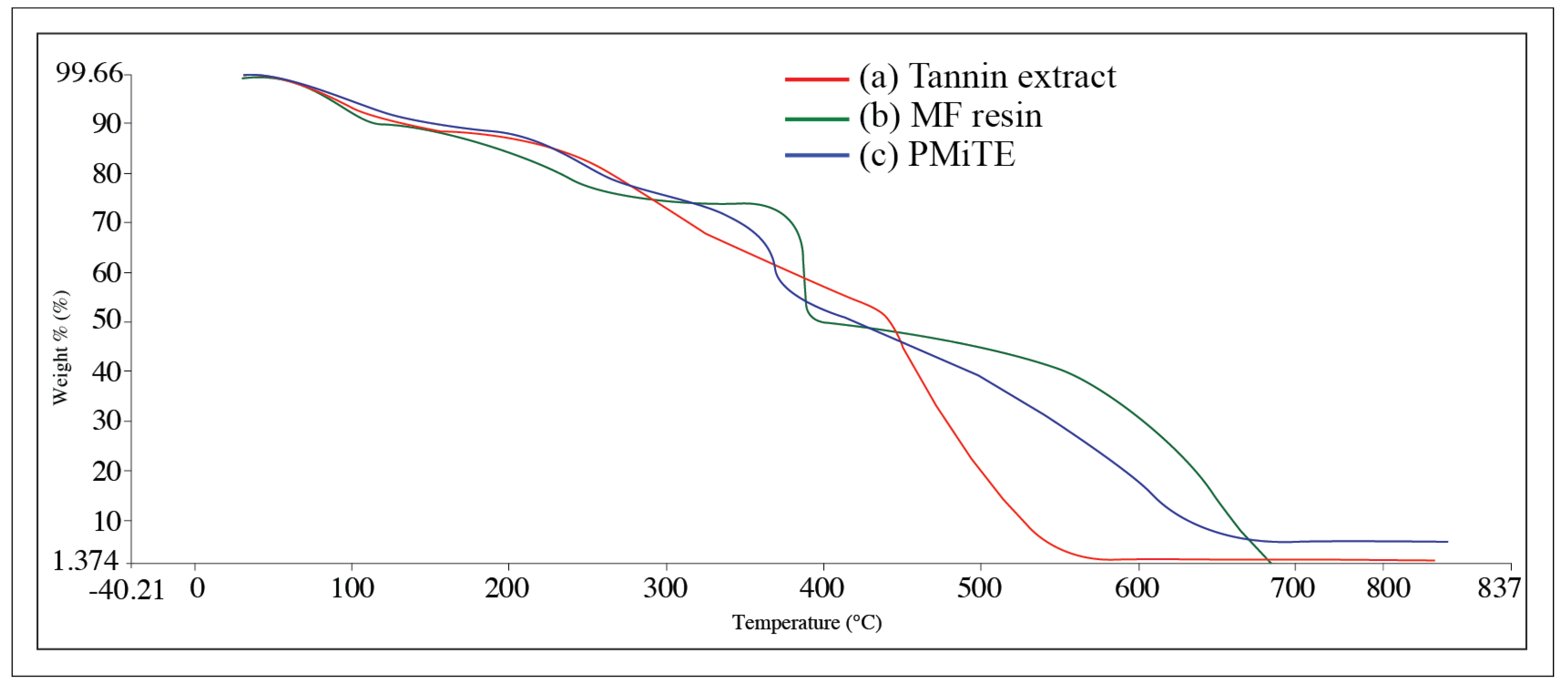
3.2. Chemical Structure and Thermal Stability
3.3. Coating Wettability and Average Roughness
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steigleder, A.P.; Roldo, L. Surface morphology and repellency mechanism investigation of Salvinia molesta plant. Rev. Mater. 2019, 24, e12480. [Google Scholar] [CrossRef]
- Hamidi, N.A.S.M.; Kamaruzzaman, W.M.I.W.M.; Nasir, N.A.M.; Shaifudin, M.S.; Ghazali, M.S.M. Potential Application of Plant-Based Derivatives as Green Components in Functional Coatings: A Review. Clean. Mater. 2022, 4, 100097. [Google Scholar] [CrossRef]
- Ghosh, S.K. Functional Coatings and Microencapsulation: A General Perspective. In Functional Coatings: By Polymer Microencapsulation, 1st ed.; Ghosh, S.K., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 1–28. [Google Scholar]
- Shrimali, K.; Dedhia, E.M. Microencapsulation for Textile Finishing. J. Polym. Text. Eng. 2015, 2, 1–4. [Google Scholar] [CrossRef]
- Noor Idora, M.S.; Ferry, M.; Wan Nik, W.B.; Jasnizat, S. Evaluation of tannin from Rhizophora apiculata as natural antifouling agents in epoxy paint for marine application. Prog. Org. Coat. 2015, 81, 125–131. [Google Scholar] [CrossRef]
- Peres, R.S.; Armelin, E.; Alemán, C.; Ferreira, C.A. Modified tannin extracted from black wattle tree as an environmentally friendly antifouling pigment. Ind. Crop. Prod. 2015, 65, 506–514. [Google Scholar] [CrossRef]
- Monteiro, J.M.; de Albuquerque, U.P.; de Lima Araújo, E.; de Amorim, E.L.C. Taninos: Uma abordagem da química à ecologia. Quim. Nova. 2005, 28, 892–896. [Google Scholar] [CrossRef]
- Aristri, M.A.; Sari, R.K.; Lubis, M.A.R.; Laksana, R.P.B.; Antov, P.; Iswanto, A.H.; Mardawati, E.; Lee, S.H.; Savov, V.; Kristak, L.; et al. Eco-Friendly Tannin-Based Non-Isocyanate Polyurethane Resins for the Modification of Ramie (Boehmeria nivea L.). Fibers Polym. 2023, 15, 1492. [Google Scholar] [CrossRef]
- Barthlott, W.; Mail, M.; Neinhuis, C. Superhydrophobic hierarchically structured surfaces in biology: Evolution, structural principles and biomimetic applications. Phil. Trans. R. Soc. A 2016, 374, 20160191. [Google Scholar] [CrossRef]
