Hierarchical Porous Nickel Anode with Low Polarization at High Current Density
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Hierarchical Porous Ni Anode
2.2. Preparation of Ni-MnO2 Full Cell
2.3. Structure and Composition Characterizations
2.4. Electrochemical Measurements
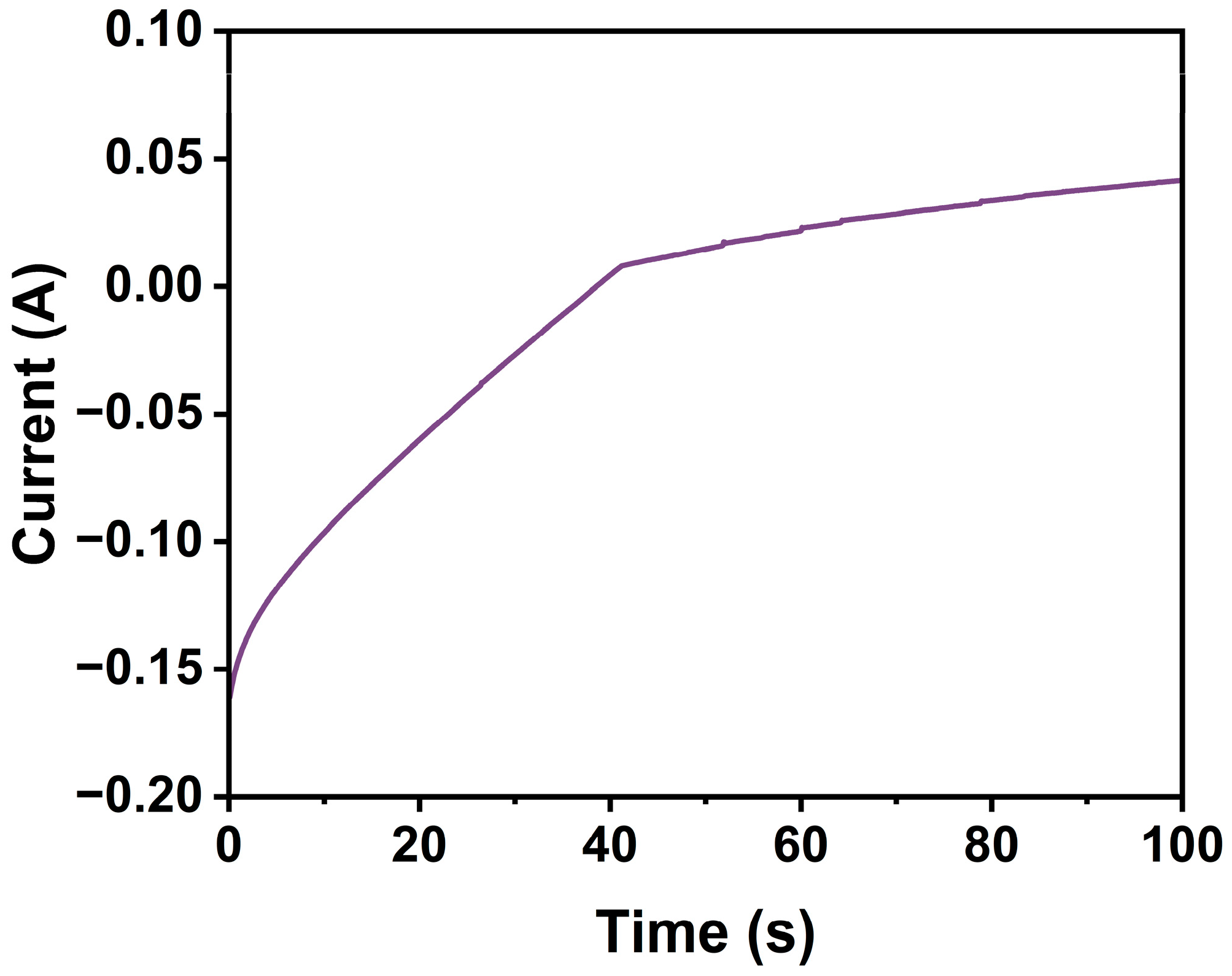
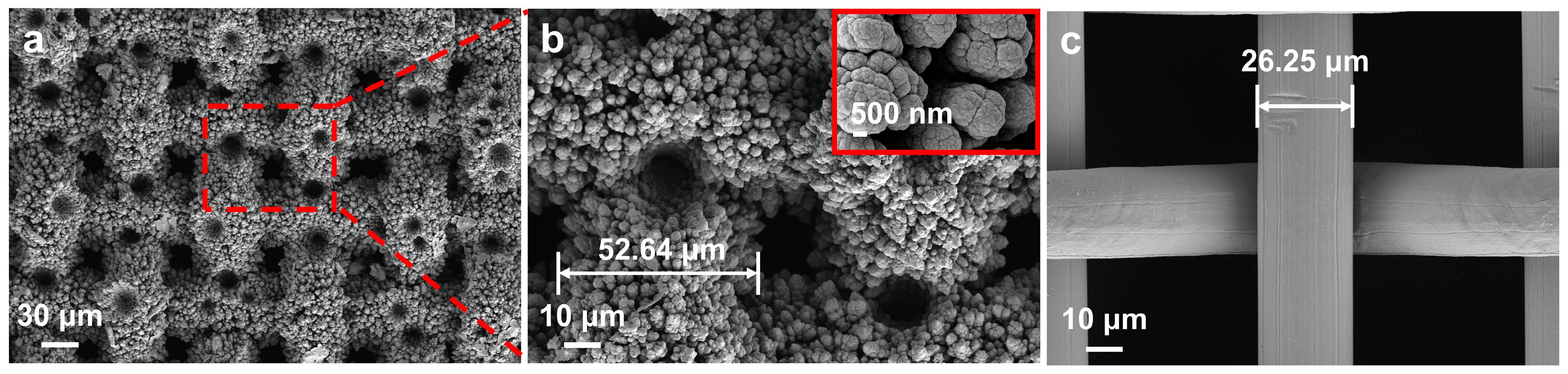
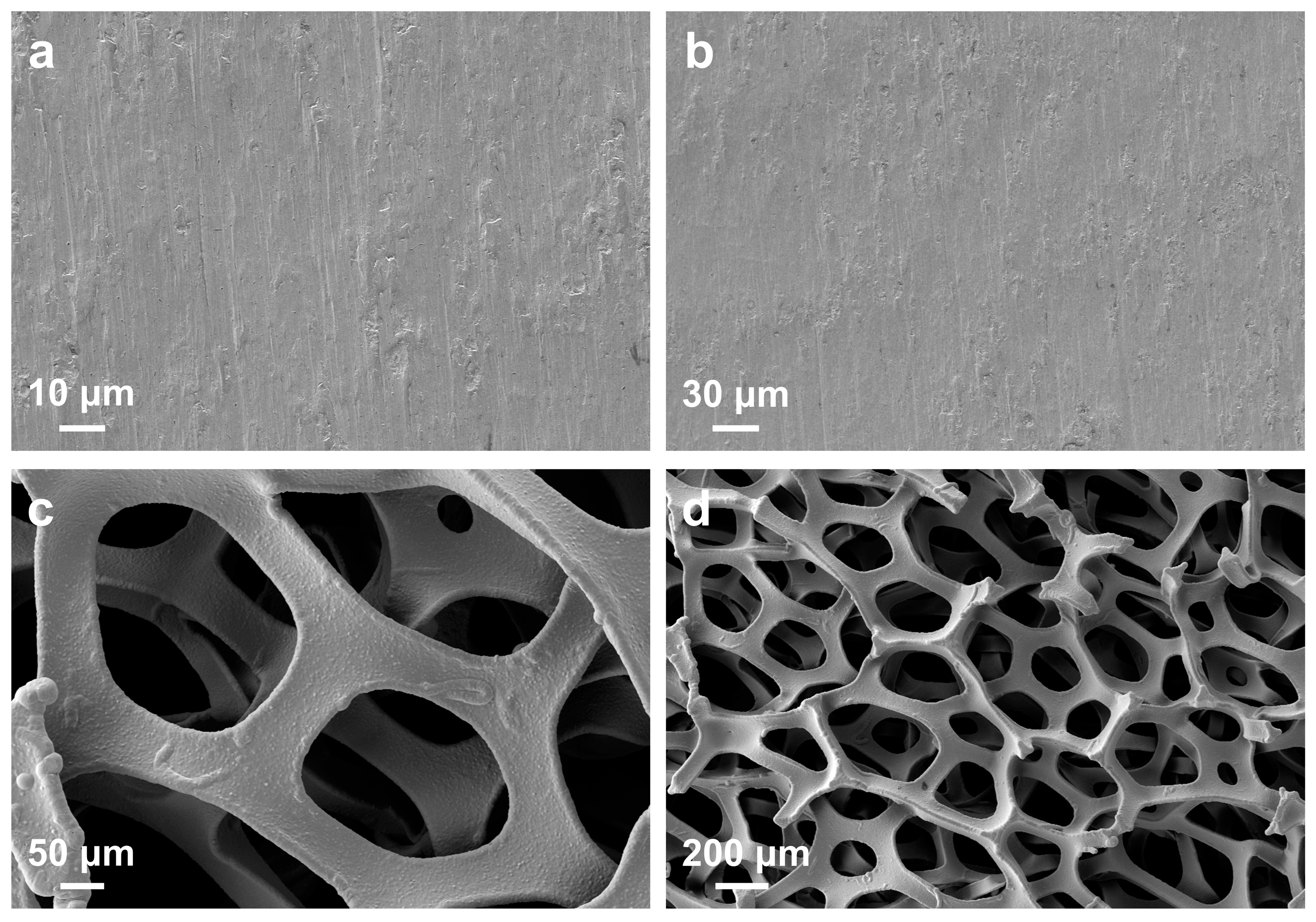
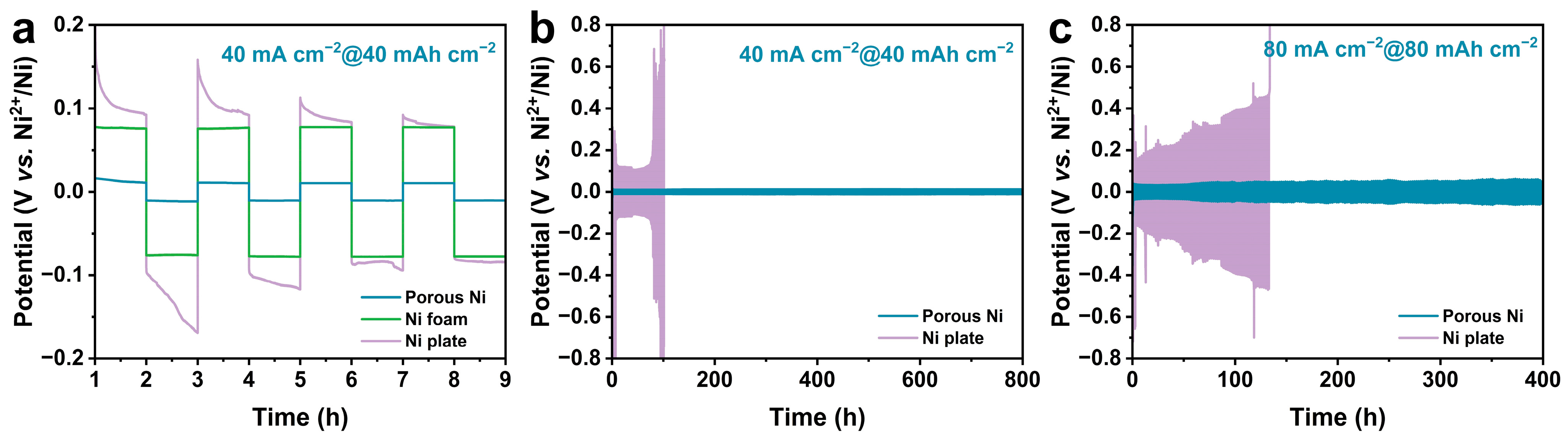
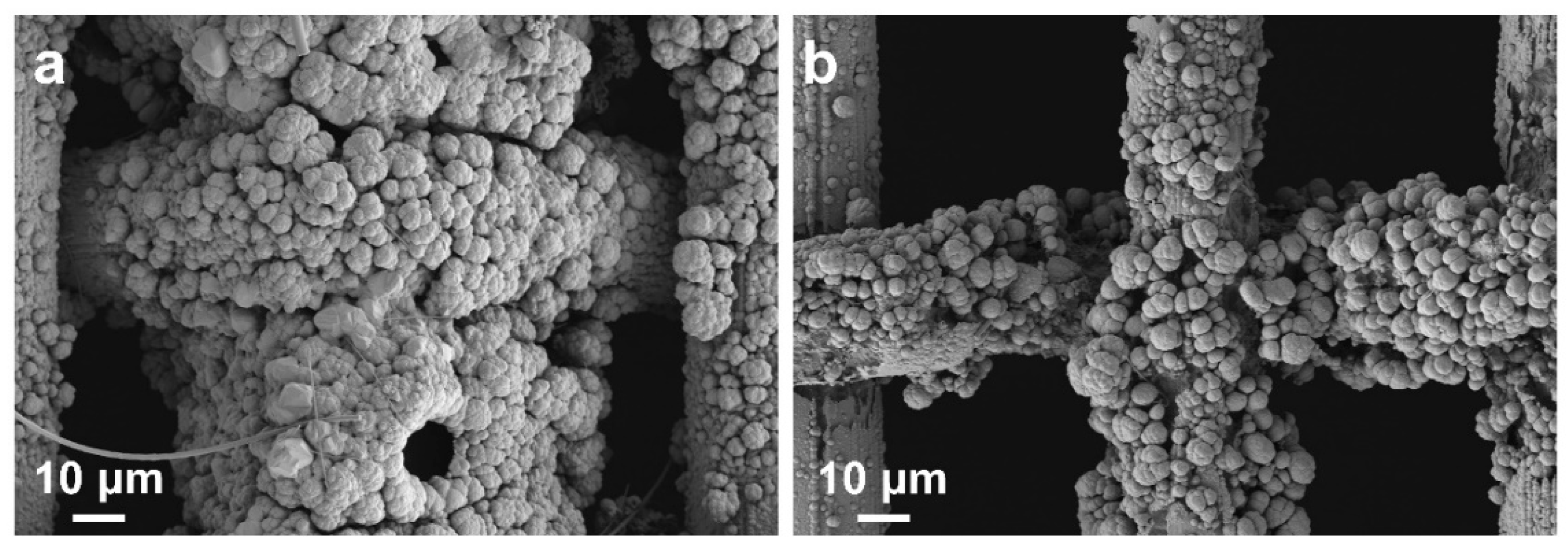
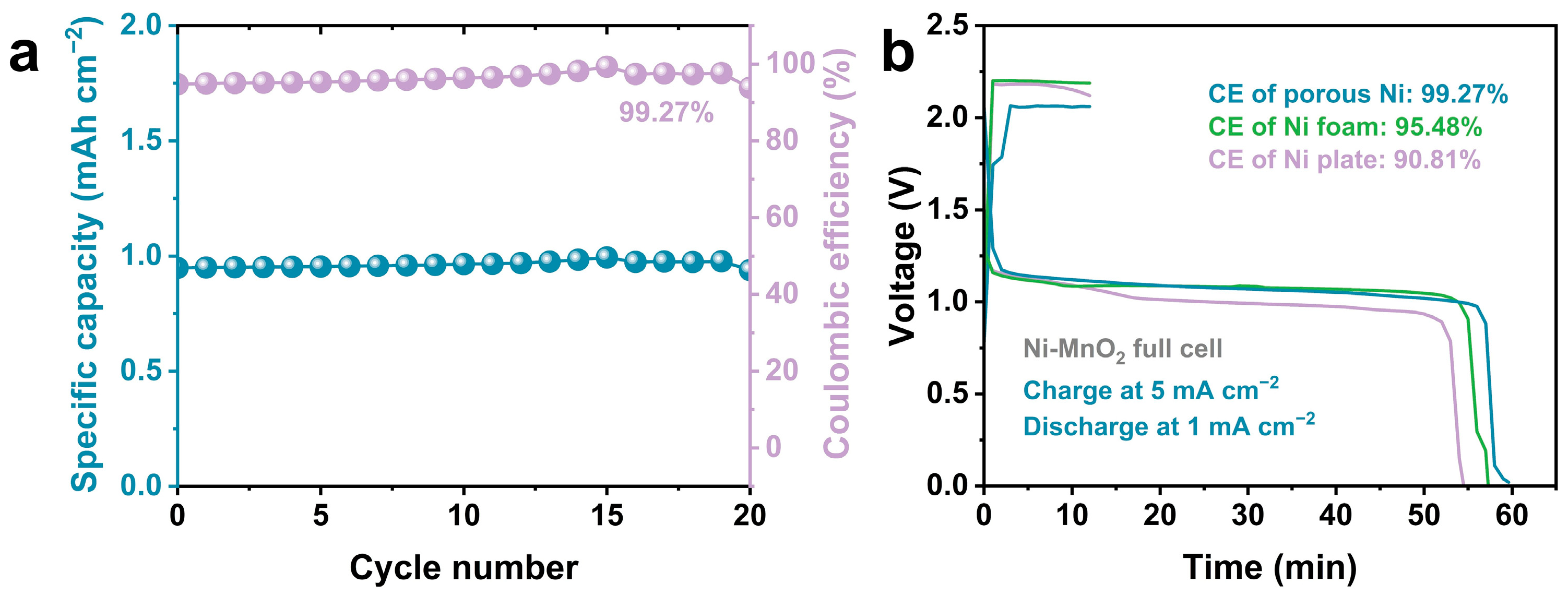
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, W.; Zhu, D.; He, J.; Li, J.; Chen, H.; Chen, Y.; Chao, D. A scalable top-down strategy toward practical metrics of Ni-Zn aqueous batteries with total energy densities of 165 W h kg−1 and 506 W h L−1. Energy Environ. Sci. 2020, 13, 4157–4167. [Google Scholar] [CrossRef]
- Lai, C.; Li, M.; Shen, Y.; Zhou, M.; Wang, W.; Jiang, K.; Li, H.; Wang, K. In Situ Coupling of Highly Dispersed Ni/Fe Metal-NC Sites and N-Doped 3D Carbon Fibers Toward Free-Standing Bifunctional Cathode for Flexible Zinc-Air Battery. Energy Environ. Mater. 2024, 7, e12541. