Near-Infrared Light-Induced Deep Curing of Thiol–Epoxy Networks Based on Upconversion Photochemistry
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
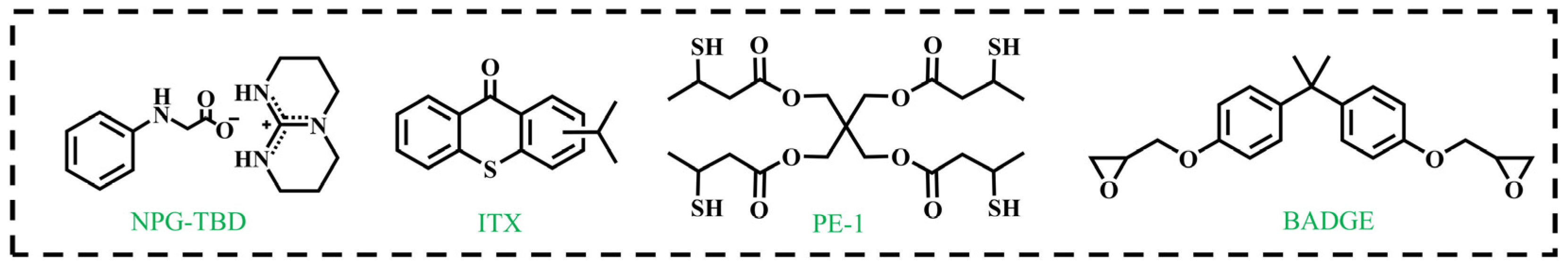
2.2.1. Synthesis of NPG-TBD
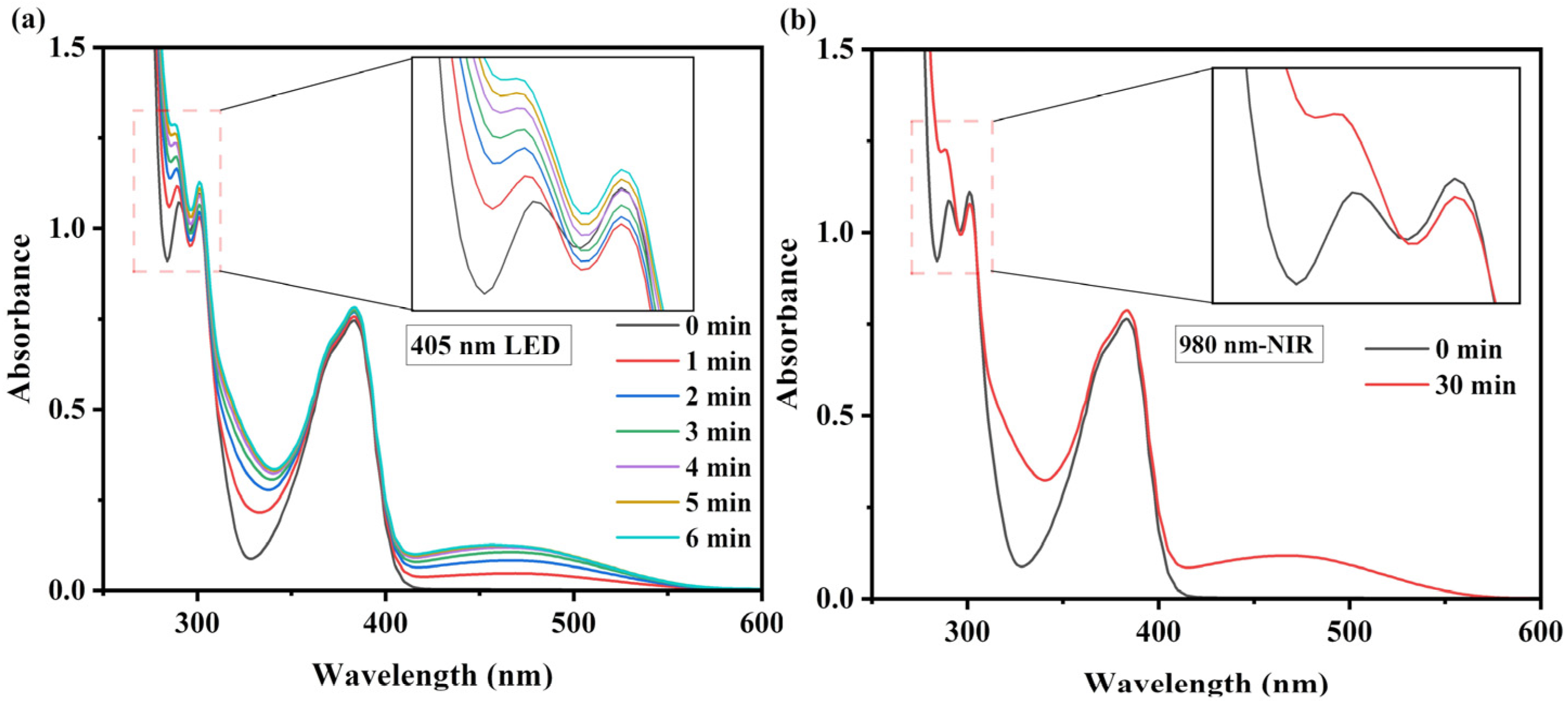
2.2.2. UV-Vis Absorption and Steady-State Photolysis
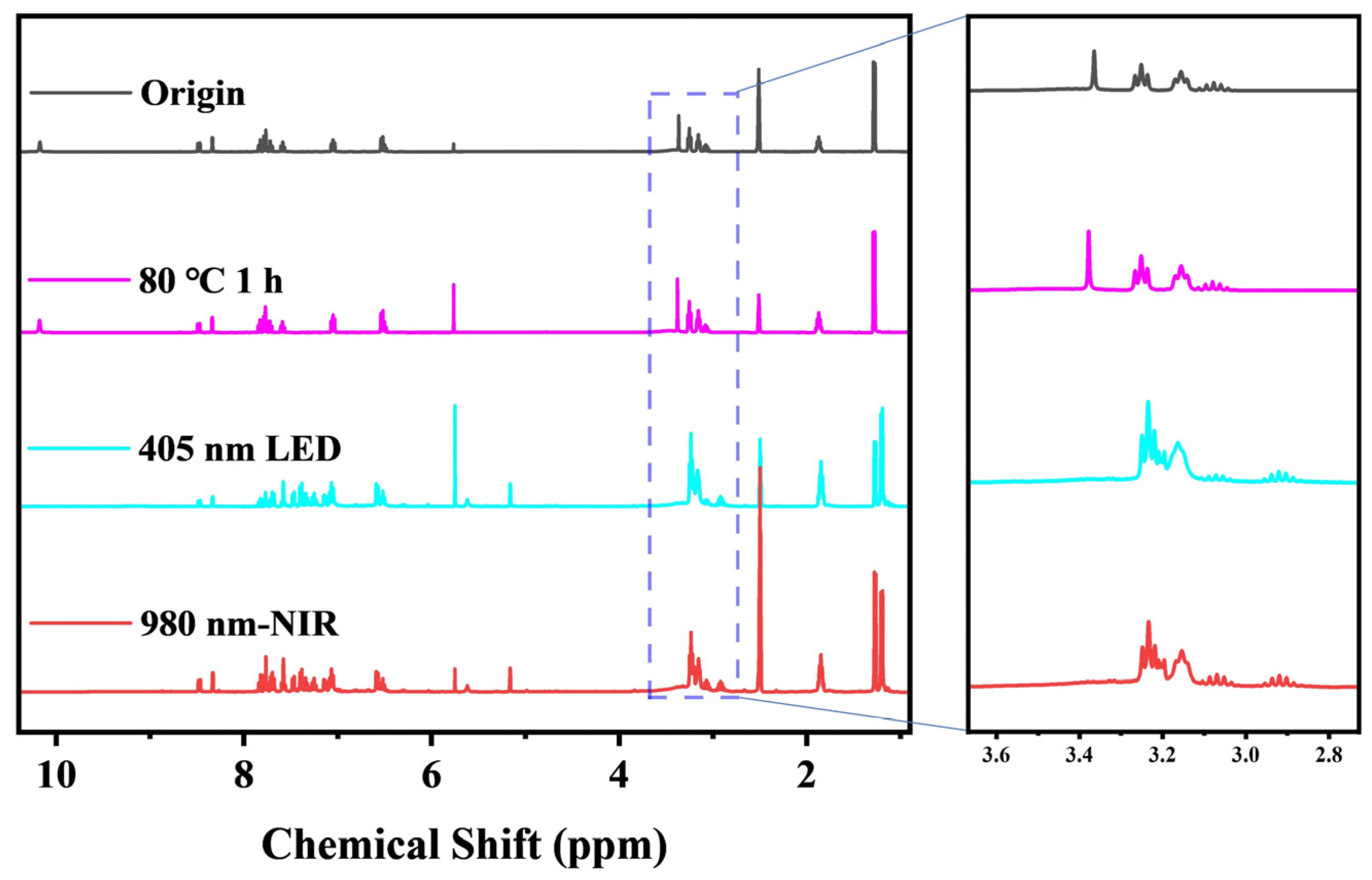
2.2.3. 1H NMR Characterization of Photolysis
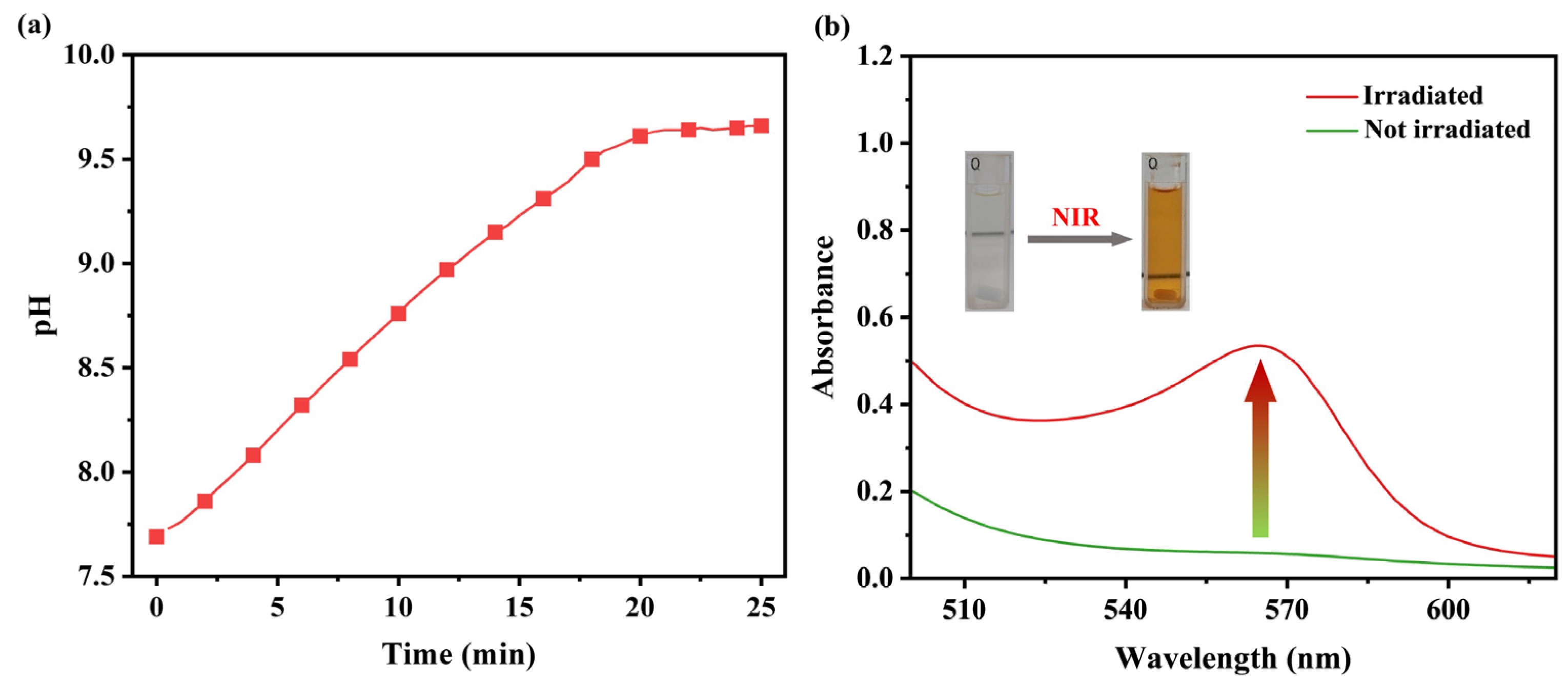
2.2.4. Evaluation of Photo-Induced Base Generation
2.2.5. Formulation of the NIR-Curable Thiol–Epoxy System
2.2.6. Monitoring of Photopolymerization by FTIR
2.2.7. DSC Analysis of Polymerization Kinetics
2.2.8. Deep Photocuring Protocol and Conversion Profiling
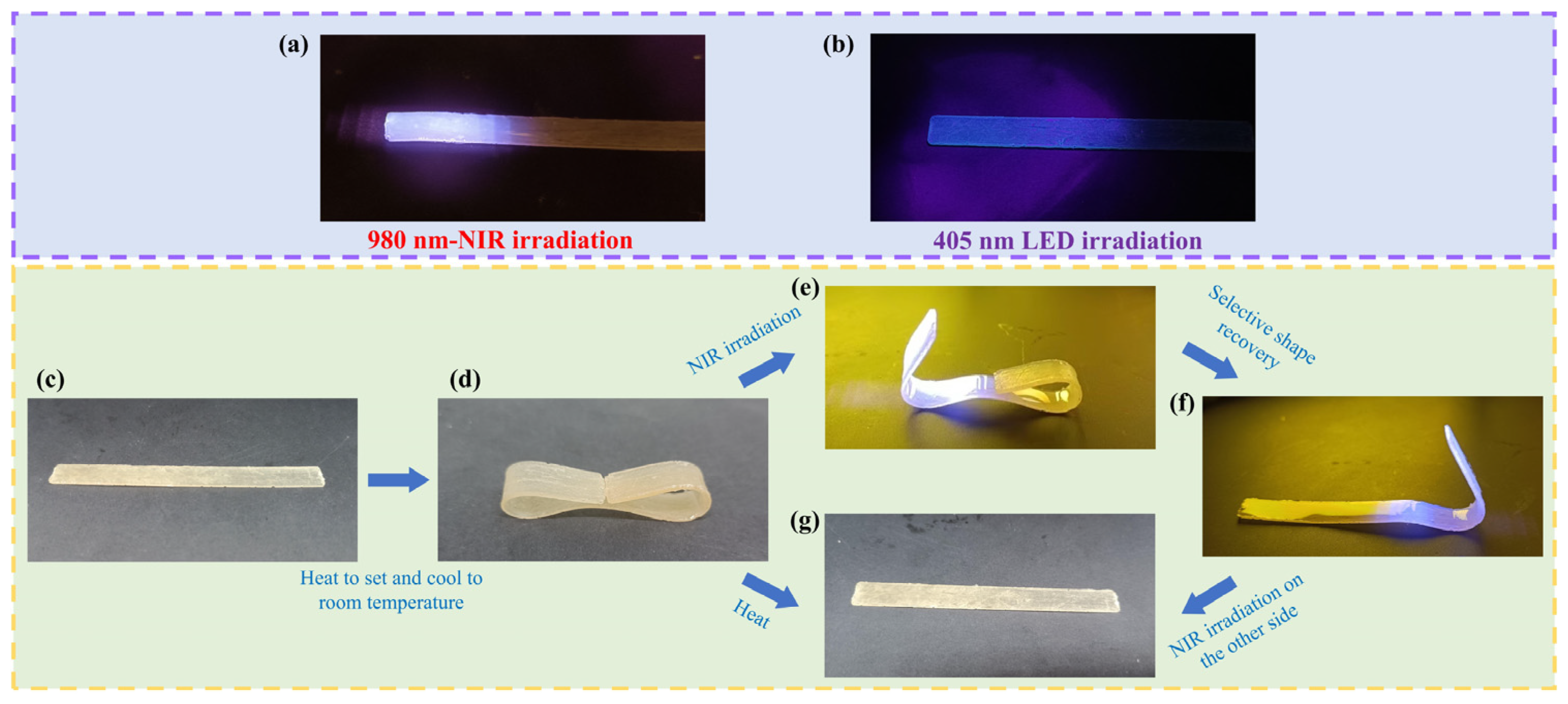
2.2.9. Preparation and Activation of Shape Memory Samples
2.2.10. Coating Application and Performance Evaluation
3. Results and Discussion
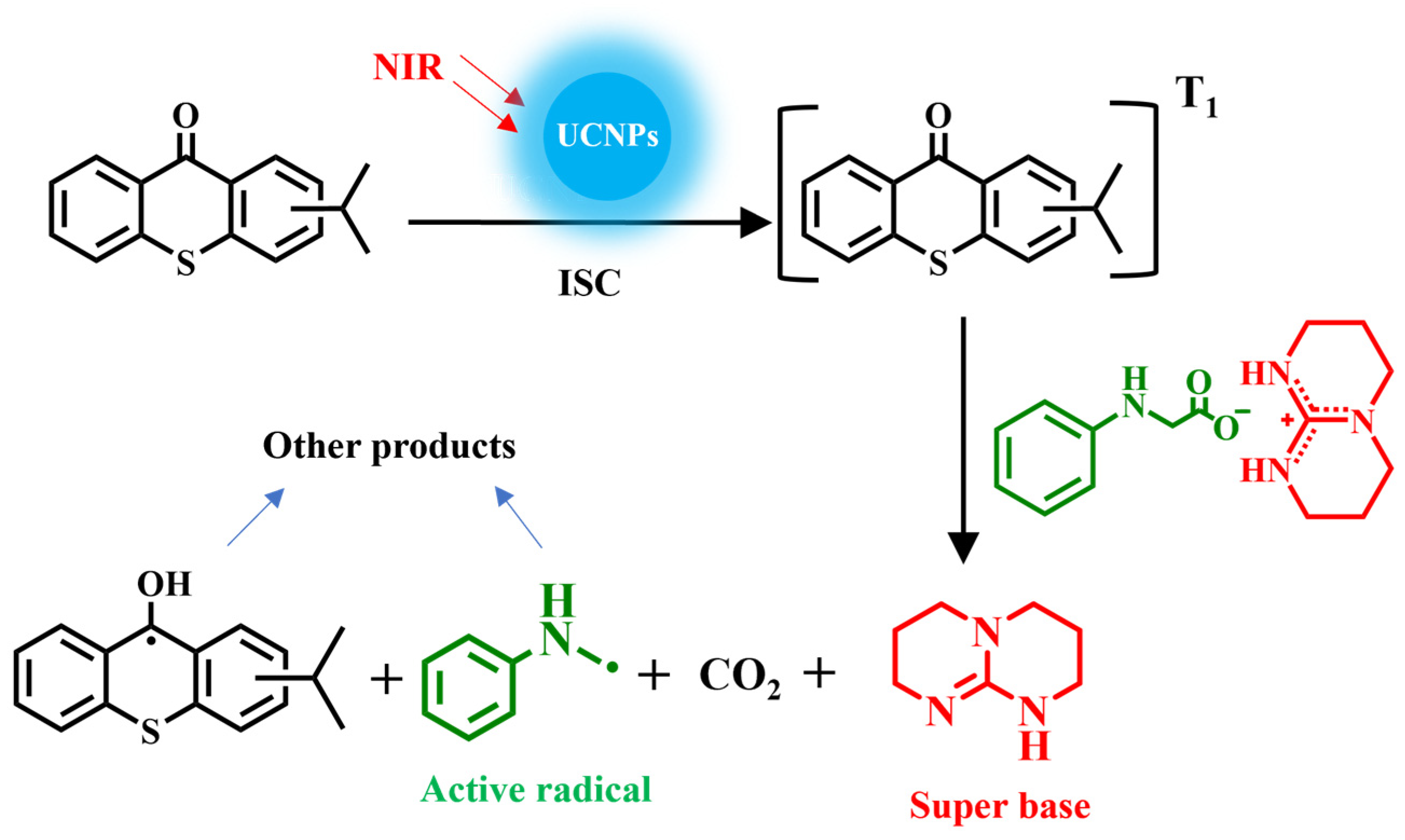
3.1. Design and Characterization of Base-Releasing System
3.2. Investigation of Photo-Induced Base Generation
3.3. Polymerization Study
3.4. Multifunctional Properties of the Polymeric Materials
