Biocompatibility of Al2O3-Doped Diamond-like Carbon Laparoscope Coatings
Abstract
:1. Introduction
2. Experimental Details
3. Results and Discussion
3.1. Visual Inspection
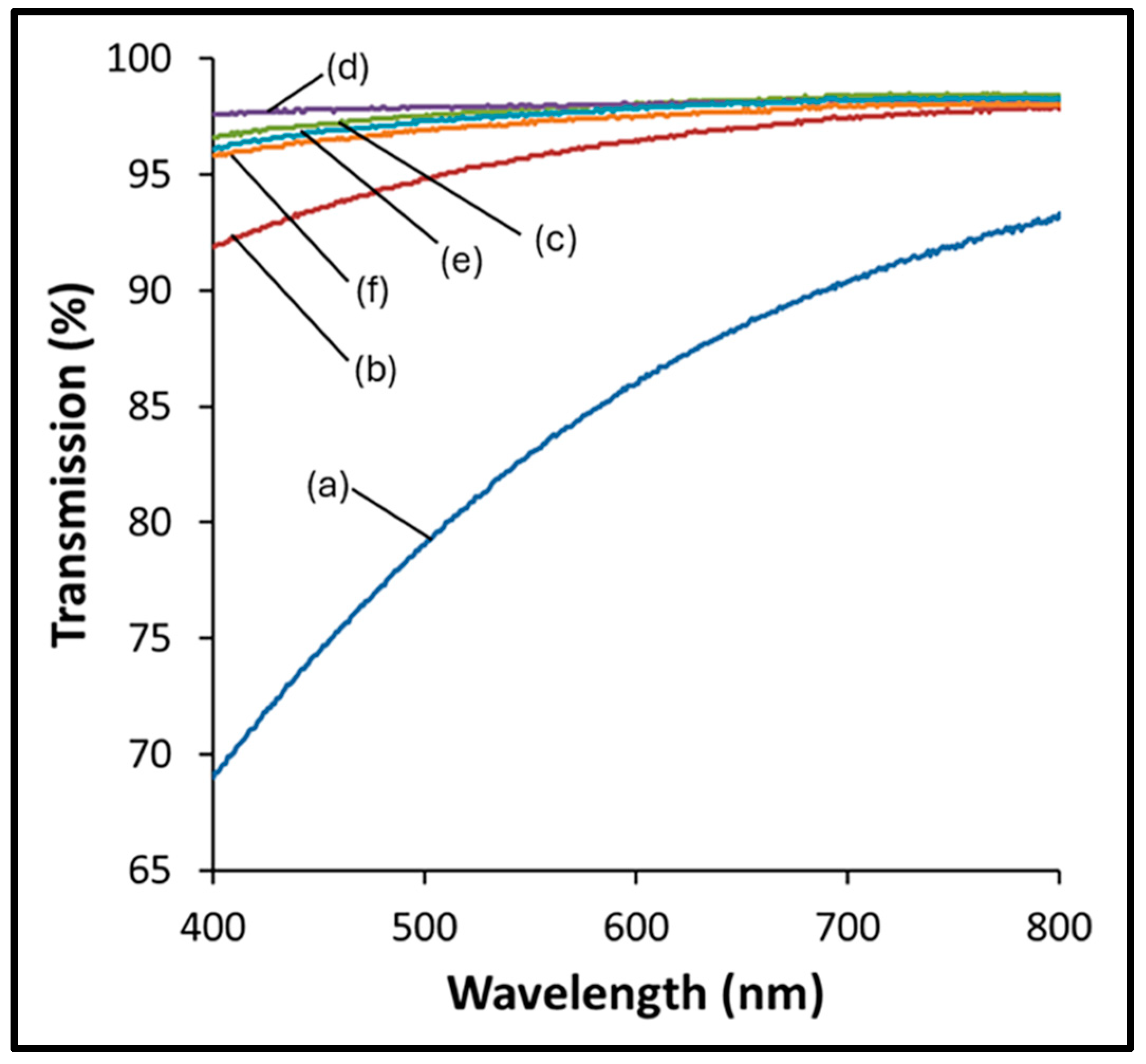
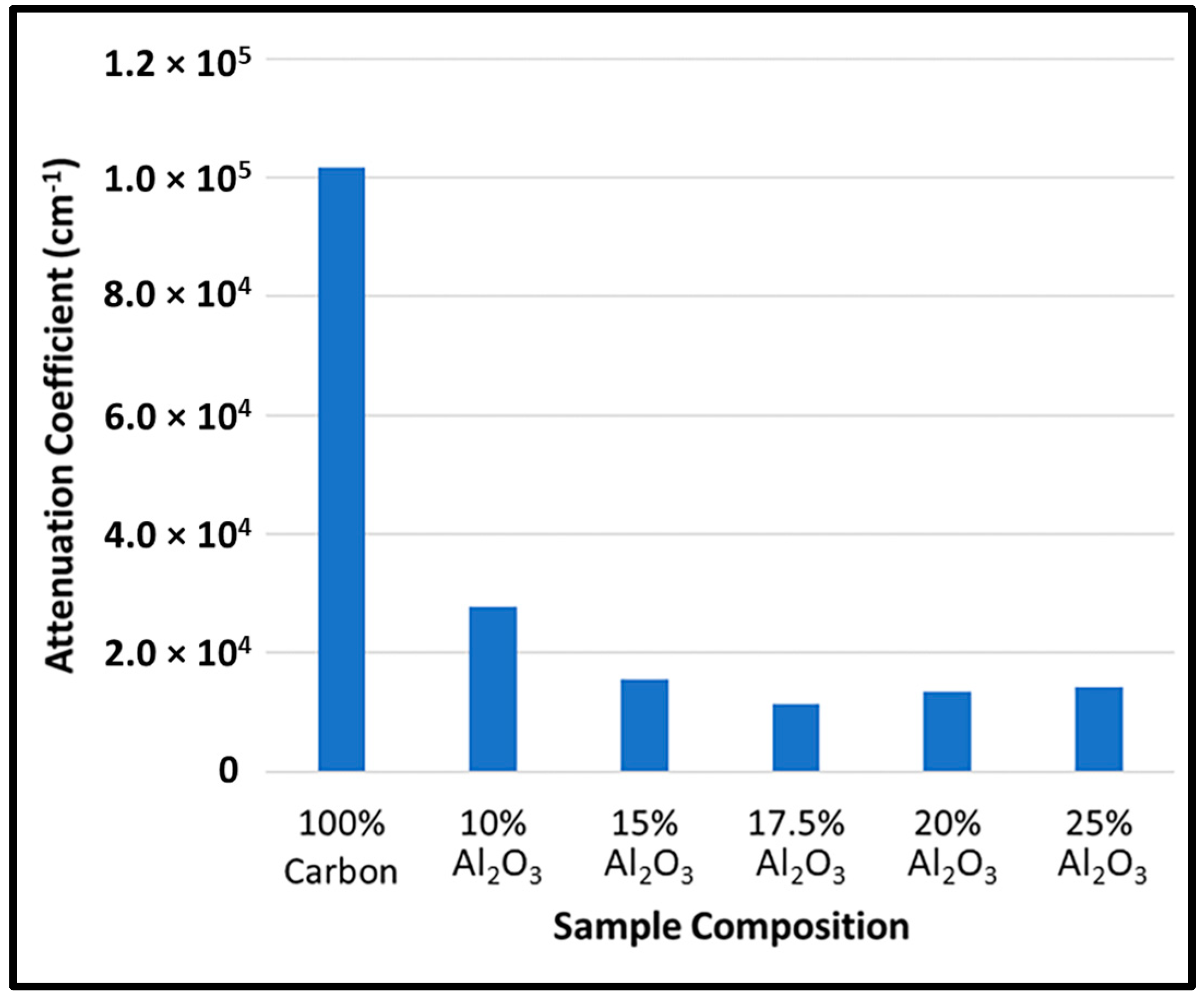
3.2. Film Thickness and Transparency
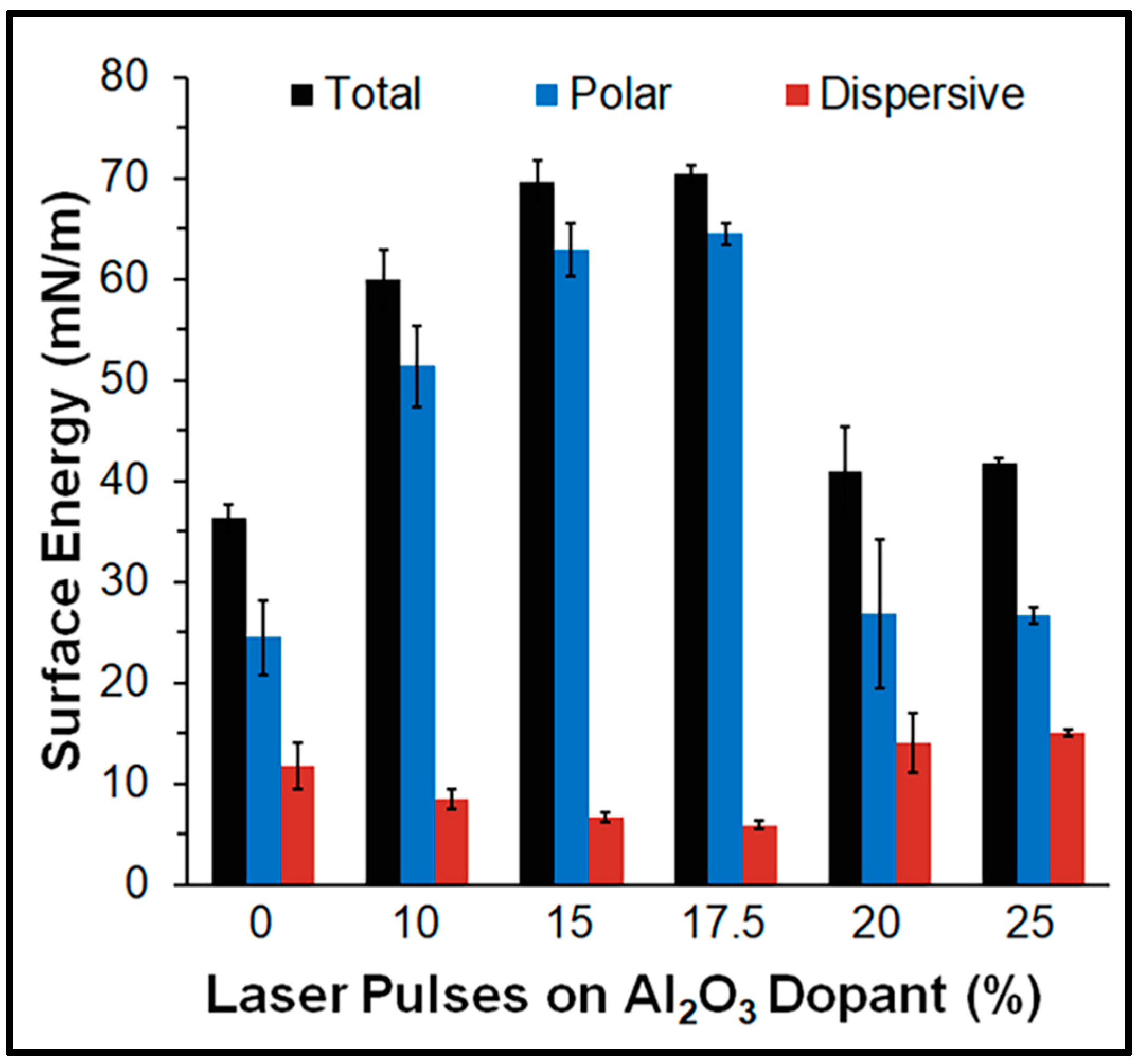
3.3. Water Contact Angle and Surface Energy
3.4. Stability and Biocompatibility
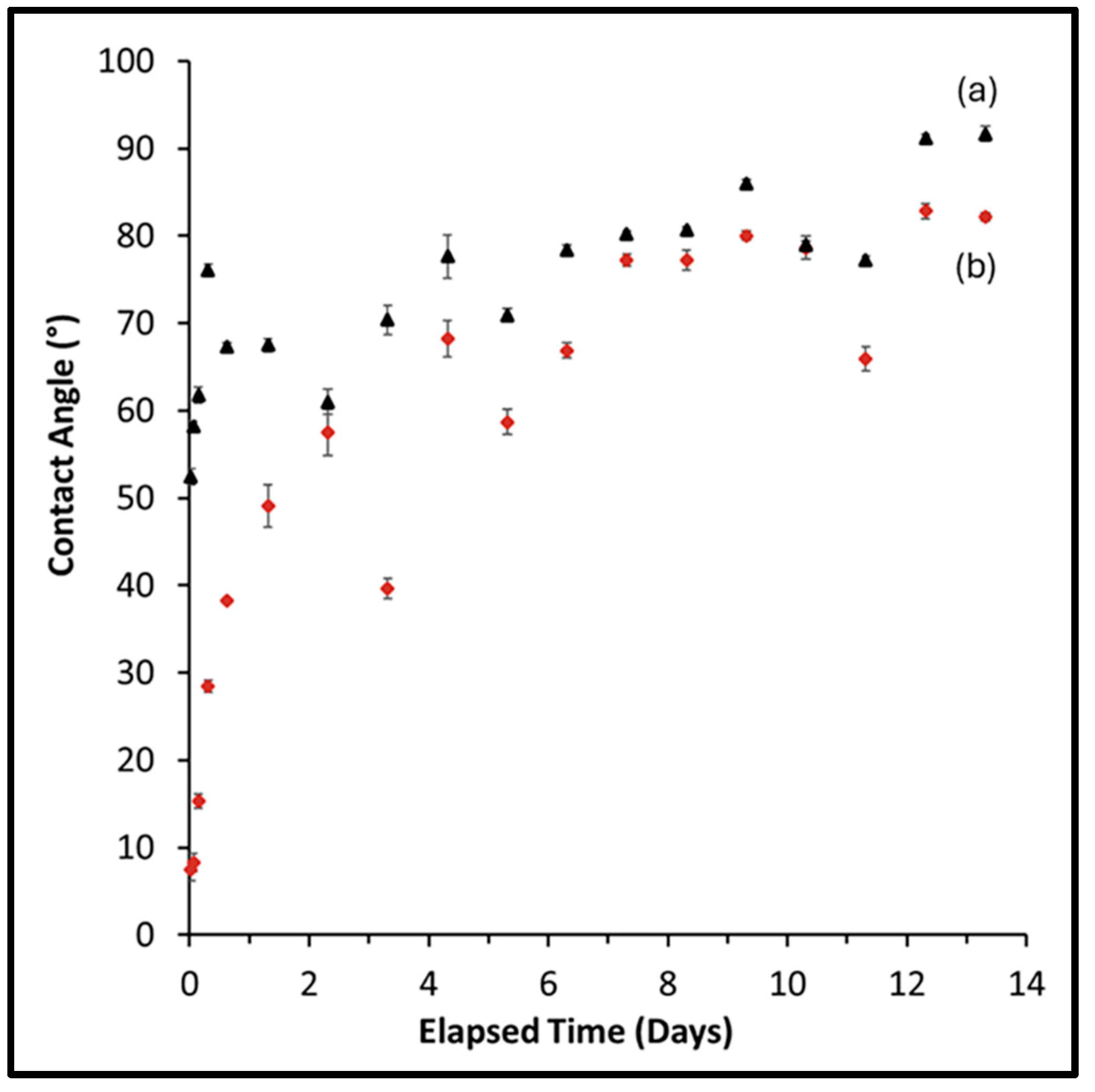
3.4.1. Simulated Body Fluid Studies
3.4.2. Assessment of Biocompatibility Using CellTiter-Glo Assays
3.4.3. Blood Compatibility Testing
3.5. Surface Roughness and Morphology
3.6. Wear Properties
3.7. Raman Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dua, A.; Aziz, A.; Desai, S.S.; McMaster, J.; Kuy, S. National Trends in the Adoption of Laparoscopic Cholecystectomy over 7 Years in the United States and Impact of Laparoscopic Approaches Stratified by Age. Minim. Invasive Surg. 2014, 2014, 635461. [Google Scholar] [CrossRef] [PubMed]
- Mattingly, A.S.; Chen, M.M.; Divi, V.; Holsinger, F.C.; Saraswathula, A. Minimally Invasive Surgery in the United States, 2022: Understanding Its Value Using New Datasets. J. Surg. Res. 2023, 281, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Spaner, S.J.; Warnock, G.L. A brief history of endoscopy, laparoscopy, and laparoscopic surgery. J. Laparoendosc. Adv. Surg. Tech. 1997, 7 Pt A, 369–373. [Google Scholar] [CrossRef]
- Drysch, A.; Schmitt, K.; Uribe, B.; Yoon, R.; Okhunov, Z.; Landman, J. Comparative analysis of techniques to prevent laparoscopic fogging. Minim. Invasive Ther. Allied Technol. 