- Jin, H.; Tian, L.; Bing, W.; Zao, J.; Ren, L. Bioinspired marine antifouling coatings: Status, prospects, and future. Prog. Mater. Sci. 2022, 124, 100889. [Google Scholar] [CrossRef]
- Lamprakou, Z.; Bi, H.; Weinell, C.E.; Dam-Johansen, K. Tannin-based inhibitive pigment for sustainable epoxy coatings formulation. Prog. Org. Coat. 2022, 167, 106841. [Google Scholar] [CrossRef]
- Eqbal, D.; Gundabala, V. Controlled fabrication of multi-core alginate microcapsules. J. Colloid. Interf. Sci. 2017, 507, 27–34. [Google Scholar] [CrossRef]
- Zhu, K.; Qi, H.; Wang, S.; Zhou, J.; Zhao, Y.; Su, J.; Yuan, X. Preparation and Characterization of Melamine-Formaldehyde Resin Micro- and Nanocapsules Filled with n-Dodecane. J. Macromol. Sci. B 2012, 51, 1976–1990. [Google Scholar] [CrossRef]
- Pires, F.Q.; Gross, I.P.; Sa-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Bao, S.N.; Cunha-Filho, M. In-situ formation of nanoparticles from drug-loaded 3D polymeric matrices. Eur. J. Pharm. Sci. 2023, 188, 106517. [Google Scholar] [CrossRef]
- Ollier, R.P.; Alvarez, V.A. Synthesis of epoxy-loaded poly(melamine-formaldehyde) microcapsules: Effect of pH regulation method and emulsifier selection. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 872–882. [Google Scholar] [CrossRef]
- Urdl, K.; Weiss, S.; Karpa, A.; Perić, M.; Zikulnig-Rusch, E.; Brecht, M.; Kandelbauer, A.; Müller, U.; Kern, W. Furan-functionalised melamine-formaldehyde particles performing Diels-Alder reactions. Eur. Polym. J. 2018, 108, 225–234. [Google Scholar] [CrossRef]
- Yataganbaba, A.; Ozkahraman, B.; Kurtbas, I. Worldwide trends on encapsulation of phase change materials: A bibliometric analysis (1990–2015). Appl. Energ. 2017, 185, 720–731. [Google Scholar] [CrossRef]
- Sturzenegger, P.N.; Gonzenbach, U.T.; Koltzenburg, S.; Martynczuk, J.; Gauckler, L.J. Particle-stabilized gel-core microcapsules: Synthesis and properties. Colloids Surf. A Physicochem. Eng. Asp. 2014, 447, 44–50. [Google Scholar] [CrossRef]
- Rajput, A.; Mishra, A.; Ali, A.; Goswami, R.; Bhatt, N. Transparent superhydrophobic coating for copper metal and study of surface morphology and antimicrobial characteristics. Colloid Surf. A Physicochem. Eng. Asp. 2024, 684, 133067. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Barthlott, W. Diversity of structure, morphology and wetting of plant surfaces. Soft Matter. 2008, 4, 1943–1963. [Google Scholar] [CrossRef]
