Stabilization of Epitaxial NiO(001) Ultra-Thin Films on Body-Centered-Cubic Ni(001)-p(1x1)O
Abstract
1. Introduction
2. Experimental Details
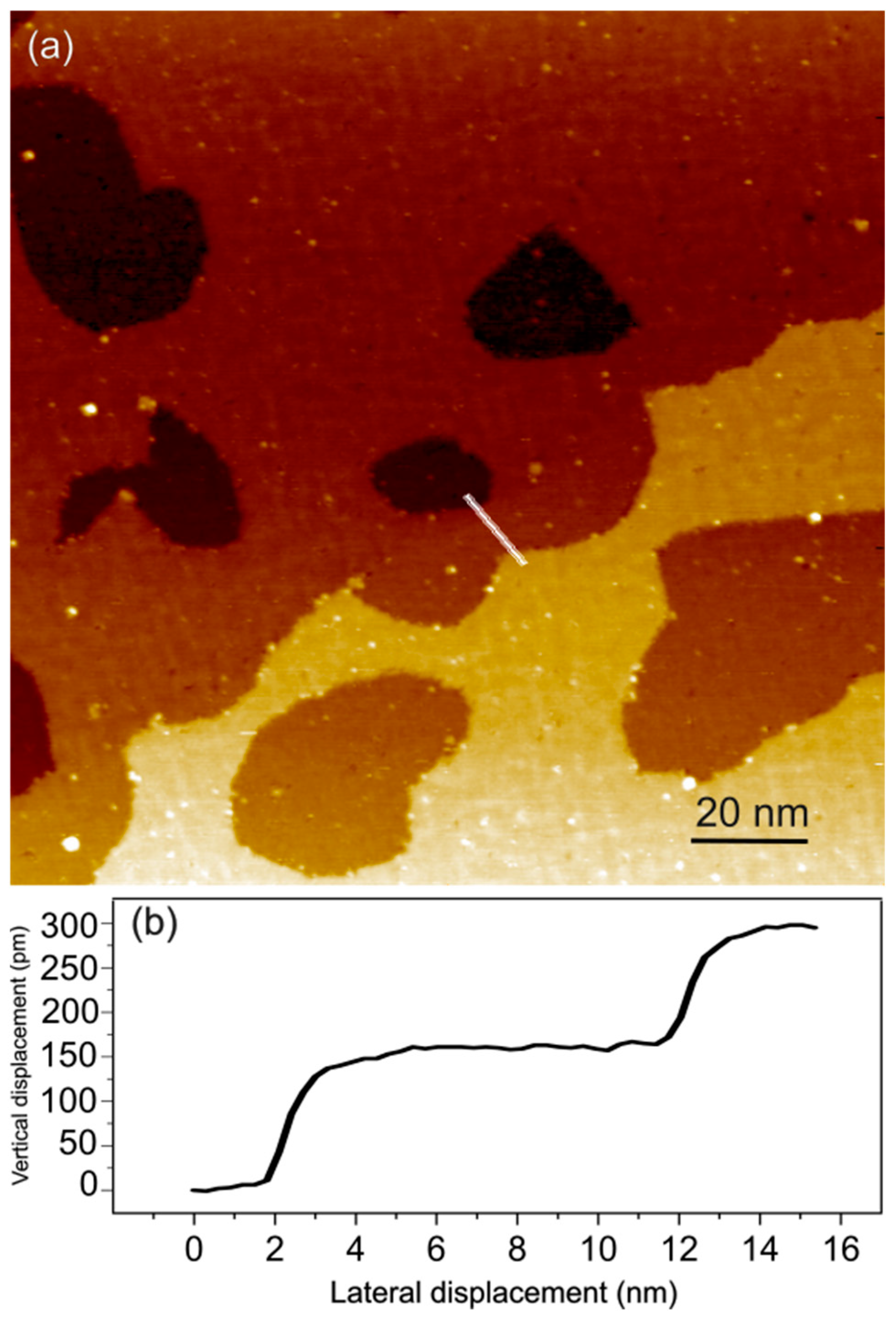
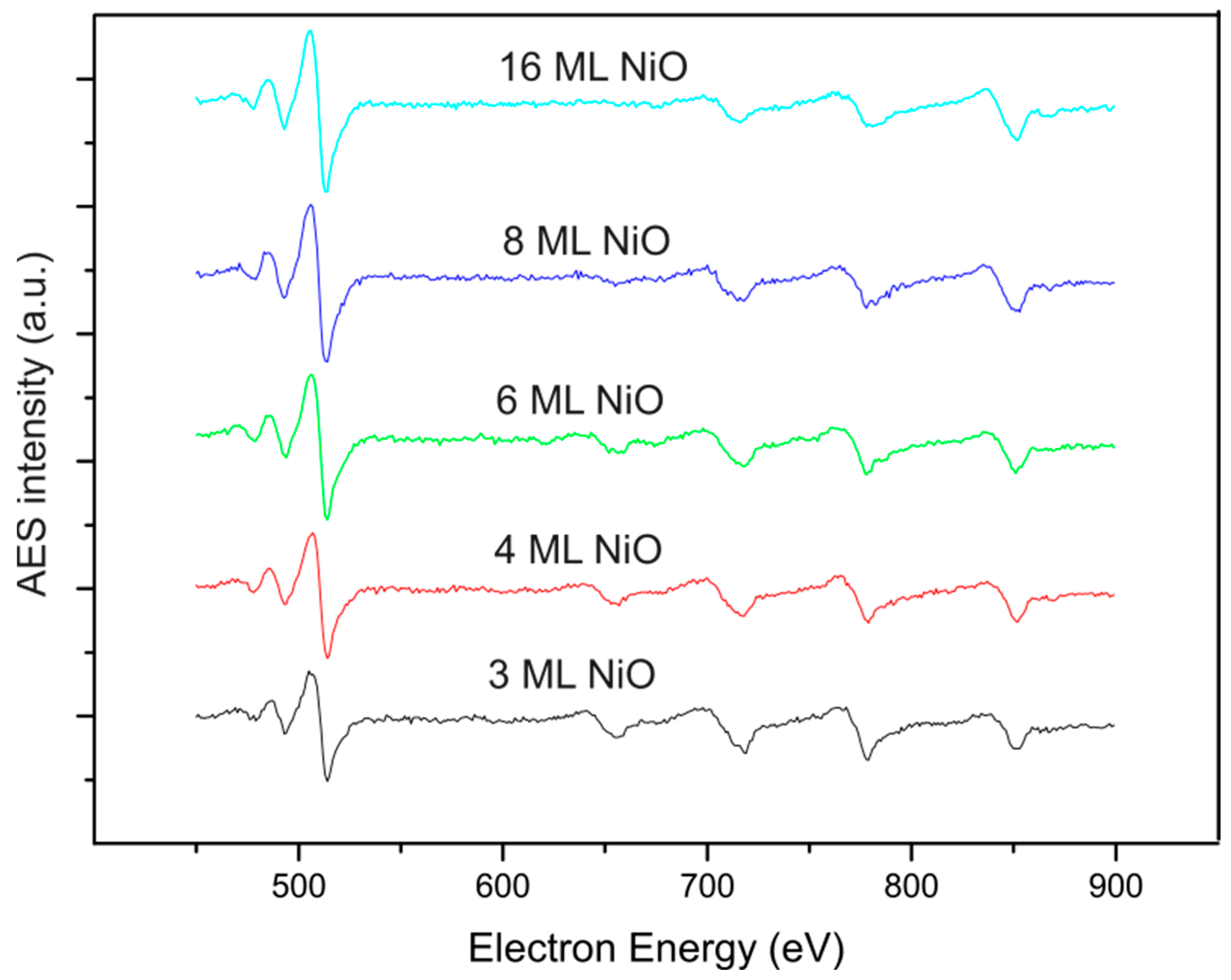
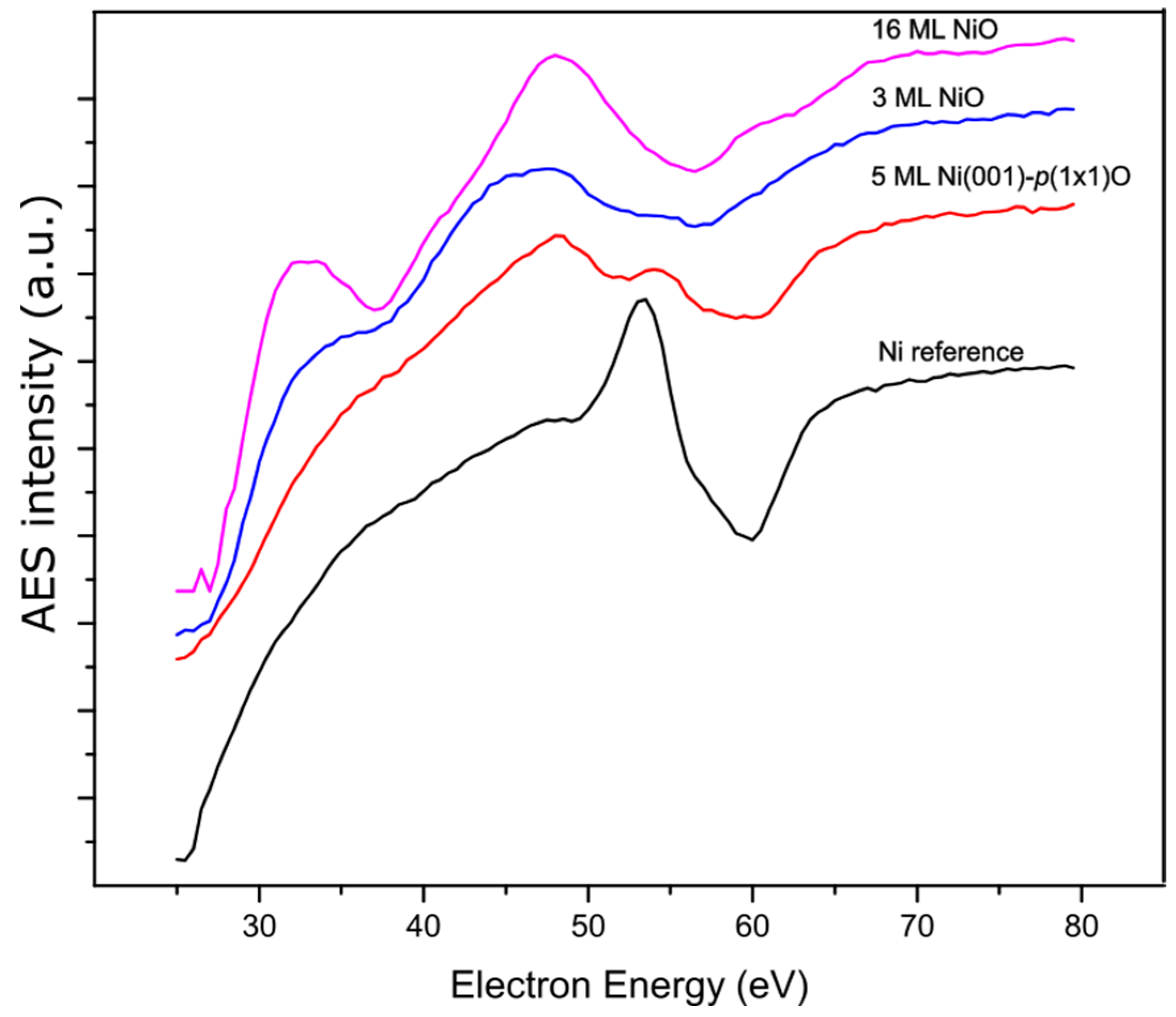
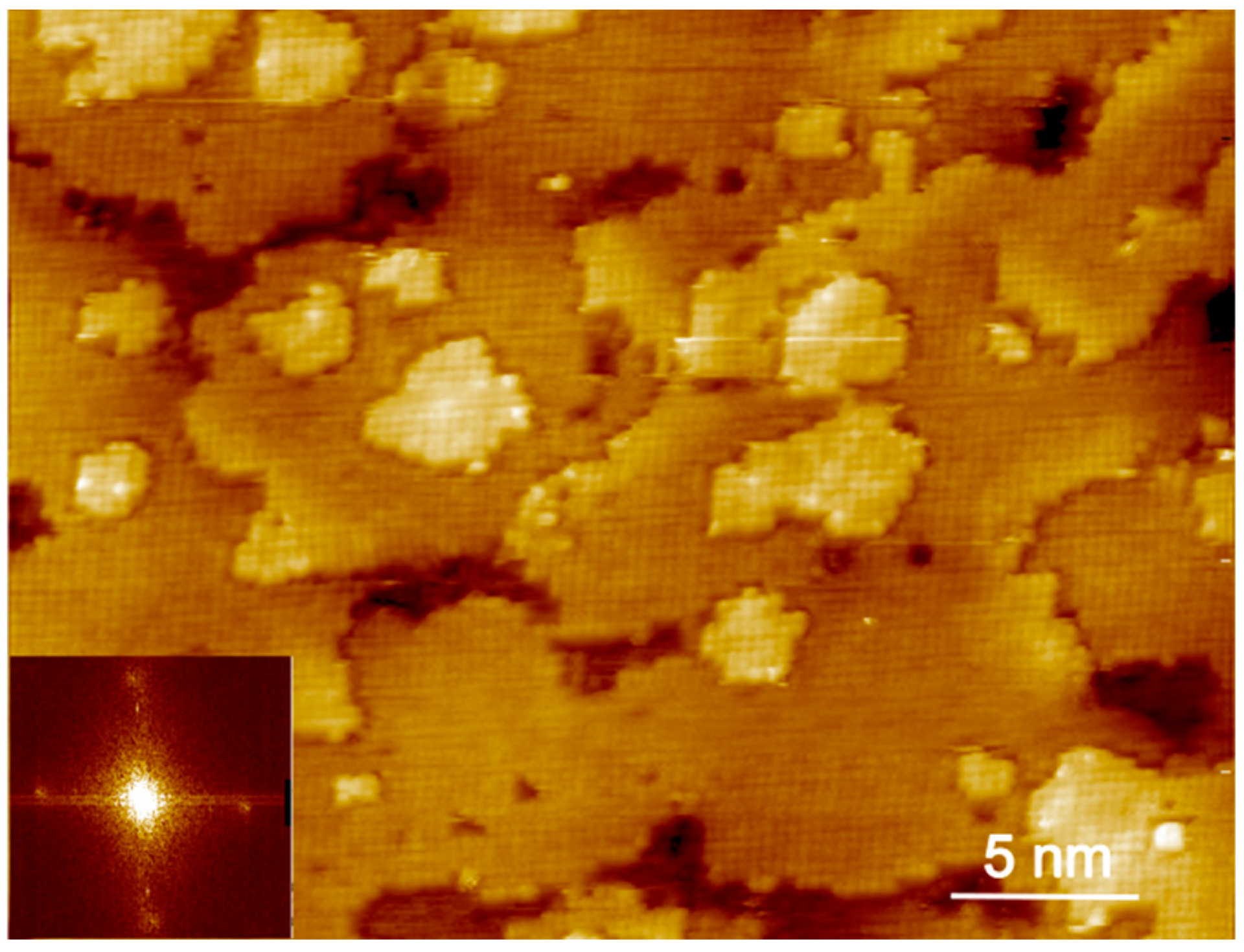
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henrich, V.E.; Cox, P.A. The Surface Science of Metal Oxides; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Jayaraj, M.K. Nanostructured Metal Oxides and Devices; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Siedliska, K. Metal Oxides: Crystal Structure, Synthesis and Characterization. Crystals 2024, 14, 991. [Google Scholar] [CrossRef]
- Bu, Z.; Xue, Y.; Chen, J.; Zhang, L.; Li, J.; Wang, L.; Wang, X.; Wang, Z.; Liu, Y.; Li, Y. Exploring the Crystal Structure and Electronic Properties of γ-Al2O3: Machine Learning Drives Future Material Innovations. ACS Appl. Mater. Interfaces 2024, 16, 60458–60471. [Google Scholar] [CrossRef] [PubMed]
- Jbara, A.S.; Othaman, Z.; Aliabad, H.A.R.; Saeed, M.A. Electronic and Optical Properties of γ- and θ-Alumina by First Principle Calculations. Adv. Sci. Eng. Med. 2017, 9, 287–293. [Google Scholar] [CrossRef]
- Hoat, D.M.; Van On, V.; Nguyen, D.K.; Naseri, M.; Ponce-Pérez, R.; Vu, T.V.; Rivas-Silva, J.F.; Hieu, N.N.; Cocoletzi, G.H. Structural, Electronic, and Optical Properties of Pristine and Functionalized MgO Monolayers: A First Principles Study. RSC Adv. 2020, 10, 40411–40420. [Google Scholar] [CrossRef]