2016, 25, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.; Edwards, J.; Culver, D.; Emori, T.G.; Tolson, J.; Gaynes, R.; National Nosocomial Infections Surveillance (NNIS) System, Centers for Disease Control and Prevention. Does using a laparoscopic approach to cholecystectomy decrease the risk of surgical site infection? Ann. Surg. 2003, 237, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Jallu, S.; Pasricha, K.; Basumatary, B.; Parmar, B.P.S.; Sahani, A.K. Laparoscopic Lens Defogging: A Review of Methods to Maintain a Clear Operating Field. Indian J. Surg. 2022, 84, 698–706. [Google Scholar] [CrossRef]
- Manning, T.G.; Perera, M.; Christidis, D.; Kinnear, N.; McGrath, S.; O’Beirne, R.; Zotov, P.; Bolton, D.; Lawrentschuk, N. Visual Occlusion During Minimally Invasive Surgery: A Contemporary Review of Methods to Reduce Laparoscopic and Robotic Lens Fogging and Other Sources of Optical Loss. J. Endourol. 2017, 31, 327–333. [Google Scholar] [CrossRef]
- Nabeel, A.; Al-Sabah, S.K.; Ashrafian, H. Effective cleaning of endoscopic lenses to achieve visual clarity for minimally invasive abdominopelvic surgery: A systematic review. Surg. Endosc. 2022, 36, 2382–2392. [Google Scholar] [CrossRef]
- Naguib, S.M.; Abdelaal, Y.A.E.-A.; Khodary, A.R.; Fahmi, K.S.; Abdalla, W.M. Nano-Ceramic Coating: A Novel Idea to Reduce Laparoscope Lens Fogging—Experimental Study. Silicon 2022, 14, 7693–7700. [Google Scholar] [CrossRef]
- Leonard, R.L.; Bull, A.B.; Xue, F.; Haycook, C.P.; Gray, S.K.; Bond, C.W.; Bond, P.E.; McDearman, J.C.; Woods, D.P.; Mayfield, J.; et al. Biocompatibility of antifogging SiO-doped Diamond-Like carbon laparoscope coatings. Appl. Surf. Sci. 2023, 634, 157606. [Google Scholar] [CrossRef]
- Leonard, R.L.; Terekhov, A.Y.; Thompson, C.; Erck, R.A.; Johnson, J.A. Antifog coating for bronchoscope lens. Surf. Eng. 2012, 28, 468–472. [Google Scholar] [CrossRef]
