Aluminum Oxide Coatings as Nanoadsorbents for the Treatment of Effluents Colored with Eriochrome Black T
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the Nanoporous Anodic Aluminum Oxide
2.2. Preparation of EBT Dye Solutions
2.3. Characterization of AAO Coatings
2.4. Adsorption Assays
2.4.1. Preliminary Studies
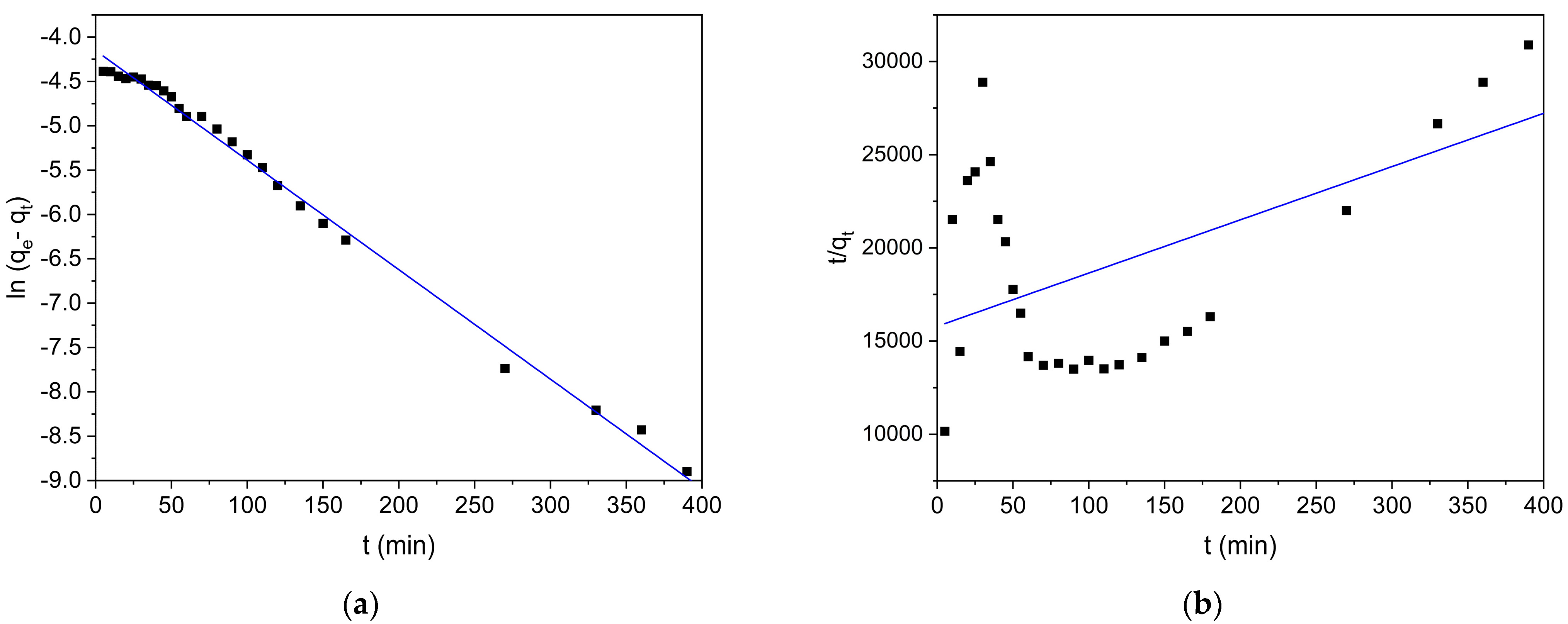
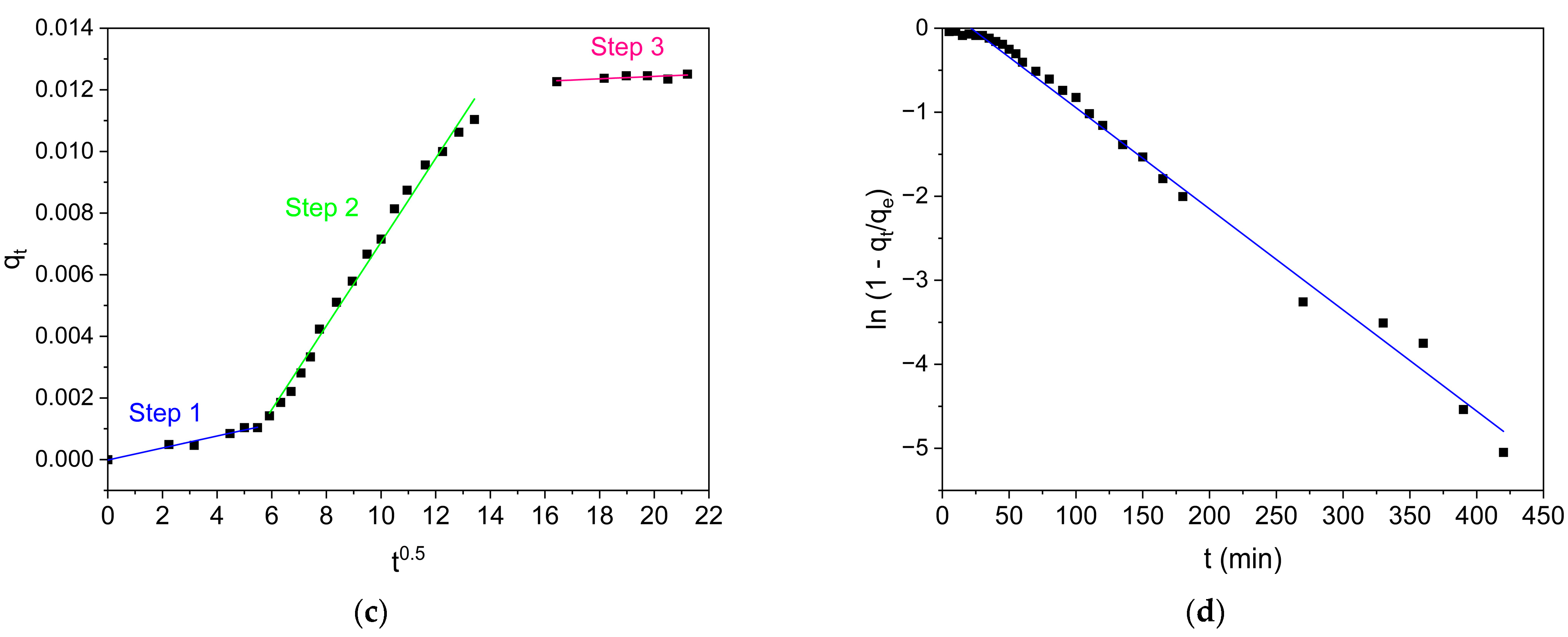
2.4.2. Kinetic Adsorption Models
2.4.3. Isotherm Adsorption Models
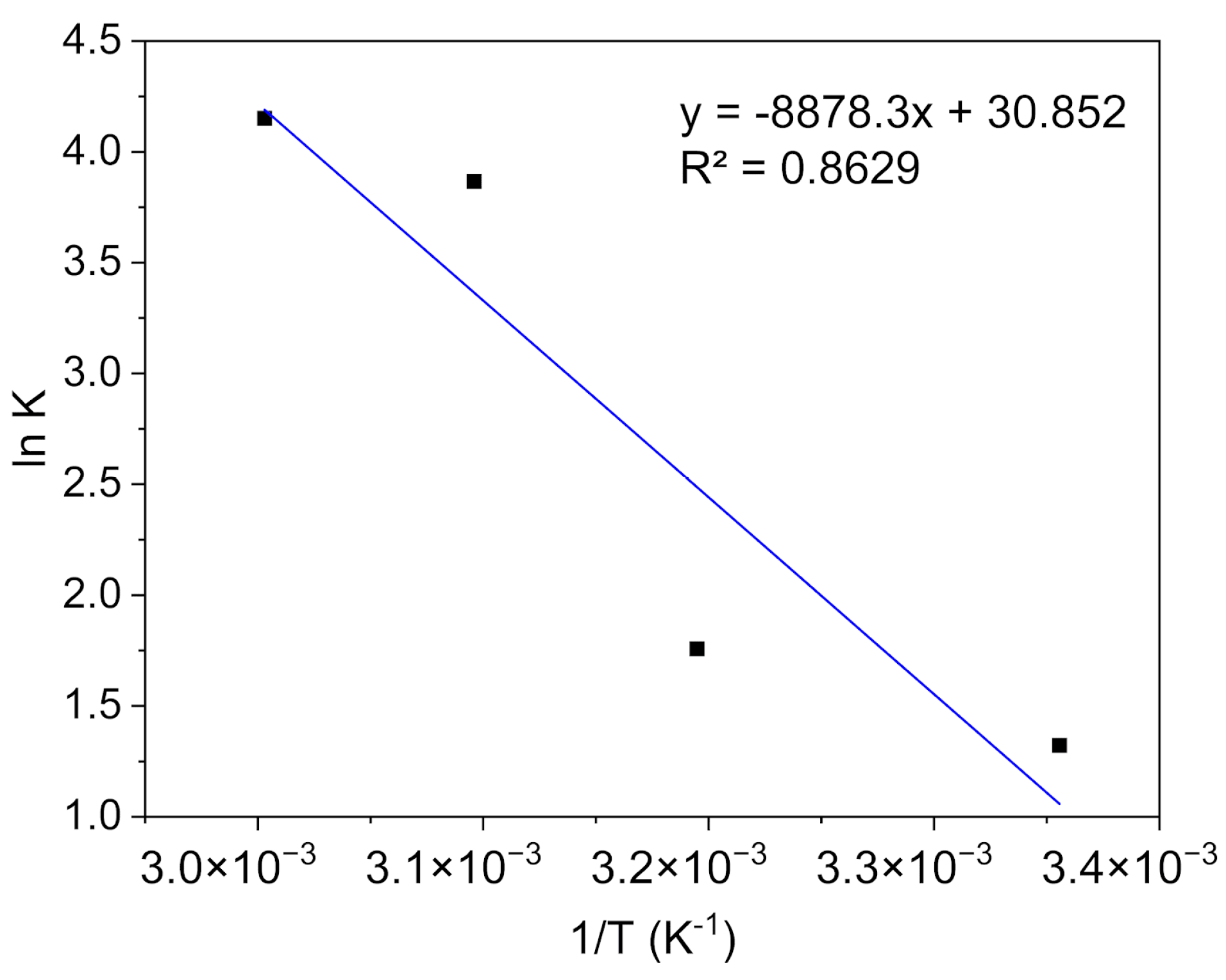
2.4.4. Thermodynamic Studies
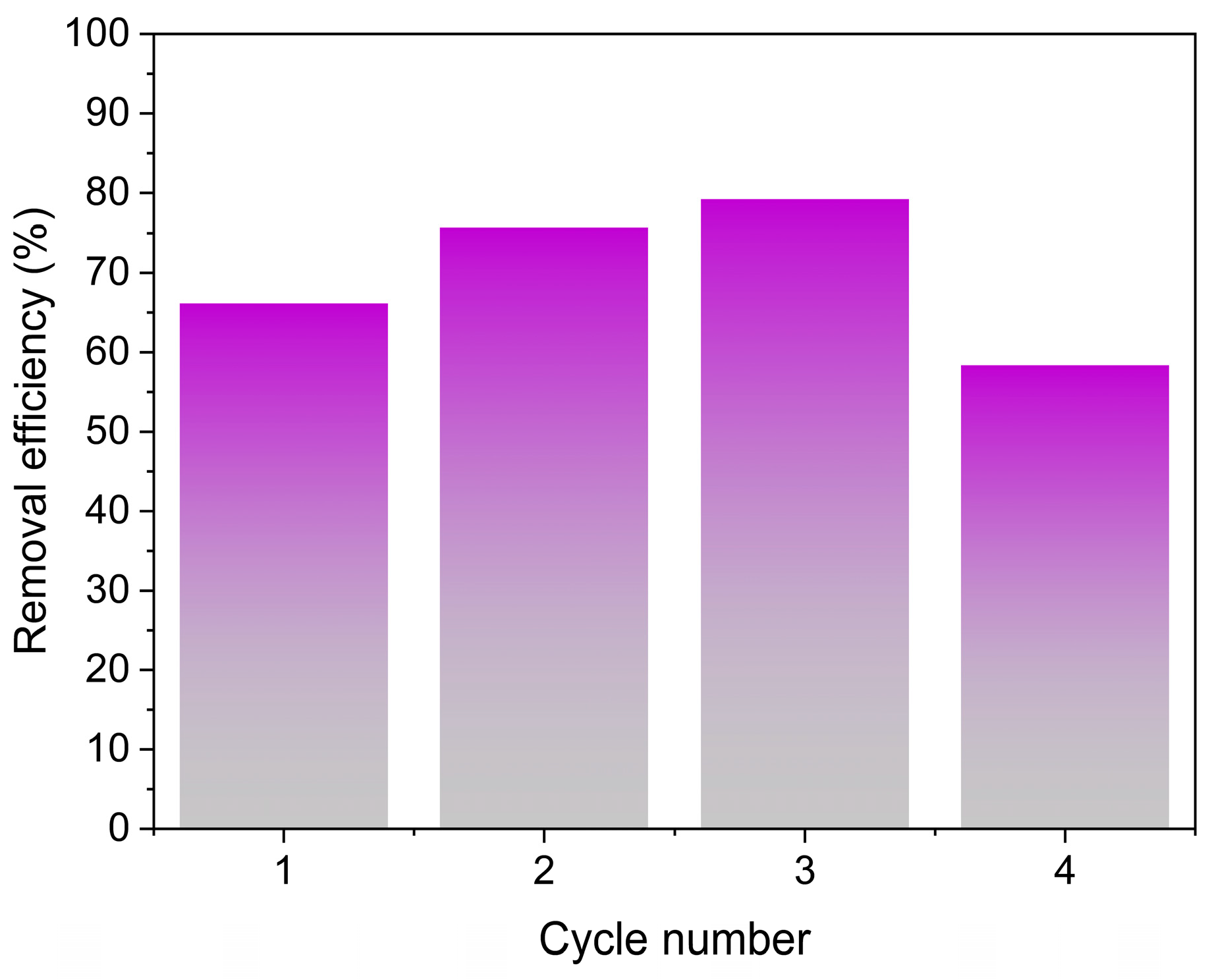
2.4.5. Reuse Performance
3. Results and Discussion
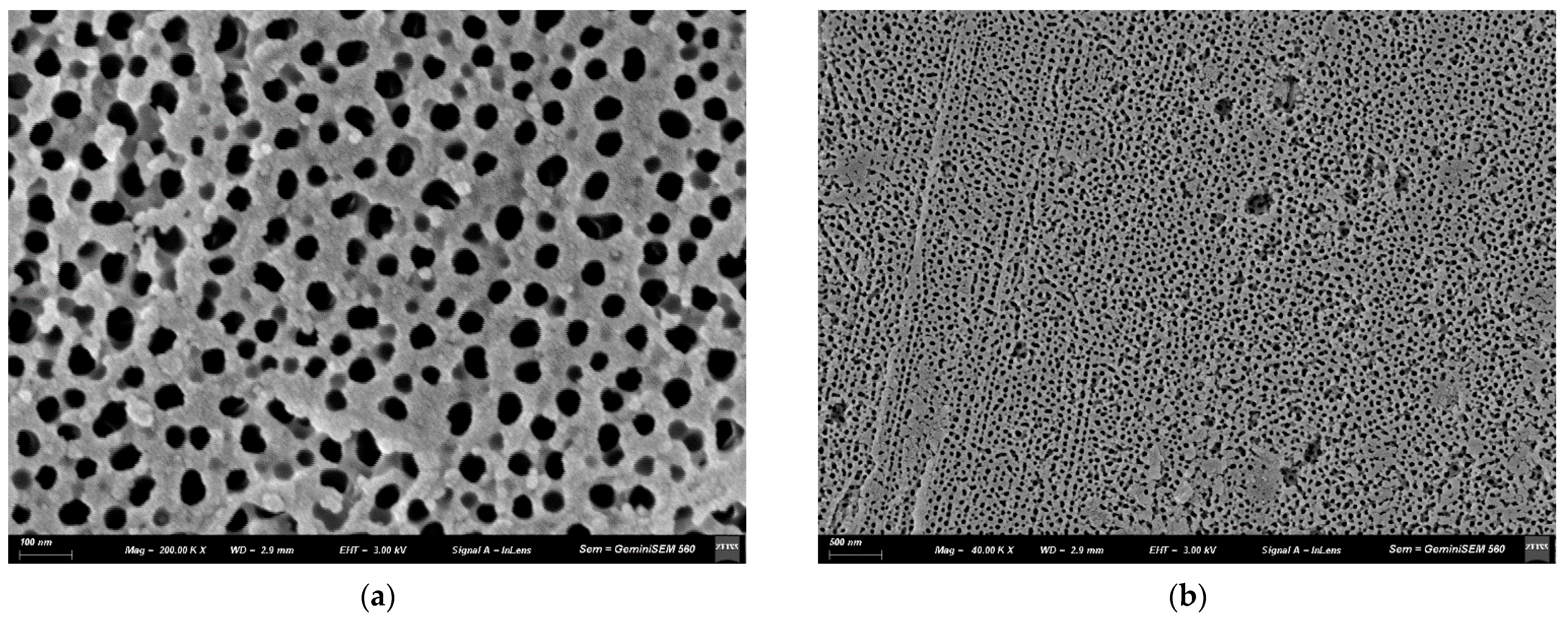
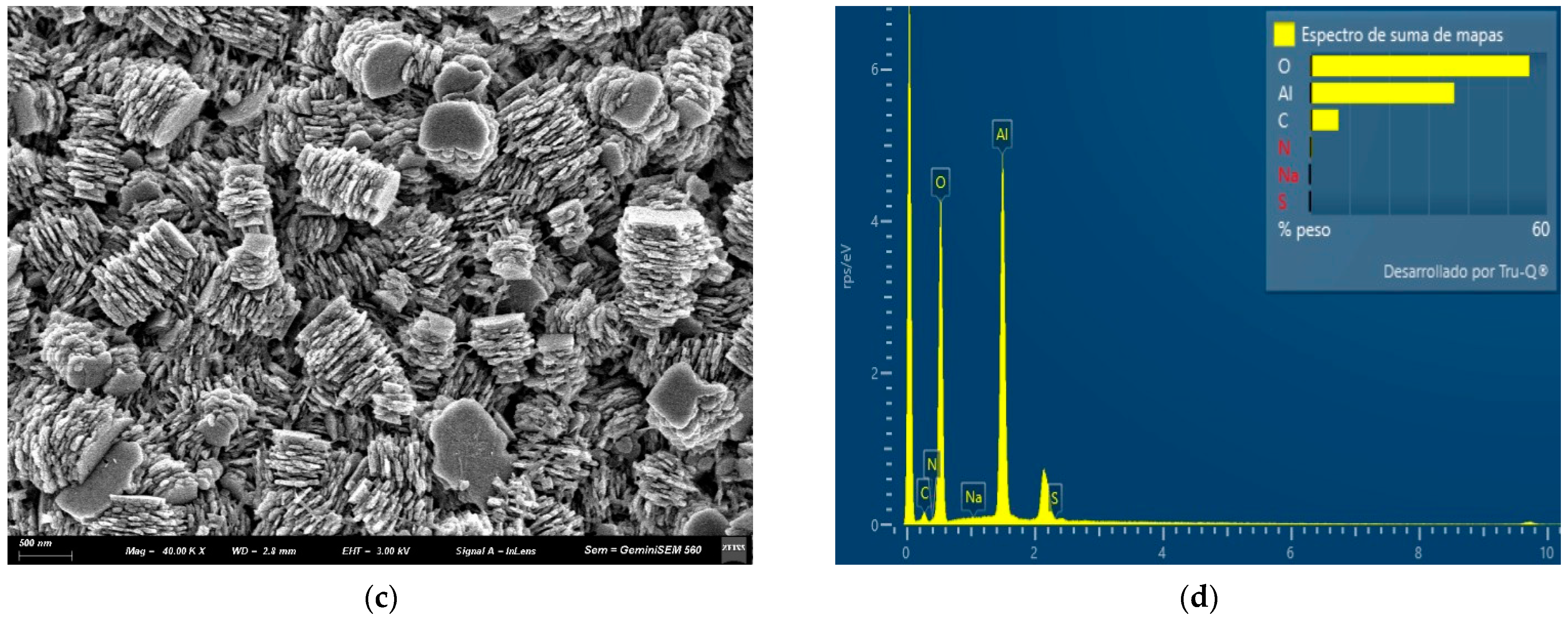
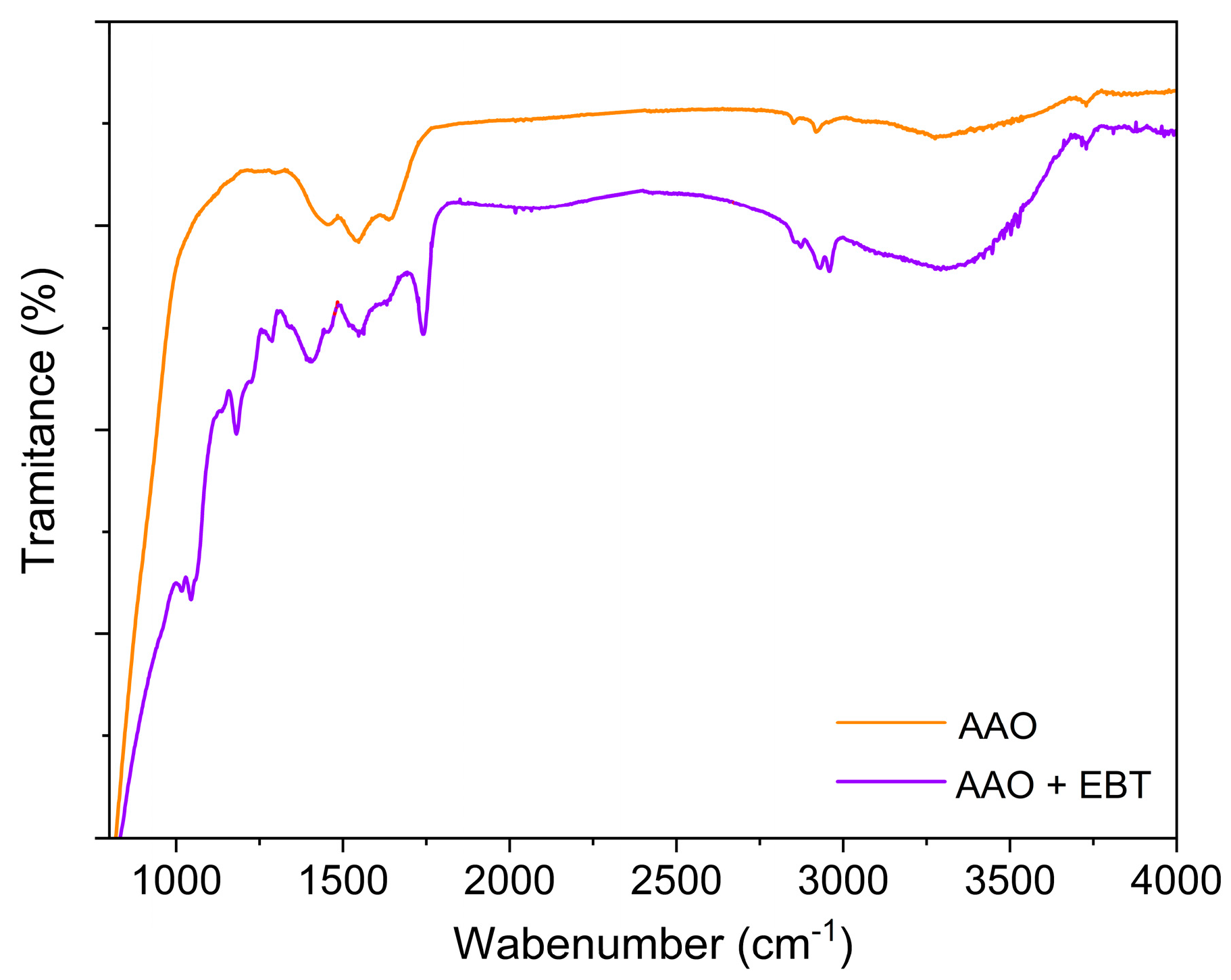
3.1. Characteristic of AAO Films
3.2. Adsorption Studies
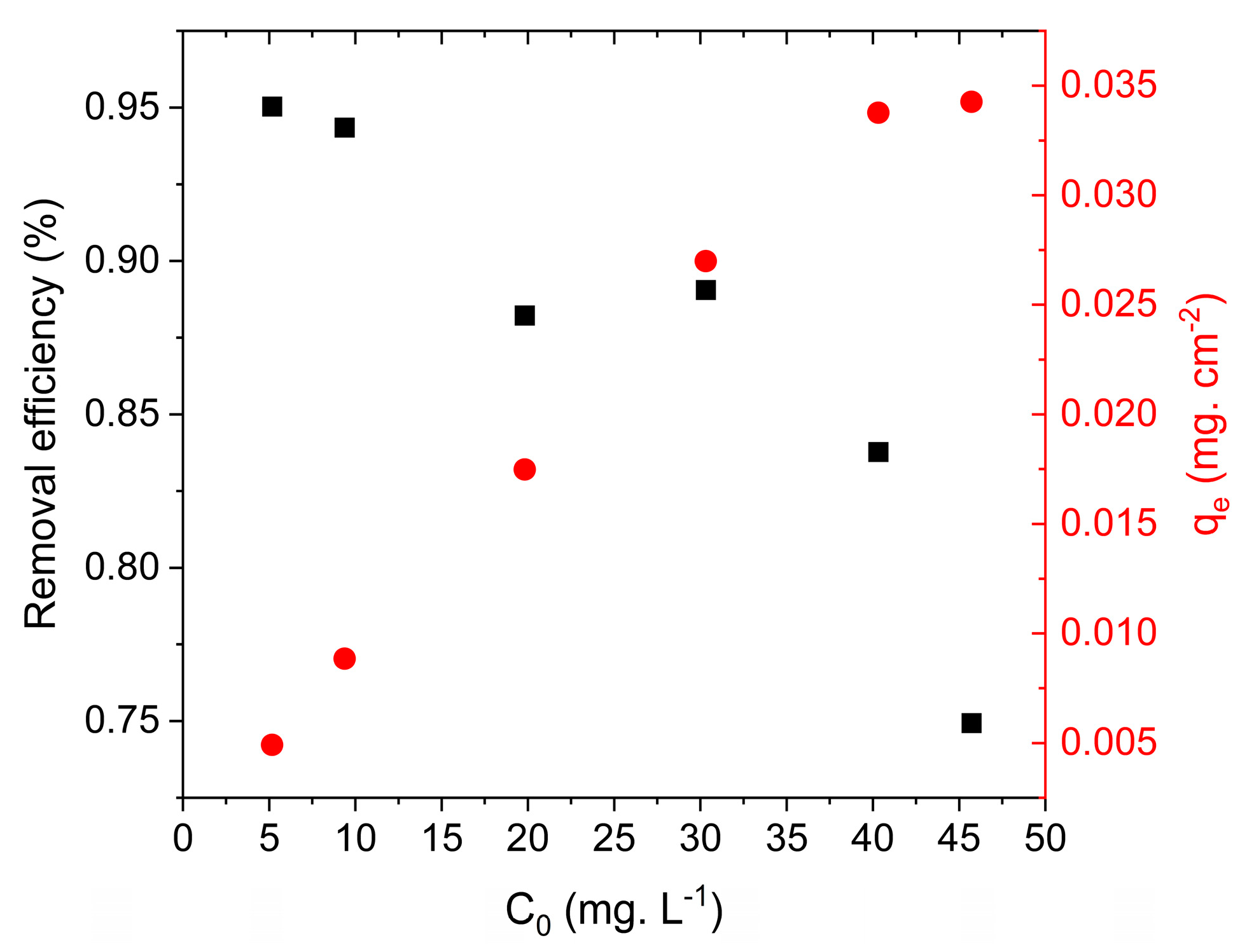
3.2.1. Influence of Agitation, Initial Concentration, and Temperature on EBT Adsorption
3.2.2. Adsorption Kinetics
3.2.3. Adsorption Isotherm
3.2.4. Thermodynamic Analysis of the Process
3.2.5. Adsorbent Reuse
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAO | Anodic aluminum oxide |
| EBT | Eriochrome black T |
| PD | Pore Diameter |
| D | Pore density |
| P | Porosity |
| SEM | Scanning electron microscopy |
| EDS | Energy-dispersive spectrometer |
| T | Thickness |
| PFO | Pseudo first-order model |
| PSO | Pseudo second-order model |
| IPD | Intraparticle diffusion model |
| ED | Boyd’s external diffusion model |
References
- Solmaz, A.; Sari, Z.A.; Karta, M.; Turna, T.; Yücel, A.; Depci, T. Production and Characterization of Activated Carbon from Pomegranate Peel for Pharmaceutical Waste (Paracetamol) Removal: Response Surface Methodology Application. Water Air Soil Poll. 2023, 234, 645. [Google Scholar] [CrossRef]
- Solmaz, A. Adsorption of Methylene Blue and Eriochrome Black T onto Pinecone Powders (Pinus Nigra Arn.): Equilibrium, Kinetics, and Thermodynamic Studies. Processes 2024, 12, 2044. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A State-of-the-Art Review on Wastewater Treatment Techniques: The Effectiveness of Adsorption Method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- Diorio, L.A.; Fréchou, D.M.S.; Levin, L.N. Removal of Dyes by Immobilization of Trametes Versicolor in a Solid-State Micro-Fermentation System. Rev. Argent. Microbiol. 