- Schönberger, U.; Aryasetiawan, F. Bulk and Surface Electronic Structures of MgO. Phys. Rev. B 1995, 52, 8788–8793. [Google Scholar] [CrossRef]
- Park, M.; Chung, S.B. Effects of Homogeneous Doping on Electron–Phonon Coupling in SrTiO3. Nanomaterials 2025, 15, 137. [Google Scholar] [CrossRef]
- Gastiasoro, M.N.; Ruhman, J.; Fernandes, R.M. Superconductivity in dilute SrTiO3: A review. Ann. Phys. 2020, 417, 168107. [Google Scholar] [CrossRef]
- Namsar, O.; Watcharapasorn, A.; Jiansirisomboon, S. Structure–property relations of ferroelectric BaTiO3 ceramics containing nano-sized Si3N4 particulates. Ceram. Int. 2012, 38, S95–S99. [Google Scholar] [CrossRef]
- Hirose, T.; Furukawa, K. Dielectric anomaly of tungsten trioxide WO3 with giant dielectric constant. Phys. Status Solidi A 2006, 203, 608–615. [Google Scholar] [CrossRef]
- Solovyev, I.V.; Kashin, I.V.; Mazurenko, V.V. Mechanisms and origins of half-metallic ferromagnetism in CrO2. Phys. Rev. B 2015, 92, 144407. [Google Scholar] [CrossRef]
- Li, L.; Kanai, Y. Antiferromagnetic structures and electronic energy levels at reconstructed NiO(111) surfaces: A DFT+U study. Phys. Rev. B 2015, 91, 235304. [Google Scholar] [CrossRef]
- Singh, R.; Ahmad, F.; Kumar, S.; Kumar, N.; Kumar, R.; Kumar, P. Magnetic, Optical and I-V Characteristics of MoO3 thin films. J. Phys. Conf. Ser. 2021, 1947, 012048. [Google Scholar] [CrossRef]
