Abstract
The tribological behavior of molybdenum disulfide (MoS2) coatings was systematically investigated under various controlled gas environments in a vacuum chamber. A hemispherical steel pin was slid cyclically over a MoS2-coated steel disk, prepared via high-speed powder spraying. The study measured both dynamic and static friction coefficients under different gaseous atmospheres, including high vacuum, helium, argon, dry air, and water vapor. In high vacuum (10−5 Pa), an ultra-low dynamic friction coefficient (µ ≈ 0.01) was observed, while increasing values were recorded with helium (µ ≈ 0.03), argon (µ ≈ 0.04), dry air (µ ≈ 0.17), and water vapor (µ ≈ 0.30). Static friction coefficients followed a similar trend, decreasing significantly upon evacuation of water vapor or injection of inert gases. Surface analyses revealed that friction in vacuum or inert gases promoted smooth wear tracks and basal plane alignment of MoS2 crystallites, while exposure to water vapor led to rougher, more disordered wear surfaces. Mass spectrometry and energetic modeling of physisorption interactions provided further insights into gas–solid interfacial mechanisms. These results demonstrate that the tribological performance of MoS2 coatings is highly sensitive to the surrounding gas environment, with inert and vacuum conditions favoring low friction through enhanced basal plane orientation and minimal gas–surface interactions. In contrast, water vapor disrupts this structure, increasing friction and surface degradation. Understanding these interactions is crucial for optimizing MoS2-based lubrication systems in varying atmospheric or sealed environments.
1. Introduction
Layered transition metal disulfides (TMDs), such as MoS2 and WS2, have garnered significant attention for their exceptional tribological properties, making them highly promising candidates as solid lubricants. They are characterized by their layered crystal structures, where weak van der Waals forces between the layers allow for easy slippage, enabling a reduction in friction and wear under various operating conditions [1]. The primary tribological benefit of TMDs is they can form a lubricating film on metal surfaces, reducing direct contact between the surfaces and minimizing wear. Their low shear strength between layers allows for smooth sliding, while their ability to withstand high pressures and temperatures further enhances their applicability in demanding industrial processes, including automotive and machinery applications. MoS2 coatings also exhibit unique tribological properties in inert gas and vacuum, making them valuable, especially in aeronautics and space applications where conventional liquid lubricants may fail [2,3]. Indeed, MoS2 coatings demonstrate superlubricating properties in vacuum environments. As gas pressure decreases, the coefficient of friction (μ) also decreases. Researchers such as M. Martin and A. Erdemir, among others [4,5,6,7,8,9,10,11], have demonstrated friction coefficients as low as μ = 0.001 in ultra-high vacuum conditions. Transmission electron microscopy (TEM) analysis revealed a well-organized structure on the worn surface sheets. Gradt et al. illustrated that under high-vacuum conditions, MoS2 coatings can achieve friction coefficients as low as 0.02 at 77 K. They also highlighted that PVD-MoS2 coatings maintain a friction coefficient of 0.06 for more than 350,000 cycles. In general, higher loads and speeds tend to lower the friction coefficient [12].
The tribological behavior of MoS2 coatings in dry nitrogen and other inert gases is also noteworthy: Babuska et al. [13] illustrated the role of basally oriented surfaces, reporting that sliding in dry nitrogen forms transfer films oriented along the (002) basal plane, which exhibit lower initial friction compared to amorphous or nanocrystalline structures. Oxidation resistance and friction reduction after aging are improved by raster patches formed in dry nitrogen.
A significant phenomenon affecting MoS2 coatings is the dwell effect, which refers to the difference between static and dynamic friction after a stationary period. The dwell effect is characterized by an increase in friction and a prolonged run-in period when sliding resumes. Babuska et al. [14] demonstrated a pressure dependance: In low vacuum (2.2 × 10−1 to 1 × 10−3 torr), friction increases more rapidly compared to high or ultra-high vacuum (1 × 10−6 to 5 × 10−8 torr). They also showed a time dependence: the friction coefficient increases by 0.005 after 300 s and 0.01 after 1800 s in low vacuum conditions. The influence of the microstructure is important: spray deposited MoS2 films show twice the dwell effect compared to sputtered films after a 28,800 s dwell time in dry nitrogen.
The performance of molybdenum disulfide (MoS2) coatings in ambient air is affected by humidity, which increases the coefficient of friction [15,16,17,18,19,20,21,22,23,24,25]. Numerous surface analysis studies have highlighted structural alterations in rubbed surfaces [13,26,27,28,29,30,31,32,33,34,35,36,37,38,39].
Several factors contribute to the tribological performance of MoS2 coatings:
- i.
- Crystal Structure: MoS2’s lubricity stems from its layered crystal structure, where weakly bonded lamellae easily slide over each other.
- ii.
- Surface Contamination: The dwell effect is affected by the interactions between water vapor and the sliding interface.
- iii.
- Shear-Induced Modifications: Sliding can lead to structural changes on the surface, with sputtered coatings exhibiting fewer defects and greater resistance to water-induced alterations.
A critical gap remains in the dynamic evolution of the static friction coefficient under varying gas environments. Understanding this factor is essential for optimizing the performance of MoS2-coated screw/nut assemblies, particularly in terms of tightening torque and long-term durability. Additionally, the use of MoS2-coated assemblies in aerospace applications, such as aeronautics and satellites, introduces potential risks to structural safety, especially in high-altitude conditions where gas composition undergoes extreme changes, such as in very low temperatures (−60 °C) or in a complete absence of water vapor. Water vapor plays a critical role in influencing the dynamic friction coefficient of MoS2 coatings. In vacuum and inert gas environments, MoS2 coatings are known for their low friction coefficients and extended wear life. However, the dwell effect introduces complexity to their tribological behavior, necessitating an in-depth understanding of both dynamic and static friction in intermittent motion applications. Although previous studies have investigated the effects of water vapor and nitrogen on MoS2 tribology [40], no experimental work has been conducted under helium or argon. This paper outlines friction tests performed in different environments, such as high vacuum, pure helium, pure argon, ambient air with low relative humidity (RH = 25%), and in the presence of water vapor. The study delves into the physical and chemical interactions between gases and the sulfide sheet, examining their consequences on the tribological behavior of molybdenum disulfide.
2. Materials and Methods
2.1. Tribological Tests
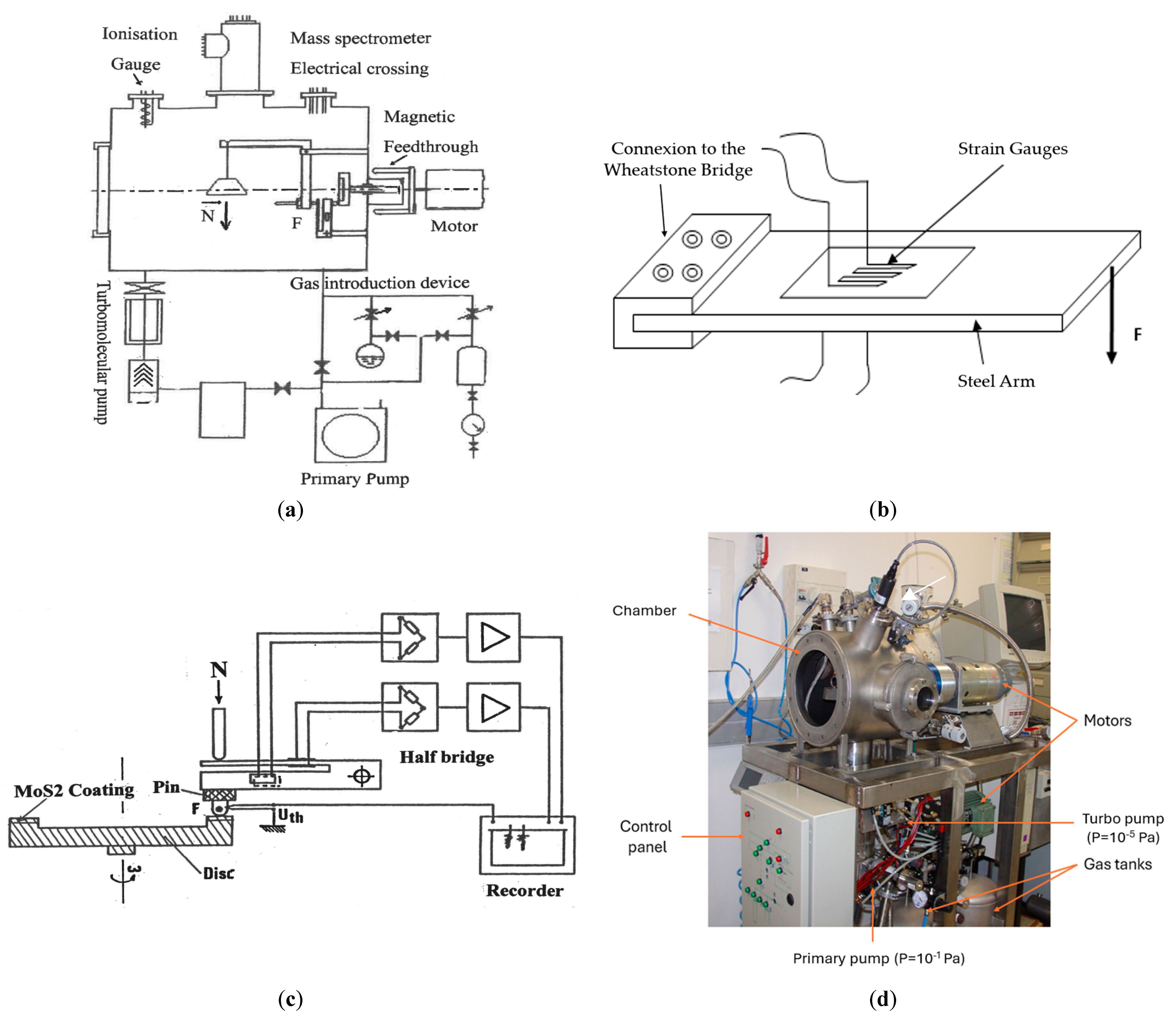
A vacuum tribometer was meticulously designed and fabricated within the laboratory to facilitate the tribological investigation of solid lubricants. This research was conducted within a precisely controlled gaseous environment surrounding the tribocontact, specifically tailored for aeronautical industrial applications. The selection of tribometric parameters, including normal load and relative sliding speed, aligns with the ASTM G-99-23 standard [41].
The experimental device (Figure 1d) comprises a pin-on-disk tribometer placed inside a vacuum chamber equipped with a turbine pump, achieving an ultimate pressure in the 10−6 Pa range. The disk rotates via a direct current motor through a magnetic feedthrough capable of transmitting a torque of 10 N.m without slippage. The rotational speed is adjustable within the range of 2 × 10−4 to 100 rad s−1. Load application was accomplished through dead weights positioned inside the vacuum chamber. Calibrated strain gauges affixed to steel arms (Figure 1b,c) measured the normal and tangential forces. The pin was cylindrical in shape with a diameter of 5 mm, a length of 15 mm, and a hemispherical end. Both the pin and disk were fabricated from hot-rolled and standardized AISI 1045 steel. The chemical composition and the mechanical properties of both the pin and the disk are presented in Table 1.
