Synergistic Corrosion Inhibition and UV Protection via TTA-Loaded LDH Nanocontainers in Epoxy Coatings
Abstract
1. Introduction
2. Experiments
2.1. Materials
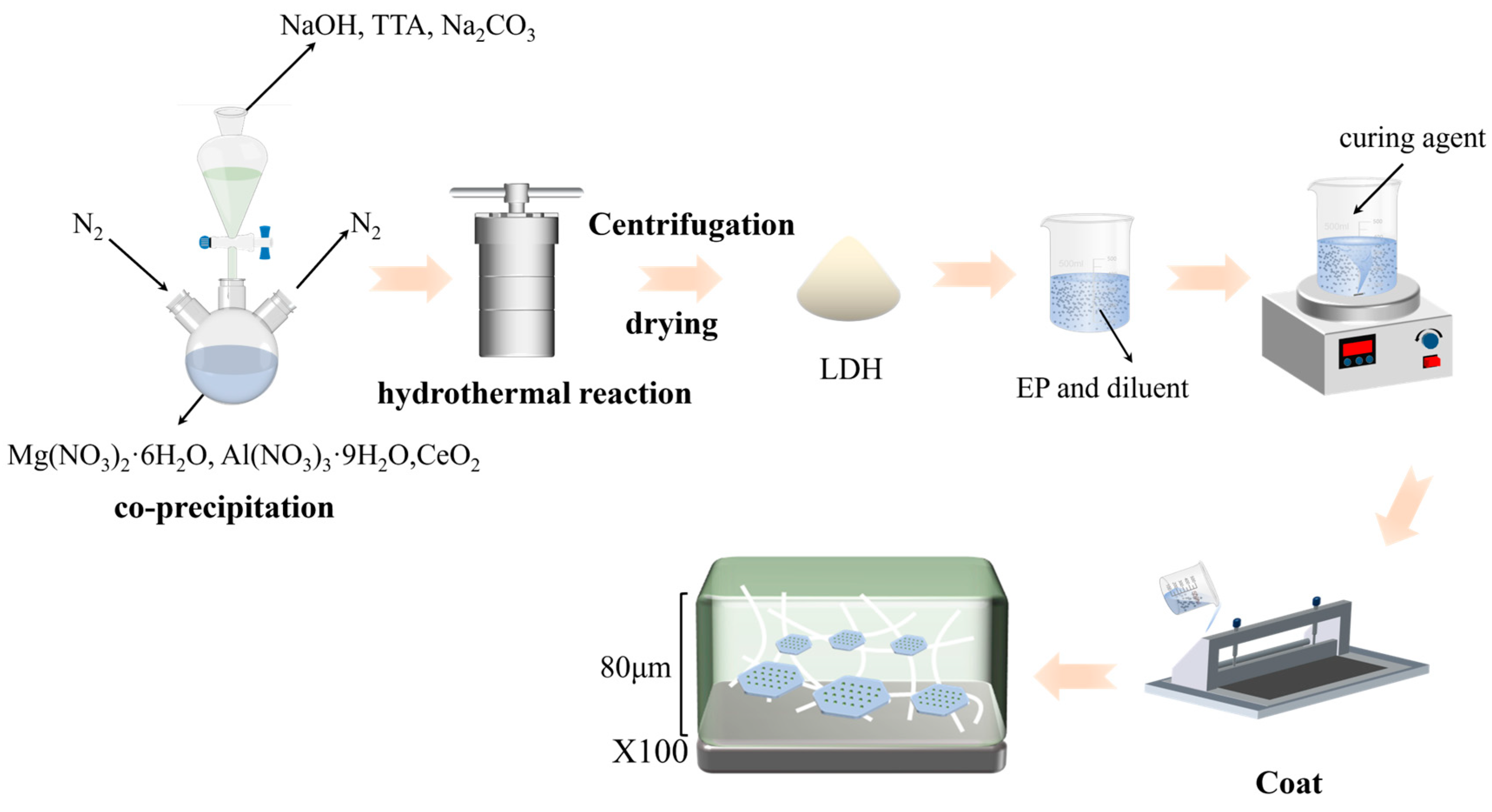
2.2. MgAl-TTA LDH Preparation
2.3. MgAl-TTA LDH@CeO2 Preparation
2.4. Epoxy-Based MgAl-TTA LDH and MgAl-TTA LDH@CeO2 Composite Coating Preparation
2.5. Characterization
2.6. Evaluation of Corrosive Properties
3. Results and Discussion
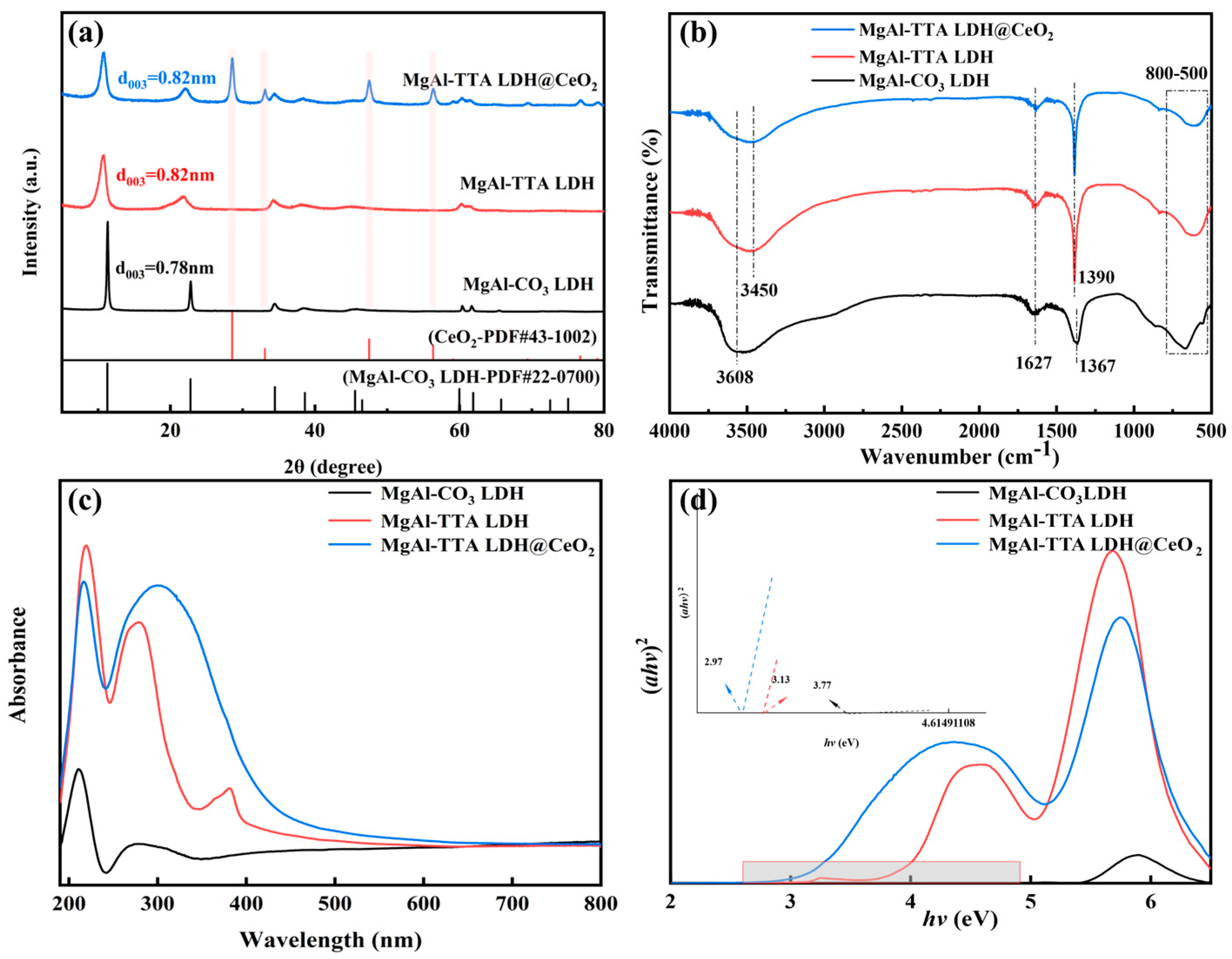
3.1. Characterization
3.2. Effects of Hydrothermal Conditions
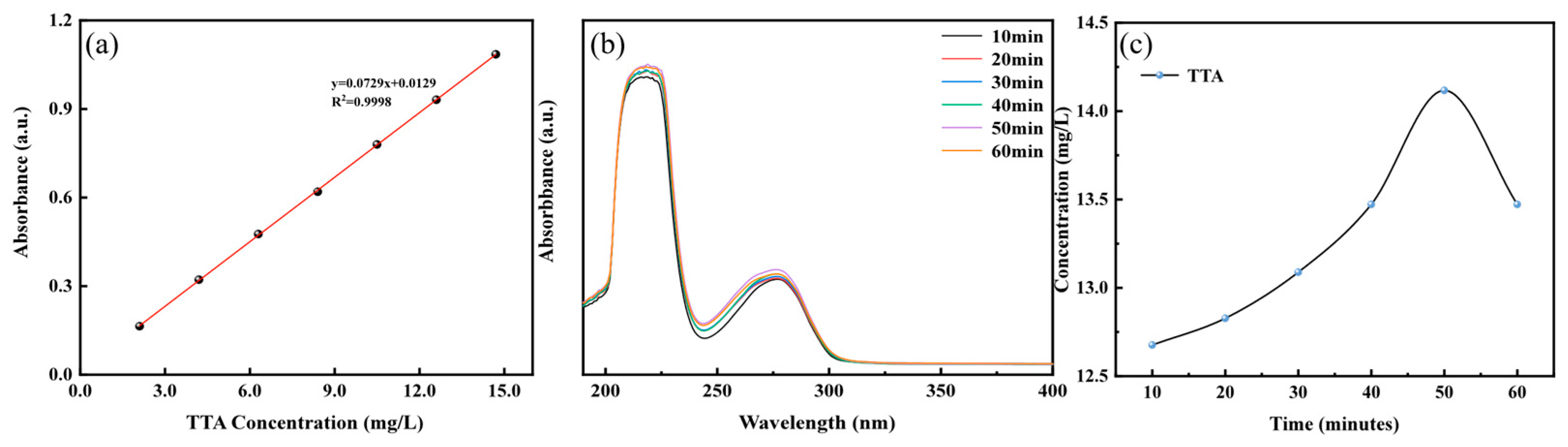
3.3. Corrosion Inhibitor Release Kinetics
3.4. Dynamic Polarization Curve
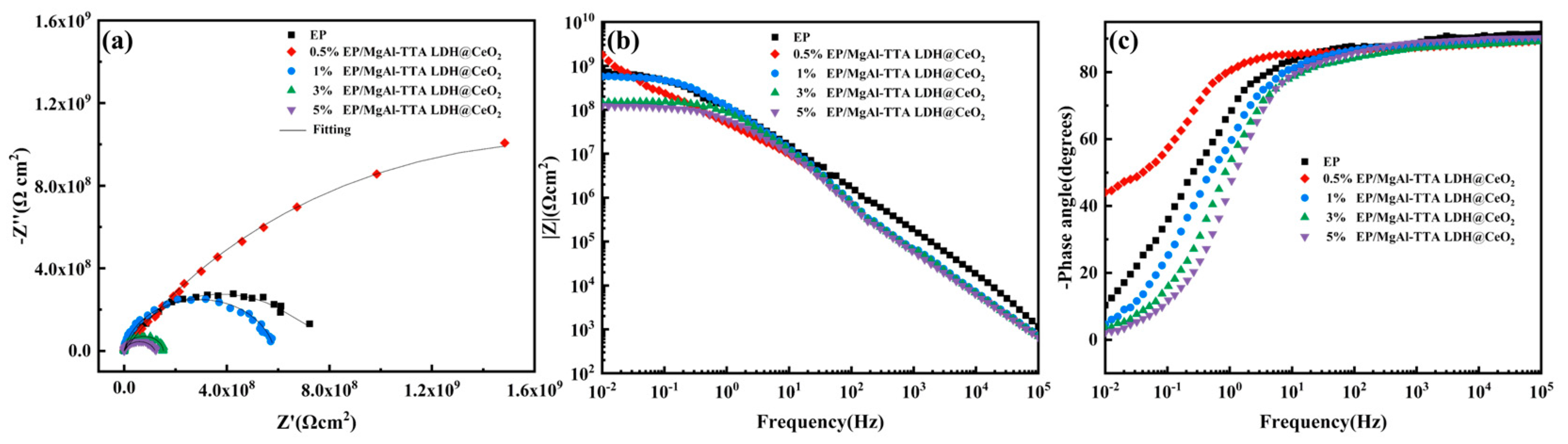
3.5. Effect of the MgAl-TTA LDH@CeO2 Addition Amount
3.6. Coating Durability Testing
3.7. Anti-Aging Testing
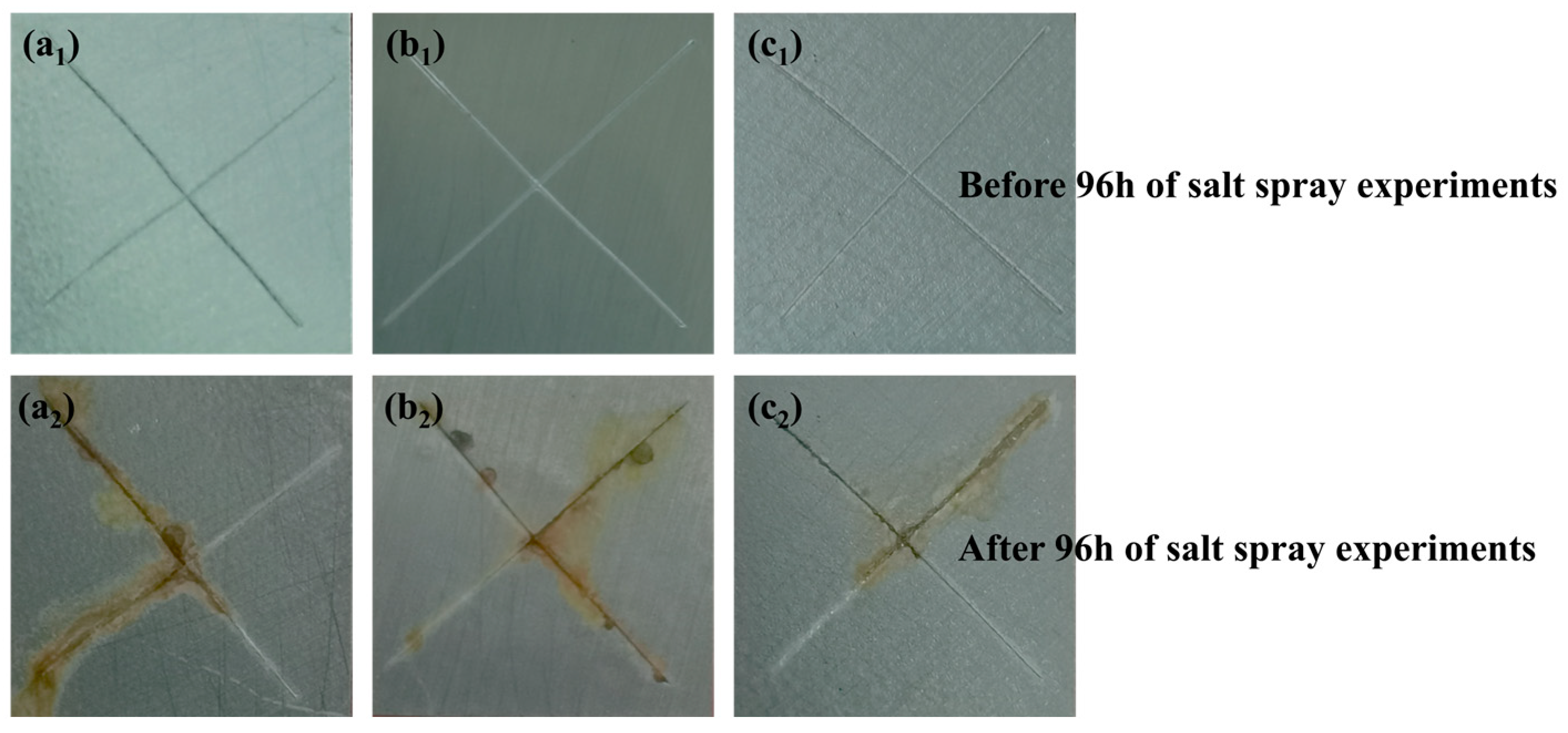
3.8. Salt Spray Resistance Testing
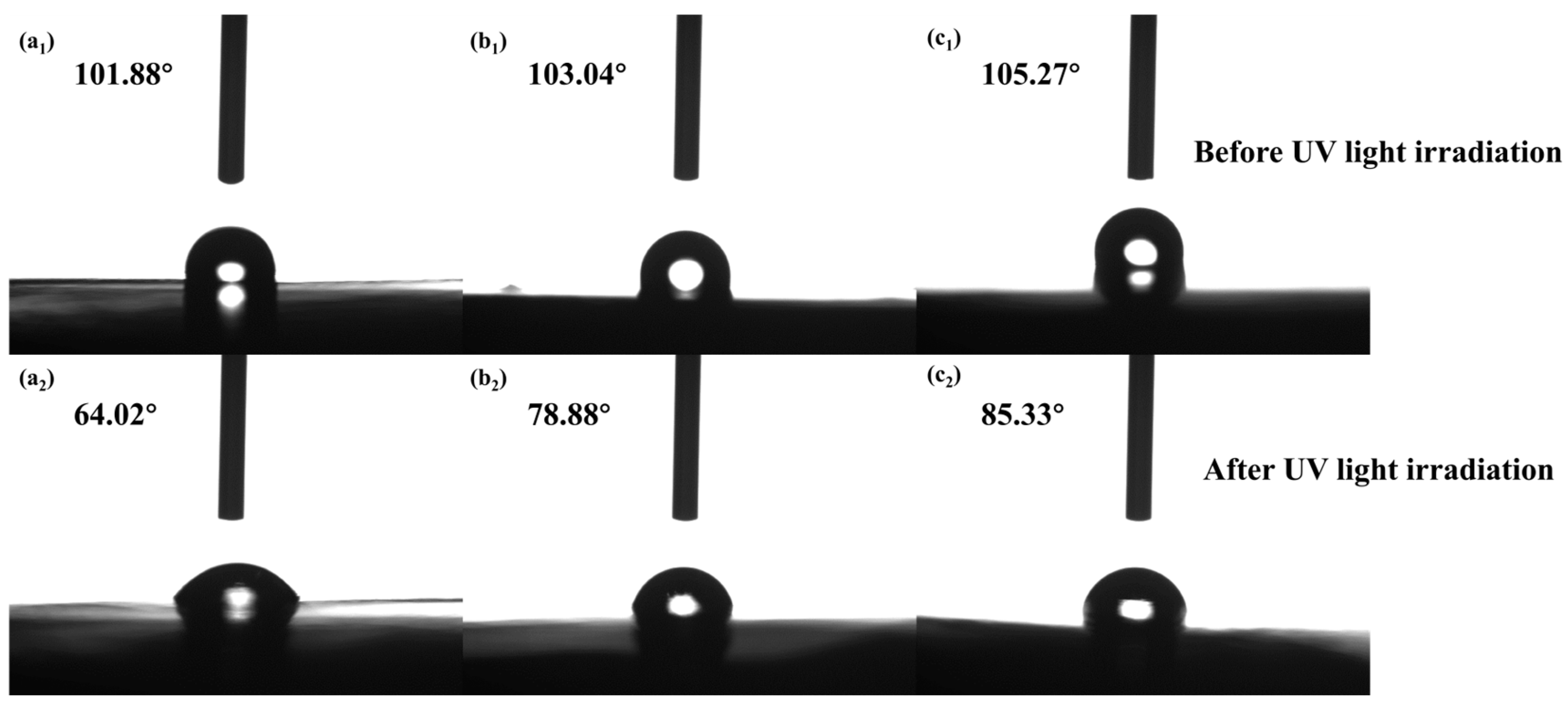
3.9. Contact Aangle Testing
3.10. Adhesion Strength Testing
3.11. Mechanisms
4. Conclusions
- (1)
- Characterization using techniques such as XRD, SEM, XPS, UV–vis DRS, and FT-IR confirmed that TTA was successfully intercalated into the LDH layers, and CeO2 was successfully loaded onto the LDH surface. The preparation process of MgAl-TTA LDH@CeO2 was optimized through XRD, with the optimal process parameters identified as a hydrothermal temperature of 140 °C and a hydrothermal time of 48 h;