- Keßler, P.; Garcia-Gassull, L.; Suter, A.; Prokscha, T.; Salman, Z.; Khalyavin, D.; Manuel, P.; Orlandi, F.; Mazin, I.I.; Valentí, R.; et al. Absence of magnetic order in RuO2: Insights from μSR spectroscopy and neutron diffraction. npj Spintron. 2024, 2, 50. [Google Scholar] [CrossRef]
- Halley, D.; Najjari, N.; Majjad, H.; Joly, L.; Ohresser, P.; Scheurer, F.; Ulhaq-Bouillet, C.; Berciaud, S.; Doudin, B.; Henry, Y. Size-Induced Enhanced Magnetoelectric Effect and Multiferroicity in Chromium Oxide Nanoclusters. Nat. Commun. 2014, 5, 3167. [Google Scholar] [CrossRef]
- Picone, A.; Riva, M.; Brambilla, A.; Calloni, A.; Bussetti, G.; Finazzi, M.; Ciccacci, F.; Duò, L. Reactive Metal-Oxide Interfaces: A Microscopic View. Surf. Sci. Rep. 2016, 71, 32–52. [Google Scholar] [CrossRef]
- Wang, T.; Hu, J.; Ouyang, R.; Wang, Y.; Huang, Y.; Hu, S.; Li, W.-X. Nature of Metal-Support Interaction for Metal Catalysts on Oxide Supports. Science 2024, 386, 915–920. [Google Scholar] [CrossRef]
- Leybo, D.; Etim, U.J.; Monai, M.; Bare, S.R.; Zhong, Z.; Vogt, C. Metal–Support Interactions in Metal Oxide-Supported Atomic, Cluster, and Nanoparticle Catalysis. Chem. Soc. Rev. 2024, 53, 10450–10490. [Google Scholar] [CrossRef]
- Mohapatra, J.; Xing, M.; Wu, R.; Yang, J.; Liu, J.P. Giant Exchange Bias by Tuning Co/CoO Core/Shell Structure. Scr. Mater. 2023, 230, 115400. [Google Scholar] [CrossRef]