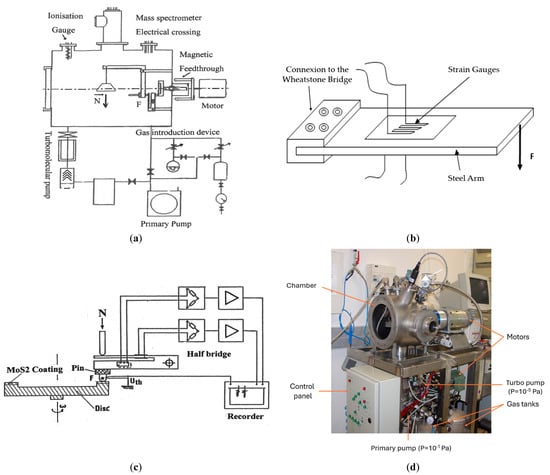
Figure 1.
(a) Schematic view of the pin-on-disk tribometer in vacuum chamber. (b) Strain gauges bonded on steel arms and Wheatstone bridge for friction force measurement. (c) Steel arm for measuring the friction force by bending deformation. (d) General view of the tribometer [15].

Table 1.
Chemical composition and mechanical properties of standardized carbon steel AISI 1045.
The thin film coating was achieved through the cold spray deposition processing of molybdenum disulfide powder, featuring an average particle size of 0.1 μm. This high-speed spraying was executed on an AISI 1045 steel disk within a controlled environment chamber, under argon at 50 bars and at room temperature. The MoS2 powder from Schaeffer distribution had a high purity (99.9%). The coated disk measures 50 mm in diameter and 5 mm in thickness. It was polished to a surface finish of 0.4 µm (Ra). This facilitated the accommodation of powder to the surface roughness for a better mechanical adhesion of the coating on the substrate. To ensure a uniform coating thickness of 5 μm, the distance between the projection source and the disk was carefully maintained at 200 mm. The resulting 5 µm thick MoS2 coating was polycrystalline with a random orientation, matte color, and Hv = 50 Vickers microhardness. It adhered strongly to the substrate. It is noteworthy that the hemispherical end pin remained polished and uncoated.
The closed tribometer chamber was purged of air through a system of pumps. The pumping group mounted on the vacuum chamber was composed of a primary pump which made it possible to go down to 10−3 Pa in one hour. Then, a Leybold turbomolecular pump with a high gas flow rate allowed for a high secondary vacuum of 10 −7 Pa in four hours.
Once the high vacuum was attained, friction tests in the high vacuum could start. In dry pure gases, a high vacuum was first established. Valves were sealed, and the introduction of pure dry gases into the tribometer chamber was initiated at the given pressure.
The gas pressure was measured with a Pirani gauge, specifically calibrated for gases, and compared versus a MacLeod gauge, within the pressure range of 10−3 to 1 torr. Lower pressures were quantified using a millitorr and an ultra-high vacuum (UHV) gauge. The partial pressures were monitored by employing a quadrupole mass spectrometer installed in the tribometer chamber.
The two pure dry gases, argon, and helium, utilized in the experimental tests were sourced from Air Liquide, with a purity level of 99.9999%. Water was introduced into the chamber from a tank initially placed under vacuum and then filled with demineralized water. No sparging of the water with nitrogen to separate the dissolved oxygen was performed.
The temperature remained relatively stable at around 25 °C. The disk rotated at a speed of approximately 200 rpm, while the relative sliding speed was maintained at about 0.4 m·s−1. The normal load applied to the pin was 4.6 N. The contact pressure P0 was approximately 37 MPa calculated via the elastic Hertzian relation:
In Equation (1), is the equivalent reduced Young’s modulus of steel = 210 GPa and that of MoS2 is = 46.2 GPa. R = 5 mm and denotes the pin radius.
For every tribological test, a pristine coated disk was employed. The test duration was 60 to 80 min. Each test was iterated a minimum of three times under identical conditions. The displayed mean value of the friction coefficient is the average of these three tests. These values are simply averages of the data from the three test graphs. Error values, all below 10%, indicate the standard deviation across the three repeated tests. Notably, tests conducted in the same gaseous environment but under varying applied normal loads gave identical results.
2.2. Analyses of Worn Surfaces
Microscopic analysis of the wear tracks was not performed in situ. It was carried out after the test was stopped, the vacuum chamber was opened to air, and the pin and the disk were disassembled. The analysis of coatings in various wear tests was conducted using a Leica Light Optical Microscope (LOM) with a digital camera and a Talysurf CCI 6000 interferometric profilometer.
To identify the crystallographic orientation of the MoS2 rubbed under different environments, a small probe (almost a monocrystal) was extracted from the wear track coating, parallel to the friction surface. The analysis was carried out by X-ray diffraction with a Cu-Kα radiation (λ = 1.5406 Å) filtered by Ni (BRUKER D8 X-ray diffractometer) equipped with a high-performance linear detector, between 20° and 60°, with a 0.02° step and 0.6 s exposition time.
2.3. Study of Van Der Waals Forces Between Molybdenum Disulfide and Gas
The forces governing Van der Waals interactions between atoms, molecules, or between a molecule and a crystal hold substantial significance. Describing these interactions involves considering the dipolar forces that arise from electric charges forming atoms and molecules. The impact of Van der Waals strength (proportional to ) [35] becomes particularly pronounced when the distance (r) is exceptionally low, as observed in the adsorption of gas in molybdenum disulfide (MoS2).
In this scenario, both molybdenum disulfide (MoS2) and the surrounding gases exhibit dipolar interactions between the MoS2 dipole and that of the gases. Each atom possesses a permanent dipole moment, varying in strength, arising from the separation between the positive charge of the nucleus and the negatively charged electron. This separation is a result of quantum fluctuations in the instantaneous charge density of valence.
Furthermore, the inter-plane distance in the molybdenum disulfide (MoS2) crystal measures approximately 3.08 Å, which is less than 4 Å. The Van der Waals force attraction between the gas and the MoS2 dipoles is notably robust in this context.
Molybdenum disulfide (MoS2) exhibits a layered structure akin to graphite, but with each layer comprising either sulfur or molybdenum. The hybridization of MoS2 is sd4p5, where each molybdenum atom is symmetrically surrounded by six sulfur atoms arranged in a hexagonal shape, resulting in saturated valences. The hexagonal configuration of MoS2 is both symmetrical and auto equilibrated. This structural arrangement contributes to the material’s ability to maintain a low coefficient of friction.
MoS2 is recognized as a microporous material with a capacity for gas adsorption. In this adsorption process, dipolar gas molecules can interact with MoS2, which possesses dipolar potential energy (U). The total potential energy is the summation of all individual potential energy components. The quantity of adsorbed gas is influenced by various factors, including the partial pressure of the gas and the reactivity of gas molecules. On the surface, the rotational and vibrational freedom of adsorbed molecules is typically restricted, and these molecules may be desorbed under the influence of temperature.
2.4. Influence of Gas on the Tribological Behavior of Molybdenum Disulfide (MoS2)
To delve into the gas adsorption process in molybdenum disulfide, it is essential to elucidate its character as a dipolar adsorbent within the layers of sulfur. The interaction between the metal layer of molybdenum (Mo) and the sulfur (S) layer involves a robust covalent bond with an energy of 250 kJ·mol−1. This energy value is significantly larger when compared to the potential energy of Van der Waals forces, which typically ranges from 4 to 25 kJ·mol−1. The impact of adsorbed gas molecules on these covalent bonds is deemed negligible.
Within consecutive and identical dipolar sulfur layers, the binding is notably weak, resembling Van der Waals forces. This characteristic ensures the self-lubricating properties of molybdenum disulfide in a vacuum. However, in the presence of chemically active gases around the tribocontact, such as water or oxygen molecules, the interplanar interaction undergoes modification due to the interaction between the gas and sulfur atoms.
In inert gases, the Van der Waals interactions between the gases and sulfur layers result from Keesom, Debye, or London forces. These forces contribute to an increase in shearing stress between the layered structures of molybdenum disulfide. Consequently, they subtly alter the intrinsic nature of the self-lubricating properties of this material.
Subsequently, we can elucidate the impact of adsorbent gas molecules on the tribological behavior of MoS2 as follows [9]: when the difference in electronegativity between two bonded elements surpasses 0.5 units on the Pauling scale, the bond exhibits permanent polarization.
In the layered and hexagonal structure of molybdenum disulfide (MoS2), the electronegativity of the sulfur atom is χS = 2.5, while that of molybdenum is χMo = 1.8. The covalent radius of the sulfur atom is rS = 104 pm, and the covalent radius of the molybdenum atom is rMo = 129 pm. The distance between the two atoms, calculated by the relation l(Mo-S) = rMo + rS − 0.09|χMo − χS|, amounts to l(Mo-S) = 2.267 Å. Additionally, the fractional charge δ of the sulfur atom is approximately δ = −0.24, resulting in a dipolar electrical charge q = −0.352 × 10−19 C.
Subsequently, the polarization of the Mo-S bond, connecting the molybdenum atom with sulfur atoms, can be assessed through the permanent dipolar moment μ1 = |q|. l(Mo-S) = 2.369 D = 7.89 × 10−30 Cm. In comparison, the water molecule, known for its strong dipolarity, possesses a dipole moment μ2 = 1.86 D = 6.19 × 10−30 Cm.
The electron cloud of inert gases (Ar or He) lacks a permanent dipole moment, and it can undergo momentary polarization in response to the electric field E induced by the permanent dipole of molybdenum disulfide. The induced temporary dipole moment μi in the inert gas is denoted as β. Under the influence of the polarization of bonds between the molybdenum atom and a sulfur atom, the Van der Waals forces primarily stem from the Mo-S dipole of each layer. Its value can be calculated as outlined below [9,10].
µi is expressed in Cm, electrical field E is in V·m−1, and the gas atom polarizability β is in C·m2·V−1. The following parameter α = is expressed in m3 and designates the volumetric atom polarization of the atom.
2.5. Van Der Waals Attractive Forces Between Two Molybdenum Disulfide Layers [42,43]
is the pure potential energy of attraction under ultra-high vacuum between two consecutive layers of sulfur (Van der Waals gap), and m is the number of pairs of interactions.
The dipole moment of connection between the molybdenum atom and a sulfur atom is = 2.369 D [15], r is the distance between two dipoles, k is the Boltzmann constant, T is absolute temperature, and is the constant dielectric vacuum; = 8.854 × 10−12 C2J−1m−1. Numerically, = 9835 × 10−9 N/atom and the experimental friction coefficient = 0.012 were obtained.
2.6. Calculation of the Van Der Waals Forces Under the Action of Argon and Helium Gases
The friction coefficient in vacuum is slightly lower than under argon, = 0.042. Argon is an inert gas; however, it was found that it could form a very small induced dipolar moment and then London forces. This force depends on the polarizability of argon = 1.66 × 10−30 m3 16 and on the ionization energy of argon atoms = 15.759 eV18
where r is the average distance between the molecules considered. Thus, = 2.899 × 10−8 N and the ratio = 3.95.
Similarly, the helium fills in the chamber and is adsorbed by the micropores on the molybdenum disulfide, and its isothermal adsorption curve is type II [9,10]. The layer adsorbs physically by dipolar interaction. Helium is a rare gas with a very low atomic mass, and it has no permanent multipolar moment. It has a very low polarizability = 0.205 × 10−30 m3; however, it has the capacity to form very weak forces because of the influence of the permanent dipole Mo-S, which polarizes the helium atoms.
where r is the average distance between the molecules considered. Thus, we obtain an attraction force = 15.6 × 10−9 N/atom and the ratio ( = 2.6 times. The coefficient of friction under helium is slightly greater than under secondary vacuum under the same test conditions, and its mean value µHe = 0.03.
