Bioactive Hydroxyapatite–Carboplatin–Quercetin Coatings for Enhanced Osteointegration and Antitumoral Protection in Hip Endoprostheses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Hydroxyapatite Nanoparticles
2.2.2. MAPLE Deposition of Hydroxyapatite-Based Coatings
2.3. Powder and Coatings Investigations
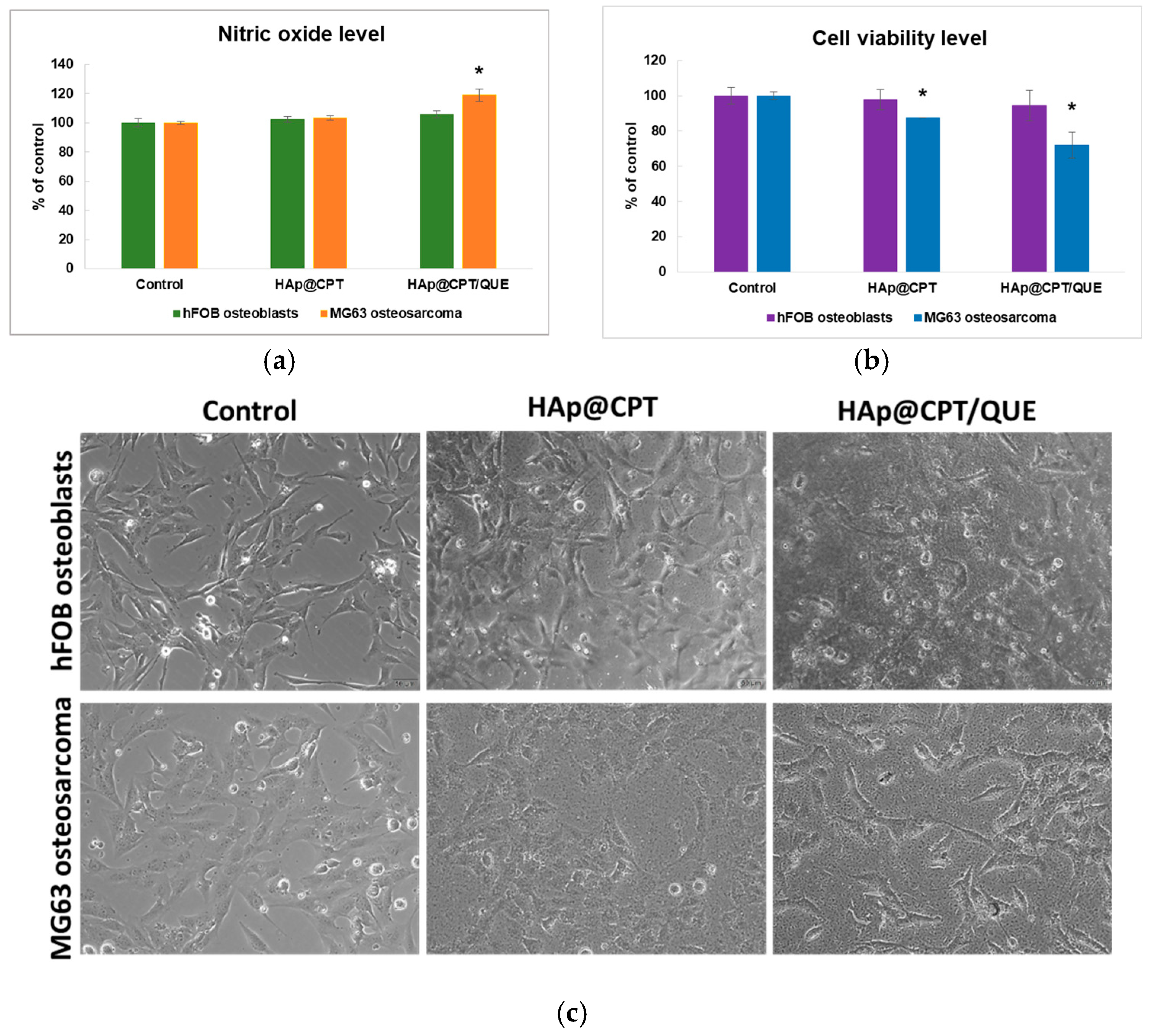
2.4. In Vitro Cell-Based Assays—Biocompatibility and Oxidative Stress Production Assessment
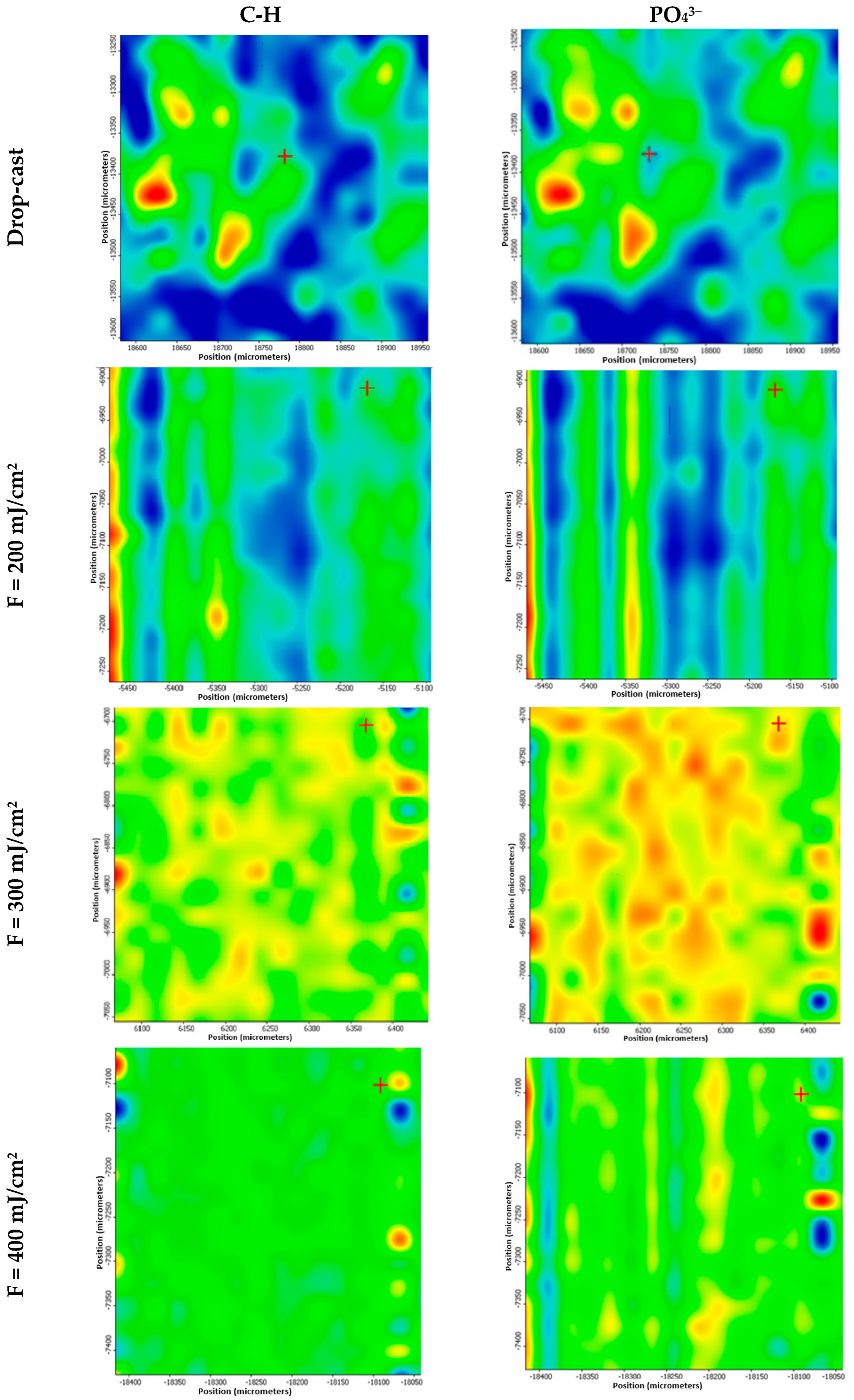
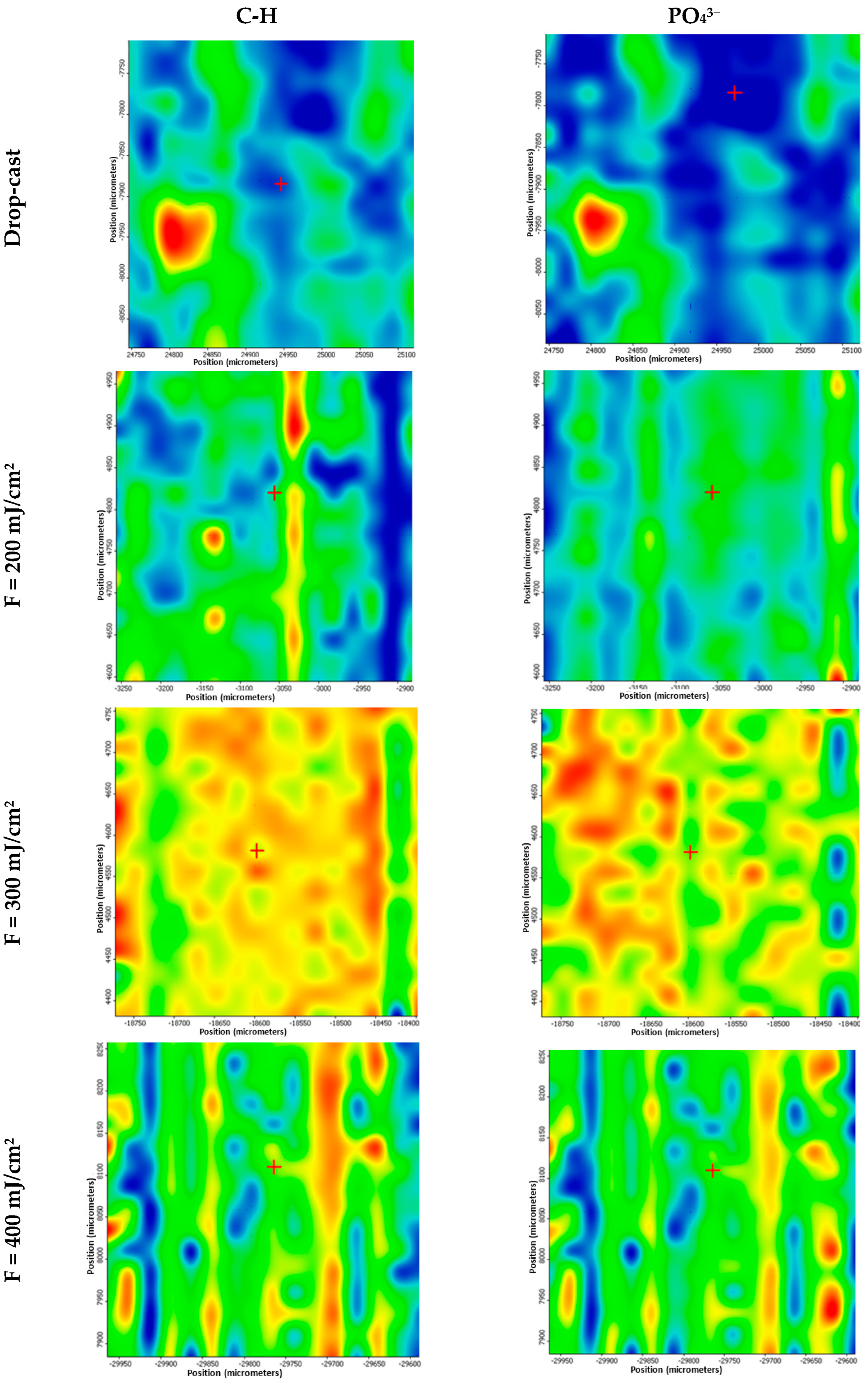
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levett, J.J.; Turcotte, R.E.; Jung, S.; Antoniou, J.; Huk, O.L. Osteosarcoma Around a Ceramic-on-Ceramic Total Hip Arthroplasty. Arthroplast. Today 2023, 19, 101094. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.-L.; Su, H.-B.; Du, S.-H.; Hou, C.-H.; Lu, M.; Dai, S.-W.; Lei, Z.-X.; Chen, W.; Li, H.-M. Revision surgery for periprosthetic fracture of distal femur after endoprosthetic replacement of knee joint following resection of osteosarcoma. Front. Oncol. 2024, 14, 1328703. [Google Scholar] [CrossRef] [PubMed]
- Callan, A.K.; Alexander, J.H.; Montgomery, N.I.; Lindberg, A.W.; Scharschmidt, T.J.; Binitie, O. Contemporary surgical management of osteosarcoma and Ewing sarcoma. Pediatr. Blood Cancer 2025, 72, e31374. [Google Scholar] [CrossRef]
- Khakzad, T.; Putzier, M.; Paksoy, A.; Rau, D.; Thielscher, L.; Taheri, N.; Wittenberg, S.; Märdian, S. Outcome of Endoprosthetic Hip Reconstruction Following Resection of Malignant Bone Tumors. Cancers 2024, 16, 2890. [Google Scholar] [CrossRef]
- Latała, B.; Kamiński, P.; Jagielski, P.; Kłosiński, M.; Wilk-Frańczuk, M. Evaluation of functional capacity, pain level and quality of life in patients following hip arthroplasty due to degenerative changes depending on the type of implanted endoprosthesis. Acta Neuropsychol. 2024, 22, 417–433. [Google Scholar] [CrossRef]
- Tepper, S.C.; Lee, L.; Kasson, L.B.; Herbst, L.R.; Vijayakumar, G.; Colman, M.W.; Gitelis, S.; Blank, A.T. Hip Arthroplasty Outcomes in Patients with Metastatic Bone Disease. Orthop. Rev. 2024, 16, 94568. [Google Scholar] [CrossRef]
- Radunović, A.; Radunović, O.; Vulović, M.; Aksić, M. Bioceramics in Orthopedic Surgery. In Bioceramics, Biomimetic and Other Compatible Materials Features for Medical Applications; Najman, S., Mitić, V., Groth, T., Barbeck, M., Chen, P.-Y., Sun, Z., Randjelović, B., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 53–65. [Google Scholar]
- Meng, M.; Wang, J.; Huang, H.; Liu, X.; Zhang, J.; Li, Z. 3D printing metal implants in orthopedic surgery: Methods, applications and future prospects. J. Orthop. Transl. 2023, 42, 94–112. [Google Scholar] [CrossRef]