- Mitrofanov, O.; Wang, J.; Liu, Y.; Nagaoka, M.; Mizukami, S.; Ohno, H. Exchange Bias without Directional Anisotropy in Permalloy/CoO Bilayers. Phys. Rev. B 2021, 104, 144413. [Google Scholar] [CrossRef]
- Campbell, C.T.; Sauer, J. Introduction: Surface Chemistry of Oxides. Chem. Rev. 2013, 113, 3859–3862. [Google Scholar] [CrossRef]
- Diebold, U.; Li, S.-C.; Schmid, M. Oxide Surface Science. Annu. Rev. Phys. Chem. 2010, 61, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Picone, A.; Brambilla, A.; Riva, M.; Calloni, A.; Bussetti, G.; Finazzi, M.; Ciccacci, F.; Duò, L. Self-organized chromium oxide monolayers on Fe(001). Phys. Rev. B 2013, 87, 085403. [Google Scholar] [CrossRef]
- Gubo, M.; Ebensperger, C.; Meyer, W.; Hammer, L.; Heinz, K. Substoichiometric cobalt oxide monolayer on Ir(100)-(1 × 1). J. Phys. Condens. Matter 2009, 21, 474211. [Google Scholar] [CrossRef] [PubMed]
- Kostov, K.L.; Schumann, F.O.; Polzin, S.; Sander, D.; Widdra, W. NiO growth on Ag(001): A layer-by-layer vibrational study. Phys. Rev. B 2016, 94, 075438. [Google Scholar] [CrossRef]
- Jaouen, T.; Tricot, S.; Delhaye, G.; Lépine, B.; Sébilleau, D.; Jézéquel, G.; Schieffer, P. Layer-Resolved Study of Mg Atom Incorporation at MgO/Ag(001) Buried Interface. Phys. Rev. Lett. 2013, 111, 266801. [Google Scholar] [CrossRef]
- Netzer, F.P. “Small and Beautiful”—The Novel Structures and Phases of Nano-Oxides. Surf. Sci. 2010, 604, 485–489. [Google Scholar] [CrossRef]
