MOF-Derived Hollow Dodecahedral Carbon Structures with Abundant N Sites and Co Nanoparticle-Modified Cu Foil for Dendrite-Free Lithium Metal Battery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of ZIF-8
2.2. Preparation of ZIF-8@ZIF-67
2.3. Preparation of NC-Co-CNTs
2.4. Preparation of NC-Co-CNT-Modified Copper Foil
2.5. Electrochemical Measurements
2.6. Characterization
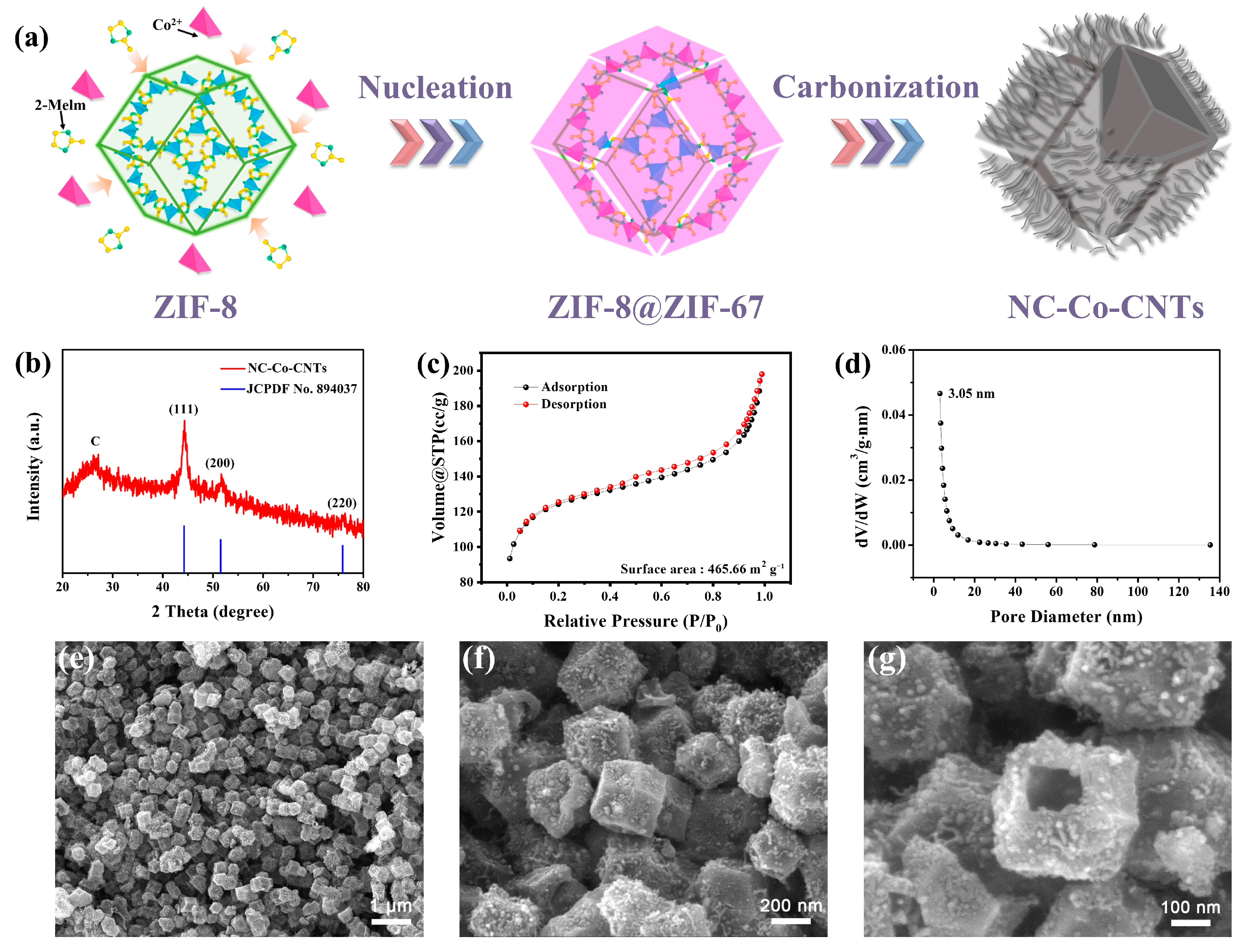
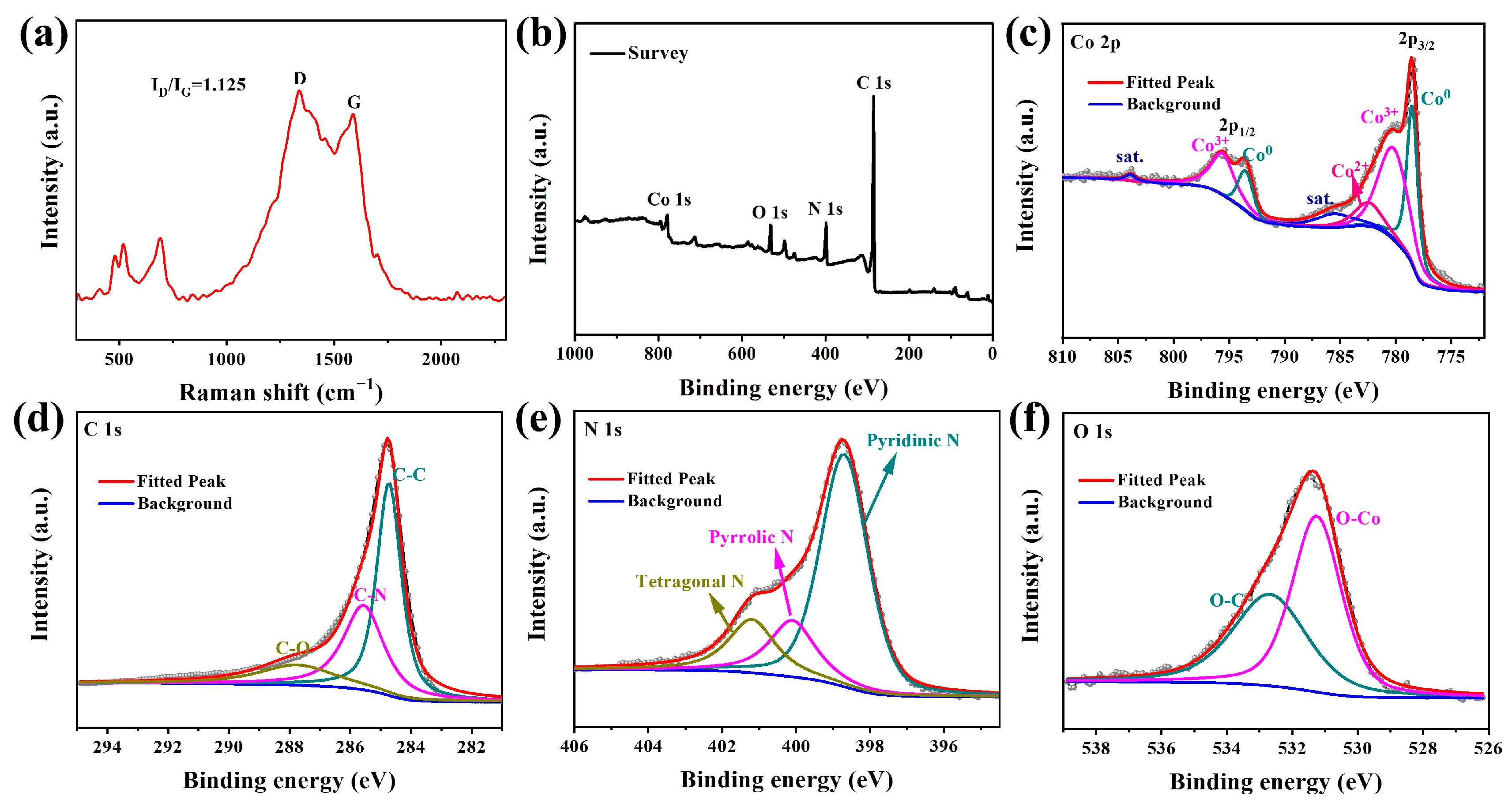
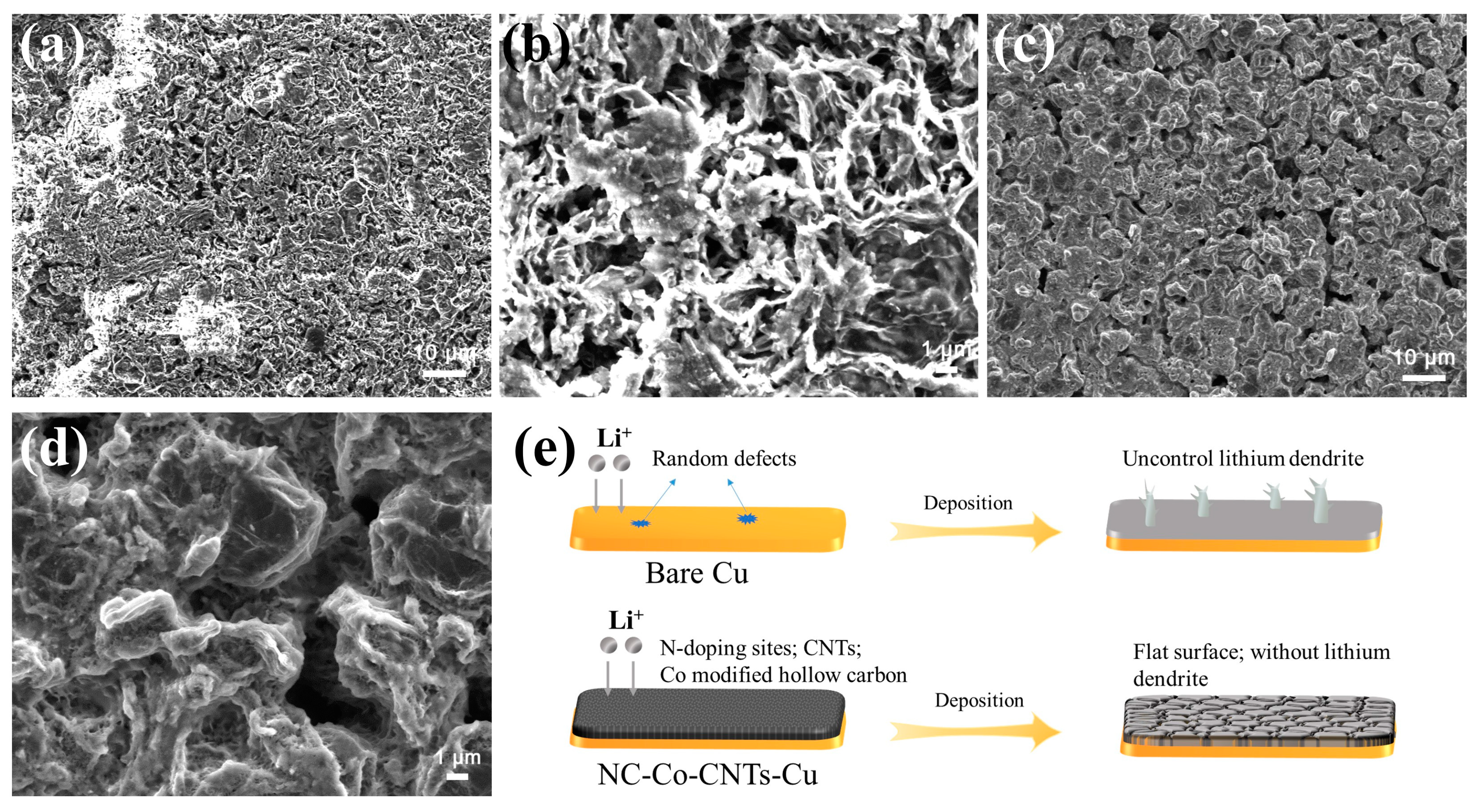
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Z.-L.; Liu, X.; Luo, Y.; Zhou, L.; Kim, J.-K. Nanosilicon anodes for high performance rechargeable batteries. Prog. Mater. Sci. 2017, 90, 1–44. [Google Scholar] [CrossRef]
- Gao, Y.-M.; Liu, Y.; Feng, K.-J.; Ma, J.-Q.; Miao, Y.-J.; Xu, B.-R.; Pan, K.-M.; Akiyoshi, O.; Wang, G.-X.; Zhang, K.-K.; et al. Emerging WS2/WSe2@graphene nanocomposites: Synthesis and electrochemical energy storage applications. Rare Met. 2024, 43, 1–19. [Google Scholar] [CrossRef]
- Wu, N.; Shen, J.; Zhou, X.; Li, S.; Li, J.; Liu, G.; Guo, D.; Deng, W.; Yuan, C.; Liu, X.; et al. Constructing Iron Vacancies in Thiospinel FeIn2S4 to Modulate Fe D-Band Center and Accelerate Sodiation Kinetics Enabling High-Rate and Durable Sodium Storage. Adv. Energy Mater. 2025, 2405729. [Google Scholar] [CrossRef]
- Liu, D.; Wu, B.; Xu, Y.; Ellis, J.; Baranovskiy, A.; Lu, D.; Lochala, J.; Anderson, C.; Baar, K.; Qu, D.; et al. Controlled large-area lithium deposition to reduce swelling of high-energy lithium metal pouch cells in liquid electrolytes. Nat. Energy 2024, 9, 559–569. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, T.; Li, Y.; Luo, Z.; Liao, X.; Wang, X.; Pan, J. Growing mulberry-like copper on copper current collector for stable lithium metal battery anodes. J. Colloid Interface Sci. 2025, 680, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Wang, F.; Wang, H.-B.; Kong, C.-Y.; Wang, G.-X.; Liu, X.-M.; Liu, Y. Shining Light on Fillers Uniform Dispersion of PVDF/Garnet Composite Electrolytes for High-performance Solid-State Li batteries: Fundamentals, Progress, and Perspectives. Rare Met. 2025. [Google Scholar]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Wang, F.; Gao, J.; Liu, Y.; Ren, F. An amorphous ZnO and oxygen vacancy modified nitrogen-doped carbon skeleton with lithiophilicity and ionic conductivity for stable lithium metal anodes. J. Mater. Chem. A 2022, 10, 17395–17405. [Google Scholar] [CrossRef]
- Ma, L.; Cui, J.; Yao, S.; Liu, X.; Luo, Y.; Shen, X.; Kim, J.-K. Dendrite-free lithium metal and sodium metal batteries. Energy Storage Mater. 2020, 27, 522–554. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef]
- Qiu, J.; Qiu, R.; Mao, Z.; Han, Y.; Madhusudan, P.; Wang, X.; Wang, C.; Qi, C.; Yu, X.; Zeng, S.; et al. A review on copper current collector used for lithium metal batteries: Challenges and strategies. J. Energy Storage 2024, 100, 113683. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.; Wang, J.; Sun, Y.; Feng, X.; Xiang, H. Regulated lithium deposition behavior by an artificial coating of Cu foil for dendrite-free lithium metal batteries. Mater. Today Sustain. 2022, 18, 100127. [Google Scholar] [CrossRef]
- Zhai, P.; He, Q.; Jiang, H.; Gao, B.; Zhang, B.; Chen, Q.; Yang, Z.; Wang, T.; Gong, Y. Thickness-Dependence of 2D g-C3N4 Artificial Interface Layers on Lithium Metal Deposition. Adv. Energy Mater. 2024, 14, 2302730. [Google Scholar] [CrossRef]