- Leonard, R.L.; Hasan, S.A.; Terekhov, A.Y.; Thompson, C.; Erck, R.A.; Dickerson, J.H.; Johnson, J.A. Protective coatings for enhanced performance in biomedical applications. Surf. Eng. 2012, 28, 473–479. [Google Scholar] [CrossRef]
- Ohtake, N.; Hiratsuka, M.; Kanda, K.; Akasaka, H.; Tsujioka, M.; Hirakuri, K.; Hirata, A.; Ohana, T.; Inaba, H.; Kano, M.; et al. Properties and Classification of Diamond-Like Carbon Films. Materials 2021, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, J.C.; Fernández, A. Doping and Alloying Effects on DLC Coatings. In Tribology of Diamond-Like Carbon Films: Fundamentals and Applications; Donnet, C., Erdemir, A., Eds.; Springer: Boston, MA, USA, 2008; pp. 311–338. [Google Scholar]
- Zhang, M.; Xie, T.; Qian, X.; Zhu, Y.; Liu, X. Mechanical Properties and Biocompatibility of Ti-doped Diamond-like Carbon Films. ACS Omega 2020, 5, 22772–22777. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.M.; Huang, G.J.; Wang, S.; Mi, C.W.; Wei, S.F.; Tian, F.T.; Li, W.; Cao, H.Y.; Cheng, Y. A review on diamond-like carbon films grown by pulsed laser deposition. Appl. Surf. Sci. 2021, 541, 12. [Google Scholar] [CrossRef]
- Antuzevics, A.; Elsts, E.; Kemere, M.; Lushchik, A.; Moskina, A.; Scherer, T.A.; Popov, A.I. Thermal annealing of neutron irradiation generated paramagnetic defects in transparent Al2O3 ceramics. Opt. Mater. 2023, 135, 6. [Google Scholar] [CrossRef]
- Deng, N.M.; Zhao, J.; Yang, L.L.; Zheng, Z.Q. Effects of Brazing Technology on Hermeticity of Alumina Ceramic-Metal Joint Used in Nuclear Power Plants. Front. Mater. 2021, 7, 7. [Google Scholar] [CrossRef]
- Houska, J.; Blazek, J.; Rezek, J.; Proksova, S. Overview of optical properties of Al2O3 films prepared by various techniques. Thin Solid Film. 2012, 520, 5405–5408. [Google Scholar] [CrossRef]