- (2)
- The release results of the corrosion inhibitor indicated that the MgAl-TTA LDH@CeO2 system has a fast TTA release rate during the early stage of immersion in NaCl solution, reaching a peak at around 50 min and reaching dynamic equilibrium after 60 min;
- (3)
- The optimal addition amount of MgAl-TTA LDH@CeO2 in the coating is 0.5%. At this optimal addition amount, the EP/MgAl-TTA LDH@CeO2 coating exhibits good adhesion. After 240 h of UV aging, the interface impedance of the EP/MgAl-TTA LDH@CeO2 coating is 3.10 × 108 Ω·cm2, which is four orders of magnitude higher than that of the EP coating. Compared to the EP/MgAl-TTA LDH coating, the EP/MgAl-TTA LDH@CeO2 coating has a higher water contact angle and better hydrophobicity. After immersion in corrosion solution for 60 days, the impedance of the EP/MgAl-TTA LDH@CeO2 coating is 3.88 × 108 Ω·cm2, which is two orders of magnitude higher than that of the EP coating. After 96 h of salt spray testing, the corrosion products at the scratch sites of the EP/MgAl-TTA LDH@CeO2 coating are significantly fewer than those on the EP/MgAl-TTA LDH coating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, D.; Yan, Z.; Gao, L.; Xin, Z.; Zhu, Y.; Wu, W. Corrosion behavior of AA5052 aluminum alloy in the presence of heavy metal ions in 3.5% NaCl solution under negative pressure. Desalination 2024, 570, 117082. [Google Scholar] [CrossRef]