- Fenske, F.; Krause, L.; Meyer, S.; Kujat, B.; Repmann, J.; Neuhaus, M.; Zimmerer, R.; Roth, A.; Lethaus, B.; Ziebolz, D.; et al. Oral Health Screening for Risk Reduction for Early Periprosthetic Joint Infections of Hip and Knee Endoprostheses—Results of a Prospective Cohort Study. J. Clin. Med. 2023, 12, 4451. [Google Scholar] [CrossRef]
- Liang, W.; Zhou, C.; Bai, J.; Zhang, H.; Jiang, B.; Wang, J.; Fu, L.; Long, H.; Huang, X.; Zhao, J.; et al. Current advancements in therapeutic approaches in orthopedic surgery: A review of recent trends. Front. Bioeng. Biotechnol. 2024, 12, 1328997. [Google Scholar] [CrossRef]
- Hariharan, K.; Selvakumar, M.; Ramkumar, T.; Chandramohan, P. Cell viability and bioactive coating on additive manufactured polycaprolactone substrate for osteointegration applications. Chem. Phys. Impact 2024, 8, 100409. [Google Scholar] [CrossRef]
- Uklejewski, R.; Winiecki, M.; Dąbrowski, M.; Rogala, P. Towards the First Generation of Biomimetic Fixation for Resurfacing Arthroplasty Endoprostheses. Biomimetics 2024, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhou, C.; Fu, L.; Huang, X.; Liu, Z.; Zhao, J.; Liang, W.; Shao, H. A mini-review on the emerging role of nanotechnology in revolutionizing orthopedic surgery: Challenges and the road ahead. Front. Bioeng. Biotechnol. 2023, 11, 1191509. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Cardoso, F.N.; Souza, F.; Montreuil, J.; Pretell-Mazzini, J.; Temple, H.T.; Hornicek, F.; Crawford, B.; Subhawong, T.K. Failure Modes in Orthopedic Oncologic Reconstructive Surgery: A Review of Imaging Findings and Failure Rates. Curr. Oncol. 2024, 31, 6245–6266. [Google Scholar] [CrossRef] [PubMed]
- Uklejewski, R.; Uklejewski, R.; Rogala, P.; Winiecki, M.; Winiecki, M. Structural and Hydroxyapatite-like Surface Functionalization of Advanced Biomimetic Prototype Interface for RA Endoprostheses to Enhance Osteoconduction and Osteointegration. Adv. Surf. Eng. Mater. 2016, 175–240. [Google Scholar]
- Rios-Pimentel, F.F.; Méndez-González, M.M.; García-Rocha, M. A short review: Hydroxyapatite coatings for metallic implants. Heat. Treat. Surf. Eng. 2023, 5, 2202002. [Google Scholar] [CrossRef]
- Ioniță-Radu, F.; Nicolau, I.-N.; Petrache, O.-G.; Groșeanu, M.-L.; Bojincă, V.-C.; Negru, M.-M.; Bucurică, S.; Anghel, D. Correlation Between Trabecular Bone Score and Homocysteine Level in Rheumatoid Arthritis Patients on Anti-TNF Inhibitors. Life 2024, 14, 463. [Google Scholar] [CrossRef]