- Song, R.; Ge, Y.; Wang, B.; Lv, Q.; Wang, F.; Ruan, T.; Wang, D.; Dou, S.; Liu, H. A new reflowing strategy based on lithiophilic substrates towards smooth and stable lithium metal anodes. J. Mater. Chem. A 2019, 7, 18126–18134. [Google Scholar] [CrossRef]
- Li, J.; Ma, Z.; Yang, K.; Zhao, F.; Yang, H.; Wang, H.; He, Y. Laser-Generated Au nanoparticles as lithophilic sites in self-supported film host for anode-free lithium metal battery. J. Colloid Interface Sci. 2025, 678, 578–587. [Google Scholar] [CrossRef]
- Yang, K.; Li, L.; Xiao, Y.; Zhang, Q.; Xi, C.; Li, B.; Yu, Y.; Yang, C. Congener-derived template to construct lithiophilic organic-inorganic layer/interphase for high volumetric capacity dendrite-free Li metal batteries. Chin. Chem. Lett. 2024, 35, 108451. [Google Scholar] [CrossRef]
- Xia, H.-y.; Wang, D.-l.; Wang, Y.-k.; Fu, Z.-w. Study on Stable Lithiophilic Ag Modification Layer on Copper Current Collector for High Coulombic-Efficiency Lithium Metal Anode. J. Electrochem. Soc. 2023, 170, 060546. [Google Scholar] [CrossRef]
- Liu, Z.; Ha, S.; Liu, Y.; Wang, F.; Tao, F.; Xu, B.; Yu, R.; Wang, G.; Ren, F.; Li, H. Application of Ag-based materials in high-performance lithium metal anode: A review. J. Mater. Sci. Technol. 2023, 133, 165–182. [Google Scholar] [CrossRef]
- Song, Y.-X.; Lu, W.-Y.; Chen, Y.-J.; Yang, H.; Wu, C.; Wei, W.-F.; Chen, L.-B.; Ouyang, X.-P. Coating highly lithiophilic Zn on Cu foil for high-performance lithium metal batteries. Rare Met. 2022, 41, 1255–1264. [Google Scholar] [CrossRef]