2021, 53, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Hoppen, M.I.; Carvalho, K.Q.; Ferreira, R.C.; Passig, F.H.; Pereira, I.C.; Rizzo-Domingues, R.C.P.; Lenzi, M.K.; Bottini, R.C.R. Adsorption and Desorption of Acetylsalicylic Acid onto Activated Carbon of Babassu Coconut Mesocarp. J. Environ. Chem. Eng. 2019, 7, 102862. [Google Scholar] [CrossRef]
- Slama, H.B.; Chenari Bouket, A.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Gupta, V.K.; Kumar, R.; Nayak, A.; Saleh, T.A.; Barakat, M.A. Adsorptive Removal of Dyes from Aqueous Solution onto Carbon Nanotubes: A Review. Adv. Colloid Interface Sci. 2013, 193–194, 24–34. [Google Scholar] [CrossRef]
- Yiğit Avdan, Z.; Demirtaş, İ.; Suvacı, E. Improved Photocatalytic Degradation of Methylene Blue by Novel Hexagonal ZnO Particles. Water SA 2024, 50, 392–403. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of Dyes in Textile Effluent: A Critical Review on Current Treatment Technologies with a Proposed Alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Kusumlata; Ambade, B.; Kumar, A.; Gautam, S. Sustainable Solutions: Reviewing the Future of Textile Dye Contaminant Removal with Emerging Biological Treatments. Limnol. Rev. 2024, 24, 126–149. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and Its Removal from Aqueous Solution by Adsorption: A Review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Sarkar, S.; Banerjee, A.; Halder, U.; Biswas, R.; Bandopadhyay, R. Degradation of Synthetic Azo Dyes of Textile Industry: A Sustainable Approach Using Microbial Enzymes. Water Conserv. Sci. Eng. 2017, 2, 121–131. [Google Scholar] [CrossRef]
- Zafar, S.; Bukhari, D.A.; Rehman, A. Azo Dyes Degradation by Microorganisms—An Efficient and Sustainable Approach. Saudi J. Biol. Sci. 2022, 29, 103437. [Google Scholar] [CrossRef]
- Khalid, A.; Zubair, M.; Ihsanullah. A Comparative Study on the Adsorption of Eriochrome Black T Dye from Aqueous Solution on Graphene and Acid-Modified Graphene. Arab. J. Sci. Eng. 2018, 43, 2167–2179. [Google Scholar] [CrossRef]