- Madadi, F.; Rezaeian, A.; Edris, H.; Zhiani, M. Influence of surface roughness and hydrophobicity of bipolar plates on PEM performance. Surf. Coat Tech. 2020, 389, 125676. [Google Scholar] [CrossRef]
- Wiesener, M.; Regenspurger, R.; Pilz, M.; Shchukin, D.; Latnikova, A.; Yang, J.; Grundmeier, G. In-situ contact angle studies of the release of water displacing agents from capsule filled organic coatings. Surf. Coat Tech. 2012, 206, 4481–4487. [Google Scholar] [CrossRef]
- Pantoja, M.; Velasco, F.; Abenojar, J.; Martinez, M.A. Development of superhydrophobic coatings on AISI 304 austenitic stainless steel with different surface pretreatments. Thin Solid Films 2019, 671, 22–30. [Google Scholar] [CrossRef]
- Avossa, J.; Bifulco, A.; Amendola, E.; Gesuele, F.; Oscurato, S.L.; Gizaw, Y.; Mensitieri, G.; Branda, F. Forming nanostructured surfaces through Janus colloidal silica particles with nanowrinkles: A new strategy to superhydrophobicity. Appl. Surf. Sci. 2019, 465, 73–81. [Google Scholar] [CrossRef]
- Noreljaleel, A.E.M.; Wilhelm, A.; Bonnet, S.L. Analysis of commercial proanthocyanidins. part 6: Sulfitation of flavan-3-ols catechin and epicatechin, and procyanidin B-3. Molecules 2020, 25, 4980. [Google Scholar] [CrossRef]
- Escobar, C.F.; Roldo, L.; Rocha, T.L.A.; Kindlein, W., Jr. Nanoencapsulation mechanism of fragrant oil: Effect of formaldehyde−melamine molar ratio. Polym. Int. 2018, 67, 220–226. [Google Scholar] [CrossRef]
- Cheong, I.W.; Shin, J.S.; Kim, J.H.; Lee, S.J. Preparation of Monodisperse Melamine-Formaldehyde Microspheres via Dispersed Polycondensation. Macromol. Res. 2004, 12, 225–232. [Google Scholar] [CrossRef]
- Leskovšek, M.; Kortnik, J.; Elesini, U.S.; Šumiga, B. Characterisation of melamine formaldehyde microspheres synthesised with prolonged microencapsulated reaction time. J. Polym. Eng. 2022, 42, 288–297. [Google Scholar] [CrossRef]
- ASTM D2247; Standard Practice for Testing Water Resistance of Coatings in 100 % Relative Humidity. American Society for Testing and Materials: Montgomery County, PA, USA, 2015.