- Shi, Y.; Nie, Y.; Xu, H.; Bi, P.; Chen, B.; Hou, X.; Ma, H.; Cao, M. Fabrication of silane-based surface coating with Ag/TiO2 hybridization and its anti-corrosion performance on metal surface. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132241. [Google Scholar] [CrossRef]
- Rao Kotni, T.; Pandey, S.; Shekhar, S.; Ranjan, R.; Sarthi Srivastava, P. Corrosion of different metals/alloys in soil environment: A review. Mater. Today Proc. 2023, 102, 203–205. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, T.; He, Y.; Wang, Y.; Bi, Y. Corrosion and aging of organic aviation coatings: A review. Chin. J. Aeronaut. 2023, 36, 1–35. [Google Scholar] [CrossRef]
- Croll, S.G. Stress and embrittlement in organic coatings during general weathering exposure: A review. Prog. Org. Coat. 2022, 172, 107085. [Google Scholar] [CrossRef]
- Chen, Z.; Scharnagl, N.; Zheludkevich, M.L.; Ying, H.; Yang, W. Micro/nanocontainer-based intelligent coatings: Synthesis, performance and applications—A review. Chem. Eng. J. 2023, 451, 138582. [Google Scholar] [CrossRef]
- Feng, C.; Zhu, L.; Cao, K.; Yu, Z.; Song, Y. Difunctional Silicon Dioxide Combined with Graphene Oxide Nanocomposite to Enhance the Anticorrosion Performance of Epoxy Coatings. ACS Omega 2022, 7, 24134–24144. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, C.; Li, Y.; Wang, C.; Shen, T.; Wu, C.; Hu, Z.; Cheng, D.; Yang, H. Enhanced corrosion resistance and self-healing effect of sol–gel coating incorporating one-pot-synthesized corrosion inhibitor-encapsulated silica nanocontainers. J. Sol-Gel Sci. Technol. 2022, 104, 78–90. [Google Scholar] [CrossRef]
- Chen, X.; Hu, D.; Zhang, Z.; Ma, W. In situ assembly of halloysite nanotubes@cerium oxide nanohybrid for highly UV-shielding and superhydrophobic coating. J. Alloys Compd. 2019, 811, 151986. [Google Scholar] [CrossRef]
- Cao, Y.; Zheng, D.; Li, X.; Lin, J.; Wang, C.; Dong, S.; Lin, C. Enhanced Corrosion Resistance of Superhydrophobic Layered Double Hydroxide Films with Long-Term Stability on Al Substrate. ACS Appl. Mater. Interfaces 2018, 10, 15150–15162. [Google Scholar] [CrossRef]
- Wang, Z.; Xiong, Z.; Li, X.; Wang, D.; Wang, Y.; Wu, S.; Ying, L.; Wang, Z.; Wang, G. Effect of modified MgAl-LDH coating on corrosion resistance and friction properties of aluminum alloy. Chin. J. Chem. Eng. 2023, 63, 81–95. [Google Scholar] [CrossRef]
