Electrochemical One-Step Synthesis of Cu2O with Tunable Oxygen Defects and Their Electrochemical Performance in Li-Ion Batteries
Abstract
1. Introduction
2. Experimental Section
2.1. The Synthesis of Cu2O Nanoparticles with Oxygen Defects
2.2. Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
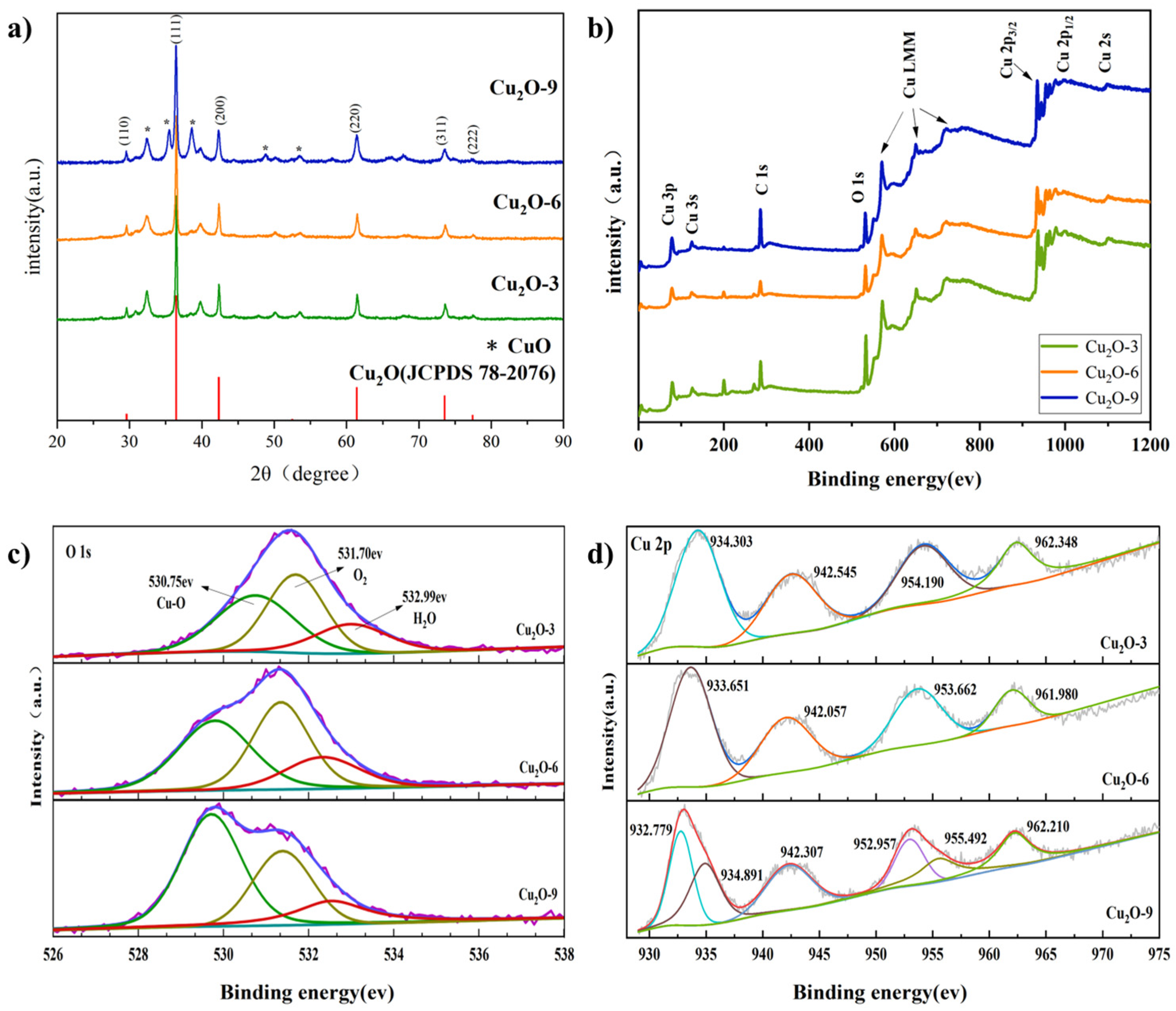
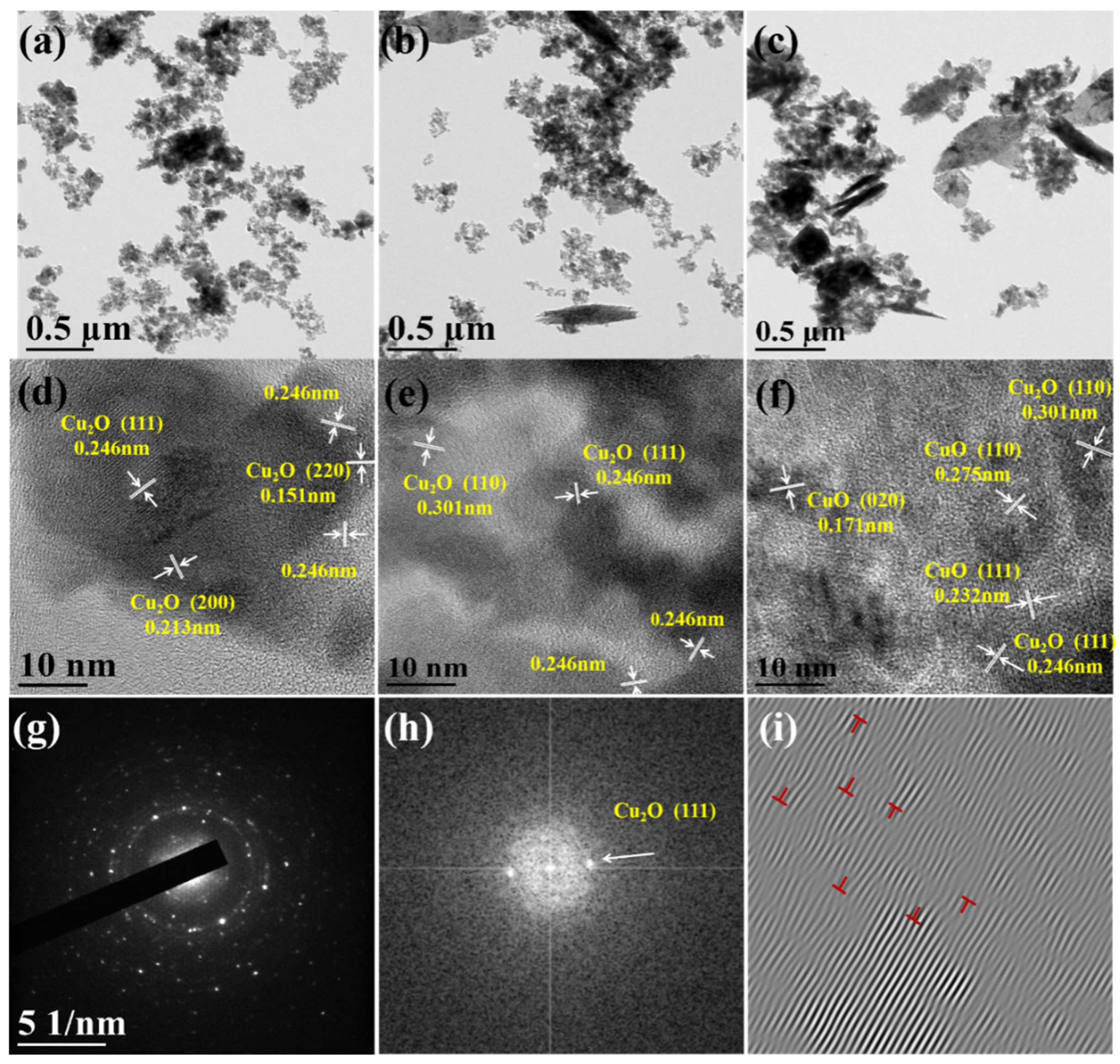
3.1. Materials Characterization
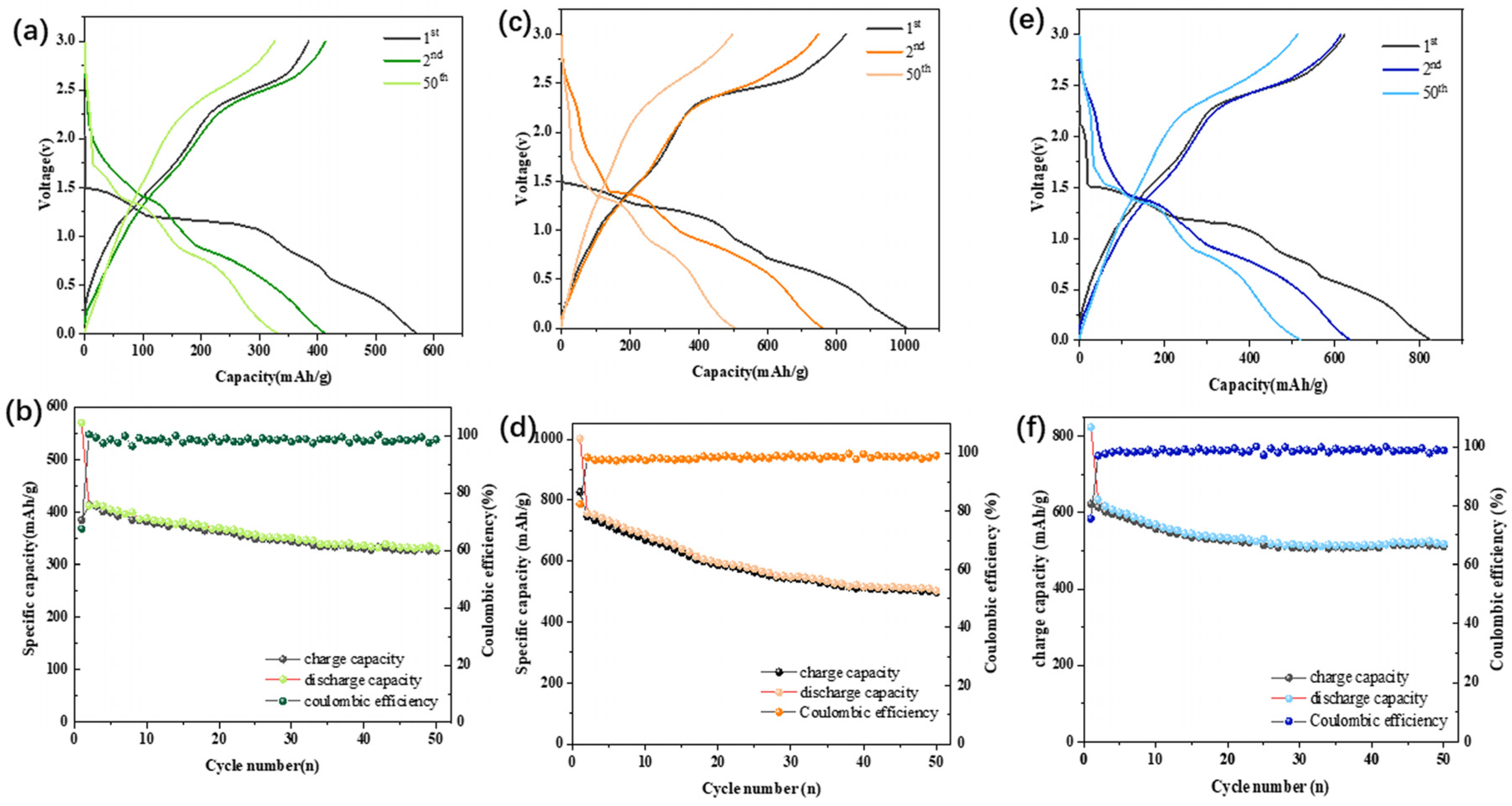
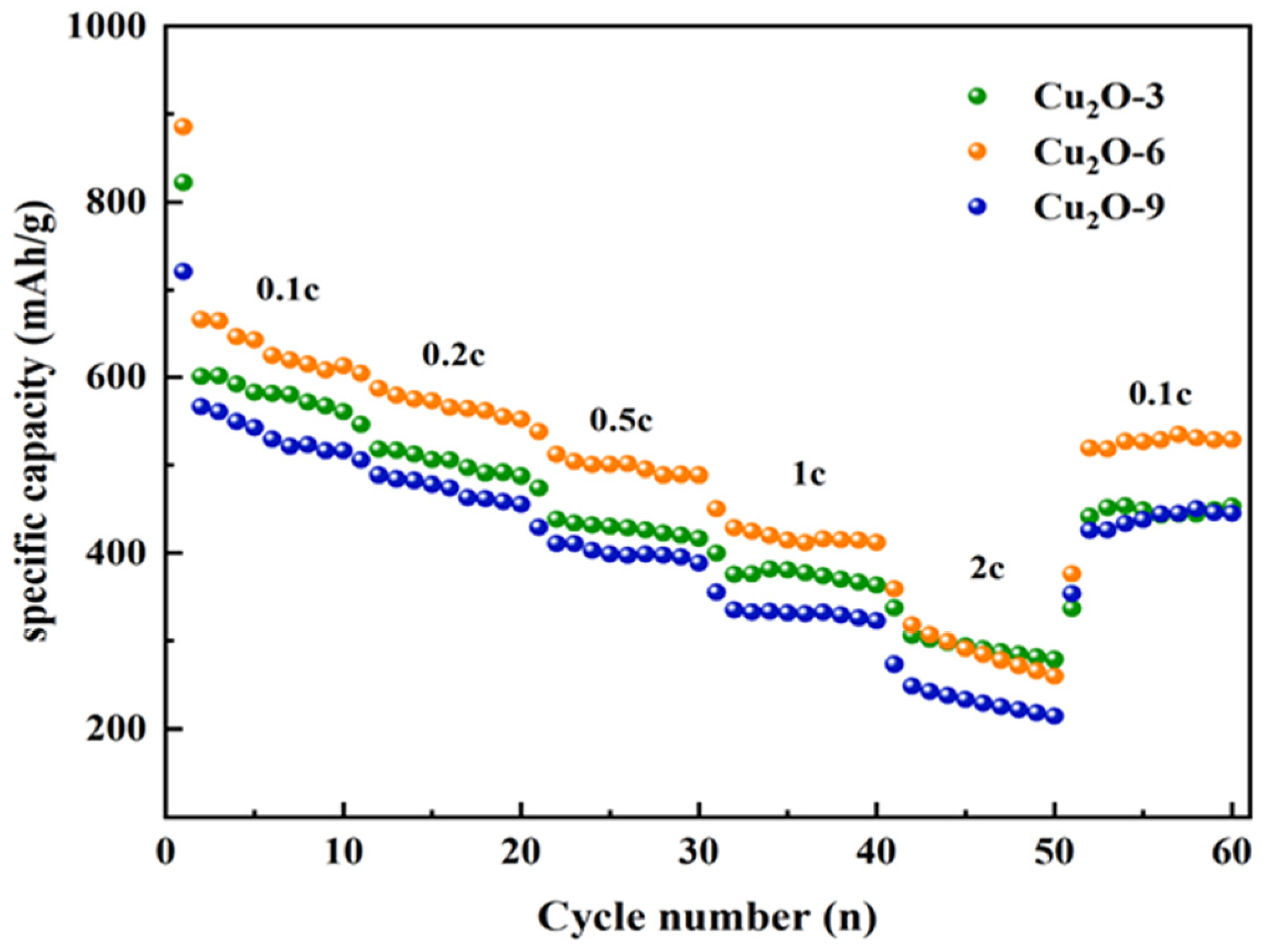
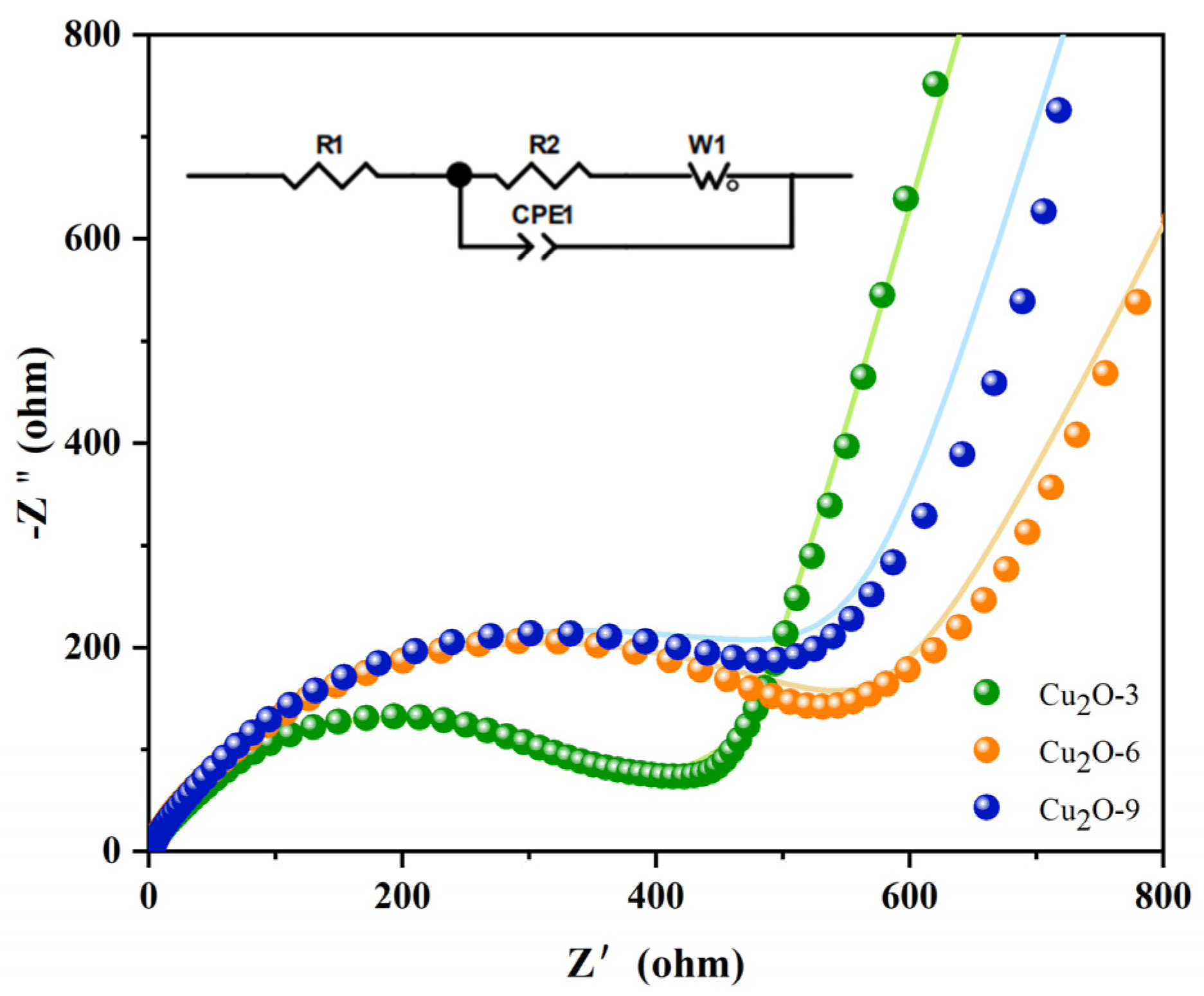
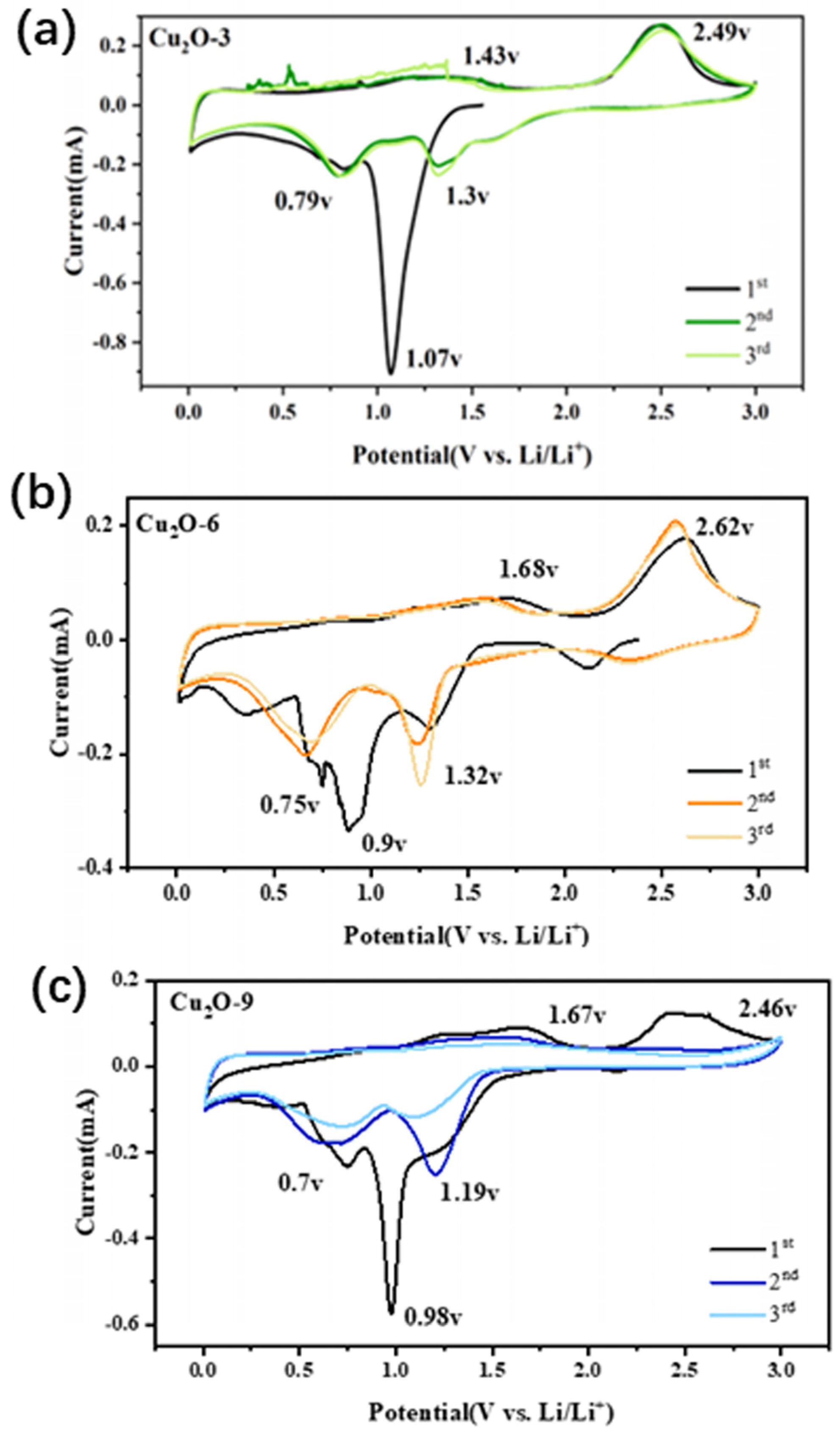
3.2. Electrochemical Investigation
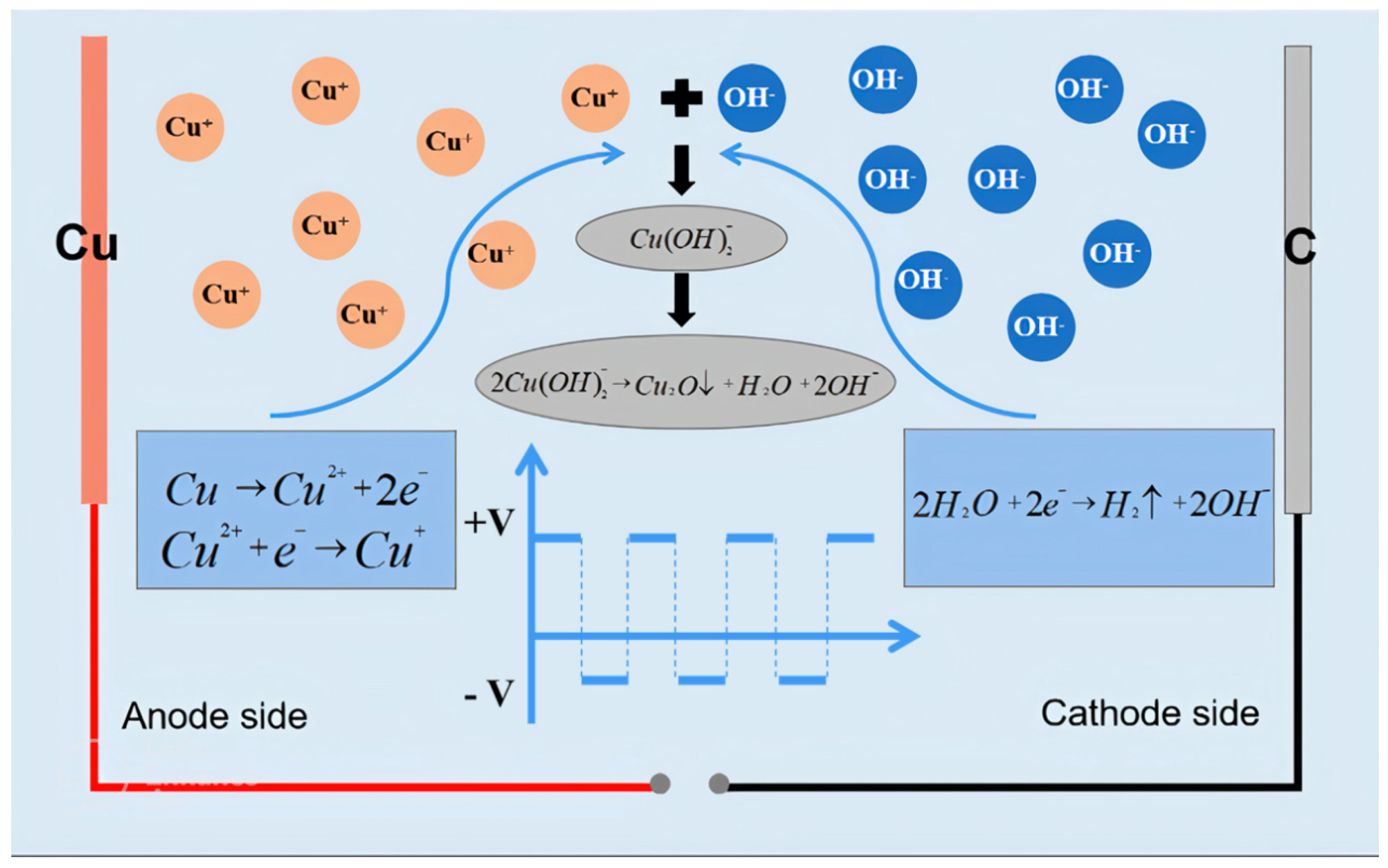
3.3. Reaction Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Venkata, S.A.; Mewin, V.; Ivan, J.F.; Steven, J.H.; Vinodkumar, E. Unusual pseudocapacitive lithium-ion storage on defective Co3O4 nanosheets. Nanotechnology 2022, 33, 225403. [Google Scholar] [CrossRef]
- Nzereogu, P.U.; Omah, A.D.; Ezema, F.I.; Iwuoha, E.I.; Nwanya, A.C. Anode materials for lithium-ion batteries: A review. Appl. Surf. Sci. Adv. 2022, 9, 100233. [Google Scholar] [CrossRef]
- Chang, H.; Wu, Y.-R.; Han, X.; Yi, T.-F. Recent developments in advanced anode materials for lithium-ion batteries. Energy Mater. 2022, 1, 100003. [Google Scholar] [CrossRef]
- Arnaud, B.L.; Tobias, L.; Sebastian, M.; Karoline, S.; Sebastian, R.; Christian, H.; Alexander, M. Impact of Electrode Defects on Battery Cell Performance: A Review. Batter. Supercaps 2022, 5, 202200239. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Rojaee, R.; Shahbazian-Yassar, R. Two-Dimensional Materials to Address the Lithium Battery Challenges. ACS Nano 2020, 14, 2628–2658. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, H.; Fu, L.; Ye, F.; Zhang, Y.; Luo, W.; Huang, Y. Promises, Challenges, and Recent Progress of Inorganic Solid-State Electrolytes for All-Solid-State Lithium Batteries. Adv. Mater. 2018, 30, 1705702. [Google Scholar] [CrossRef]
- Liu, W.; Chen, G.; He, G.; Zhang, W. Synthesis of starfish-like Cu2O nanocrystals through γ-irradiation and their application in lithium-ion batteries. J. Nanopart. Res. 2011, 13, 2705–2713. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.-M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shen, S.; Guo, P.; Wang, M.; Wu, P.; Wang, X.; Guo, L. In-situ reduction synthesis of nano-sized Cu2O particles modifying g-C3N4 for enhanced photocatalytic hydrogen production. Appl. Catal. B Environ. 2014, 152–153, 335–341. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, K.; Yang, Z.; Zhang, Y.; Gu, H.; Zhang, W.; Li, E.; Zhou, C. An efficient route to Cu2O nanorod array film for high-performance Li-ion batteries. Thin Solid Film. 2016, 608, 79–87. [Google Scholar] [CrossRef]
- Grugeon, S.; Laruelle, S.; Herrera-Urbina, R.; Dupont, L.; Poizot, P.; Tarascon, J. Particle size effects on the electrochemical performance of copper oxides toward lithium. J. Electrochem. Soc. 2001, 148, A285. [Google Scholar] [CrossRef]