3.5. Coating Performance Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garra, P.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Photopolymerization Processes of Thick Films and in Shadow Areas: A Review for the Access to Composites. Polym. Chem. 2017, 8, 7088–7101. [Google Scholar] [CrossRef]
- Li, Z.; Chen, H.; Wang, C.; Chen, L.; Liu, J.; Liu, R. Efficient Photopolymerization of Thick Pigmented Systems Using Upconversion Nanoparticles-Assisted Photochemistry. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 994–1002. [Google Scholar] [CrossRef]
- Allonas, X.; Ibrahim, A.; Charlot, V.; Retailleau, M.; Karasu, F.; Croutxé-Barghorn, C. Development of New Photoinitiating Systems for Depth Curing of Thick Materials. J. Photopolym. Sci. Technol. 2015, 28, 25–29. [Google Scholar] [CrossRef]
- Liu, R.; Chen, H.; Li, Z.; Shi, F.; Liu, X. Extremely Deep Photopolymerization Using Upconversion Particles as Internal Lamps. Polym. Chem. 2016, 7, 2457–2463. [Google Scholar] [CrossRef]
- Ding, A.; Zhang, P.; Zhou, B.; Liu, G.; Xu, P.; Luo, Y.; Zhuang, Y.; Zhang, P.; Chen, A.; Liu, Y.; et al. Structural Design and Performance Study of a Biobased UV/Moisture Dual-Curing Coating for UV Inadequate Curing Conditions. Mater. Today Commun. 2025, 44, 111863. [Google Scholar] [CrossRef]