- Molla, J.; Heidinger, R.; Ibarra, A. Alumina ceramics for heating-systems. J. Nucl. Mater. 1994, 212, 1029–1034. [Google Scholar] [CrossRef]
- Merola, M.; Affatato, S. Materials for Hip Prostheses: A Review of Wear and Loading Considerations. Materials 2019, 12, 495. [Google Scholar] [CrossRef]
- Rahmati, M.; Mozafari, M. Biocompatibility of alumina-based biomaterials–A review. J. Cell. Physiol. 2019, 234, 3321–3335. [Google Scholar] [CrossRef] [PubMed]
- Spajic, I.; Rodi, P.; Sekularac, G.; Lekka, M.; Fedrizzi, L.; Milosev, I. The effect of surface preparation on the protective properties of Al2O3 and HfO2 thin films deposited on cp-titanium by atomic layer deposition. Electrochim. Acta 2021, 366, 16. [Google Scholar] [CrossRef]
- Cheng, Y.; Lu, Y.; Huang, G.; Guo, Y.; Xi, L.; Wang, S.; Tian, F. Infrared and mechanical properties of the oxygen-doped non-hydrogenated diamond-like carbon film prepared by pulsed laser deposition. Infrared Phys. Technol. 2019, 102, 102983. [Google Scholar] [CrossRef]
- Safaie, P.; Eshaghi, A.; Bakhshi, S.R. Structure and mechanical properties of oxygen doped diamond-like carbon thin films. Diam. Relat. Mater. 2016, 70, 91–97. [Google Scholar] [CrossRef]
- Yoshitake, T.; Nishiyama, T.; Nagayama, K. The role of hydrogen and oxygen gas in the growth of carbon thin films by pulsed laser deposition. Diam. Relat. Mater. 2000, 9, 689–692. [Google Scholar] [CrossRef]
- Bouabibsa, I.; Lamri, S.; Sanchette, F. Structure, Mechanical and Tribological Properties of Me-Doped Diamond-Like Carbon (DLC) (Me = Al, Ti, or Nb) Hydrogenated Amorphous Carbon Coatings. Coatings 2018, 8, 370. [Google Scholar] [CrossRef]
- Ghadai, R.K.; Shanmugasundar, G.; Cepova, L.; Das, S.; Kumar Mahto, P.; Kalita, K. A temperature-based synthesis and characterization study of aluminum-incorporated diamond-like carbon thin films. Front. Mech. Eng. 2023, 9, 1325040. [Google Scholar] [CrossRef]