2.7. Van Der Waals Attractive Forces Between Sulfur Layer and Molecule of Water
The Kessom interaction between the molybdenum disulfide dipole and the water vapor molecular dipole µ2 is expressed as follows:
Numerically, = 28 × 10−8 N and the friction coefficient under water vapor can be obtained as = (28.983/0.983), = 0.35.
The computation of this Van der Waals force is significantly contingent on the distance (r) between the gas molecule and the sulfur atom of MoS2, characterized by a dependence on r−7. Determining this distance involves the consideration of various parameters, including orientation, the interplay of the two layers, the nature of the adsorbed gas, and more.
Furthermore, in a dry sliding contact, diverse mechanical shear forces come into play, contingent on the gaseous environment. These forces interact with the relatively weak electrical dipole forces within the contact. Consequently, we can infer that the relationship between the average friction coefficients obtained under inert gases or in a vacuum evolves in tandem with the relationship between the calculated dipolar forces.
3. Results and Discussion
3.1. Tribological Behavior of Molybdenum Disulfide in High Vacuum (10−5 Pa)
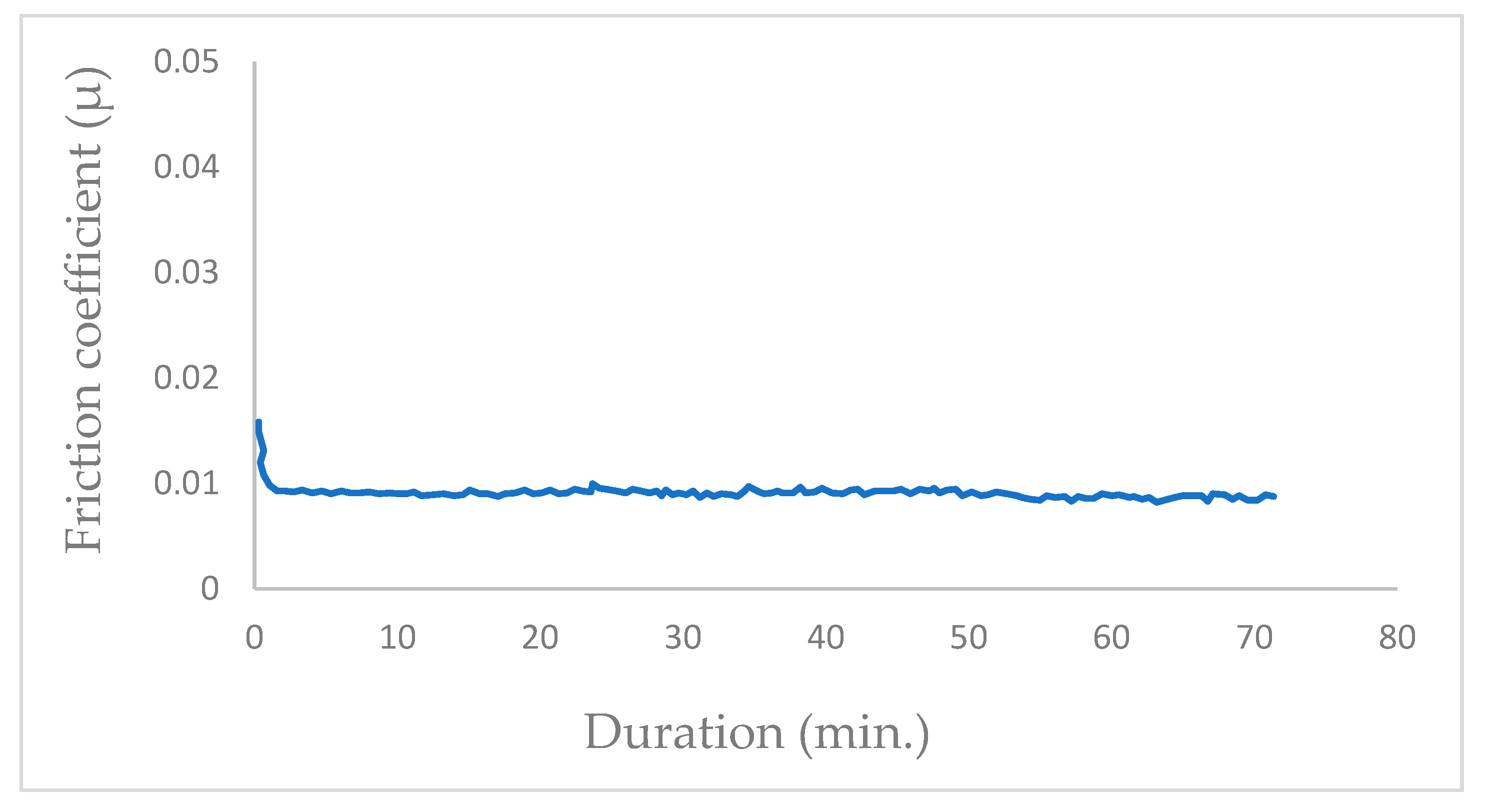
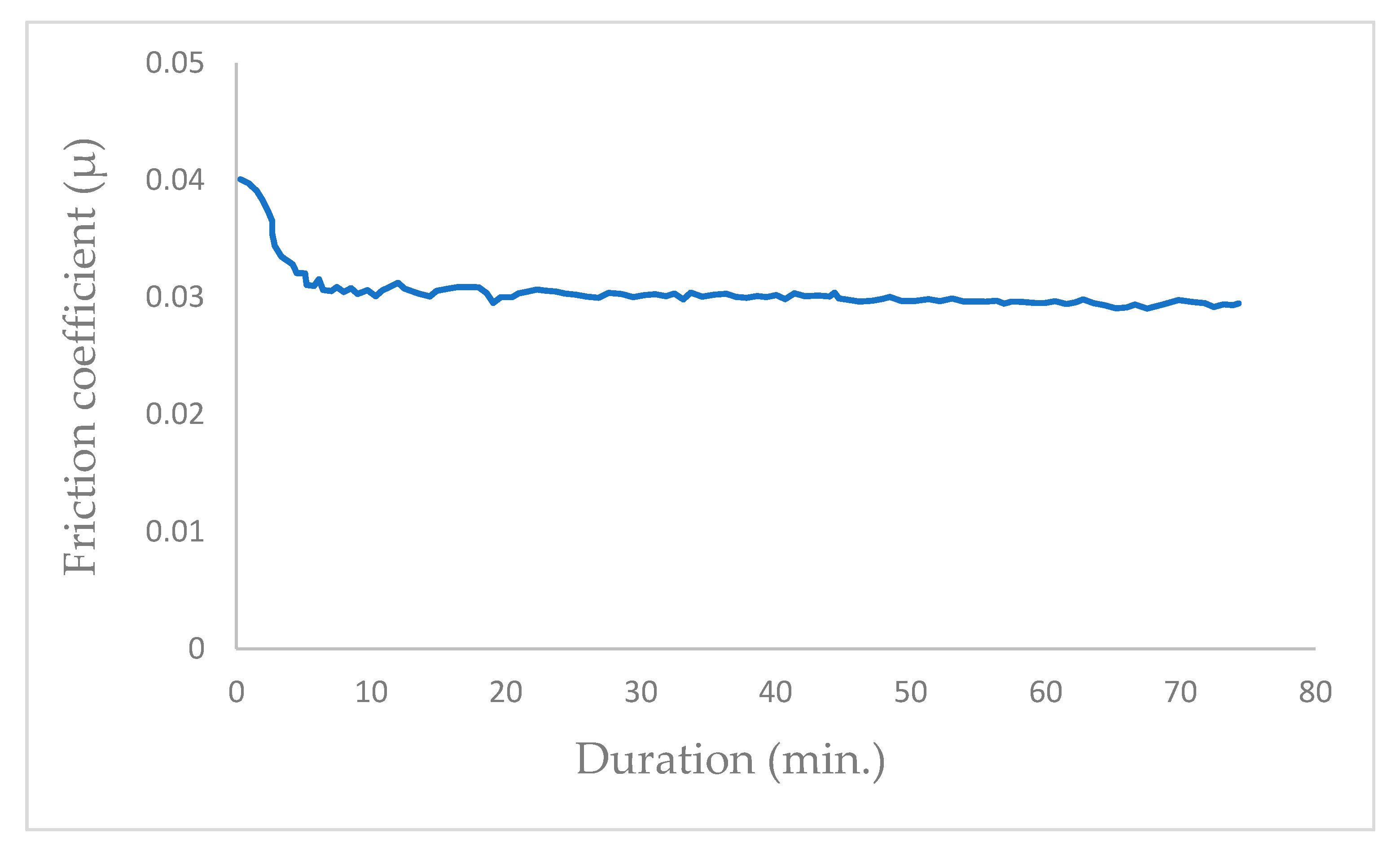
In vacuum conditions (P = 10−5 Pa), the tribological tests exhibit a progressive reduction in the mean friction coefficient (μ), stabilizing at μ = 0.01 after a few hundred cycles (350 cycles; Figure 2). The friction coefficient transitions from μ = 0.017 to the permanent value of μ = 0.01 at the initiation of the friction test in vacuum with the newly coated MoS2 surfaces. Once the contact track is established, the friction coefficient remains consistently constant.
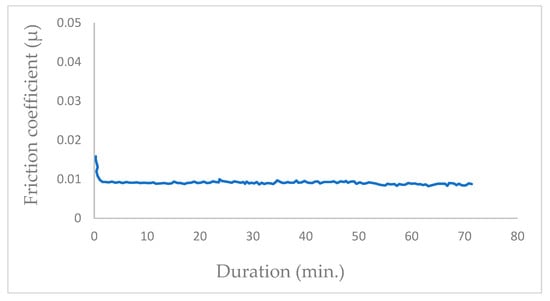
Figure 2.
Evolution of friction coefficient of the steel/molybdenum disulfide/steel high vacuum (P = 10−5 Pa) (v = 0.4 m.s−1, T = 25 °C, N = 4.6 N).
Figure 3a [15] shows the pristine surface of the MoS2 coating sprayed onto the disk surface as reference. Figure 3b illustrates the wear track on the coated steel disk under vacuum conditions, revealing a shiny surface after sliding.
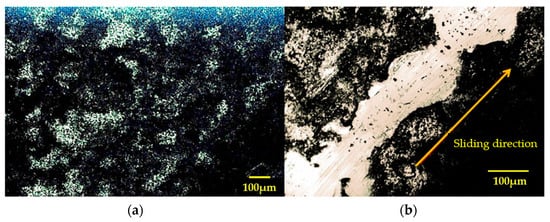
Figure 3.
LOM images: (a) morphology of the fresh surface of the MoS2 coating (black, random, and rough profile), (b) reflected white wear track of coated steel disk, rubbed under vacuum.
This is the result of the smoothing of the MoS2 surface due to the friction removing irregularities and microscopic defects, resulting in a shinier or smoother appearance. In addition, under the effect of pressure and frictional movement, the crystalline structure of MoS2 may be altered, creating more aligned or ordered areas that reflect light more uniformly, making the surface appear brighter. Figure 3b shows an example of a highly oriented thin film along the sliding direction.