- Kanokwijitsilp, T.; Körner, M.; Prucker, O.; Anton, A.; Lübke, J.; Rühe, J. Kinetics of Photocrosslinking and Surface Attachment of Thick Polymer Films. Macromolecules 2021, 54, 6238–6246. [Google Scholar] [CrossRef]
- Zhang, D.; Yuan, L.; Yu, J. Effect of Phenolic Resin Coating Thickness on Mechanical Properties and Corrosion Resistance of Metal Materials. Mater. Plast. 2024, 60, 31–43. [Google Scholar] [CrossRef]
- Hamulić, D.; Medoš, G.; Korte, D.; Rodič, P.; Milošev, I. The Effect of Curing Temperature and Thickness of Polybutyl Methacrylate Siloxane Coatings on the Corrosion Protection of Structural Steel S355. Coatings 2023, 13, 675. [Google Scholar] [CrossRef]
- Alam, A.; Chowdhury, A.F.M.A.; Yamauti, M.; Saikaew, P.; Hoshika, S.; Carvalho, R.M.; Sano, H.; Sidhu, S.K. Cause-Effect Relationship of Varying Bonding Thicknesses in Dentin Adhesion of Universal Adhesives. J. Adhes. Dent. 2022, 24, b3240695. [Google Scholar] [CrossRef]
- Hamano, R.; Niidome, Y.; Tanaka, N.; Shiraki, T.; Fujigaya, T. Temperature Response of Defect Photoluminescence in Locally Functionalized Single-Walled Carbon Nanotubes. RSC Adv. 2025, 15, 4137–4148. [Google Scholar] [CrossRef]