- Salahuddin, N.; Ibrahim, E.M.; El-Kemary, M. Different methods for preparation of hydroxyapatite nanostructures. Biointerface Res. Appl. Chem. 2023, 13, 236. [Google Scholar]
- Alberts, A.; Bratu, A.G.; Niculescu, A.-G.; Grumezescu, A.M. Collagen-Based Wound Dressings: Innovations, Mechanisms, and Clinical Applications. Gels 2025, 11, 271. [Google Scholar] [CrossRef]
- Buradagunta, R.S.; Madiga, J.; Dumpala, R. Producing Calcium Deficient Nano-hydroxyapatite from Silver pomfret Fish Bones for Biomedical Applications. Lett. Appl. NanoBioScience 2024, 13, 130. [Google Scholar] [CrossRef]
- Liu, C.; Xu, M.; Wang, Y.; Yin, Q.; Hu, J.; Chen, H.; Sun, Z.; Liu, C.; Li, X.; Zhou, W.; et al. Exploring the potential of hydroxyapatite-based materials in biomedicine: A comprehensive review. Mater. Sci. Eng. R Rep. 2024, 161, 100870. [Google Scholar] [CrossRef]
- Fendi, F.; Abdullah, B.; Suryani, S.; Usman, A.N.; Tahir, D. Development and application of hydroxyapatite-based scaffolds for bone tissue regeneration: A systematic literature review. Bone 2024, 183, 117075. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.; Morris, M.A.; Baez, J. Development of Hydroxyapatite Coatings for Orthopaedic Implants from Colloidal Solutions: Part 2—Detailed Characterisation of the Coatings and Their Growth Mechanism. Nanomaterials 2023, 13, 2606. [Google Scholar] [CrossRef] [PubMed]
- Shoeib, M.A.; Abdel-Gawad, S.A. High performance nano hydroxyapatite coating on zinc for biomedical applications. J. Mater. Sci. 2023, 58, 740–756. [Google Scholar] [CrossRef]
- Murugesan, V.; Govindarasu, M.; Manoharadas, S.; Pandiaraj, S.; Thiruvengadam, M.; Govindasamy, R.; Vaiyapuri, M. Combinatorial anticancer effects of multi metal ion and drug substitute with hydroxyapatite coatings on surgical grade 316LSS stainless steel alloys towards biomedical applications. J. Mater. Res. Technol. 2023, 27, 7244–7258. [Google Scholar] [CrossRef]
- Youness, R.A.; Saleh, H.A.; Taha, M.A. Microstructure and elastic properties of hydroxyapatite/alumina nanocomposites prepared by mechanical alloying technique for biomedical applications. Biointerface Res. Appl. Chem. 2023, 13, 395. [Google Scholar]
- Awasthi, S.; Pandey, S.K.; Arunan, E.; Srivastava, C. A review on hydroxyapatite coatings for the biomedical applications: Experimental and theoretical perspectives. J. Mater. Chem. B 2021, 9, 228–249. [Google Scholar] [CrossRef]
- Florea, D.A.; Grumezescu, V.; Bîrcă, A.C.; Vasile, B.Ș.; Iosif, A.; Chircov, C.; Stan, M.S.; Grumezescu, A.M.; Andronescu, E.; Chifiriuc, M.C. Bioactive Hydroxyapatite-Magnesium Phosphate Coatings Deposited by MAPLE for Preventing Infection and Promoting Orthopedic Implants Osteointegration. Materials 2022, 15, 7337. [Google Scholar] [CrossRef]