- Jung, W.-B.; Chae, O.B.; Kim, M.; Kim, Y.; Hong, Y.J.; Kim, J.Y.; Choi, S.; Kim, D.Y.; Moon, S.; Suk, J.; et al. Effect of Highly Periodic Au Nanopatterns on Dendrite Suppression in Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2021, 13, 60978–60986. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, X.-R.; Chen, X.; Cheng, X.-B.; Zhang, X.-Q.; Yan, C.; Zhang, Q. Lithiophilic Sites in Doped Graphene Guide Uniform Lithium Nucleation for Dendrite-Free Lithium Metal Anodes. Angew. Chem. Int. Ed. 2017, 56, 7764–7768. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.-J.; Wang, J.; Liu, F.; Cao, Y.; Deng, B.; Li, J.; Xie, H.; Zhang, J.; Tong, H.; Liang, C. Self-supporting heteroatomic S/N co-doped carbon scaffold for robust lithium metal anodes. Carbon 2025, 236, 120106. [Google Scholar] [CrossRef]
- Li, P.; Liu, Y.; Bao, X.; Xie, J.; Li, Z.; Li, H.; Ren, Q.; Feng, X.; Hu, Y.; Ma, Y. Nitrogen and fluorine co-doped graphene for ultra-stable lithium metal anodes. Nano Res. 2024, 17, 7212–7220. [Google Scholar] [CrossRef]
- Patrike, A.; Karbhal, I.; Wasnik, K.; Torris, A.; Maibam, A.; Krishnamurty, S.; Shelke, M.V. High rate, high temperature, dendrite free plating/stripping of Li in 3-dimensional honeycomb boron carbon nitride to realize an ultrastable lithium metal anode. J. Energy Storage 2023, 68, 107547. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Wei, H.-J.; Li, T.-F.; Xiong, X.-H.; Wei, S.-Z.; Ren, F.-Z.; Volinsky, A.A. Recent advances and perspective in metal coordination materials-based electrode materials for potassium-ion batteries. Rare Met. 2021, 40, 448–470. [Google Scholar] [CrossRef]
- Cheng, N.-C.; Wu, Y.C.; Chu, Y.-C.; Hsu, H.-Y.; Chen, W.-C.; Wang, P.-H.; Chang, T.-L.; Chang, J.-K.; Wang, C.-Y. A modulated MOF as a modification layer on copper foil for lithium dendrite suppression. J. Mater. Chem. A 2024, 12, 8474–8486. [Google Scholar] [CrossRef]