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhou, C.; Hua, J.H.; Hong, X.F.; Sun, C.L.; Li, H.W.; Xu, X.; Mai, L.Q. Engineering Oxygen Vacancies in a Polysulfide-Blocking Layer with Enhanced Catalytic Ability. Adv. Mater. 2020, 32, 1907444. [Google Scholar] [CrossRef]
- Song, J.; Jin, Y.Q.; Zhang, L.; Dong, P.; Li, J.; Xie, F.; Zhang, H.; Chen, J.; Jin, Y.; Meng, H.; et al. Phase-Separated Mo-Ni Alloy for Hydrogen Oxidation and Evolution Reactions with High Activity and Enhanced Stability. Adv. Energy Mater. 2021, 11, 2003511. [Google Scholar] [CrossRef]
- Shi, W.; Lee, W.S.V.; Xue, J. Recent Development of Mn-based Oxides as Zinc-Ion Battery Cathode. ChemSusChem 2021, 14, 1634–1658. [Google Scholar] [CrossRef]
- Ding, X.; Zhu, Q.; Fan, Y.; Yang, Y.; Liu, L.; Shao, Y.; Xiao, Y.; Wu, C.-H.; Han, L. High-entropy V-based cathode for high-capacity and long-life aqueous zinc-ion battery. Nano Energy 2025, 136, 110701. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, R.; Dai, Y.; Wu, X.; Chen, J.; Zong, W.; Zhang, M.; Du, Z.; Dong, H.; Zhao, F.; et al. Asymmetric acceptor-donor—Donor small organic molecule enabling versatile and highly-stable aqueous zinc batteries. Mater. Today 2024, 78, 32–45. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, Z.; He, X.; Song, R.; Liao, X.; Wu, L.; Duan, Y.; Zhao, C.; Tahir, M.; Dai, J.; et al. High-performance zinc metal anode enabled by large-scale integration of superior ion transport layer. Chem. Eng. J. 2024, 492, 152114. [Google Scholar] [CrossRef]
- He, X.; Zhu, Z.; Liao, X.; Yang, K.; Duan, Y.; Lv, L.; Zhao, C.; Zhao, W.; Chen, J.; Tian, P.; et al. In-situ construction of epitaxial phase for boosting zinc nucleation on three-dimensional interface. Prog. Nat. Sci. Mater. 2024, 34, 578–584. [Google Scholar] [CrossRef]
- Zhao, C.L.; Lu, Y.X.; Yue, J.M.; Pan, D.; Qi, Y.R.; Hu, Y.S.; Chen, L.Q. Advanced Na metal anodes. J. Energy Chem. 2018, 27, 1584–1596. [Google Scholar] [CrossRef]
- Wang, M.; Meng, Y.; Gao, P.; Li, K.; Liu, Z.; Zhu, Z.; Ali, M.; Ahmad, T.; Chen, N.; Yuan, Y.; et al. Anions Regulation Engineering Enables a Highly Reversible and Dendrite-Free Nickel-Metal Anode with Ultrahigh Capacities. Adv. Mater. 2023, 35, 2305368. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, W.; Zhang, Q.; Liu, Y.; Yuan, G.; Braunstein, P.; Pang, H. Ultrasmall metal (Fe, Co, Ni) nanoparticles strengthen silicon oxide embedded nitrogen-doped carbon superstructures for long-cycle-life Li-ion-battery anodes. Chem. Eng. J. 2022, 432, 134413. [Google Scholar] [CrossRef]