- Tabish, M.; Zhao, J.; Wang, J.; Anjum, M.J.; Qiang, Y.; Yang, Q.; Mushtaq, M.A.; Yasin, G. Improving the corrosion protection ability of epoxy coating using CaAl LDH intercalated with 2-mercaptobenzothiazole as a pigment on steel substrate. Prog. Org. Coat. 2022, 165, 106765. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, H.; Pan, X.; Li, J.; Chen, B.; Gong, W.; Yang, Q.; Luo, X.; Zeng, H.; Liu, Y. Smart waterborne composite coating with passive/active protective performances using nanocontainers based on metal organic frameworks derived layered double hydroxides. J. Colloid Interface Sci. 2022, 619, 132–147. [Google Scholar] [CrossRef]
- Tarzanagh, Y.J.; Seifzadeh, D.; Samadianfard, R. Combining the 8-hydroxyquinoline intercalated layered double hydroxide film and sol—Gel coating for active corrosion protection of the magnesium alloy. Int. J. Miner. Metall. Mater. 2022, 29, 536–546. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, S.; Liu, Y.; Huangfu, H.; Guo, X.; Zhang, J. Enhancement of corrosion resistance of AZ31B magnesium alloy by preparing MgAl-LDHs coatings modified with imidazolium based dicationic ionic liquids. Surf. Coat. Technol. 2022, 440, 128504. [Google Scholar] [CrossRef]
- Fathabadi, H.E.; Ghorbani, M.; Ghartavol, H.M. Corrosion Inhibition of Mild Steel with Tolyltriazole. Mater. Res. 2021, 24. [Google Scholar] [CrossRef]