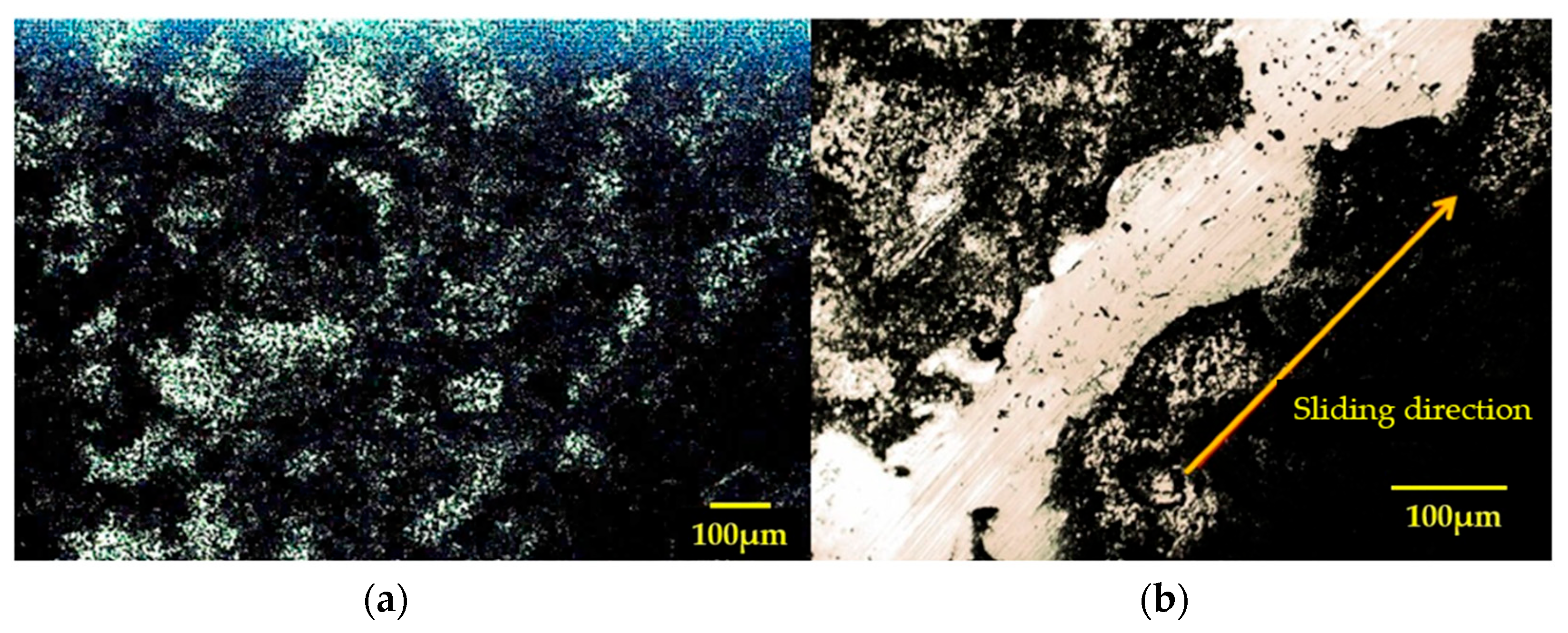
The morphologies and profiles of the wear tracks distinctly depict the orientation and smoothness of the contact surface (Figure 4).
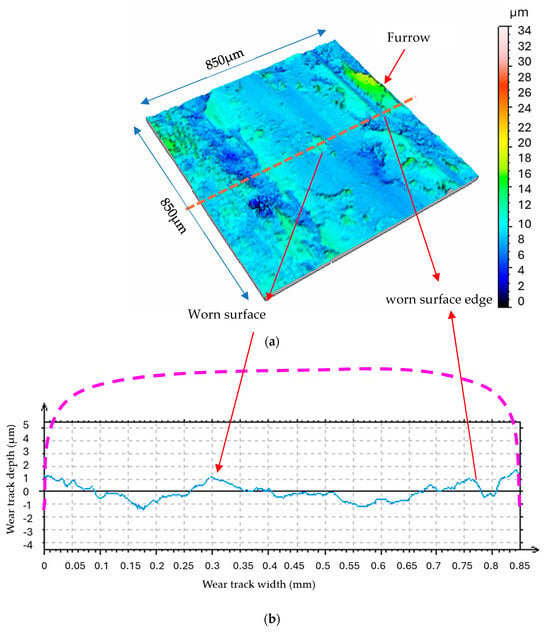
Figure 4.
Morphology and profile of rubbed disk surface in vacuum (N = 4.6N, v = 0.4 m.s−1, t = 40 mn); (a) morphology; (b) 2D profile of worn disk surface [15].
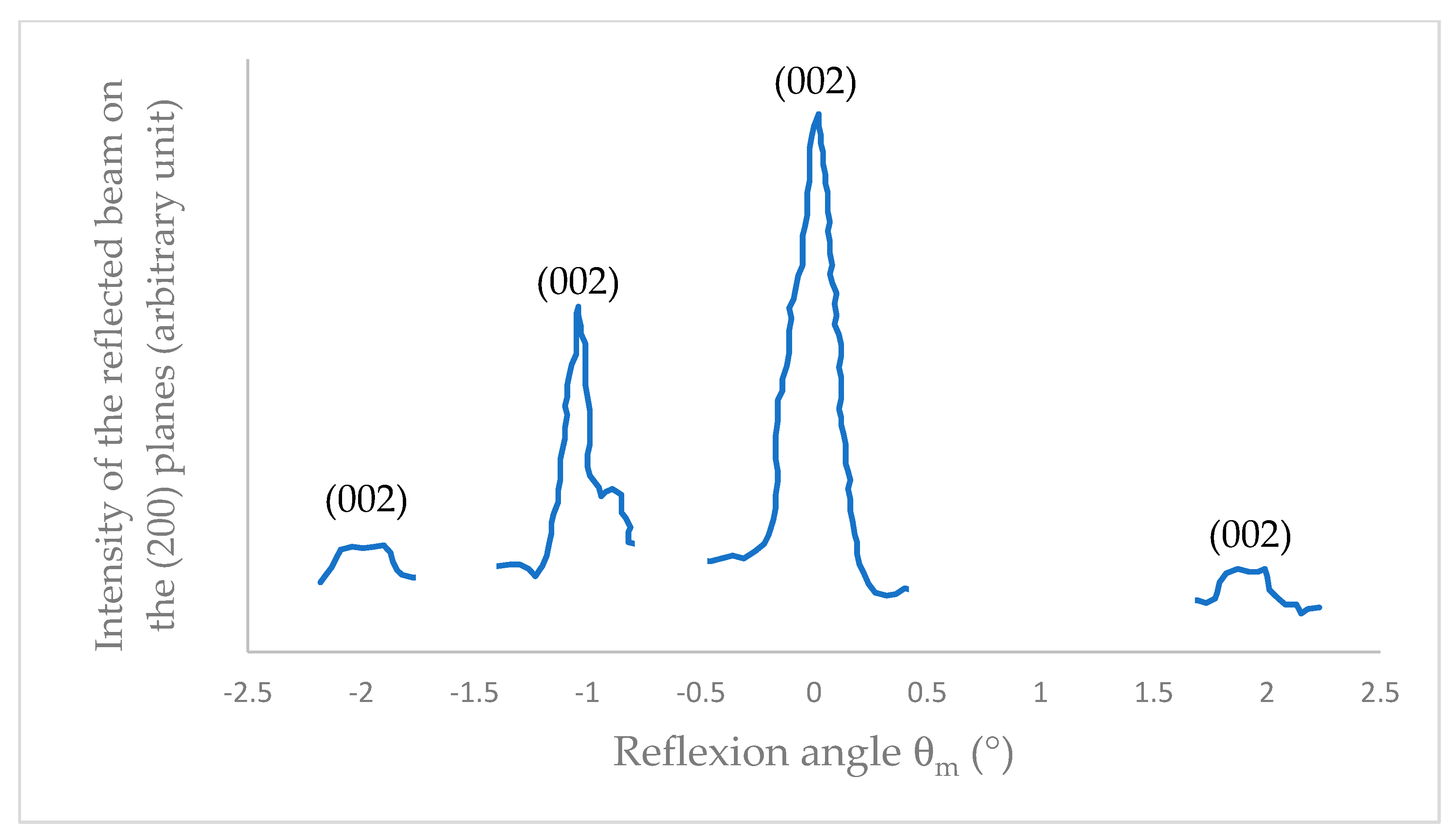
X-ray diffraction analysis of a probe taken from the surface of the worn disk, which was rubbed under high vacuum at µ = 0.01, revealing the pronounced orientation of the basal plane (002) parallel to the sliding plane, with a dispersion of less than 2° (Figure 5). The surface orientation serves to mitigate defects on the surface, reduce the surface work function, enhance passive resistance to tribochemical reactions, and result in a low and stabilized friction state (glazing/fading effect) [3,13].
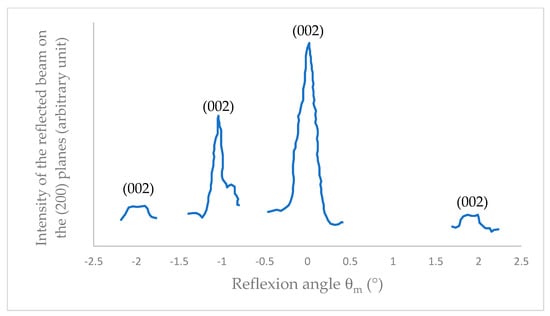
Figure 5.
X-ray diffraction diagram of MoS2 surface crystallites which have rubbed under high vacuum.
3.2. Friction Behavior Under Pure Helium (105 Pa)
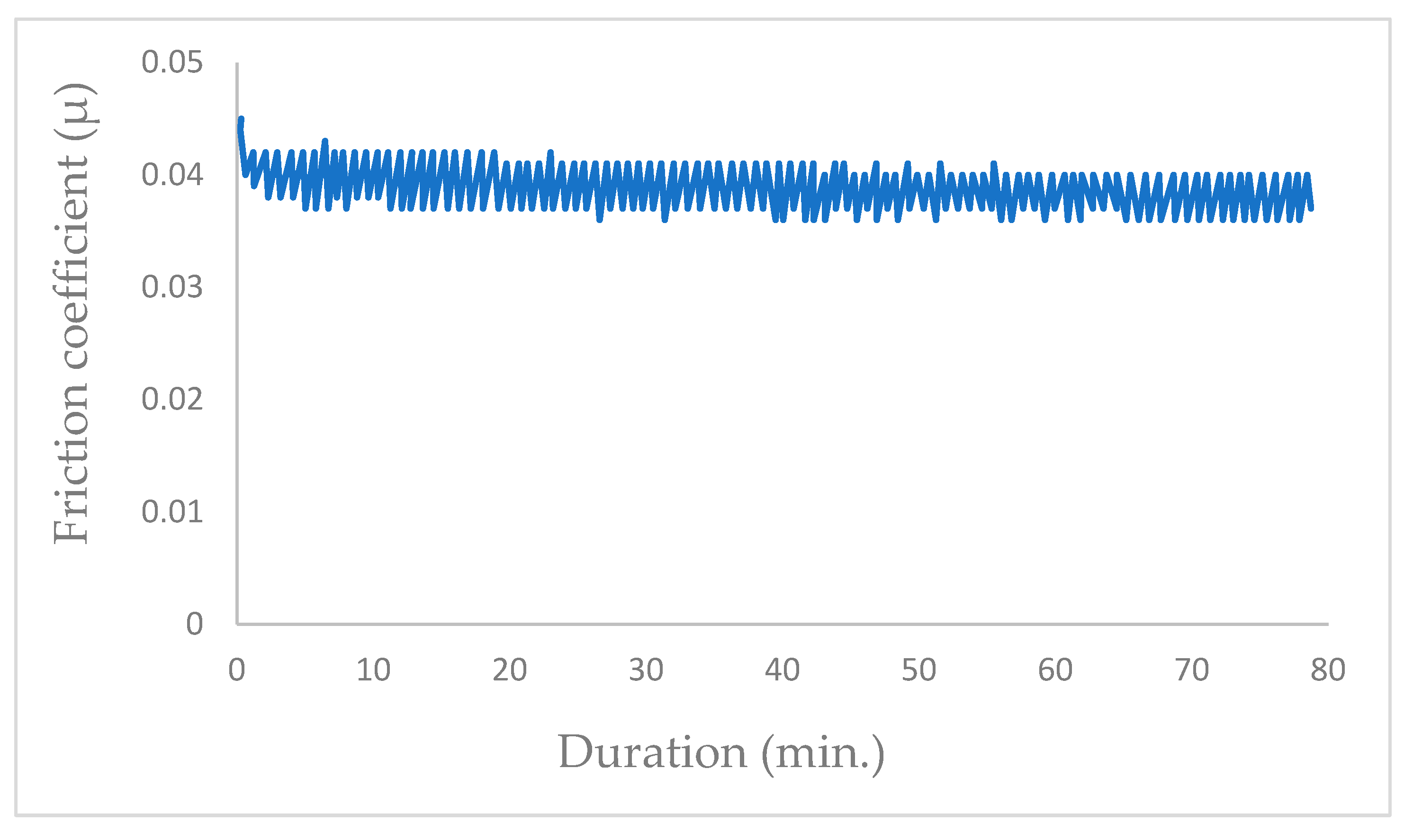
To conduct the tests, the chamber was evacuated to a pressure of 10−5 Pa, after which it was filled with helium at 105 Pa pressure. Subsequently, the test was initiated upon the introduction of helium. Figure 6 graph illustrates the progression of the coefficient of friction, culminating in an approximate value of μ = 0.03.