- Zhao, F.; Chen, T.; Zeng, Y.; Chen, J.; Zheng, J.; Liu, Y.; Han, G. Nickel Oxide Electrochromic Films: Mechanisms, Preparation Methods, and Modification Strategies—A Review. J. Mater. Chem. C 2024, 12, 7126–7145. [Google Scholar] [CrossRef]
- Gagaoudakis, E.; Tsakirakis, A.; Moschogiannaki, M.; Sfakianou, A.; Binas, V. Room-Temperature Nitric Oxide Gas Sensors Based on NiO/SnO2 Heterostructures. Sensors 2023, 23, 8583. [Google Scholar] [CrossRef]
- Gong, M.; Zhou, W.; Tsai, M.-C.; Zhou, J.; Guan, M.; Lin, M.-C.; Zhang, B.; Hu, Y.; Wang, D.-Y.; Yang, J.; et al. Nanoscale Nickel Oxide/Nickel Heterostructures for Active Hydrogen Evolution Electrocatalysis. Nat. Commun. 2014, 5, 4695. [Google Scholar] [CrossRef]
- Kasinathan, D.; Prabhakar, P.; Muruganandam, P.; Wiston, B.R.; Mahalingam, A.; Sriram, G. Solution Processed NiO/MoS2 Heterostructure Nanocomposite for Supercapacitor Electrode Application. Energies 2023, 16, 335. [Google Scholar] [CrossRef]
- Vinodh, R.; Babu, R.S.; Atchudan, R.; Kim, H.-J.; Yi, M.; Samyn, L.M.; de Barros, A.L.F. Fabrication of High-Performance Asymmetric Supercapacitor Consists of Nickel Oxide and Activated Carbon (NiO//AC). Catalysts 2022, 12, 375. [Google Scholar] [CrossRef]
- Ni, J.; Zhang, Y.; Li, J.; Ren, Y.; Zhou, J.; Dai, B. Electric Control of NiFe/NiO Exchange Bias through Resistive Switching under Zero Magnetic Field. J. Mater. Sci. Mater. Electron. 2023, 34, 552. [Google Scholar] [CrossRef]