- Qiu, F.; Gong, J.; Tong, G.; Han, S.; Zhuang, X.; Zhu, X. Near-Infrared Light-Induced Polymerizations: Mechanisms and Applications. ChemPlusChem 2024, 89, e202300782. [Google Scholar] [CrossRef]
- Li, Q.; Yuan, S.; Liu, F.; Zhu, X.; Liu, J. Lanthanide-Doped Nanoparticles for Near-Infrared Light Activation of Photopolymerization: Fundamentals, Optimization and Applications. Chem. Rec. 2021, 21, 1681–1696. [Google Scholar] [CrossRef]
- Wang, K.; Peña, J.; Xing, J. Upconversion Nanoparticle-Assisted Photopolymerization. Photochem. Photobiol. 2020, 96, 741–749. [Google Scholar] [CrossRef]
- Meng, X.; Lu, H.; Li, Z.; Wang, C.; Liu, R.; Guan, X.; Yagci, Y. Near-Infrared Light Induced Cationic Polymerization Based on Upconversion and Ferrocenium Photochemistry. Polym. Chem. 2019, 10, 5574–5577. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Wong, K.-L.; All, A.H. Lanthanide-Doped Upconversion Nanoparticles as Nanoprobes for Bioimaging. Biomater. Sci. 2024, 12, 4650–4663. [Google Scholar] [CrossRef] [PubMed]
- Borse, S.; Rafique, R.P.; Murthy, Z.V.; Jung Park, T.; Kumar Kailasa, S. Applications of Upconversion Nanoparticles in Analytical and Biomedical Sciences: A Review. Analyst 2022, 147, 3155–3179. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yang, Y.; Yang, H.; Han, F.; Li, Z. Thioxanthone-Based Amidine: An Efficient Nonionic Photobase Generator for Thiol-Based Click Polymerization under Visible LED Light. Prog. Org. Coat. 2020, 148, 105842. [Google Scholar] [CrossRef]
- Brändle, A.; Khan, A. Thiol–Epoxy ‘Click’ Polymerization: Efficient Construction of Reactive and Functional Polymers. Polym. Chem. 2012, 3, 3224–3227. [Google Scholar] [CrossRef]
- Kandeloos, A.J.; Bastani, S.; Ghahari, M.; Jalili, M.; Lalevée, J. NIR-Induced Upconversion-Assisted Photopolymerization: Key Factors, Challenges, and Future Directions. Polym. Eng. Sci. 2024, 64, 4630–4652. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tanaka, N.; Aoki, D.; Arimitsu, K. Rational Design of a Room-Temperature Curing Method, Based on the Epoxy–Thiol Click Reaction, for UV-Curable Hard Coatings with Ultrahigh Strength and Adhesion. J. Polym. Sci. 2024, 62, 5497–5506. [Google Scholar] [CrossRef]
- Kim, D.; Yu, C.; Kwon, Y.; Kim, J.; Chung, K.; Kim, H.-J.; Kwon, M.S. Correlation between the Structural Variations in Thiol-Based Hardeners and Properties of Thiol–Epoxy Polymers. ACS Appl. Polym. Mater. 2023, 5, 9046–9055. [Google Scholar] [CrossRef]