- El Gaayda, J.; Akbour, R.A.; Titchou, F.E.; Afanga, H.; Zazou, H.; Swanson, C.; Hamdani, M. Uptake of an Anionic Dye from Aqueous Solution by Aluminum Oxide Particles: Equilibrium, Kinetic, and Thermodynamic Studies. Groundw. Sustain. Dev. 2021, 12, 100540. [Google Scholar] [CrossRef]
- Wong, S.; Ghafar, N.A.; Ngadi, N.; Razmi, F.A.; Inuwa, I.M.; Mat, R.; Amin, N.A.S. Effective Removal of Anionic Textile Dyes Using Adsorbent Synthesized from Coffee Waste. Sci. Rep. 2020, 10, 2928. [Google Scholar] [CrossRef] [PubMed]
- Ballou, I.; Naja, J.; Bakher, Z.; Kholtei, S. Adsorption of Eriochrome Black T on Pseudo Boehmite and Gamma Alumina Synthesized from Drinking Water Treatment Sludge: A Waste-to-Recycling Approach. Recycling 2024, 9, 49. [Google Scholar] [CrossRef]
- Abhinaya, M.; Parthiban, R.; Kumar, P.S.; Vo, D.-V.N. A Review on Cleaner Strategies for Extraction of Chitosan and Its Application in Toxic Pollutant Removal. Environ. Res. 2021, 196, 110996. [Google Scholar] [CrossRef]
- Mittal, A.; Gupta, V.K. Adsorptive Removal and Recovery of the Azo Dye Eriochrome Black T. Environ. Toxicol. Chem. 2010, 92, 1813–1823. [Google Scholar] [CrossRef]
- Khan, A.; Ju, P.; Han, Z.; Ni, C. A Comprehensive Review on Adsorptive Removal of Azo Dyes Using Functional Materials. AQUA Water Infrastruct. Ecosyst. Soc. 2024, 73, 266–285. [Google Scholar] [CrossRef]
- Sardar, M.; Manna, M.; Maharana, M.; Sen, S. Remediation of Dyes from Industrial Wastewater Using Low-Cost Adsorbents. In Green Adsorbents to Remove Metals, Dyes and Boron from Polluted Water; Inamuddin, A.M.I., Lichtfouse, E., Asiri, A.M., Eds.; Environmental Chemistry for a Sustainable World; Springer International Publishing: Cham, Switzerland, 2021; Volume 49, pp. 377–403. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Isotherm Models: Classification, Physical Meaning, Application and Solving Method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, L.C.; De Carvalho, K.Q.; Bassetti, F.J.; Coral, L.A.A. Kinetic, Isothermal and Thermodynamic Studies of Reactive Black 5 Removal Using Rice Husk Ashes and Powdered Activated Carbon. Desalin. Water. Treat. 2024, 320, 100606. [Google Scholar] [CrossRef]