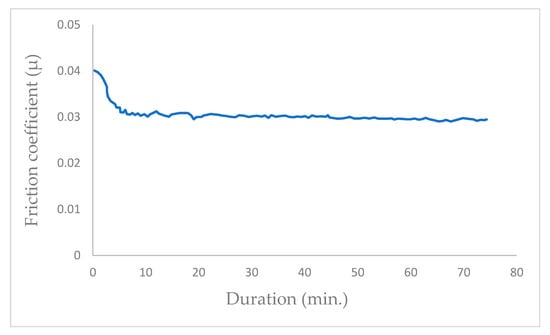
Figure 6.
Evolution of friction coefficient of the steel/molybdenum disulfide/steel under pure helium (99.9999%) under a gas pressure of 105 Pa (v = 0.4 m·s−1, T = 25 °C, N = 4.6 N).
3.3. Friction Behavior Under Pure Argon (105 Pa)
Once the chamber was under a vacuum of 10−5 Pa, the valve was opened to introduce argon and fill the chamber with argon to a pressure of 105 Pa.
The introduction of argon around the tribocontact at 105 Pa resulted in a mean friction coefficient value of µ = 0.04 (Figure 7). While inert gases exhibit non-reactivity with molybdenum disulfide surfaces, they significantly influence the evolution of the friction coefficient compared to vacuum conditions [44,45].
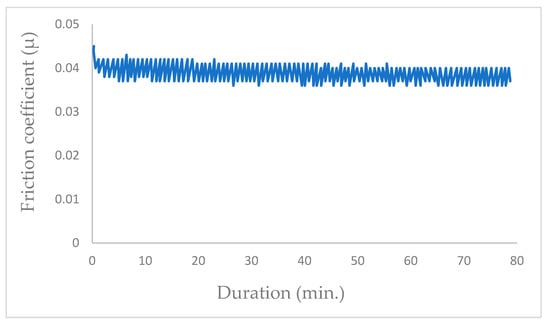
Figure 7.
Evolution of friction coefficient of the steel/molybdenum disulfide/steel under pure argon (99.9999%) under a gas pressure of 105 Pa (v = 0.4 m·s−1, T = 25 °C, N = 4.6 N).
Failure in dry argon is usually caused by the gradual depletion of MoS2 from the contact surfaces rather than chemical degradation.
In the literature, the friction coefficient of MoS2 in pure helium is generally higher than in vacuum or dry argon. Indeed, helium atoms can penetrate between MoS2 layers, slightly increasing interlayer friction. Nevertheless, the friction coefficients are the same amplitude and are low [44,45].
3.4. Friction Behavior Under Pure Water Vapor (102 Pa) and in Ambient Air
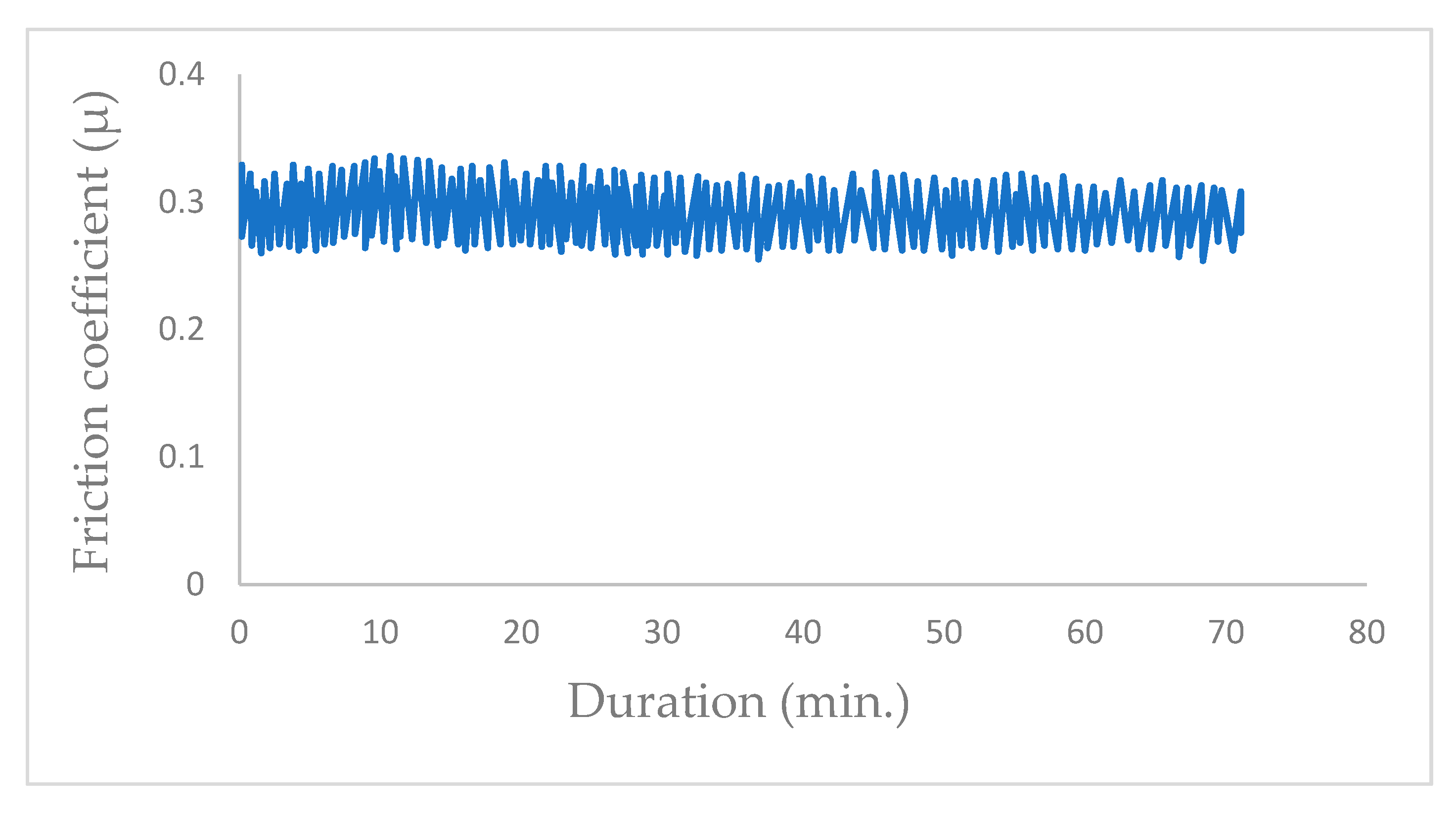
Once the chamber is under a vacuum of 10−5 Pa, the valve is opened to introduce water vapor at a pressure of 102 Pa. The presence of water vapor in the vacuum chamber instantaneously increased the friction coefficient from µ = 0.06 to μ = 0.3 (Figure 8) when the friction test was started with a new MoS2 coating surface.
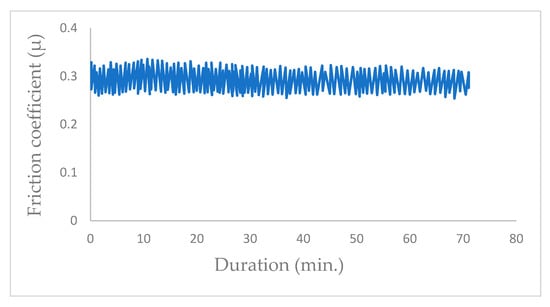
Figure 8.
Evolution of friction coefficient of the steel/molybdenum disulfide/steel under water vapor at pressure of 102 Pa (v = 0.4 m·s−1, T = 25 °C, N = 4.6 N).
The ongoing monitoring of water vapor pressure within the tribometer chamber revealed a discernible decline during the friction test. Notably, the extent of this decrease amplified proportionally with the relative sliding speed of the contact. This observation serves as a clear indication of water vapor consumption throughout the sliding friction process of the coating.
Additionally, mass spectrometry analysis of the tribochemical gases emitted by the coating during friction, conducted under low pressure conditions (P = 2 × 10−4) with water vapor, reveals the presence of molecular hydrogen (H2) and sulfur dihydrogen (H2S) gases. The emergence of these gases during friction under water vapor unmistakably underscores the chemisorption of water vapor by the molybdenum disulfide, a phenomenon elucidated by the tribochemical reactions. If the disk is rotated without contact with the pin and therefore without sliding friction, the water vapor pressure in the chamber remains almost constant. The H2 signal recorded by the mass spectrometer is very weak and the H2S signal is almost non-existent. This proves the consumption of water vapor by the coating-worn sliding surface, and its transformation into molecular hydrogen and to H2S.