- Wu, X.; Li, S.; Xu, Y.; Wang, B.; Liu, J.; Yu, M. Hierarchical heterostructures of NiO nanosheet arrays grown on pine twig-like β-NiS@Ni3S2 frameworks as free-standing integrated anode for high-performance lithium-ion batteries. Chem. Eng. J. 2019, 356, 245–254. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Li, X.; Wei, Y.-S.; Fu, Z.; Wei, W.; Wu, X.-T.; Zhu, Q.-L.; Xu, Q. Ordered Macroporous Superstructure of Nitrogen-Doped Nanoporous Carbon Implanted with Ultrafine Ru Nanoclusters for Efficient pH-Universal Hydrogen Evolution Reaction. Adv. Mater. 2021, 33, 2006965. [Google Scholar] [CrossRef]
- Chen, B.; Chao, D.; Liu, E.; Jaroniec, M.; Zhao, N.; Qiao, S.-Z. Transition metal dichalcogenides for alkali metal ion batteries: Engineering strategies at the atomic level. Energy Environ. Sci. 2020, 13, 1096–1131. [Google Scholar] [CrossRef]
- Leng, J.; Wang, Z.; Wang, J.; Wu, H.-H.; Yan, G.; Li, X.; Guo, H.; Liu, Y.; Zhang, Q.; Guo, Z. Advances in nanostructures fabricated via spray pyrolysis and their applications in energy storage and conversion. Chem. Soc. Rev. 2019, 48, 3015–3072. [Google Scholar] [CrossRef]