- Fernandes, E.P.; Silva, T.S.; Carvalho, C.M.; Selvasembian, R.; Chaukura, N.; Oliveira, L.M.T.M.; Meneghetti, S.M.P.; Meili, L. Efficient Adsorption of Dyes by γ-Alumina Synthesized from Aluminum Wastes: Kinetics, Isotherms, Thermodynamics and Toxicity Assessment. J. Environ. Chem. Eng. 2021, 9, 106198. [Google Scholar] [CrossRef]
- Wang, B.; Hu, Y.; Xie, L.; Peng, K. Biosorption Behavior of Azo Dye by Inactive CMC Immobilized Aspergillus Fumigatus Beads. Bioresour. Technol. 2008, 99, 794–800. [Google Scholar] [CrossRef]
- Uddin, M.J.; Ampiaw, R.E.; Lee, W. Adsorptive Removal of Dyes from Wastewater Using a Metal-Organic Framework: A Review. Chemosphere 2021, 284, 131314. [Google Scholar] [CrossRef]
- Bruera, F.A.; Kramer, G.R.; Vera, M.L.; Ares, A.E. Synthesis and Morphological Characterization of Nanoporous Aluminum Oxide Films by Using a Single Anodization Step. Coatings 2019, 9, 115. [Google Scholar] [CrossRef]
- Bruera, F.A.; Kramer, G.R.; Vera, M.L.; Ares, A.E. Evaluation of the Influence of Synthesis Conditions on the Morphology of Nanostructured Anodic Aluminum Oxide Coatings on Al 1050. Surf. Interfaces 2020, 18, 100448. [Google Scholar] [CrossRef]
- Bruera, F.A.; Kramer, G.R.; Velázquez, J.E.; Sadañoski, M.A.; Fonseca, M.I.; Ares, A.E.; Zapata, P.D. Laccase Immobilization on Nanoporous Aluminum Oxide for Black Liquor Treatment. Surf. Interfaces 2022, 30, 101879. [Google Scholar] [CrossRef]
- Li, N.; Li, X.; Yang, Y.; Liu, Y.; Zhao, L.; Zhou, Z. Adsorption-Desorption Behavior of Silver Ions on Stainless Steel as a Proxy for Disinfection of Domestic Hot Water. Desalin. Water. Treat. 2019, 151, 230–241. [Google Scholar] [CrossRef]
- Sharififard, H.; Soleimani, M. Performance Comparison of Activated Carbon and Ferric Oxide-Hydroxide–Activated Carbon Nanocomposite as Vanadium (v) Ion Adsorbents. RSC Adv. 2015, 5, 80650–80660. [Google Scholar] [CrossRef]
- Muliwa, A.M.; Oyewo, O.A.; Maity, A. Recent Progress on the Removal of Aqueous Mercury by Carbon-Based Adsorbents: A Review. Inorg. Chem. Commun. 2023, 156, 111207. [Google Scholar] [CrossRef]