- Komarova, E.G.; Senkina, E.I.; Lozhkomoev, A.S.; Kazantseva, E.A.; Prosolov, K.A.; Kazantsev, S.O.; Akimova, E.B.; Tolkacheva, T.V.; Khimich, M.A.; Sharkeev, Y.P. Controlled anticancer 5-Fluorouracil release from functionalized 5-FU/PLGA/CaP coating on titanium implants: Characterization, in vitro drug delivery and cytotoxicity. Mater. Today Commun. 2024, 39, 109332. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Eshaghi, M.M.; Shaghaghi, M.; Das, S.S.; Arshad, R.; Ghotekar, S.; Rahdar, A.; Manicum, A.-L.E.; Pandey, S. Nano-scale drug delivery systems for carboplatin: A comprehensive review. OpenNano 2023, 13, 100175. [Google Scholar] [CrossRef]
- Choeyprasert, W.; Natesirinilkul, R.; Charoenkwan, P.; Sittipreechacharn, S. Carboplatin and doxorubicin in treatment of pediatric osteosarcoma: A 9-year single institute experience in the Northern Region of Thailand. Asian Pac. J. Cancer Prev. 2013, 14, 1101–1106. [Google Scholar] [CrossRef]
- Skverchinskaya, E.; Levdarovich, N.; Ivanov, A.; Mindukshev, I.; Bukatin, A. Anticancer Drugs Paclitaxel, Carboplatin, Doxorubicin, and Cyclophosphamide Alter the Biophysical Characteristics of Red Blood Cells, In Vitro. Biology 2023, 12, 230. [Google Scholar] [CrossRef] [PubMed]
- Hain, B.A.; Xu, H.; Wilcox, J.R.; Mutua, D.; Waning, D.L. Chemotherapy-induced loss of bone and muscle mass in a mouse model of breast cancer bone metastases and cachexia. JCSM Rapid Commun. 2019, 2, 1–12. [Google Scholar] [CrossRef]
- Szefler, B.; Makarewicz, A.; Obońska, E.; Rutkowska, D.; Czeleń, P. Docking of carboplatin towards chosen nanostructures. Biointerface Res. Appl. Chem. 2022, 13, 109. [Google Scholar]
- Mirza, M.A.; Mahmood, S.; Hilles, A.R.; Ali, A.; Khan, M.Z.; Zaidi, S.A.; Iqbal, Z.; Ge, Y. Quercetin as a Therapeutic Product: Evaluation of Its Pharmacological Action and Clinical Applications—A Review. Pharmaceuticals 2023, 16, 1631. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Lotfi, M.-S.; Sheibani, M.; Jafari-Sabet, M. Quercetin-based biomaterials for enhanced bone regeneration and tissue engineering. Tissue Cell 2024, 91, 102626. [Google Scholar] [CrossRef]
- Durmaz, B.; Gunes, N.; Koparal, M.; Gul, M.; Dundar, S.; Bingul, M.B. Investigation of the effects of quercetin and xenograft on the healing of bone defects: An experimental study. J. Oral. Biol. Craniofacial Res. 2023, 13, 22–27. [Google Scholar] [CrossRef]
- Sharma, S.; Cwiklinski, K.; Mahajan, S.D.; Schwartz, S.A.; Aalinkeel, R. Combination Modality Using Quercetin to Enhance the Efficacy of Docetaxel in Prostate Cancer Cells. Cancers 2023, 15, 902. [Google Scholar] [CrossRef]