- An, K.; Sui, Y.; Qing, Y.; Yang, C.; Long, C.; Wang, L.; Liu, C. Synergistic reinforcement coating with anti-corrosion and UV aging resistance by filling modified CeO2 nanoflakes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126904. [Google Scholar] [CrossRef]
- GB/T 10125-2021; Corrosion Tests in Artificial Atmospheres—Salt Spray Tests. Standardization Administration of China: Beijing, China, 2021.
- GB/T 9286-2021; Paints and Varnishes—Cross-Cut Test. Standardization Administration of China: Beijing, China, 2021.
- Tabish, M.; Zhao, J.; Kumar, A.; Yan, J.; Wang, J.; Shi, F.; Zhang, J.; Peng, L.; Mushtaq, M.A.; Yasin, G. Developing epoxy-based anti-corrosion functional nanocomposite coating with CaFe-Tolyl-triazole layered double hydroxide@g-C3N4 as nanofillers on Q235 steel substrate against NaCl corrosive environment. Chem. Eng. J. 2022, 450, 137624. [Google Scholar] [CrossRef]
- Guo, X.; Fan, Z.; Wang, Y.; Jin, Z. CeO2 nanoparticles dispersed on CoAl-LDH hexagonal nanosheets as 0D/2D binary composite for enhanced photocatalytic hydrogen evolution. Surf. Interfaces 2021, 24, 101105. [Google Scholar] [CrossRef]
- Zhang, Q. Preparation and Corrosion Resistance of Superhydrophobic Ni- P-Al2O3 Coating on Pipeline Steel in simulated alkaline soil solution. Int. J. Electrochem. Sci. 2021, 16, 211234–211248. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, Y.; Liao, W.; Li, J.; Yin, C.; Zhou, J.; Chen, Z.; Zhang, P.; Ning, Z. Preparation and corrosion resistance of superhydrophobic Ni-Co-Al(2)O(3) coating on X100 steel. RSC Adv. 2023, 13, 6847–6860. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xue, Z.; Sun, W.; Chu, J.; Wang, Q.; Tong, L.; Wang, K. Bio-inspired self-healing MXene/polyurethane coating with superior active/passive anticorrosion performance for Mg alloy. Chem. Eng. J. 2023, 454, 140187. [Google Scholar] [CrossRef]
- Kokalj, A.; Lozinsek, M.; Kapun, B.; Taheri, P.; Neupane, S.; Losada-Perez, P.; Xie, C.; Stavber, S.; Crespo, D.; Renner, F.U.; et al. Simplistic correlations between molecular electronic properties and inhibition efficiencies: Do they really exist? Corros. Sci. J. Environ. Degrad. Mater. Control 2021, 179, 108856. [Google Scholar] [CrossRef]




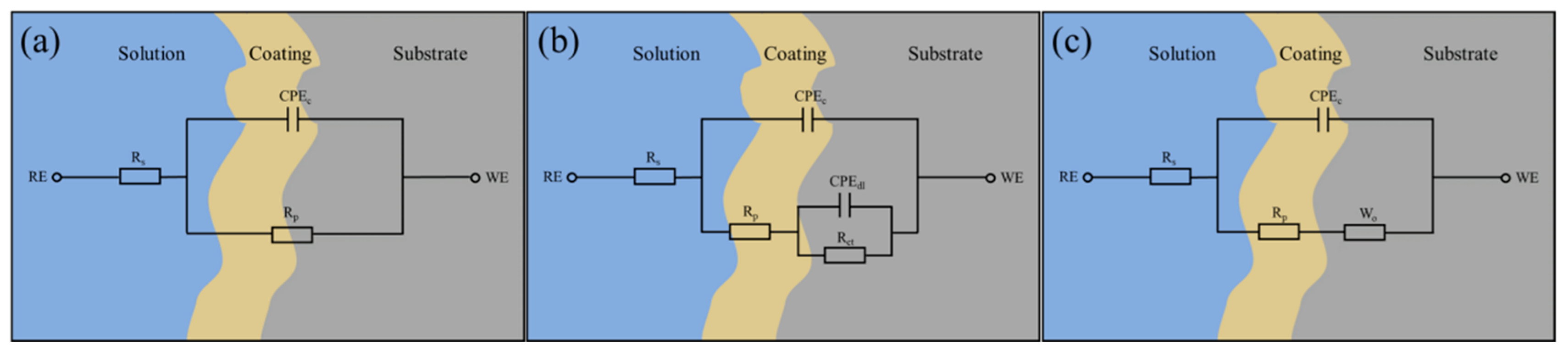


| Solution | Ecorr (V) | Icorr (A·cm−2) | vcorr (mm·a−1) | η (%) |
|---|---|---|---|---|
| 3.5 wt.% NaCl | −0.6239 | 3.6358 × 10−5 | 0.4265 | / |
| MgAl-TTA LDH 3.5 wt.% NaCl | −0.2509 | 9.6451 × 10−6 | 0.1132 | 73.47% |
| Addition Amount | Rs (Ω·cm2) | CPEc (S·sn·cm−2) | Rp (Ω·cm2) | |
|---|---|---|---|---|
| Y0 | n | |||
| 0 | 1.08 × 10−6 | 3.59 × 10−9 | 0.80 | 7.56 × 108 |
| 0.5% | 3.13 × 10−4 | 2.27 × 10−9 | 0.66 | 3.52 × 109 |