- Hong, S.-M.; Kim, O.Y.; Hwang, S.-H. Chemistry of Polythiols and Their Industrial Applications. Materials 2024, 17, 1343. [Google Scholar] [CrossRef] [PubMed]
- Kotb, Y.; Cagnard, A.; Houston, K.R.; Khan, S.A.; Hsiao, L.C.; Velev, O.D. What Makes Epoxy-Phenolic Coatings on Metals Ubiquitous: Surface Energetics and Molecular Adhesion Characteristics. J. Colloid Interface Sci. 2022, 608, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Koehler, J.; Kuehne, A.J.C.; Piermattei, A.; Qiu, J.; Keul, H.A.; Dirks, T.; Keul, H.; Moeller, M. Polyglycidol-Based Metal Adhesion Promoters. J. Mater. Chem. B 2015, 3, 804–813. [Google Scholar] [CrossRef]
- Patil, H.A.; More, A.P. High-Performance Polyurethane Coatings From Cardanol-Derived Polyols: A Sustainable Approach for Mild Steel Protection. J. Appl. Polym. Sci. 2025, 142, e56715. [Google Scholar] [CrossRef]
- Huang, Y.; Li, L.; Liu, X.; Li, Z. Photobase-Catalysed Anionic Thiol-Epoxy Click Photopolymerization under NIR Irradiation: From Deep Curing to Shape Memory. Polym. Chem. 2022, 13, 3048–3052. [Google Scholar] [CrossRef]
- Available online: https://std.samr.gov.cn/search/std?q=GB%2FT%209754%20%20-2007 (accessed on 9 April 2025).