- Zhuang, Z.; Zhang, F.; Gandla, D.; Jadhav, V.V.; Liu, Z.; Hu, L.; Lu, F.; Tan, D.Q. Metal–Organic Framework-Derived ZnO, N Dually Doped Nanocages as an Efficient Host for Stable Li Metal Anodes. ACS Appl. Mater. Interfaces 2023, 15, 38530–38539. [Google Scholar] [CrossRef]
- Zhou, T.; Shen, J.; Wang, Z.; Liu, J.; Hu, R.; Ouyang, L.; Feng, Y.; Liu, H.; Yu, Y.; Zhu, M. Regulating Lithium Nucleation and Deposition via MOF-Derived Co@C-Modified Carbon Cloth for Stable Li Metal Anode. Adv. Funct. Mater. 2020, 30, 1909159. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Yun, J.; Choi, S.H.; Han, S.A.; Moon, J.; Kim, J.H.; Lee, J.-W.; Park, M.-S. Functionality of Dual-Phase Lithium Storage in a Porous Carbon Host for Lithium-Metal Anode. Adv. Funct. Mater. 2020, 30, 1910538. [Google Scholar] [CrossRef]
- Lee, J.; Choi, S.H.; Qutaish, H.; Hyeon, Y.; Han, S.A.; Heo, Y.-U.; Whang, D.; Lee, J.-W.; Moon, J.; Park, M.-S.; et al. Structurally stabilized lithium-metal anode via surface chemistry engineering. Energy Storage Mater. 2021, 37, 315–324. [Google Scholar] [CrossRef]
- Lin, J.; Chen, Z.; Zhao, W.; Han, J.; Chen, B.; Chen, Y.; Liu, Q.; Wu, H.B. A 3D mixed ion-electron conducting framework for dendrite-free lithium metal anodes. Nanoscale 2025, 17, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Seo, H.Y.; Senthamaraikannan, T.G.; Cho, J.S.; Kang, Y.C.; Lim, D.-H.; Park, G.D. One-step synthesis of zinc oxide-carbon microspheres decorated with multi-voids and carbon nanotubes via spray pyrolysis for enhanced stability in lithium metal anodes. J. Mater. Sci. Technol. 2024, 192, 95–107. [Google Scholar] [CrossRef]