- Zou, L.; Kitta, M.; Hong, J.; Suenaga, K.; Tsumori, N.; Liu, Z.; Xu, Q. Fabrication of a Spherical Superstructure of Carbon Nanorods. Adv. Mater. 2019, 31, 1900440. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.; Yang, M.; Wu, J.; Chen, F.; Huang, W.; Han, N.; Ye, H.; Zhao, F.; Li, Y.; et al. Hierarchical VS2 Nanosheet Assemblies: A Universal Host Material for the Reversible Storage of Alkali Metal Ions. Adv. Mater. 2017, 29, 1702061. [Google Scholar] [CrossRef]
- Wang, H.-F.; Chen, L.; Wang, M.; Liu, Z.; Xu, Q. Hollow Spherical Superstructure of Carbon Nanosheets for Bifunctional Oxygen Reduction and Evolution Electrocatalysis. Nano Lett. 2021, 21, 3640–3648. [Google Scholar] [CrossRef]
- Hou, C.-C.; Zou, L.; Xu, Q. A Hydrangea-Like Superstructure of Open Carbon Cages with Hierarchical Porosity and Highly Active Metal Sites. Adv. Mater. 2019, 31, 1904689. [Google Scholar] [CrossRef]
- He, Y.; Li, M.; Zhang, Y.; Shan, Z.; Zhao, Y.; Li, J.; Liu, G.; Liang, C.; Bakenov, Z.; Li, Q. All-Purpose Electrode Design of Flexible Conductive Scaffold toward High-Performance Li-S Batteries. Adv. Funct. Mater. 2020, 30, 2000613. [Google Scholar] [CrossRef]