- Available online: https://std.samr.gov.cn/gb/search/gbDetailed?id=CA6C0E542B4AC983E05397BE0A0AED11 (accessed on 9 April 2025).
- Available online: https://std.samr.gov.cn/gb/search/gbDetailed?id=F159DFC2A95D47EFE05397BE0A0AF334 (accessed on 9 April 2025).
- Available online: https://std.samr.gov.cn/gb/search/gbDetailed?id=71F772D7861BD3A7E05397BE0A0AB82A (accessed on 9 April 2025).
- Yu, R.; Li, S.; Chen, G.; Zuo, C.; Zhou, B.; Ni, M.; Peng, H.; Xie, X.; Xue, Z. Monochromatic “Photoinitibitor”-Mediated Holographic Photopolymer Electrolytes for Lithium-Ion Batteries. Adv. Sci. 2019, 6, 1900205. [Google Scholar] [CrossRef]
- Mousawi, A.A.; Garra, P.; Schmitt, M.; Toufaily, J.; Hamieh, T.; Graff, B.; Fouassier, J.P.; Dumur, F.; Lalevee, J. 3-Hydroxyflavone and N-Phenylglycine in High Performance Photoinitiating Systems for 3D Printing and Photocomposites Synthesis. J. Mater. Sci. Eng. 2018, 51, 4633–4641. [Google Scholar] [CrossRef]
- Hamidi, A.S.; Hadis, M.A.; Palin, W.M. Alternative Co-Initiators for Photocurable Dental Resins: Polymerisation, Quantum Yield of Conversion and Cytotoxicity. Dent. Mater. 2022, 38, 1330–1343. [Google Scholar] [CrossRef]
- Díaz-Hernández, D.; Cañete, Á.; Pavez, L.; Pérez-Sanhueza, A.; Günther, G.; Szreder, T.; De la Fuente, J.R. Spectral and Kinetic Study of 3-Styrylquinoxalin-2(1H)-Ones Photoreduced by N-Phenylglycine and Amines. J. Phys. Chem. B 2019, 123, 3688–3698. [Google Scholar] [CrossRef]
- Huang, T.-L.; Chen, Y.-C. Ketone Number and Substitution Effect of Benzophenone Derivatives on the Free Radical Photopolymerization of Visible-Light Type-II Photoinitiators. Polymers 2021, 13, 1801. [Google Scholar] [CrossRef]
- Ullrich, G.; Burtscher, P.; Salz, U.; Moszner, N.; Liska, R. Phenylglycine Derivatives as Coinitiators for the Radical Photopolymerization of Acidic Aqueous Formulations. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 115–125. [Google Scholar] [CrossRef]
- Ikeda, S.; Murata, S.; Ishii, K.; Hamaguchi, H. Mechanistic Studies of the Pyrene-Sensitized Photodecomposition of N-Phenylglycine: Acceleration of the Photodecomposition by the Addition of an Electron Acceptor. Bull. Chem. Soc. Jpn. 2000, 73, 2783–2792. [Google Scholar] [CrossRef]
- De la Fuente, J.R.; Cañete, Á.; Jullian, C.; Saitz, C.; Aliaga, C. Unexpected Imidazoquinoxalinone Annulation Products in the Photoinitiated Reaction of Substituted-3-Methyl-Quinoxalin-2-Ones with-Phenylglycine. Photochem. Photobiol. 2013, 89, 1335–1345. [Google Scholar] [CrossRef]
- Shi, F.; Wang, J.; Zhai, X.; Zhao, D.; Qin, W. Facile Synthesis of β-NaLuF4: Yb/Tm Hexagonal Nanoplates with Intense Ultraviolet Upconversion Luminescence. CrystEngComm 2011, 13, 3782–3787. [Google Scholar] [CrossRef]
- Boyer, J.-C.; Veggel, F.C.J.M. van Absolute Quantum Yield Measurements of Colloidal NaYF4: Er3+, Yb3+ Upconverting Nanoparticles. Nanoscale 2010, 2, 1417–1419. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Würth, C.; Kraft, M.; Hyppänen, I.; Soukka, T.; Resch-Genger, U. Power-Dependent Upconversion Quantum Yield of NaYF4:Yb3+,Er3+ Nano- and Micrometer-Sized Particles—Measurements and Simulations. Nanoscale 2017, 9, 10051–10058. [Google Scholar] [CrossRef]
- Wisser, M.D.; Chea, M.; Lin, Y.; Wu, D.M.; Mao, W.L.; Salleo, A.; Dionne, J.A. Strain-Induced Modification of Optical Selection Rules in Lanthanide-Based Upconverting Nanoparticles. Nano Lett. 2015, 15, 1891–1897. [Google Scholar] [CrossRef]
- Tessitore, G.; Mandl, G.A.; Brik, M.G.; Park, W.; Capobianco, J.A. Recent Insights into Upconverting Nanoparticles: Spectroscopy, Modeling, and Routes to Improved Luminescence. Nanoscale 2019, 11, 12015–12029. [Google Scholar] [CrossRef]