- Fu, L.J.; Gao, J.; Zhang, T.; Cao, Q.; Yang, L.C.; Wu, Y.P.; Holze, R.; Wu, H.Q. Preparation of Cu2O particles with different morphologies and their application in lithium ion batteries. J. Power Sources 2007, 174, 1197–1200. [Google Scholar] [CrossRef]
- Chen, X.; Yu, C.; Guo, X.; Bi, Q.; Sajjad, M.; Ren, Y.; Zhou, X.; Liu, Z. Cu2O Nanoparticles and Multi-Branched Nanowires as Anodes for Lithium-Ion Batteries. Nano 2018, 13, 1850103. [Google Scholar] [CrossRef]
- Shen, X.; Chen, S.; Mu, D.; Wu, B.; Wu, F. Novel synthesis and electrochemical performance of nano-structured composite with Cu2O embedment in porous carbon as anode material for lithium ion batteries. J. Power Sources 2013, 238, 173–179. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.-H.; Qi, M.-Y.; Tang, Z.-R.; Xu, Y.-J. Boosting the activity and stability of Ag-Cu2O/ZnO nanorods for photocatalytic CO2 reduction. Appl. Catal. B Environ. 2020, 268, 118380. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, L.; Li, J.; Kou, L.; Huang, J.; Feng, Y.; Chen, S. Cu/Cu2O@Ppy nanowires as a long-life and high-capacity anode for lithium ion battery. Chem. Eng. J. 2020, 391, 123597. [Google Scholar] [CrossRef]
- Kang, W.; Liu, F.; Su, Y.; Wang, D.; Shen, Q. The catanionic surfactant-assisted syntheses of 26-faceted and hexapod-shaped Cu2O and their electrochemical performances. CrystEngComm 2011, 13, 4174. [Google Scholar] [CrossRef]
- He, Y.; Pu, Y.; Zhu, B.; Zhu, H.; Wang, C.; Tang, W.; Tang, H. Electrochemical one-step synthesis of Mn3O4 with tunable oxygen defects for high-performance aqueous zinc-ion batteries. J. Alloys Compd. 2023, 934, 167933. [Google Scholar] [CrossRef]