- Sun, X.; Man, J.; Liu, K.; Liu, W.; Sun, J.; Zhang, N.; Zhou, Y.; Geng, Z.; Li, S.; Sun, J. Uniform lithium deposition enabled by a carbon nanotubes framework modified with nanosized ZIF-8 particles for dendrite-free lithium metal anode. Appl. Surf. Sci. 2023, 616, 156474. [Google Scholar] [CrossRef]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application to CO2 Capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef]
- Li, Z.; He, H.; Cao, H.; Sun, S.; Diao, W.; Gao, D.; Lu, P.; Zhang, S.; Guo, Z.; Li, M.; et al. Atomic Co/Ni dual sites and Co/Ni alloy nanoparticles in N-doped porous Janus-like carbon frameworks for bifunctional oxygen electrocatalysis. Appl. Catal. B Environ. 2019, 240, 112–121. [Google Scholar] [CrossRef]
- Li, Y.; Wang, G.; Yu, H.; Liu, F.; Miao, L.; Wang, L.; Song, Y. Yolk-shell structured ZnO@N-doped carbon with controllable cavity as anode of lithium ion battery. J. Energy Storage 2025, 109, 115143. [Google Scholar] [CrossRef]
- Sun, J.; Li, P.; Cheng, Z.; Tang, C.; Du, A.; Zhang, H. Bacteria-Derived Carbon Composite Anode for Highly Durable Lithium-Ion Storage Enabled by Heteroatom Doping and Pore Construction. Adv. Funct. Mater. 2025, 2500154. [Google Scholar] [CrossRef]
- Zhao, J.; Konh, M.; Teplyakov, A. Surface chemistry of thermal dry etching of cobalt thin films using hexafluoroacetylacetone (hfacH). Appl. Surf. Sci. 2018, 455, 438–445. [Google Scholar] [CrossRef]
- Zang, W.; Sumboja, A.; Ma, Y.; Zhang, H.; Wu, Y.; Wu, S.; Wu, H.; Liu, Z.; Guan, C.; Wang, J.; et al. Single Co Atoms Anchored in Porous N-Doped Carbon for Efficient Zinc−Air Battery Cathodes. ACS Catal. 2018, 8, 8961–8969. [Google Scholar] [CrossRef]