- El-Far, M.; Essam, A.; Fardous, F.; Abd El-Azim, A.O.; Yahia, S.; El-Sherbiny, I.M. Newly Modified Nanoformulation of Quercetin as Promising Chemotherapeutic Anticancer Agent. Biointerface Res. Appl. Chem. 2023, 13, 387. [Google Scholar]
- Alberts, A.; Moldoveanu, E.-T.; Niculescu, A.-G.; Grumezescu, A.M. Nigella sativa: A Comprehensive Review of Its Therapeutic Potential, Pharmacological Properties, and Clinical Applications. Int. J. Mol. Sci. 2024, 25, 13410. [Google Scholar] [CrossRef]
- Pati, S.; Antara, N.S.; Gunam, I.B.W.; Sarkar, T.; Lahiri, D. Elucidating the Quorum Sensing Inhibitory Mechanism of Flavonoid Quercetin by Molecular Docking, Molecular Dynamics Simulation, and MM-GBSA Study. Lett. Appl. NanoBioScience 2024, 13, 152. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Ansary, J.; Ghoreishi, R. Sol-gel process applications: A mini-review. Proc. Nat. Res. Soc. 2018, 2, 02008–02029. [Google Scholar] [CrossRef]
- Grămadă, A.M.; Stoica, A.-E.; Niculescu, A.-G.; Bîrcă, A.C.; Vasile, B.Ș.; Holban, A.M.; Mihaiescu, T.; Șerban, A.I.; Ciceu, A.; Balta, C.; et al. Zinc Oxide-Loaded Recycled PET Nanofibers for Applications in Healthcare and Biomedical Devices. Polymers 2025, 17, 45. [Google Scholar] [CrossRef]
- Bîrcă, A.C.; Minculescu, M.A.; Niculescu, A.-G.; Hudiță, A.; Holban, A.M.; Alberts, A.; Grumezescu, A.M. Nanoparticle-Enhanced Collagen Hydrogels for Chronic Wound Management. J. Funct. Biomater. 2025, 16, 91. [Google Scholar] [CrossRef]
- Barrino, F. Hybrid Organic–Inorganic Materials Prepared by Sol–Gel and Sol–Gel-Coating Method for Biomedical Use: Study and Synthetic Review of Synthesis and Properties. Coatings 2024, 14, 425. [Google Scholar] [CrossRef]
- Shankar, D.; Jayaganesh, K.; Gowda, N.; Lakshmi, K.S.; Jayanthi, K.J.; Jambagi, S.C. Thermal spray processes influencing surface chemistry and in-vitro hemocompatibility of hydroxyapatite-based orthopedic implants. Biomater. Adv. 2024, 158, 213791. [Google Scholar] [CrossRef] [PubMed]
- Shankar, D.; Jambagi, S.C. Improvements in bioactivity, blood compatibility, and wear resistance of thermally sprayed carbon nanotube reinforced hydroxyapatite-based orthopedic implants. Tribol. Int. 2024, 197, 109809. [Google Scholar] [CrossRef]
- Constantinescu, S.; Niculescu, A.-G.; Hudiță, A.; Grumezescu, V.; Rădulescu, D.; Bîrcă, A.C.; Dorcioman, G.; Gherasim, O.; Holban, A.M.; Gălățeanu, B.; et al. Nanostructured Coatings Based on Graphene Oxide for the Management of Periprosthetic Infections. Int. J. Mol. Sci. 2024, 25, 2389. [Google Scholar] [CrossRef]