- Luches, P.; Altieri, S.; Giovanardi, C.; Moia, T.S.; Valeri, S.; Bruno, F.; Floreano, L.; Morgante, A.; Santaniello, A.; Verdini, A.; et al. Growth, Structure, and Epitaxy of Ultrathin NiO Films on Ag(001). Thin Solid Film. 2001, 400, 139–143. [Google Scholar] [CrossRef]
- Visikovskiy, A.; Mitsuhara, K.; Hazama, M.; Kohyama, M.; Kido, Y. The Atomic and Electronic Structures of NiO(001)/Au(001) Interfaces. J. Chem. Phys. 2013, 139, 144705. [Google Scholar] [CrossRef]
- Schoiswohl, J.; Agnoli, S.; Xu, B.; Surnev, S.; Sambi, M.; Ramsey, M.G.; Granozzi, G.; Netzer, F.P. Growth and Thermal Behaviour of NiO Nanolayers on Pd(100). Surf. Sci. 2005, 599, 1–13. [Google Scholar] [CrossRef]
- Finazzi, M.; Brambilla, A.; Duò, L.; Ghiringhelli, G.; Portalupi, M.; Ciccacci, F.; Zacchigna, M.; Zangrando, M. Chemical Effects at the Buried NiO/Fe(001) Interface. Phys. Rev. B 2004, 70, 235420. [Google Scholar] [CrossRef]
- Mitchell, D.F.; Sewell, P.B.; Cohen, M. A Kinetic Study of the Initial Oxidation of the Ni(001) Surface by RHEED and X-ray Emission. Surf. Sci. 1976, 61, 355–376. [Google Scholar] [CrossRef]
- Bussetti, G.; Riva, M.; Picone, A.; Brambilla, A.; Duò, L.; Finazzi, M.; Ciccacci, F. Martensitic Transition During Ni Growth on Fe(001): Evidence of a Precursor Phase. New J. Phys. 2012, 14, 053048. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.Q.; Li, Y.S.; Jona, F.; Marcus, P.M. Epitaxial Growth of Body-Centered-Cubic Nickel on Iron. Solid State Commun. 1987, 61, 623–626. [Google Scholar] [CrossRef]