- Peckus, D.; Meškinis, Š.; Vasiliauskas, A.; Rajackaitė, E.; Andrulevičius, M.; Kopustinskas, V.; Tamulevičius, S. Structure and optical properties of diamond like carbon films containing aluminium and alumina. Appl. Surf. Sci. 2020, 529, 147040. [Google Scholar] [CrossRef]
- Voevodin, A.A.; O’Neill, J.P.; Zabinski, J.S. Nanocomposite tribological coatings for aerospace applications. Surf. Coat. Technol. 1999, 116–119, 36–45. [Google Scholar] [CrossRef]
- ISO 10993-4:2017; Biological Evaluation of Medical Devices, in: Part 4: Selection of Tests for Interactions with Blood. International Organization for Standardization: Geneva, Switzerland, 2017.
- Jung, F.; Braune, S.; Lendlein, A. Haemocompatibility testing of biomaterials using human platelets. Clin. Hemorheol. Microcirc. 2013, 53, 97–115. [Google Scholar] [CrossRef]
- ASTM D3359-23; Standard Test Methods for Rating Adhesion by Tape Test. ASTM International: West Conshohocken, PA, USA, 2023.
- Panzer, J. Components of solid surface free energy from wetting measurements. J. Colloid Interface Sci. 1973, 44, 142–161. [Google Scholar] [CrossRef]
- Fernández, V.; Khayet, M. Evaluation of the surface free energy of plant surfaces: Toward standardizing the procedure. Front. Plant Sci. 2015, 6, 510. [Google Scholar] [CrossRef]
- Cho, S.-B.; Nakanishi, K.; Kokubo, T.; Soga, N.; Ohtsuki, C.; Nakamura, T.; Kitsugi, T.; Yamamuro, T. Dependence of Apatite Formation on Silica Gel on Its Structure: Effect of Heat Treatment. J. Am. Ceram. Soc. 2005, 78, 1769–1774. [Google Scholar] [CrossRef]
- Nama Manjunatha, K.; Paul, S. Investigation of optical properties of nickel oxide thin films deposited on different substrates. Appl. Surf. Sci. 2015, 352, 10–15. [Google Scholar] [CrossRef]