- Park, J.C.; Kim, J.; Kwon, H.; Song, H. Gram-Scale Synthesis of Cu2O Nanocubes and Subsequent Oxidation to CuO Hollow Nanostructures for Lithium-Ion Battery Anode Materials. Adv. Mater. 2009, 40, 803–807. [Google Scholar] [CrossRef]
- Yan, G.-C.; Li, X.-H.; Wang, Z.-X.; Guo, H.-J.; Zhang, Q.; Peng, W.-J. Synthesis of Cu2O/reduced graphene oxide composites as anode materials for lithium ion batteries. Trans. Nonferrous Met. Soc. China 2013, 23, 3691–3696. [Google Scholar] [CrossRef]
- He, X.; He, Y.; Wang, C.; Zhu, B.; Liu, A.; Tang, H. Oxygen vacancy-rich Fe3O4 nanoparticle synthesized via facile electrochemical method as anode material for high-performance lithium-ion batteries. J. Phys. Chem. Solids 2022, 171, 111028. [Google Scholar] [CrossRef]
- Xu, Y.; Jian, G.; Liu, Y.; Zhu, Y.; Zachariah, M.R.; Wang, C. Superior electrochemical performance and structure evolution of mesoporous Fe2O3 anodes for lithium-ion batteries. Nano Energy 2014, 3, 26–35. [Google Scholar] [CrossRef]
- Cao, K.; Jin, T.; Yang, L.; Jiao, L. Recent progress in conversion reaction metal oxide anodes for Li-ion batteries. Mater. Chem. Front. 2017, 1, 2213–2242. [Google Scholar] [CrossRef]
- Yang, G.; Yang, S.; Shen, J.; Cui, Y.; Sun, J.; Duan, G.; Cao, B.; Liu, Z. KOH-Induced Oxygen-Deficient VO2 for High-Rate Aqueous Zn-Ion Batteries. ACS Appl. Energy Mater. 2023, 6, 1871–1876. [Google Scholar] [CrossRef]