- Latthe, S.S.; Gurav, A.B.; Maruti, C.S.; Vhatkar, R.S. Recent Progress in Preparation of Superhydrophobic Surfaces: A Review. J. Surf. Eng. Mat. Adv. Tech. 2012, 2, 76–94. [Google Scholar] [CrossRef]
- Verplanck, N.; Coffinier, Y.; Thomy, V.; Boukherroub, R. Wettability Switching Techniques on Superhydrophobic Surfaces. Nanoscale Res. Lett. 2007, 2, 577–596. [Google Scholar] [CrossRef]
- Bandeira, R.M.; van Drunen, J.; Garcia, A.C.; Tremiliosi-Filho, G. Influence of the thickness and roughness of polyaniline coatings on corrosion protection of AA7075 aluminum alloy. Electrochim. Acta 2017, 240, 215–224. [Google Scholar] [CrossRef]
- ABNT NBR ISO 4287:2002; Especificações Geométricas do Produto (GPS)—Rugosidade: Método do Perfil—Termos, Definições e Parâmetros da Rugosidade. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2002.
- Porras-Saavedra, J.; Alamilla-Beltrán, L.; Lartundo-Rojas, L.; Perea-Flores, M.J.; Yáñez-Fernandez, J.; Palacios-González, E.; Gutierrez-López, G.F. Chemical components distribution and morphology of microcapsules of paprika oleoresin by microscopy and spectroscopy. Food Hydrocolloid. 2018, 81, 6–14. [Google Scholar] [CrossRef]
- bin Hussein, M.Z.; Ghotbi, M.Y.; Yahaya, A.H.; Rahman, M.Z.A. Synthesis and characterization of (zinc-layered-gallate) nanohybrid using structural memory effect. Mater. Chem. Phys. 2009, 113, 491–496. [Google Scholar] [CrossRef]
- Viswanath, V.; Leo, V.V.; Sabna Prabha, S.; Prabhakumari, C.; Potty, V.P.; Jisha, M.S. Thermal properties of tannin extracted from Anacardium occidentale L. using TGA and FT-IR spectroscopy. Nat. Prod. Res. 2015, 30, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Baaka, N.; Ammar, M.; Saad, M.K.; Khiari, R. Properties of tannin-glyoxal resins prepared from lyophilized and condensed tannin. J. Text. Eng. Fashion Technol. 2017, 4, 705–711. [Google Scholar] [CrossRef]
- Merline, D.J.; Vukusic, S.; Abdala, A.A. Melamine formaldehyde: Curing studies and reaction mechanism. Polym. J. 2013, 45, 413–419. [Google Scholar] [CrossRef]
- Mangrich, A.S.; Doumer, M.E.; Mallmann, A.S.; Wolf, C.R. Química verde no tratamento de águas: Uso de coagulante derivado de tanino de Acacia mearnsii. Rev. Virtual Quim. 2014, 6, 2–15. [Google Scholar] [CrossRef]
- Luo, C.; Grigsby, W.; Edmonds, N.; Easteal, A.; Al-Hakkak, J. Synthesis, characterization, and thermal behaviors of tannin stearates prepared from quebracho and pine bark extracts. J. Appl. Polym. Sci. 2010, 117, 352–360. [Google Scholar] [CrossRef]