- Yi, J.W.; Moon, M.-W.; Ahmed, S.F.; Kim, H.; Cha, T.-G.; Kim, H.-Y.; Kim, S.-S.; Lee, K.-R. Long-Lasting Hydrophilicity on Nanostructured Si-Incorporated Diamond-Like Carbon Films. Langmuir 2010, 26, 17203–17209. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.H.; Rice, A.S.; Garrett, K.; Stein, S.F. Gender, race and diet affect platelet function tests in normal subjects, contributing to a high rate of abnormal results. Br. J. Haematol. 2014, 165, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, S.; Hasebe, T.; Kamijo, A.; Yoshimoto, Y.; Hotta, A.; Morita, H.; Terada, H.; Tanaka, M.; Takahashi, K.; Suzuki, T. Effect of oxygen plasma treatment on non-thrombogenicity of diamond-like carbon films. Diam. Relat. Mater. 2010, 19, 861–865. [Google Scholar] [CrossRef]
- Cappelli, E.; Scilletta, C.; Mattei, G.; Valentini, V.; Orlando, S.; Servidori, M. Critical role of laser wavelength on carbon films grown by PLD of graphite. Appl. Phys. A 2008, 93, 751–758. [Google Scholar] [CrossRef]
- Jadhav, H.; Singh, A.K.; Sinha, S. Pulsed laser deposition of graphite in air and in vacuum for field emission studies. Mater. Chem. Phys. 2015, 162, 279–285. [Google Scholar] [CrossRef]
- Ferrari, A.C. Determination of bonding in diamond-like carbon by Raman spectroscopy. Diam. Relat. Mater. 2002, 11, 1053–1061. [Google Scholar] [CrossRef]
- Pang, H.; Wang, X.; Zhang, G.; Chen, H.; Lv, G.; Yang, S. Characterization of diamond-like carbon films by SEM, XRD and Raman spectroscopy. Appl. Surf. Sci. 2010, 256, 6403–6407. [Google Scholar] [CrossRef]
- Balon, F.; Stolojan, V.; Silva, S.R.P.; Michalka, M.; Kromka, A. Diamond-like carbon thin films for high-temperature applications prepared by filtered pulsed laser deposition. Vacuum 2005, 80, 163–167. [Google Scholar] [CrossRef]