- Uddin, M.J.; Alaboina, P.K.; Cho, S.J. Nanostructured cathode materials synthesis for lithium-ion batteries. Mater. Today Energy 2017, 5, 138–157. [Google Scholar] [CrossRef]
- Duan, Y.; You, G.; Zhu, Z.; Lv, L.; Liao, X.; He, X.; Yang, K.; Song, R.; Tian, P.; He, L. Reconstructed NiCo Alloy Enables High-Rate Ni-Zn Microbattery with High Capacity. Coatings 2023, 13, 603. [Google Scholar] [CrossRef]
- You, G.; Zhu, Z.; Duan, Y.; Lv, L.; Liao, X.; He, X.; Yang, K.; Song, R.; Yang, Y.; He, L. Alkaline Ni-Zn Microbattery Based on 3D Hierarchical Porous Ni Microcathode with High-Rate Performance. Micromachines 2023, 14, 927. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Zhao, Y.; Yang, Y.; Lv, H.; Zhong, R.; Ding, F.; Mo, F.; Hu, H.; Zhi, C.; Liang, G. Development of Inverse-Opal-Structured Charge-Deficient Co9S8@nitrogen-Doped-Carbon to Catalytically Enable High Energy and High Power for the Two-Electron Transfer I+/I- Electrode. Adv. Mater. 2024, 36, 2312246. [Google Scholar] [CrossRef]
- Petrii, O.A.; Nazmutdinov, R.R.; Bronshtein, M.D.; Tsirlina, G.A. Life of the Tafel equation: Current understanding and prospects for the second century. Electrochim. Acta 2007, 52, 3493–3504. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lim, J.H.; Chun, H.S. Creep mechanism of porous MCFC Ni anodes strengthened by Ni3Al. AIChE J. 2006, 52, 359–365. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, R.; Ma, Z.; Shao, Q.; Wu, L.; Liao, X.; Wang, W.; Wang, J.; Liang, H.; Tahir, M.; Lu, D.; et al. Hierarchical Porous Nickel Anode with Low Polarization at High Current Density. Coatings 2025, 15, 480. https://doi.org/10.3390/coatings15040480
Song R, Ma Z, Shao Q, Wu L, Liao X, Wang W, Wang J, Liang H, Tahir M, Lu D, et al. Hierarchical Porous Nickel Anode with Low Polarization at High Current Density. Coatings. 2025; 15(4):480. https://doi.org/10.3390/coatings15040480
Chicago/Turabian StyleSong, Ruiqi, Zeyu Ma, Qi Shao, Leixin Wu, Xiaoqiao Liao, Wenwu Wang, Jiangwang Wang, Huimin Liang, Muhammad Tahir, Dan Lu, and et al. 2025. "Hierarchical Porous Nickel Anode with Low Polarization at High Current Density" Coatings 15, no. 4: 480. https://doi.org/10.3390/coatings15040480
APA StyleSong, R., Ma, Z., Shao, Q., Wu, L., Liao, X., Wang, W., Wang, J., Liang, H., Tahir, M., Lu, D., & He, L. (2025). Hierarchical Porous Nickel Anode with Low Polarization at High Current Density. Coatings, 15(4), 480. https://doi.org/10.3390/coatings15040480