- Chatla, A.; Almanassra, I.W.; Kochkodan, V.; Laoui, T.; Alawadhi, H.; Atieh, M.A. Efficient Removal of Eriochrome Black T (EBT) Dye and Chromium (Cr) by Hydrotalcite-Derived Mg-Ca-Al Mixed Metal Oxide Composite. Catalysts 2022, 12, 1247. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Laxmi Gayatri, S. Batch Adsorption of 4-Nitrophenol by Acid Activated Jute Stick Char: Equilibrium, Kinetic and Thermodynamic Studies. Chem. Eng. J. 2010, 158, 173–180. [Google Scholar] [CrossRef]
- Jamhour, R.M.A.Q.; Al-Msiedeen, A.; Al-Faraheed, R.; Esaifan, M.; Jamhour, M. Adsorptive Removal of Hazardous Eriochrome Black T and Its Metal Complexes from Aqueous Media Using Spent Coffee Grounds. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Sangor, F.I.M.S.; Al-Ghouti, M.A. Waste-to-Value: Synthesis of Nano-Aluminum Oxide (Nano-γ-Al2O3) from Waste Aluminum Foils for Efficient Adsorption of Methylene Blue Dye. Case Stud. Chem. Environ. Eng. 2023, 8, 100394. [Google Scholar] [CrossRef]
- Goswami, R.; Goswami, Y.C.; Shahrestasni, M. Synthesis and Characterization of Aluminium Oxide Nano Adsorbents via a Sustainable Combustion Method for Methyl Red Remediation. Rev. Roum. Chim. 2024, 69, 595–603. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Favvas, E.P.; Kostoglou, M.; Mitropoulos, A.C. Effect of Agitation on Batch Adsorption Process Facilitated by Using Nanobubbles. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125440. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Świątkowski, A. The Influence of Different Agitation Techniques on the Adsorption Kinetics of 4-Chlorophenol on Granular Activated Carbon. Reac. Kinet. Mech. Cat. 2015, 116, 261–271. [Google Scholar] [CrossRef]
- Dharmarathna, S.P.; Priyantha, N. Investigation of Boundary Layer Effect of Intra-Particle Diffusion on Methylene Blue Adsorption on Activated Carbon. Energy Nexus 2024, 14, 100294. [Google Scholar] [CrossRef]
- Boudouaia, N.; Bengharez, Z.; Jellali, S. Preparation and Characterization of Chitosan Extracted from Shrimp Shells Waste and Chitosan Film: Application for Eriochrome Black T Removal from Aqueous Solutions. Appl. Water Sci. 2019, 9, 91. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Gulcan, H.O.; Saber-Samandari, S.; Gazi, M. Efficient Removal of Anionic and Cationic Dyes from an Aqueous Solution Using Pullulan-Graft-Polyacrylamide Porous Hydrogel. Water Air Soil Pollut. 2014, 225, 2177. [Google Scholar] [CrossRef]