- Salmi, H.; Allonas, X.; Ley, C.; Defoin, A.; Ak, A. Quaternary Ammonium Salts of Phenylglyoxylic Acid as Photobase Generators for Thiol-Promoted Epoxide Photopolymerization. Polym. Chem. 2014, 5, 6577–6583. [Google Scholar] [CrossRef]
- Pei, H.-W.; Ye, K.; Shao, Y.; Chen, D.; Sun, Z.-Y.; Gong, T.; Liu, D.; Sun, K. Photopolymerization Activated by Photobase Generators and Applications: From Photolithography to High-Quality Photoresists. Polym. Chem. 2024, 15, 248–268. [Google Scholar] [CrossRef]
- Dong, X.; Hu, P.; Zhu, G.; Li, Z.; Liu, R.; Liu, X. Thioxanthone Acetic Acid Ammonium Salts: Highly Efficient Photobase Generators Based on Photodecarboxylation. RSC Adv. 2015, 5, 53342–53348. [Google Scholar] [CrossRef]
- Hayki, N.; Lecamp, L.; Désilles, N.; Lebaudy, P. Kinetic Study of Photoinitiated Frontal Polymerization. Influence of UV Light Intensity Variations on the Conversion Profiles. Macromolecules 2010, 43, 177–184. [Google Scholar] [CrossRef]
- Wang, X.; Seng Kai Kho, A.; Liu, J.; Mao, T.; Gilchrist, M.D.; Zhang, N. Mechanistic Modelling of Coupled UV Energy Penetration and Resin Flow Dynamics in Digital Light Processing (DLP)-Based Microfluidic Chip Printing. Micromachines 2025, 16, 115. [Google Scholar] [CrossRef]
- Angulo, G.; Grilj, J.; Vauthey, E.; Serrano-Andrés, L.; Rubio-Pons, Ò.; Jacques, P. Ultrafast Decay of the Excited Singlet States of Thioxanthone by Internal Conversion and Intersystem Crossing. ChemPhysChem 2010, 11, 480–488. [Google Scholar] [CrossRef]
- Zhao, Q.; Qi, H.J.; Xie, T. Recent Progress in Shape Memory Polymer: New Behavior, Enabling Materials, and Mechanistic Understanding. Prog. Polym. Sci. 2015, 49–50, 79–120. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, H.; Zhao, Y. Optically Triggered and Spatially Controllable Shape-Memory Polymer–Gold Nanoparticle Composite Materials. J. Mater. Chem. 2011, 22, 845–849. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y. Polymers with Dual Light-Triggered Functions of Shape Memory and Healing Using Gold Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 13069–13075. [Google Scholar] [CrossRef]



| Photo-Initiator | λmax nm | εmax L/(mol·cm) | λs nm | eV | eV | eV | eV |
|---|---|---|---|---|---|---|---|
| NPG-TBD | 299 | 2005 | - | 0.6 | −1.212 | - | - |
| ITX | 383 | 6860 | 398 | 1.441 | −0.837 | 3.116 | −0.463 |
| Parameter | Thickness/μm | |||||
|---|---|---|---|---|---|---|
| 50 | 100 | 150 | 1500 a | 1500 b | 2000 | |
| Adhesion (grade) | 0 | 0 | 0 | - | - | - |
| Pencil hardness | 3H | 3H | 3H | 3H | H | 3H |
| Flexibility (mm) | 2 | 2 | 2 | 2 | 6 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, P.; Huang, Y.; Liu, X.; Li, Z. Near-Infrared Light-Induced Deep Curing of Thiol–Epoxy Networks Based on Upconversion Photochemistry. Coatings 2025, 15, 494. https://doi.org/10.3390/coatings15040494
Yang P, Huang Y, Liu X, Li Z. Near-Infrared Light-Induced Deep Curing of Thiol–Epoxy Networks Based on Upconversion Photochemistry. Coatings. 2025; 15(4):494. https://doi.org/10.3390/coatings15040494
Chicago/Turabian StyleYang, Pin, Yaoxin Huang, Xiaoxuan Liu, and Zhiquan Li. 2025. "Near-Infrared Light-Induced Deep Curing of Thiol–Epoxy Networks Based on Upconversion Photochemistry" Coatings 15, no. 4: 494. https://doi.org/10.3390/coatings15040494
APA StyleYang, P., Huang, Y., Liu, X., & Li, Z. (2025). Near-Infrared Light-Induced Deep Curing of Thiol–Epoxy Networks Based on Upconversion Photochemistry. Coatings, 15(4), 494. https://doi.org/10.3390/coatings15040494