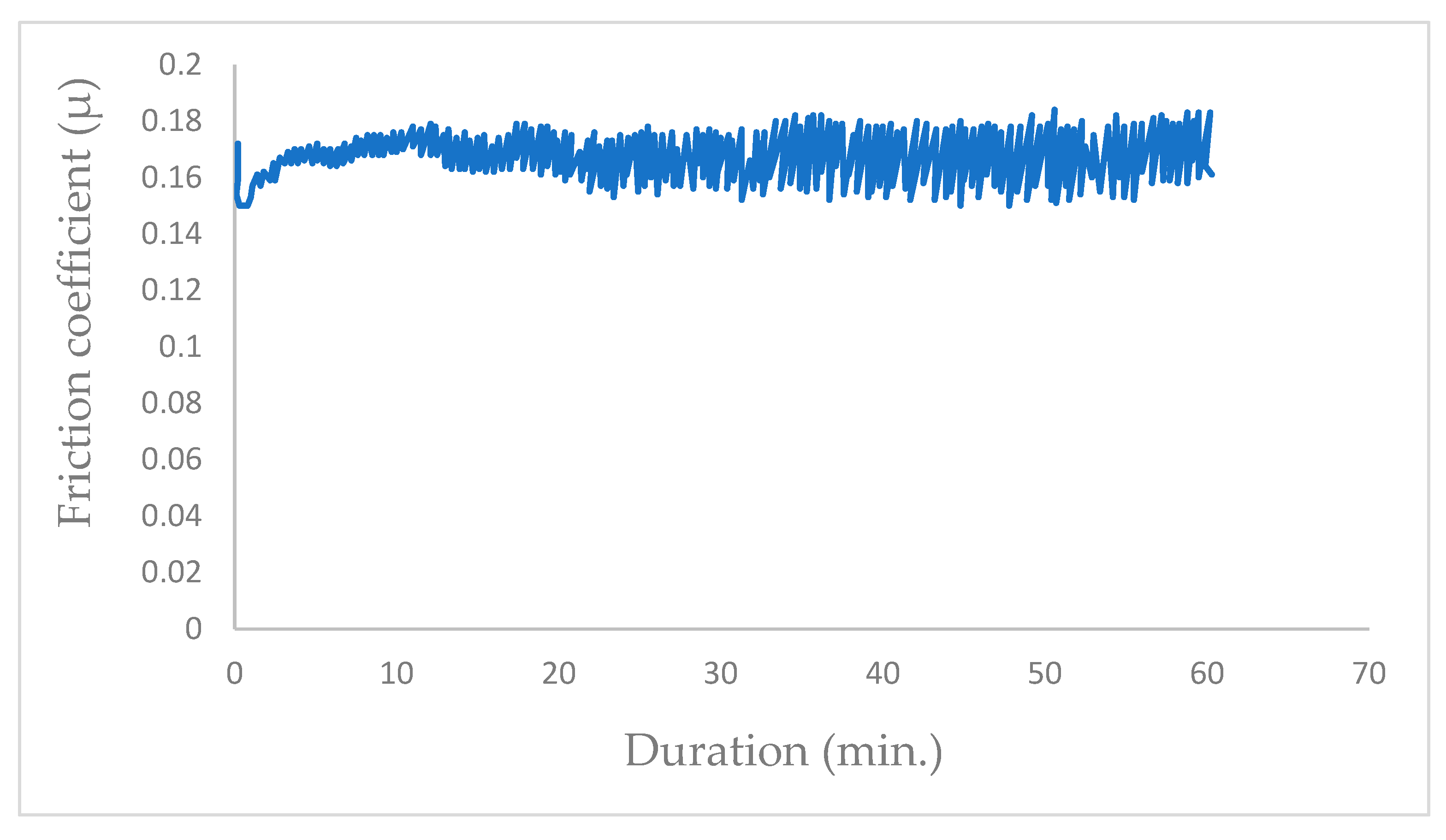
In a vacuum environment, the wear of the coating remains minimal; however, sliding under water vapor induces excessive wear damage to the coating. Bui [15], in his Ph.D. work with the same tribometer, recorded the average friction coefficient obtained in ambient air with low relative humidity (RH = 25%) at µ = 0.17 (Figure 9).
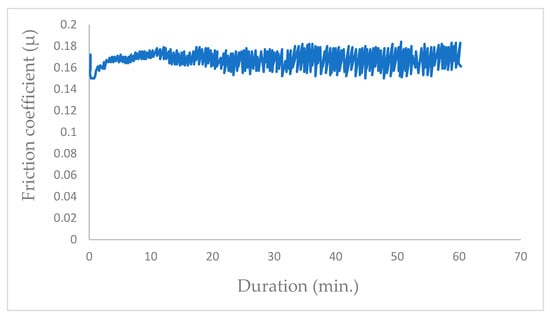
Figure 9.
Frictiogram of coupled steel/MoS2 in ambient air with RH = 25% [15].
In summary, our experimental findings indicate that the achievement of superlubricity in MoS2 does not occur instantaneously upon the initial sliding of the pin. The crystalline structure of molybdenum disulfide (MoS2) is layered, and it serves as a solid lubricant material. The lubricating properties, as documented through experimental observations in dry air and controlled gas environments and extensively reported in various publications [5,6,7,8,15,16,17,18,19,20,21,22,23,24,25], stem from the weak Van der Waals bonds existing between sulfur layers. Additionally, these properties arise from the mechanical alignment of the coating film parallel to the sliding direction, facilitated by the repeated action of a hemispherical rubber after several cycles of passage solely on the coating. A color change in the coating is observed, changing from its initially black and rough form before the tribological test. During the vacuum friction process, it gradually transforms to have a shiny, less rough appearance, as demonstrated by profilometry and X-ray analysis.
3.5. Evolution of the Static Friction Coefficient During the Gas Pumping from the Vacuum Chamber
During the sliding test, the measurement of dynamic tangential frictional shear force was conducted using half-bridge strain gauges affixed to a steel arm (Figure 1b). Upon halting the dynamic friction test while maintaining the gas around the tribocontact, the steel arm retained its deformation induced by the friction force during sliding, sustained by the static friction force.
The blade used for measuring keeps its amplitude of dynamic deformation when the test is stopped by bending, because of the tangential force of friction. This deformation is ensured by the tangential force of static friction. This makes it possible to measure the static friction coefficient of the contact under a given gaseous environment.
Stopping a tribological sliding test carried out in gas makes it possible to maintain a static friction force identical to the dynamic friction force recorded during the test as long as the gas environment remains identical around the tribocontact. However, pumping gas to evacuate the chamber results in a significant reduction in the coefficient of static friction. This result highlights the influence of gas insertion or adsorption in the layered structure of MoS2 because the modification of the tangential shear force takes place on the surface and in the subsurface of the contact.
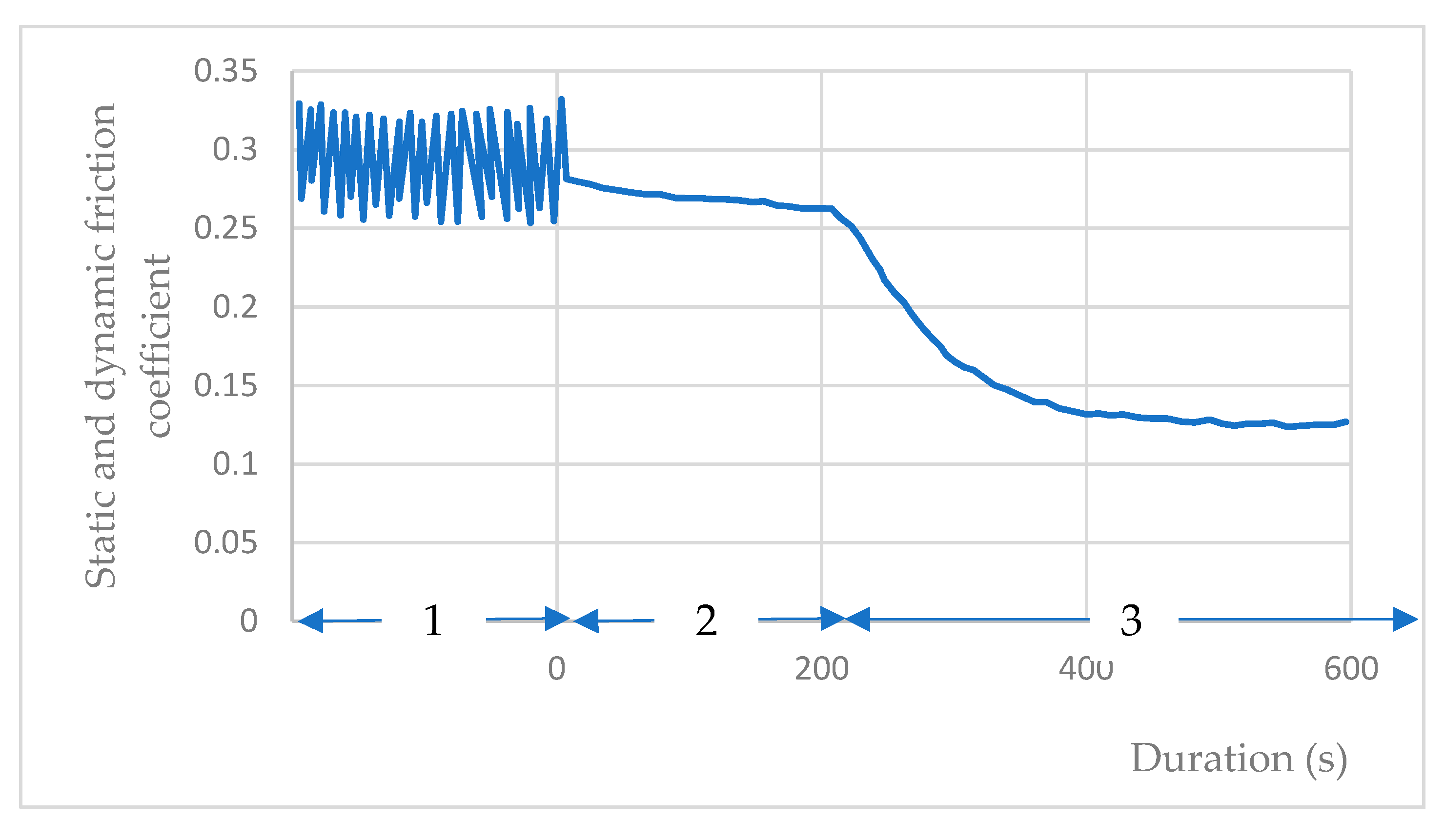
When the sliding friction test under water vapor is stopped at µ = 0.3, and the water vapor is subsequently evacuated from the chamber, a gradual reduction in the bending deformation of the steel arm becomes evident. This corresponds to a diminishing static friction force, resulting in a decline in the static friction coefficient from µs = 0.3 to µs = 0.14 under vacuum at 10−3 Pa (Figure 10). The start of phase 3 corresponds to the pumping of water vapor from the enclosure. The gas immediately pressure drops, through the pumping group, to 10−1 Pa; then, at the end of phase 3, the pressure is 10−3 Pa.
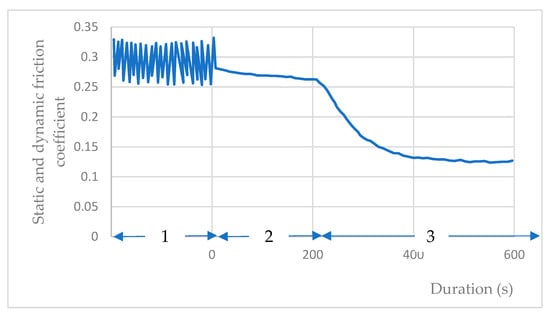
Figure 10.
Evolution of the friction coefficient of the coating during gas pumping: 1—dynamic friction coefficient under water vapor during the test; 2—static friction coefficient at the end of the test without pumping water vapor; 3—evolution of static friction coefficient during stopping the sliding and with pumping water vapor.
To validate the impact of gas pumping on the static friction coefficient of the coating, the phenomenon was systematically replicated. Additionally, dynamic friction tests were conducted between steel surfaces without a MoS2 coating. Notably, after cessation, the static contact maintained its dynamic deformation without reverting to a lower static friction force.
Upon introducing argon or helium gas following the cessation of the dynamic sliding test involving the coated contact, a significant reduction in the tangential force of static friction was observed. Consequently, the static friction coefficient decreased from µs = 0.04 to µs = 0.02 for argon gas, and from µs = 0.03 to µs = 0.02 for helium gas.