- Qiao, S.; Chen, Y.; Tang, Y.; Yuan, J.; Shen, J.; Zhang, D.; Du, Y.; Li, Z.; Yuan, D.; Tang, H.; et al. Oxygen vacancy–rich Cu2O@Cu with a hydrophobic microenvironment for highly selective C–C coupling to generate C2H4. Chem. Eng. J. 2023, 454, 140321. [Google Scholar] [CrossRef]
- Yang, S.; Li, F.; Fu, P.; Zhen, C.; Wu, J.; Lu, H.; Hou, C.; Sheng, Z. Nickel-doped δ-MnO2 abundant in oxygen vacancies as a cathode material for aqueous Zn-ion batteries with superior performance. Nanoscale 2025, 17, 7423–7433. [Google Scholar] [CrossRef]
- Zhu, M.; Ullah, I.; Zhang, X.; Long, Y.; Gao, X.; Liu, Z.; Yang, Y.; Shen, G. Phenolphthalein-Modified Multivalent Vanadium Oxide: Harnessing Oxygen Vacancies and Hydrophobicity for Improved Aqueous Zinc-Ion Battery Performance. ACS Sustain. Chem. Eng. 2024, 12, 4255–4263. [Google Scholar] [CrossRef]
- Dong, W.; Xu, J.; Wang, C.; Lu, Y.; Liu, X.; Wang, X.; Yuan, X.; Wang, Z.; Lin, T.; Sui, M.; et al. A Robust and Conductive Black Tin Oxide Nanostructure Makes Efficient Lithium-Ion Batteries Possible. Adv. Mater. 2017, 29, 1700136. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Huang, H.; Cai, J.; Ding, K.; Lin, S. Electrochemical fabrication of shape-controlled Cu2O with spheres, octahedrons and truncated octahedrons and their electrocatalysis for ORR. New J. Chem. 2018, 42, 458–464. [Google Scholar] [CrossRef]
- Wang, C.; Higgins, D.; Wang, F.; Li, D.; Liu, R.; Xia, G.; Li, N.; Li, Q.; Xu, H.; Wu, G. Controlled synthesis of micro/nanostructured CuO anodes for lithium-ion batteries. Nano Energy 2014, 9, 334–344. [Google Scholar] [CrossRef]