- Kim, J.; Young, C.; Lee, J.; Heo, Y.-U.; Park, M.-S.; Hossain, M.S.A.; Yamauchi, Y.; Kim, J.H. Nanoarchitecture of MOF-derived nanoporous functional composites for hybrid supercapacitors. J. Mater. Chem. A 2017, 5, 15065–15072. [Google Scholar] [CrossRef]
- Adams, B.D.; Zheng, J.; Ren, X.; Xu, W.; Zhang, J.-G. Accurate Determination of Coulombic Efficiency for Lithium Metal Anodes and Lithium Metal Batteries. Adv. Energy Mater. 2018, 8, 1702097. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Wei, H.; Ren, X.; Zhang, J.; Jiang, A.; Liu, Y.; Ren, F. MOF-Derived Hollow Dodecahedral Carbon Structures with Abundant N Sites and Co Nanoparticle-Modified Cu Foil for Dendrite-Free Lithium Metal Battery. Coatings 2025, 15, 490. https://doi.org/10.3390/coatings15040490
Wang F, Wei H, Ren X, Zhang J, Jiang A, Liu Y, Ren F. MOF-Derived Hollow Dodecahedral Carbon Structures with Abundant N Sites and Co Nanoparticle-Modified Cu Foil for Dendrite-Free Lithium Metal Battery. Coatings. 2025; 15(4):490. https://doi.org/10.3390/coatings15040490
Chicago/Turabian StyleWang, Fei, Huijie Wei, Xinyuan Ren, Junle Zhang, Aiyun Jiang, Yong Liu, and Fengzhang Ren. 2025. "MOF-Derived Hollow Dodecahedral Carbon Structures with Abundant N Sites and Co Nanoparticle-Modified Cu Foil for Dendrite-Free Lithium Metal Battery" Coatings 15, no. 4: 490. https://doi.org/10.3390/coatings15040490
APA StyleWang, F., Wei, H., Ren, X., Zhang, J., Jiang, A., Liu, Y., & Ren, F. (2025). MOF-Derived Hollow Dodecahedral Carbon Structures with Abundant N Sites and Co Nanoparticle-Modified Cu Foil for Dendrite-Free Lithium Metal Battery. Coatings, 15(4), 490. https://doi.org/10.3390/coatings15040490