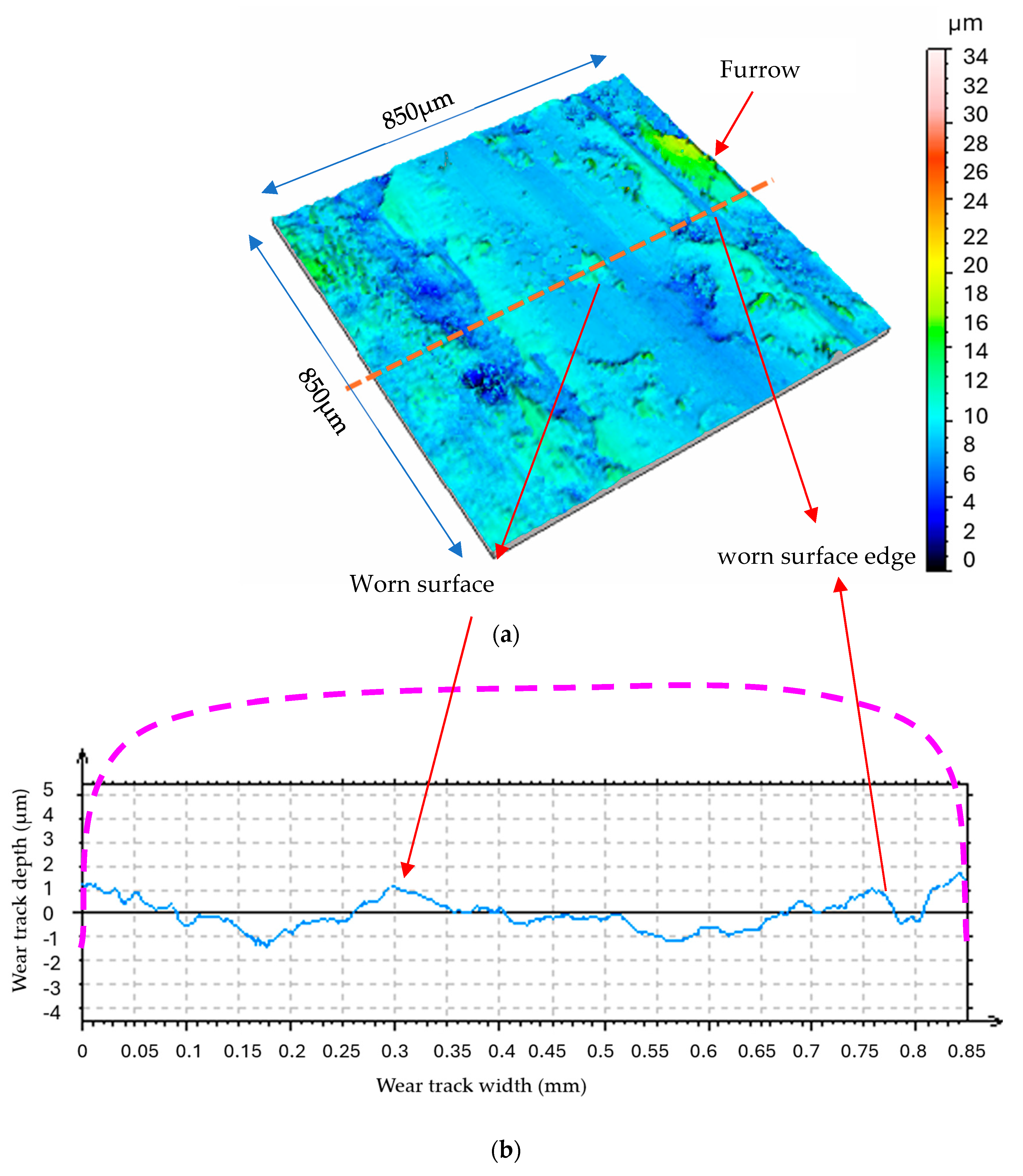
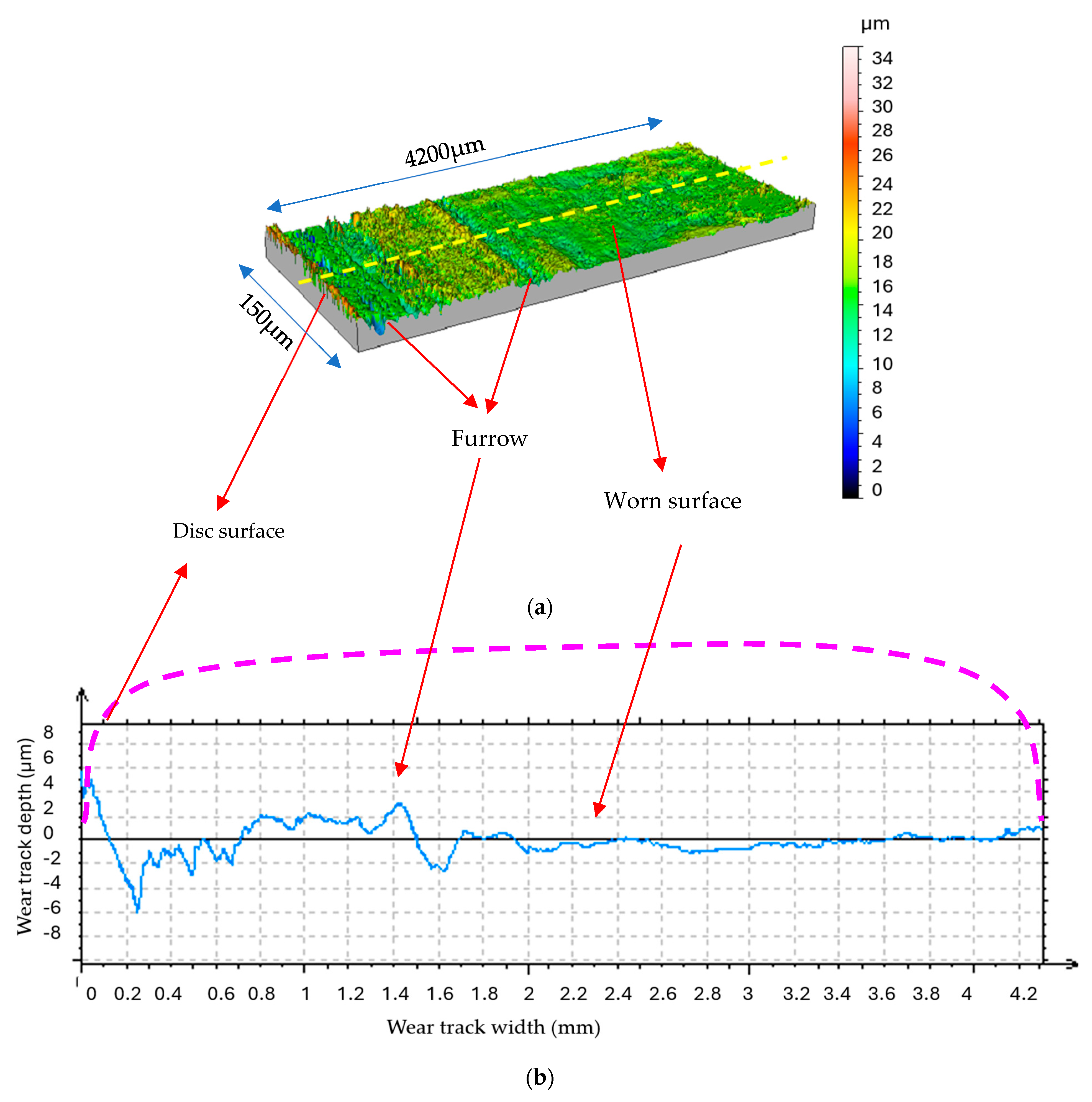
The worn surfaces utilized under water vapor display a random and rough profile, as evident in Figure 11 and Figure 12. The MoS2 coating was completely removed in certain areas, thereby exposing the underlying steel substrate. The steel substrate, being harder and more wear-resistant, appears as bright spots where the coating is no longer present (Figure 11).

Figure 11.
Wear track of coated steel disk, rubbed under water vapor environment (LOM image).
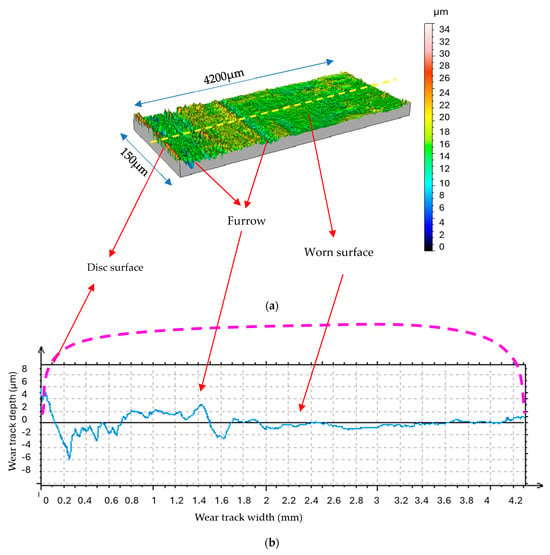
Figure 12.
Morphology and profile of the sliding surface under water vapor (N = 4.6N, v = 0.4 m/s, t = 40 mn); (a) 3D morphology; (b) 2D profile of worn disk surface [15].
The introduction of any gaseous contamination to molybdenum disulfide results in an elevation of the friction coefficient compared to the values observed under high vacuum, a phenomenon experimentally demonstrated and corroborated by numerous publications [39].
The contamination of molybdenum disulfide by water vapor significantly impacts its layered structure and weak Van der Waals-type interplane electronic bonds. This effect induces a notable increase in the material’s yield shear stress, hindering its organization and basal reorientation in the sliding direction. The friction coefficient rises from 0.01 under vacuum to 0.3 in the presence of even trace amounts of water vapor. The friction of MoS2 under water vapor is influenced by both physisorption and chemisorption.
It is worth noting that any gaseous contamination of molybdenum disulfide consistently leads to an augmented friction coefficient compared to values obtained under high vacuum, a finding supported by our experimental evidence and documented in [46].
4. Conclusions
Friction tests were carried out under various conditions: in vacuum (P = 10−5 Pa), under pure helium (P = 105 Pa), under pure argon gases (105 Pa), in the presence of water vapor (102 Pa), and in ambient air.
These comprehensive tribological investigations clearly demonstrate the profound sensitivity of molybdenum disulfide’s frictional behavior to the surrounding gaseous environment. While high vacuum conditions unveil its intrinsic superlubricating properties (μ ≈ 0.01), even inert gases such as helium and argon significantly alter this behavior, increasing friction and modifying the surface structure. Water vapor emerges as a critical contaminant, triggering tribochemical reactions that degrade lubrication. X-ray diffraction and gas analysis further reveal the structural and chemical mechanisms underpinning these effects. The correlation between frictional response and Van der Waals interactions offers a quantitative framework to understand environmental influence. Future tests in complex gas mixtures are essential to pinpoint critical partial pressures and to simulate operational conditions more accurately. Collectively, these results provide valuable insight into the design and application of MoS2-based solid lubricants in varied atmospheric contexts.
Author Contributions
Conceptualization, H.Z.; methodology, S.T. and H.S.B.; software, S.T.; writing—original draft preparation, H.Z., H.S.B. and C.R.; writing—review and editing, C.R., S.T., K.B. and M.A.; visualization, C.R. and K.B.; supervision, H.Z.; investigation, M.A.; project administration, H.Z.; funding acquisition, H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed from Institut Pprime’s own sources.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the correspondence contact information. This change does not affect the scientific content of the article.