- Chhowalla, M.; Ferrari, A.C.; Robertson, J.; Amaratunga, G.A.J. Evolution of sp2 bonding with deposition temperature in tetrahedral amorphous carbon studied by Raman spectroscopy. Appl. Phys. Lett. 2000, 76, 1419–1421. [Google Scholar] [CrossRef]
- Ding, X.; Li, Q.; Kong, X. Effect of repetition rates of laser pulses on microstructure and optical properties of diamond-like carbon films. Int. J. Mod. Phys. B—IJMPB 2009, 23, 5671–5681. [Google Scholar]
- Lan, Y.; Zou, Y.; Ma, X.; Xu, L.; Shi, L.; Zhang, J. Fabrication of amorphous Al2O3 optical film with various refractive index and low surface roughness. Mater. Res. Express 2020, 7, 086405. [Google Scholar] [CrossRef]
- Solonenko, D.; Žukauskaitė, A.; Pilz, J.; Moridi, M.; Risquez, S. Raman Spectroscopy and Spectral Signatures of AlScN/Al2O3. Micromachines 2022, 13, 1961. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Tamseel, M.; Amami, M.; Javed, M.R.; Javaid, K.; Mahmood, K.; Ikram, S.; Ali, A.; Amin, N.; Hussain, M.S.; et al. Growth and characterization of Ag–Al2O3 composites thin films for thermoelectric power generation applications. Ceram. Int. 2022, 48, 3647–3651. [Google Scholar] [CrossRef]
- Jalvo, B.; Santiago-Morales, J.; Romero, P.; Guzman de Villoria, R.; Rosal, R. Microbial colonisation of transparent glass-like carbon films triggered by a reversible radiation-induced hydrophobic to hydrophilic transition. RSC Adv. 2016, 6, 50278–50287. [Google Scholar] [CrossRef]





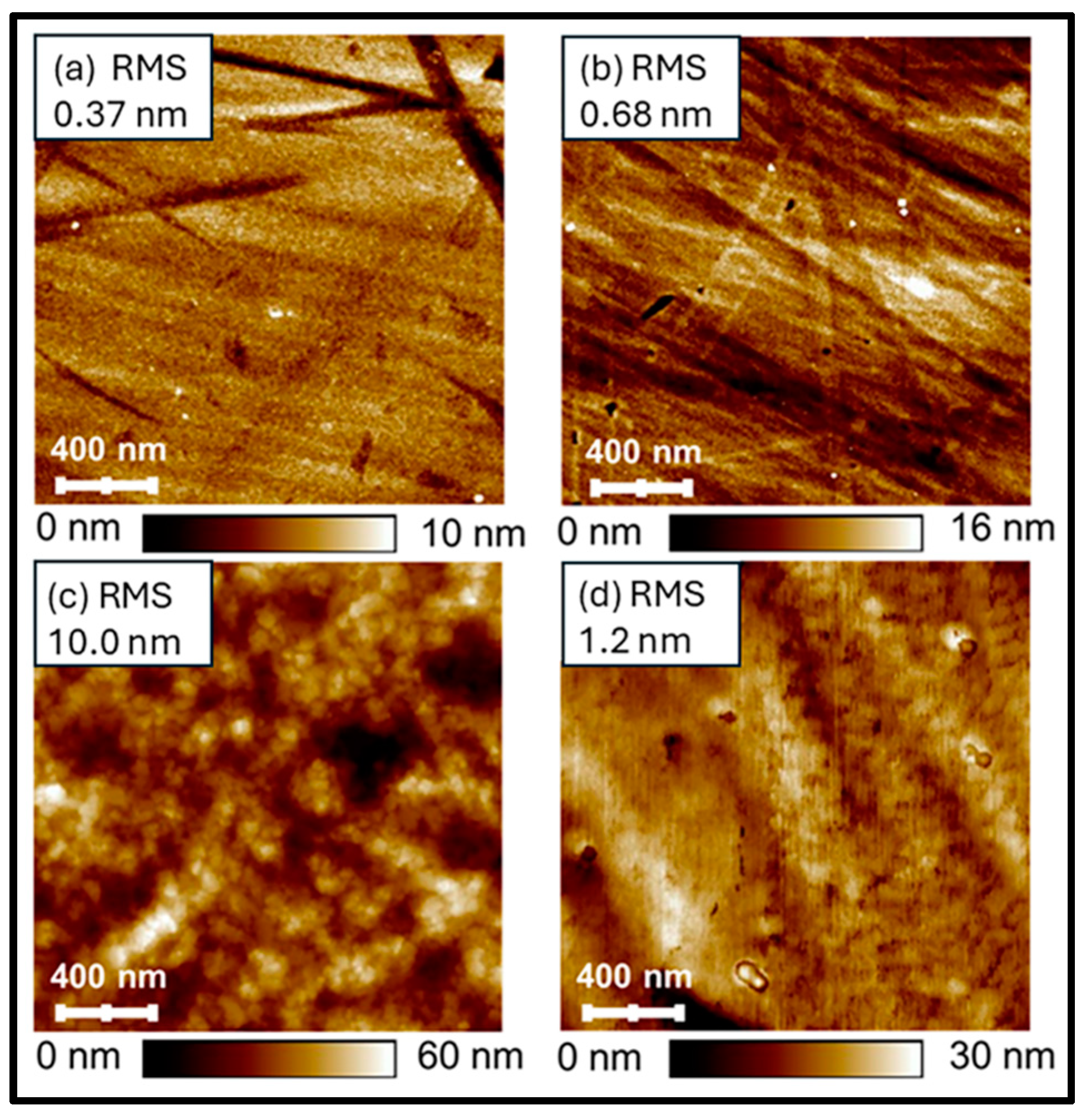
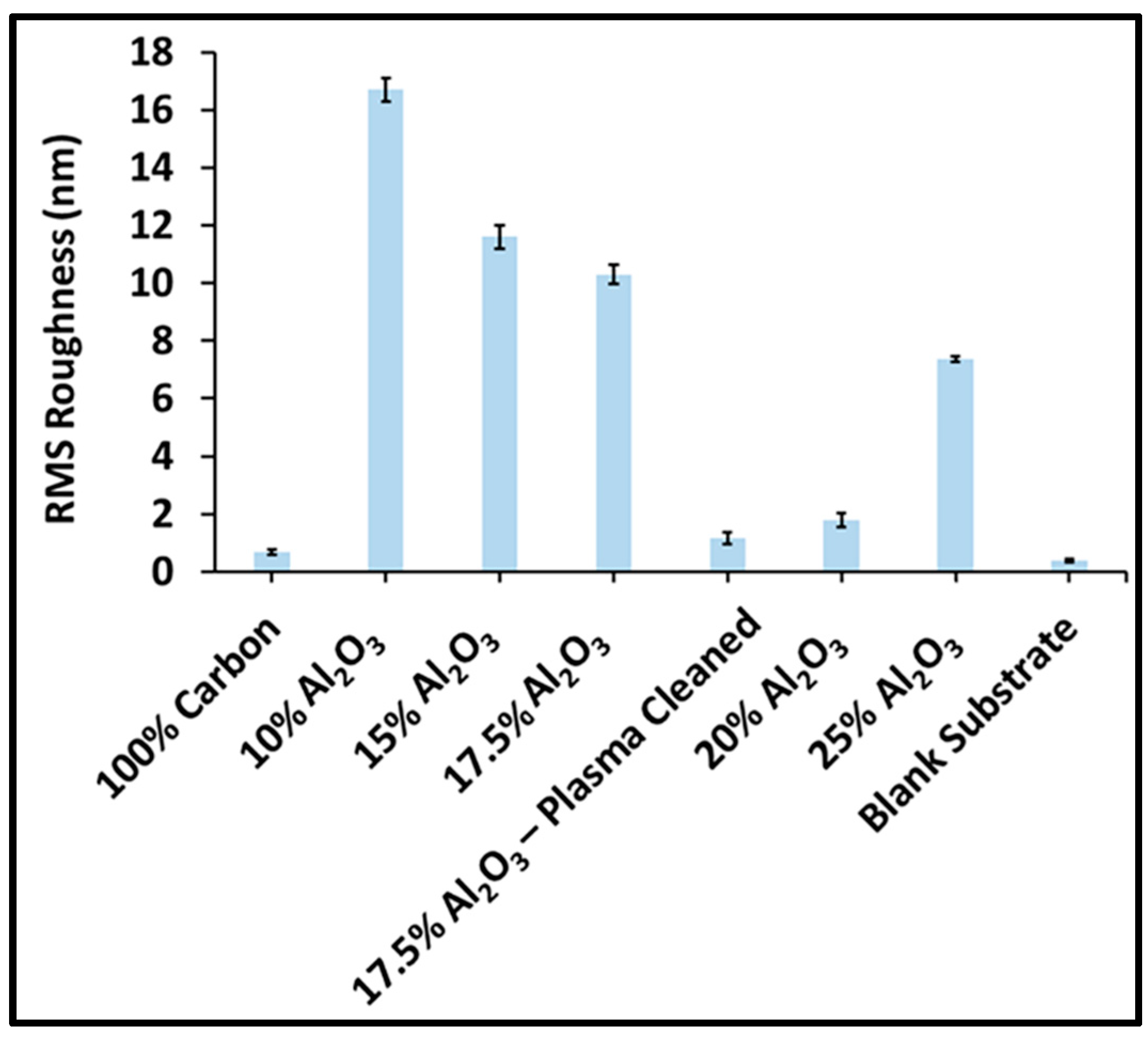

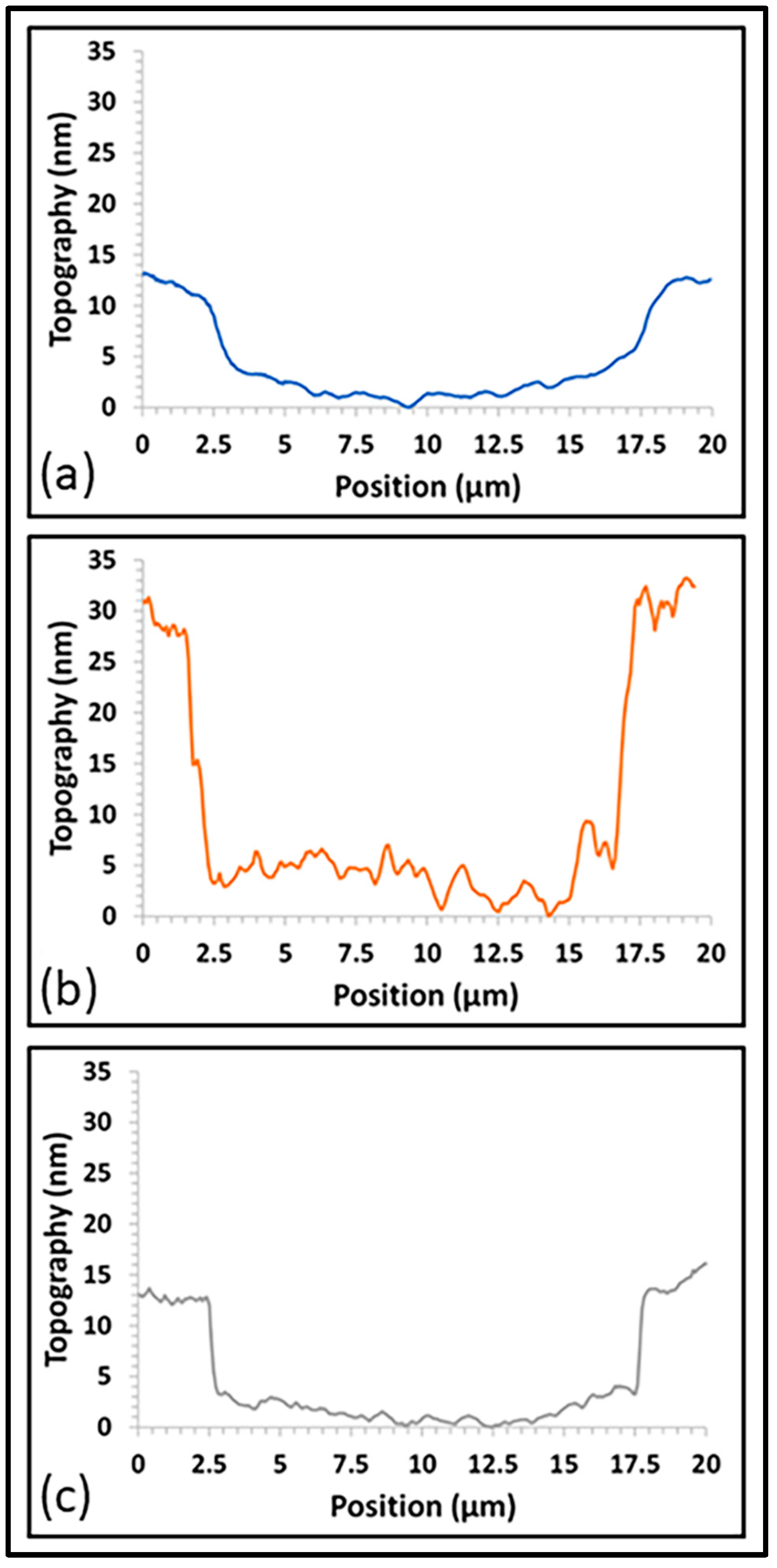

| Sample Name | Pulses on Dopant (%) | Pulses on Carbon | Pulses on Al2O3 |
|---|---|---|---|
| ABB025 | N/A | 400,000 | N/A |
| ABB019 | 10 | 360,000 | 40,000 |