- Lafi, R.; Montasser, I.; Hafiane, A. Adsorption of Congo Red Dye from Aqueous Solutions by Prepared Activated Carbon with Oxygen-Containing Functional Groups and Its Regeneration. Adsorp. Sci. Technol. 2019, 37, 160–181. [Google Scholar] [CrossRef]
- Bansal, M.; Patnala, P.K.; Dugmore, T. Adsorption of Eriochrome Black-T(EBT) Using Tea Waste as a Low Cost Adsorbent by Batch Studies: A Green Approach for Dye Effluent Treatments. Curr. Res. Green Sustain. Chem. 2020, 3, 100036. [Google Scholar] [CrossRef]
- Chebbi, M.; Ounoki, S.; Youcef, L.; Amrane, A. Synthesis and Characterization of Pine Cones Biochar for the Removal of an Antibiotic (Metronidazole) from Aqueous Solutions. J. Ind. Eng. Chem. 2023, 126, 327–339. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Kinetic Models: Physical Meanings, Applications, and Solving Methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.J.; Theydan, S.K. Microwave Assisted Preparation of Microporous Activated Carbon from Siris Seed Pods for Adsorption of Metronidazole Antibiotic. Chem. Eng. J. 2013, 214, 310–318. [Google Scholar] [CrossRef]
- Ghibate, R.; Senhaji, O.; Taouil, R. Kinetic and Thermodynamic Approaches on Rhodamine B Adsorption onto Pomegranate Peel. Case Stud. Chem. Environ. Eng. 2021, 3, 100078. [Google Scholar] [CrossRef]
- Kecira, Z.; Benturki, O.; Benturki, A.; Daoud, M.; Girods, P. High Adsorption Capacity of Nitrobenzene from Aqueous Solution Using Activated Carbons Prepared from Vegetable Waste. Env. Prog. Sustain. Energy 2020, 39, e13463. [Google Scholar] [CrossRef]
- Randhawa, N.S.; Das, N.N.; Jana, R.K. Adsorptive Remediation of Cu(II) and Cd(II) Contaminated Water Using Manganese Nodule Leaching Residue. Desalin. Water Treat. 2014, 52, 4197–4211. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, D.; Zheng, Y.; Liang, S.; Liu, Z. Sorption Isotherm and Kinetic Modeling of Aniline on Cr-Bentonite. J. Hazard. Mater. 2009, 167, 141–147. [Google Scholar] [CrossRef]