- Wang, X.H.; Wang, Z.M.; Hong, Q.L.; Zhang, Z.N.; Shi, F.; Li, D.S.; Li, S.N.; Chen, Y. Oxygen-Vacancy-Rich Cu2O Hollow Nanocubes for Nitrate Electroreduction Reaction to Ammonia in a Neutral Electrolyte. Inorg. Chem. 2022, 61, 15678–15685. [Google Scholar] [CrossRef] [PubMed]
- Grigore, M.; Biscu, E.; Holban, A.; Gestal, M.; Grumezescu, A. Methods of Synthesis, Properties and Biomedical Applications of CuO Nanoparticles. Pharmaceuticals 2016, 9, 75. [Google Scholar] [CrossRef]
- Xie, M.; Lin, M.; Feng, C.; Liu, Z.; Xu, Y.; Wang, N.; Zhang, X.; Jiao, Y.; Chen, J. Coupling Zn2+ doping and rich oxygen vacancies in MnO2 nanowire toward advanced aqueous zinc-ion batteries. J. Colloid Interface Sci. 2023, 645, 400–409. [Google Scholar] [CrossRef]
- Xu, Y.-T.; Guo, Y.; Li, C.; Zhou, X.-Y.; Tucker, M.C.; Fu, X.-Z.; Sun, R.; Wong, C.-P. Graphene oxide nano-sheets wrapped Cu2O microspheres as improved performance anode materials for lithium ion batteries. Nano Energy 2015, 11, 38–47. [Google Scholar] [CrossRef]
- Cheng, H.; Shapter, J.G.; Li, Y.; Gao, G. Recent progress of advanced anode materials of lithium-ion batteries. J. Energy Chem. 2021, 57, 451–468. [Google Scholar] [CrossRef]
- Fu, Y.; Wei, Q.; Zhang, G.; Wang, X.; Zhang, J.; Hu, Y.; Wang, D.; Zuin, L.; Zhou, T.; Wu, Y.; et al. High-Performance Reversible Aqueous Zn-Ion Battery Based on Porous MnOx Nanorods Coated by MOF-Derived N-Doped Carbon. Adv. Energy Mater. 2018, 8, 1801445. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, H.; Nanda, J.; Braun, P.V. Three-Dimensionally Mesostructured Fe2O3 Electrodes with Good Rate Performance and Reduced Voltage Hysteresis. Chem. Mater. 2015, 27, 150414100216000. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Y.; Nuli, Y.; Yu, Y.; Gao, P.; Chen, Q.; Wei, L.; Hu, N.; Yang, Z.; Gao, R. Cu2O nanowires as anode materials for Li-ion rechargeable batteries. Sci. China Technol. Sci. 2014, 57, 1073–1076. [Google Scholar] [CrossRef]
- He, X.; Guo, H.; Zhang, X.; Liao, T.; Zhu, Y.; Tang, H.; Li, T.; Chen, J.S. Facile electrochemical fabrication of magnetic Fe3O4 for electrocatalytic synthesis of ammonia used for hydrogen storage application. Int. J. Hydrogen Energy 2021, 46, 24128–24134. [Google Scholar] [CrossRef]
- Zhao, W.; Fu, W.; Yang, H.; Tian, C.; Li, M.; Li, Y.; Zhang, L.; Sui, Y.; Zhou, X.; Chen, H.; et al. Electrodeposition of Cu2O films and their photoelectrochemical properties. CrystEngComm 2011, 13, 2871. [Google Scholar] [CrossRef]
- Hossain, M.A.; Al-Gaashani, R.; Hamoudi, H.; Al Marri, M.J.; Hussein, I.A.; Belaidi, A.; Merzougui, B.A.; Alharbi, F.H.; Tabet, N. Controlled growth of Cu2O thin films by electrodeposition approach. Mater. Sci. Semicond. Process. 2017, 63, 203–211. [Google Scholar] [CrossRef]
- Kim, D.; Dimitrakopoulos, G.; Yildiz, B. Controlling the Size of Au Nanoparticles on Reducible Oxides with the Electrochemical Potential. J. Am. Chem. Soc. 2022, 144, 21926–21938. [Google Scholar] [CrossRef] [PubMed]