| ABB020 | 15 | 340,000 | 60,000 |
| ABB023 | 17.5 | 330,000 | 70,000 |
| ABB021 | 20 | 320,000 | 80,000 |
| ABB022 | 25 | 300,000 | 100,000 |
| Sample Name | Total Pulses | Pulses on Dopant (%) | Thickness (nm) |
|---|---|---|---|
| ABB025 | 400,000 | None | 45.0 |
| ABB019 | 400,000 | 10 | 29.6 |
| ABB020 | 400,000 | 15 | 41.0 |
| ABB023 | 400,000 | 17.5 | 37.4 |
| ABB021 | 400,000 | 20 | 33.0 |
| ABB022 | 400,000 | 25 | 31.8 |
| Pulses on Dopant (%) | ID/IG | G-Peak Position | FWHM G-Peak |
|---|---|---|---|
| 0 | 0.45 ± 0.04 | 1555 ± 1 | 217 ± 2 |
| 10 | 0.89 ± 0.03 | 1562 ± 1 | 150 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonard, R.L.; Bull, A.B.; Xue, F.; Haycook, C.P.; Gray, S.K.; Bond, C.W.; Bond, P.E.; Brown, L.R.; Giorgio, T.D.; Johnson, J.A. Biocompatibility of Al2O3-Doped Diamond-like Carbon Laparoscope Coatings. Coatings 2025, 15, 437. https://doi.org/10.3390/coatings15040437
Leonard RL, Bull AB, Xue F, Haycook CP, Gray SK, Bond CW, Bond PE, Brown LR, Giorgio TD, Johnson JA. Biocompatibility of Al2O3-Doped Diamond-like Carbon Laparoscope Coatings. Coatings. 2025; 15(4):437. https://doi.org/10.3390/coatings15040437
Chicago/Turabian StyleLeonard, Russell L., Anna B. Bull, Fan Xue, Christopher P. Haycook, Sharon K. Gray, Charles W. Bond, Paige E. Bond, Lesa R. Brown, Todd D. Giorgio, and Jacqueline A. Johnson. 2025. "Biocompatibility of Al2O3-Doped Diamond-like Carbon Laparoscope Coatings" Coatings 15, no. 4: 437. https://doi.org/10.3390/coatings15040437
APA StyleLeonard, R. L., Bull, A. B., Xue, F., Haycook, C. P., Gray, S. K., Bond, C. W., Bond, P. E., Brown, L. R., Giorgio, T. D., & Johnson, J. A. (2025). Biocompatibility of Al2O3-Doped Diamond-like Carbon Laparoscope Coatings. Coatings, 15(4), 437. https://doi.org/10.3390/coatings15040437