- El Amri, A.; Bensalah, J.; Kadiri, L.; Essaadaoui, Y.; Lebkiri, I.; Zarrouk, A.; Rifi, E.H.; Lebkiri, A. Investigation of the Cellulose Reed Plant as a Potential Adsorbent to Remove Pb(II): Equilibrium Isotherms and Thermodynamic Studies. Desalin. Water Treat. 2022, 262, 137–151. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, S.; Kumar, S.; Kumar, A. Development of Parthenium Based Activated Carbon and Its Utilization for Adsorptive Removal of P-Cresol from Aqueous Solution. J. Hazard. Mater. 2008, 155, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Ifelebuegu, A.O. Removal of Steriod Hormones by Activated Carbon Adsorption—Kinetic and Thermodynamic Studies. J. Environ. Prot. 2012, 03, 469–475. [Google Scholar] [CrossRef]
- Jasper, E.E.; Ajibola, V.O.; Onwuka, J.C. Nonlinear Regression Analysis of the Sorption of Crystal Violet and Methylene Blue from Aqueous Solutions onto an Agro-Waste Derived Activated Carbon. Appl. Water Sci. 2020, 10, 132. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Zhang, X.; Chen, C. Powdered Activated Carbon Adsorption of Two Fishy Odorants in Water: Trans, Trans-2,4-Heptadienal and Trans,Trans-2,4-Decadienal. J. Environ. Sci. Int. 2015, 32, 15–25. [Google Scholar] [CrossRef]
- Treybal, R.E. Mass Transfer Operations, 3rd ed.; McGraw Hill: New York, NY, USA, 1980. [Google Scholar]
- Carvalho, M.; Duque, A.; Goncalves, I.; Castro, P. Adsorption of Fluorobenzene onto Granular Activated Carbon: Isotherm and Bioavailability Studies. Bioresour. Technol. 2007, 98, 3424–3430. [Google Scholar] [CrossRef]
- Lutfullah; Rashid, M.; Haseen, U.; Rahman, N. An Advanced Cr(III) Selective Nano-Composite Cation Exchanger: Synthesis, Characterization and Sorption Characteristics. J. Ind. Eng. Chem. 2014, 20, 809–817. [Google Scholar] [CrossRef]
- Labaali, Z.; Kholtei, S.; Naja, J. Co2+ Removal from Wastewater Using Apatite Prepared through Phosphate Waste Rocks Valorization: Equilibrium, Kinetics and Thermodynamics Studies. Sci. Afr. 2020, 8, e00350. [Google Scholar] [CrossRef]
- Asoh, H.; Fukumoto, S.; Shishido, K.; Hagiwara, K. Anodization of Aluminum in a Sodium Hydroxide Solution: Effect of pH on Chemical Dissolution. J. Electrochem. Soc. 2024, 171, 123504. [Google Scholar] [CrossRef]