- Paneer Selvam, S.; Ayyappan, S.; I Jamir, S.; Sellappan, L.K.; Manoharan, S. Recent advancements of hydroxyapatite and polyethylene glycol (PEG) composites for tissue engineering applications—A comprehensive review. Eur. Polym. J. 2024, 215, 113226. [Google Scholar] [CrossRef]
- Kravanja, K.A.; Finšgar, M. A review of techniques for the application of bioactive coatings on metal-based implants to achieve controlled release of active ingredients. Mater. Des. 2022, 217, 110653. [Google Scholar] [CrossRef]
- Liao, T.-Y.; Biesiekierski, A.; Berndt, C.C.; King, P.C.; Ivanova, E.P.; Thissen, H.; Kingshott, P. Multifunctional cold spray coatings for biological and biomedical applications: A review. Prog. Surf. Sci. 2022, 97, 100654. [Google Scholar] [CrossRef]
- Zureigat, O.A.; NeamŢU, J.; Duta, L.; Icriverzi, M.; Florian, P.; Sima, L.-e.; Dorcioman, G.; Grumezescu, V.; BĂLĂŞOiu, R.; ButeicĂ, S.A. Development and characterisation of implantable sandwich structures made of lithium-doped biological-derived hydroxyapatite and ciprofloxacin. Farmacia 2024, 72, 6. [Google Scholar] [CrossRef]
- Jelinek, M. 23—Hybrid laser technology for biomaterials. In Lasers for Medical Applications; Jelínková, H., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 704–724. [Google Scholar]
- Grumezescu, V.; Grumezescu, A.M.; Ficai, A.; Negut, I.; Vasile, B.Ș.; Gălățeanu, B.; Hudiță, A. Composite Coatings for Osteoblast Growth Attachment Fabricated by Matrix-Assisted Pulsed Laser Evaporation. Polymers 2022, 14, 2934. [Google Scholar] [CrossRef] [PubMed]
- Rabiei, M.; Palevicius, A.; Monshi, A.; Nasiri, S.; Vilkauskas, A.; Janusas, G. Comparing Methods for Calculating Nano Crystal Size of Natural Hydroxyapatite Using X-Ray Diffraction. Nanomaterials 2020, 10, 1627. [Google Scholar] [CrossRef]
- Shah, S.; Joshi, R.; Rai, N.; Adhikari, R.; Pandit, R. Microstructural analysis of biowaste-derived hydroxyapatite-chitosan nanocomposites. Micro Nano Lett. 2022, 17, 369–376. [Google Scholar] [CrossRef]
- Iliescu, R.I.; Andronescu, E.; Ghitulica, C.D.; Berger, D.; Ficai, A. Montmorillonite-alginate nanocomposite beads as drug carrier for oral administration of carboplatin-preparation and characterization. UPB Sci. Bull. Ser. B 2011, 73, 3–16. [Google Scholar]
- Wang, Q.; Wei, H.; Deng, C.; Xie, C.; Huang, M.; Zheng, F. Improving Stability and Accessibility of Quercetin in Olive Oil-in-Soy Protein Isolate/Pectin Stabilized O/W Emulsion. Foods 2020, 9, 123. [Google Scholar] [CrossRef]