| 1% | 1.02 × 10−4 | 1.48 × 10−9 | 0.90 | 5.86 × 108 |
| 3% | 1.03 × 10−4 | 1.50 × 10−9 | 0.95 | 1.53 × 108 |
| 5% | 3.20 × 10−4 | 2.64 × 10−9 | 0.82 | 1.20 × 108 |
| Grading | 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| Appearance |  |  |  |  |  | The peeling condition exceeds grade 4. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Yu, Y.; Li, J.; Yin, C.; Tian, F.; Liu, J.; Zhou, J. Synergistic Corrosion Inhibition and UV Protection via TTA-Loaded LDH Nanocontainers in Epoxy Coatings. Coatings 2025, 15, 505. https://doi.org/10.3390/coatings15050505
Zhang Q, Yu Y, Li J, Yin C, Tian F, Liu J, Zhou J. Synergistic Corrosion Inhibition and UV Protection via TTA-Loaded LDH Nanocontainers in Epoxy Coatings. Coatings. 2025; 15(5):505. https://doi.org/10.3390/coatings15050505
Chicago/Turabian StyleZhang, Qiuli, Yaning Yu, Jingjing Li, Chengxian Yin, Feng Tian, Jiahui Liu, and Jun Zhou. 2025. "Synergistic Corrosion Inhibition and UV Protection via TTA-Loaded LDH Nanocontainers in Epoxy Coatings" Coatings 15, no. 5: 505. https://doi.org/10.3390/coatings15050505
APA StyleZhang, Q., Yu, Y., Li, J., Yin, C., Tian, F., Liu, J., & Zhou, J. (2025). Synergistic Corrosion Inhibition and UV Protection via TTA-Loaded LDH Nanocontainers in Epoxy Coatings. Coatings, 15(5), 505. https://doi.org/10.3390/coatings15050505