References
- Li, Q.; Pu, J.; Su, F. The study on modulation mechanism for anti-corrosion and low-friction of R0-MoS2 by Ti-/Pb-doped model. Tribol. Int. 2024, 194, 109491. [Google Scholar] [CrossRef]
- Mukhtar, S.H.; Gulzar, A.; Saleem, S.; Wani, M.F.; Sehgal, R.; Yakovenko, A.A.; Goryacheva, I.G.; Sharma, M.D. Advances in development of solid lubricating MoS2 coatings for space applications: A review of modeling and experimental approaches. J. Tribol. Int. 2024, 19, 109194. [Google Scholar] [CrossRef]
- Vazirisereshk, M.R.; Martini, A.; Strubbe, D.A.; Baykara, M.Z. Solid lubrication with MoS2: A review. Lubricants 2019, 7, 57. [Google Scholar] [CrossRef]
- Martin, J.M.; Erdemir, A. Superlubricity: Friction’s vanishing act. Phys. Today 2018, 71, 40–46. [Google Scholar] [CrossRef]
- Chen, Z.; He, X.; Xiao, C.; Kim, S.H. Effect of Humidity on Friction and Wear—A Critical Review. Lubricants 2018, 6, 74. [Google Scholar] [CrossRef]
- Martin, J.M.; Donnet, C.; Le Mogne, T.; Epicier, T. Superlubricity of molybdenum disulfide. Phys. Rev. B 1993, 48, 10583–10588. [Google Scholar] [CrossRef]
- Donnet, C.; Martin, J.; Le Mogne, T.; Belin, M. Superlow friction of oxygene-free MoS2 coatings in ultrahigh vacuum. Surf. Coat. Technol. 1993, 62, 406–411. [Google Scholar] [CrossRef]
- Takahashi, N.; Shiojiri, M.; Enomoto, S. High resolution transmission electron microscope observation of stacking faults of molybdenum disulfide in relation to lubrication. Wear 1991, 146, 107–123. [Google Scholar] [CrossRef]
- Doyle, S.E.; Mattern, N.; Pitschk, W.; Weise, G.; Kraut, D.; Bauer, H.D. X-ray diffraction studies of the structure of molybdenum sulphide thin films. Thin Solid Film 1994, 245, 255–259. [Google Scholar] [CrossRef]
- Chermette, H.; Rogemond, F.; El Beqqali, O.; Paul, J.F.; Donnet, C.; Martin, J.M.; Le Mogne, T. Lubricating properties of molybdenum disulphur: A density functional theory study. Surf. Sci. 2001, 472, 97–110. [Google Scholar] [CrossRef]
- Lieber, M.C.; Kim, Y. Characterization of structural, electronic and tribological properties of metal dichalcogenides by scanning probe microscopies. Thin Solid Film 1991, 206, 355–359. [Google Scholar] [CrossRef]
- Gradt, T.; Schneider, T. Tribological Performance of MoS2 Coatings in Various Environments. Lubricants 2016, 4, 32. [Google Scholar] [CrossRef]
- Babuska, T.F.; Curry, J.F.; Dugger, M.T.; Lu, P.; Xin, Y.; Klueter, S.; Kozen, A.C.; Grejtak, T.; Brandon, A.; Krick, B.A. Role of Environment on the Shear-Induced Structural Evolution of MoS2 and Impact on Oxidation and Tribological Properties for Space Applications. ACS Appl. Mater. Interfaces 2022, 14, 13914–13924. [Google Scholar] [CrossRef] [PubMed]
- Babuska, T.F.; Krick, B.A.; Argibay, N.; Dugger, M.T.; Chandross, M.; Curry, J.F. Revisiting the dwell effect on friction behavior of molybdenum disulfide. Wear 2023, 526, 204876. [Google Scholar] [CrossRef]
- Bui, H.S. Influence de L’environnement Sur le Comportement Thermomécanique et Tribologique du Bisulfure de Molybdène MoS2. Ph.D. Thesis, University of Poitiers, Poitiers, France, 2012. [Google Scholar]
- Pritchard, C.; Midgley, J.W. The effect of humidity on the friction and life of unbonded molybdenum disulfide films. Wear 1969, 13, 39–50. [Google Scholar] [CrossRef]
- Holinski, R.; Gansheimer, J. A study of the lubricating mechanism of molybdenum disulfide. Wear 1972, 19, 329–342. [Google Scholar] [CrossRef]
- Donnet, C.; Martin, J.; Le Mogne, T.; Belin, M. Super-low friction of MoS2 coatings in various environments. Tribol. Int. 1996, 29, 123–128. [Google Scholar] [CrossRef]
- Fleischauer, P.D.; Lince, J.R. A comparison of oxidation and oxygen substitution in MoS2 solid film lubricants. Tribol. Int. 1999, 32, 627–636. [Google Scholar] [CrossRef]
- Woollam, J.A.; Somoano, B.R. Physics and chemistry of MoS2 Intercalation compounds. Mater. Sci. Eng. 1977, 31, 289–295. [Google Scholar] [CrossRef]
- Vierneusel, B.; Schneider, T.; Tremmel, S.; Wartzack, S.; Gradt, T. Humidity resistant MoS2 coatings deposited by unbalanced magnetron sputtering. Surf. Coat. Technol. 2013, 235, 97–107. [Google Scholar] [CrossRef]
- Khare, H.S.; Burris, D.L. The effects of environmental water and oxygen on the temperature-dependent friction of sputtered molybdenum disulfide. Tribol. Lett. 2013, 52, 485–493. [Google Scholar] [CrossRef]
- Colas, G.; Saulot, A.; Regis, E.; Berthier, Y. Investigation of crystalline and amorphous MoS2 based coatings: Towards developing new coatings for space applications. Wear 2015, 330, 448–460. [Google Scholar] [CrossRef]
- Curry, J.F.; Argibay, N.; Babuska, T.F.; Nation, B.; Martini, A.; Strandwitz, N.C.; Dugger, M.T.; Krick, B.A. Highly Oriented MoS2 Coatings: Tribology and Environmental Stability. Tribol. Lett. 2016, 64, 11. [Google Scholar] [CrossRef]
- Curry, J.F.; Babuska, T.F.; Brumbach, M.T.; Argibay, N. Temperature Dependent Friction and Wear of MoS2/Sb2O3/Au Nanocomposites. Tribol. Lett. 2016, 64, 2–5. [Google Scholar] [CrossRef]
- Available online: https://www.parker.com/content/dam/Parker-com/Literature/Instrumentation-Products-Division/Technical-Articles/Moly-Coated-NutTechnology--White-Paper.pdf (accessed on 1 September 2016).
- Efeoglu, I.; Bulbul, F. Effect of crystallographic on friction and wear of MoS2. Wear 2005, 258, 852–860. [Google Scholar] [CrossRef]
- Spalvins, T. Deposition of MoS2 Films by Physical Sputtering and Their Lubrication Properties in Vacuum. Asle Trans. 1969, 12, 36–43. [Google Scholar] [CrossRef]
- Hao, R.; Tedstone, A.A.; Lewis, D.J.; Warrens, C.P.; West, K.R.; Howard, P.; Gaemers, S.; Dillon, S.J.; O’Brien, P. Property Self-Optimization During Wear of MoS2. ACS Appl. Mater. Interfaces 2017, 9, 1953–1958. [Google Scholar] [CrossRef]
- Torres, H.; Rodríguez Ripoll, M.; Prakash, B. Tribological behaviour of self-lubricating materials at high temperatures. Int. Mater. Rev. 2018, 63, 309–340. [Google Scholar] [CrossRef]
- Baykara, M.Z.; Vazirisereshk, M.R.; Martini, A. Emerging superlubricity: A review of the state of the art and perspectives on future research. Appl. Phys. Rev. 2018, 5, 041102. [Google Scholar] [CrossRef]
- Lavini, F.; Calo, A.; Gao, Y.; Albisetti, E.; Li, T.D.; Cao, T.F.; Li, G.Q.; Cao, L.Y.; Aruta, C.; Riedo, E. Friction and work function oscillatory behavior for an even and odd number of layers in polycrystalline MoS2. Nanoscale 2018, 10, 8304–8312. [Google Scholar] [CrossRef]
- Fang, L.; Liu, D.M.; Guo, Y.Z.; Liao, Z.M.; Luo, J.B.; Wen, S.Z. Thickness dependent friction on few-layer MoS2, WS2, and WSe2. Nanotechnology 2017, 28, 24503. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.A.; Gan, X.H.; Lang, H.J.; Yu, K.; Ding, S.Y.; Peng, Y.T.; Yi, W.M. Anisotropic nanofriction on MoS2 with different thicknesses. Tribol. Int. 2019, 134, 308–316. [Google Scholar] [CrossRef]
- Cho, D.H.; Jung, J.; Kim, C.; Lee, J.; Oh, S.-D.; Kim, K.-S.; Lee, C. Comparison of Frictional Properties of CVD-Grown MoS2 and Graphene Films under Dry Sliding Conditions. Nanomaterials 2019, 9, 293. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Mishchenko, A.; Carvalho, A.; Castro Neto, A.H. 2D materials and van der Waals heterostructures. Science 2016, 353, 461. [Google Scholar] [CrossRef]
- Yu, H.; Liao, M.; Zhao, W.; Liu, G.; Zhou, X.J.; Wei, Z.; Xu, X.; Liu, K.; Hu, Z.; Deng, K.; et al. Wafer-Scale Growth and Transfer of Highly-Oriented Monolayer MoS2 Continuous Films. ACS Nano 2017, 11, 12001–12007. [Google Scholar] [CrossRef]
- Huali, H.; Zhiyuan, Q.; Fanming, M.; Zhongtao, C. Tribological performances of graphite–MoS2 coating at various high temperatures. Proc. Inst. Mech. Eng. Part J J. Eng. Trib. 2019, 233, 1888–1902. [Google Scholar] [CrossRef]
- Gopal, T.J.; Pandey, C.; Mahapatra, M.M.; Mulik, R.S. Thermal barrier coatings—A state of the art review. Met. Mater. Int. 2021, 27, 1947–1968. [Google Scholar] [CrossRef]
- Gardos, M.N. Anomalous wear behavior of MoS2 films in moderate vacuum and dry nitrogen. Tribol. Lett. 1995, 1, 67–85. [Google Scholar] [CrossRef]
- G99-23-2023; Standard Test Method for Wear and Friction Testing with a Pin-on-Disk or Ball-on-Disk Apparatus. ASTM: West Conshohocken, PA, USA, 2023.
- Atkins, P.; Jones, L.; Laverman, L. Chemical Principles: The Quest for Insight, 7th ed.; W.H. Freeman and Company: Dallas, TX, USA, 2016. [Google Scholar]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Fusaro, R.L. Lubrication and Failure Mechanisms of Molybdenum Disulfide Films II—Effect of Substrate, Roughness; Nasa Technical paper; National Aeronautics and Space Administration, Scientific and Technical Information Office: Hampton, VA, USA, 1978. [Google Scholar]
- Gee de, J.H.; Salomon, G.; Zaat, J.H. Mechano-chemical factors in MoS2 film lubrication. Wear 1964, 7, 87–101. [Google Scholar] [CrossRef]
- Bobbitt, N.S.; Curry, J.F.; Babuska, T.F.; Chandross, M. Water adsorption on MoS2 under realistic atmosphere conditions and impact on tribology. RCS Adv. 2024, 14, 4717–4729. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).