- Stevenson, J.; Siddiqi, M.A.; Sheehy, V.; Kendrick, B.; Whitwell, D.; Taylor, A.; Blunn, G.; Mohammad, H.R.; Kamath, A.F.; Thoma, S. Early radiological outcomes of a fully porous bridging collar in lower-limb endoprosthetic reconstructions: A case-matched retrospective series to assess osseointegration. Arthroplasty 2024, 6, 17. [Google Scholar] [CrossRef]
- Kubiak-Mihkelsoo, Z.; Kostrzębska, A.; Błaszczyszyn, A.; Pitułaj, A.; Dominiak, M.; Gedrange, T.; Nawrot-Hadzik, I.; Matys, J.; Hadzik, J. Ionic Doping of Hydroxyapatite for Bone Regeneration: Advances in Structure and Properties over Two Decades—A Narrative Review. Appl. Sci. 2025, 15, 1108. [Google Scholar] [CrossRef]
- Tang, G.; Yang, H.; Zhao, B.; Wang, D.; Zeng, F.; Liu, Q. Microstructure, electrical properties, bioactivity, biocompatibility and osteogenic differentiation ability of bio-piezocomposite fabricated by hydroxyapatite and (Ba,Ca)(Ti,Sn)O3-based ceramics. J. Mater. Res. Technol. 2025, 35, 4482–4495. [Google Scholar] [CrossRef]
- Es-saddik, M.; Laasri, S.; Bensemlali, M.; Hariti, N.; Laghzizil, A.; Taha, M. Experimental and Finite Element Study of the Mechanical Behavior of Hydroxyapatite/Tricalcium Phosphate/Alumina Biocomposite. Biointerface Res. Appl. Chem. 2024, 14, 1–14. [Google Scholar]
- Yang, X.; Huang, P.; Wang, H.; Cai, S.; Liao, Y.; Mo, Z.; Xu, X.; Ding, C.; Zhao, C.; Li, J. Antibacterial and anti-biofouling coating on hydroxyapatite surface based on peptide-modified tannic acid. Colloids Surf. B Biointerfaces 2017, 160, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Palierse, E.; Hélary, C.; Krafft, J.-M.; Génois, I.; Masse, S.; Laurent, G.; Alvarez Echazu, M.I.; Selmane, M.; Casale, S.; Valentin, L.; et al. Baicalein-modified hydroxyapatite nanoparticles and coatings with antibacterial and antioxidant properties. Mater. Sci. Eng. C 2021, 118, 111537. [Google Scholar] [CrossRef]
- Dorcioman, G.; Hudiță, A.; Gălățeanu, B.; Craciun, D.; Mercioniu, I.; Oprea, O.C.; Neguț, I.; Grumezescu, V.; Grumezescu, A.M.; Dițu, L.M.; et al. Magnetite-Based Nanostructured Coatings Functionalized with Nigella sativa and Dicloxacillin for Improved Wound Dressings. Antibiotics 2023, 12, 59. [Google Scholar] [CrossRef]
- Pirușcă, I.A.; Balaure, P.C.; Grumezescu, V.; Irimiciuc, S.-A.; Oprea, O.-C.; Bîrcă, A.C.; Vasile, B.; Holban, A.M.; Voinea, I.C.; Stan, M.S.; et al. New Fe3O4-Based Coatings with Enhanced Anti-Biofilm Activity for Medical Devices. Antibiotics 2024, 13, 631. [Google Scholar] [CrossRef]
- Alfe, M.; Minopoli, G.; Tartaglia, M.; Gargiulo, V.; Ausanio, G. Biocompatible Hybrid Graphenic Thin Coatings on Flexible Substrates Through Matrix-Assisted Pulsed Laser Evaporation (MAPLE). ACS Appl. Mater. Interfaces 2024, 16, 38956–38967. [Google Scholar] [CrossRef]