- Yang, F.; Guo, Z. Characterization of micro-morphology and wettability of lotus leaf, waterlily leaf and biomimetic ZnO surface. J. Bionic Eng. 2015, 12, 88–97. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, W. Biomimic from the superhydrophobic plant leaves in mature: Binary structure and unitary structure. Plant Sci. 2007, 172, 1103–1112. [Google Scholar] [CrossRef]
- Sajadinia, S.H.; Sharif, F. Thermodynamic Analysis of the Wetting Behavior of Dual Scale Patterned Hydrophobic Surfaces. J. Colloid Interface Sci. 2010, 344, 575–583. [Google Scholar] [CrossRef]
- Kwon, T.W.; Jang, J.; Ambrosia, M.S.; Ha, M.Y. Molecular Dynamics Study on the Hydrophobicity of a Surface Patterned with Hierarchical Nanotextures. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 209–217. [Google Scholar] [CrossRef]
- Bormashenko, E.; Bormashenko, Y.; Whyman, G.; Pogreb, R.; Stanevsky, O. Micrometrically scaled textured metallic hydrophobic interfaces validate the Cassie–Baxter wetting hypothesis. J. Colloid Interf. Sci. 2006, 302, 308–311. [Google Scholar] [CrossRef]
- Vanithakumari, S.C.; George, R.P.; Kamachi Mudali, U. Influence of silanes on the wettability of anodized titanium. Appl. Surf. Sci. 2014, 292, 650–657. [Google Scholar] [CrossRef]

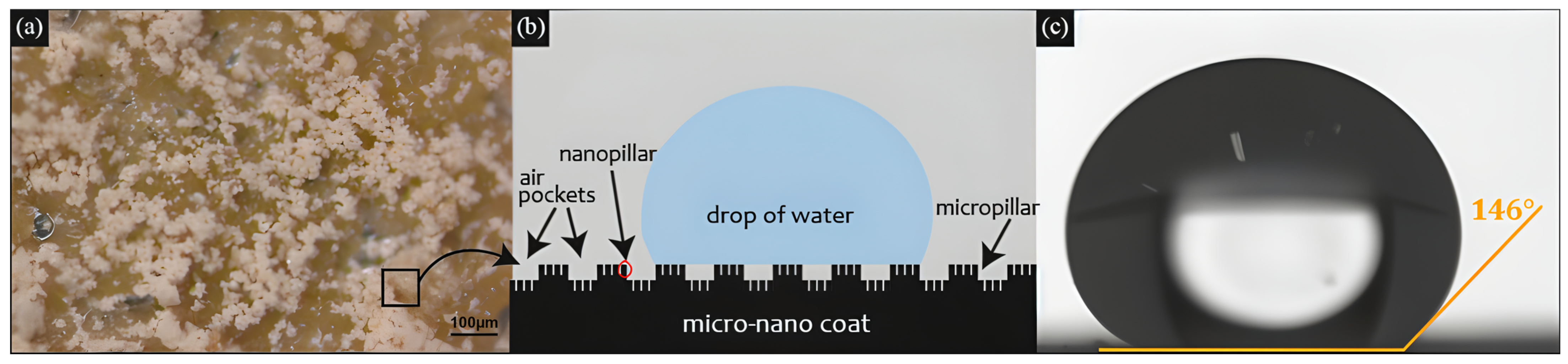
| Coated Samples Exposure Time (h) | Contact Angle | Contact Angle Hysteresis | Average Roughness (μm) |
|---|---|---|---|
| 0 | 135° | 10° | 10.9 |
| 24 | 146° | 9° | 9.9 |
| 48 | 142° | 9° | 10.2 |
| 72 | 143° | 11° | 11.3 |
| 96 | 146° | 11° | 9.4 |
| 168 | 146° | 15° | 9.7 |
| 240 | 137° | 10° | 10.2 |
| 336 | 138° | 13° | 10.4 |
| 432 | 148° | 12° | 11.1 |
| 576 | 146° | 13° | 10 |
| 744 | 139° | 6° | 9.6 |
| 1008 | 136° | 6° | 9.4 |
| Sample Mean | 141.9° | 10.8° | 10.2 |
| Standard deviation | 4.6° | 2.7° | 0.7 |
| Range | From 135° to 148° | From 65° to 15° | From 9.4 to 11.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steigleder, A.P.; Roldo, L. Fabrication of a Hydrophobic Coating Using Acacia mearnsii De Wild Tannin Melamine Formaldehyde Microcapsules. Coatings 2025, 15, 419. https://doi.org/10.3390/coatings15040419
Steigleder AP, Roldo L. Fabrication of a Hydrophobic Coating Using Acacia mearnsii De Wild Tannin Melamine Formaldehyde Microcapsules. Coatings. 2025; 15(4):419. https://doi.org/10.3390/coatings15040419
Chicago/Turabian StyleSteigleder, Ana Paula, and Liane Roldo. 2025. "Fabrication of a Hydrophobic Coating Using Acacia mearnsii De Wild Tannin Melamine Formaldehyde Microcapsules" Coatings 15, no. 4: 419. https://doi.org/10.3390/coatings15040419
APA StyleSteigleder, A. P., & Roldo, L. (2025). Fabrication of a Hydrophobic Coating Using Acacia mearnsii De Wild Tannin Melamine Formaldehyde Microcapsules. Coatings, 15(4), 419. https://doi.org/10.3390/coatings15040419