| Models | Linear Form |
|---|---|
| Lagergren Pseudo-first-order | |
| Ho-McKay Pseudo-second-order | |
| Weber-Morris Intra-particle diffusion | |
| Boyd’s external diffusion |
| Models | Linear Form |
|---|---|
| Freundlich | |
| Temkin | |
| Langmuir |
| Models | Parameters | |
|---|---|---|
| Pseudo-first-order | 0.9912 | |
| 0.0120 | ||
| 0.0165 | ||
| Pseudo-second-order | 0.3058 | |
| 0.0517 | ||
| 0.0350 | ||
| Intra-particle diffusion * | 0.9629 | |
| 0.0002 | ||
| 0.00005 | ||
| 0.9947 | ||
| 0.0014 | ||
| 0.0072 | ||
| 0.8352 | ||
| 0.0002 | ||
| 0.0082 | ||
| Boyd’s external diffusion | 0.9926 | |
| 0.0122 | ||
| 0.2936 |
| Models | Linear Fitting Formula | Parameters | |
|---|---|---|---|
| Freundlich | 0.5316x − 4.4641 | 0.9626 | |
| 0.0115 | |||
| n | 1.8811 | ||
| Temkin | 0.0084x + 0.0149 | 0.9521 | |
| 5.8932 | |||
| B | 0.0084 | ||
| Langmuir | 24.212x + 50.814 | 0.9805 | |
| 0.0413 | |||
| 0.4764 | |||
| 0.0402–0.2956 |
| T (°C) | (KJ·mol−1) | (KJ·mol−1) | (KJ·mol−1. K−1) |
|---|---|---|---|
| 25 | −3.28 | 73.81 | 0.26 |
| 40 | −4.58 | ||
| 50 | −10.39 | ||
| 60 | −11.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kramer, G.R.; Bruera, F.A.; Zapata, P.D.; Ares, A.E. Aluminum Oxide Coatings as Nanoadsorbents for the Treatment of Effluents Colored with Eriochrome Black T. Coatings 2025, 15, 488. https://doi.org/10.3390/coatings15040488
Kramer GR, Bruera FA, Zapata PD, Ares AE. Aluminum Oxide Coatings as Nanoadsorbents for the Treatment of Effluents Colored with Eriochrome Black T. Coatings. 2025; 15(4):488. https://doi.org/10.3390/coatings15040488
Chicago/Turabian StyleKramer, Gustavo R., Florencia A. Bruera, Pedro Darío Zapata, and Alicia E. Ares. 2025. "Aluminum Oxide Coatings as Nanoadsorbents for the Treatment of Effluents Colored with Eriochrome Black T" Coatings 15, no. 4: 488. https://doi.org/10.3390/coatings15040488
APA StyleKramer, G. R., Bruera, F. A., Zapata, P. D., & Ares, A. E. (2025). Aluminum Oxide Coatings as Nanoadsorbents for the Treatment of Effluents Colored with Eriochrome Black T. Coatings, 15(4), 488. https://doi.org/10.3390/coatings15040488