- Robson, H.; Meyer, S.; Shalet, S.M.; Anderson, E.; Roberts, S.; Eden, O.B. Platinum agents in the treatment of osteosarcoma: Efficacy of cisplatin vs. carboplatin in human osteosarcoma cell lines. Med. Pediatr. Oncol. 2002, 39, 573–580. [Google Scholar] [CrossRef]
- Liang, W.; Li, X.; Li, C.; Liao, L.; Gao, B.; Gan, H.; Yang, Z.; Liao, L.; Chen, X. Quercetin-mediated apoptosis via activation of the mitochondrial-dependent pathway in MG-63 osteosarcoma cells. Mol. Med. Rep. 2011, 4, 1017–1023. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Liu, X.; Yin, L.; Shen, S.; Hou, Y. Inflammation and cancer: Paradoxical roles in tumorigenesis and implications in immunotherapies. Genes. Dis. 2023, 10, 151–164. [Google Scholar] [CrossRef]
- Nigam, M.; Mishra, A.P.; Deb, V.K.; Dimri, D.B.; Tiwari, V.; Bungau, S.G.; Bungau, A.F.; Radu, A.F. Evaluation of the association of chronic inflammation and cancer: Insights and implications. Biomed. Pharmacother. 2023, 164, 115015. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iosub, G.; Tudorache, D.-I.; Iova, I.M.; Duta, L.; Grumezescu, V.; Bîrcă, A.C.; Niculescu, A.-G.; Balaure, P.C.; Voinea, I.C.; Stan, M.S.; et al. Bioactive Hydroxyapatite–Carboplatin–Quercetin Coatings for Enhanced Osteointegration and Antitumoral Protection in Hip Endoprostheses. Coatings 2025, 15, 489. https://doi.org/10.3390/coatings15040489
Iosub G, Tudorache D-I, Iova IM, Duta L, Grumezescu V, Bîrcă AC, Niculescu A-G, Balaure PC, Voinea IC, Stan MS, et al. Bioactive Hydroxyapatite–Carboplatin–Quercetin Coatings for Enhanced Osteointegration and Antitumoral Protection in Hip Endoprostheses. Coatings. 2025; 15(4):489. https://doi.org/10.3390/coatings15040489
Chicago/Turabian StyleIosub, Gheorghe, Dana-Ionela Tudorache (Trifa), Ionuț Marinel Iova, Liviu Duta, Valentina Grumezescu, Alexandra Cătălina Bîrcă, Adelina-Gabriela Niculescu, Paul Cătălin Balaure, Ionela Cristina Voinea, Miruna S. Stan, and et al. 2025. "Bioactive Hydroxyapatite–Carboplatin–Quercetin Coatings for Enhanced Osteointegration and Antitumoral Protection in Hip Endoprostheses" Coatings 15, no. 4: 489. https://doi.org/10.3390/coatings15040489
APA StyleIosub, G., Tudorache, D.-I., Iova, I. M., Duta, L., Grumezescu, V., Bîrcă, A. C., Niculescu, A.-G., Balaure, P. C., Voinea, I. C., Stan, M. S., Rădulescu, D. M., Bădilă, A. E., Vasile, B. Ș., Grumezescu, A. M., & Rădulescu, A. R. (2025). Bioactive Hydroxyapatite–Carboplatin–Quercetin Coatings for Enhanced Osteointegration and Antitumoral Protection in Hip Endoprostheses. Coatings, 15(4), 489. https://doi.org/10.3390/coatings15040489