- Taher, S.J.; Barzinjy, A.A.; Hamad, S.M. The Effect of Deposition Time on the Properties of Cu2O Nanocubes Using an Electrochemical Deposition Method. J. Electron. Mater. 2020, 49, 7532–7540. [Google Scholar] [CrossRef]
- Guo:, D.; Li, W.; Wang, D.; Meng, B.; Fang, D.; Wei, Z. High performance Cu2O film/ZnO nanowires self-powered photodetector by electrochemical deposition. Chin. Phys. B 2020, 29, 098504. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Huang, L.; Jian, F.; Zhao, S.; Tang, W.; Tang, H. Electrochemical One-Step Synthesis of Cu2O with Tunable Oxygen Defects and Their Electrochemical Performance in Li-Ion Batteries. Coatings 2025, 15, 510. https://doi.org/10.3390/coatings15050510
Zheng Y, Huang L, Jian F, Zhao S, Tang W, Tang H. Electrochemical One-Step Synthesis of Cu2O with Tunable Oxygen Defects and Their Electrochemical Performance in Li-Ion Batteries. Coatings. 2025; 15(5):510. https://doi.org/10.3390/coatings15050510
Chicago/Turabian StyleZheng, Yu, Lanxiang Huang, Feiyu Jian, Shujia Zhao, Wu Tang, and Hui Tang. 2025. "Electrochemical One-Step Synthesis of Cu2O with Tunable Oxygen Defects and Their Electrochemical Performance in Li-Ion Batteries" Coatings 15, no. 5: 510. https://doi.org/10.3390/coatings15050510
APA StyleZheng, Y., Huang, L., Jian, F., Zhao, S., Tang, W., & Tang, H. (2025). Electrochemical One-Step Synthesis of Cu2O with Tunable Oxygen Defects and Their Electrochemical Performance in Li-Ion Batteries. Coatings, 15(5), 510. https://doi.org/10.3390/coatings15050510