- Picone, A.; Brambilla, A.; Calloni, A.; Duò, L.; Finazzi, M.; Ciccacci, F. Oxygen-Induced Effects on the Morphology of the Fe(001) Surface in Out-of-Equilibrium Conditions. Phys. Rev. B Condens. Matter Mater. Phys. 2011, 83, 235402. [Google Scholar] [CrossRef]
- Tange, A.; Gao, C.L.; Yavorsky, B.Y.; Maznichenko, I.V.; Etz, C.; Ernst, A.; Hergert, W.; Mertig, I.; Wulfhekel, W.; Kirschner, J. Electronic Structure and Spin Polarization of the Fe(001)-p(1×1)O Surface. Phys. Rev. B 2010, 81, 195410. [Google Scholar] [CrossRef]
- Kim, S.K.; Petersen, C.; Jona, F.; Marcus, P.M. Ultrathin Films of Cobalt on Fe{001} and the Effect of Oxygen. Phys. Rev. B 1996, 54, 2184–2190. [Google Scholar] [CrossRef] [PubMed]
- Riva, M.; Picone, A.; Giannotti, D.; Brambilla, A.; Fratesi, G.; Bussetti, G.; Duò, L.; Finazzi, M.; Ciccacci, F. Mesoscopic Organization of Cobalt Thin Films on Clean and Oxygen-Saturated Fe(001) Surfaces. Phys. Rev. B 2015, 92, 115434. [Google Scholar] [CrossRef]
- Riva, M.; Picone, A.; Bussetti, G.; Brambilla, A.; Calloni, A.; Berti, G.; Duò, L.; Ciccacci, F.; Finazzi, M. Oxidation Effects on Ultrathin Ni and Cr Films Grown on Fe(001): A Combined Scanning Tunneling Microscopy and Auger Electron Spectroscopy Study. Surf. Sci. 2021, 713, 121894. [Google Scholar] [CrossRef]
- Brambilla, A.; Picone, A.; Giannotti, D.; Riva, M.; Bussetti, G.; Berti, G.; Calloni, A.; Finazzi, M.; Ciccacci, F.; Duò, L. Self-Organized Nano-Structuring of CoO Islands on Fe(001). Appl. Surf. Sci. 2016, 362, 374–379. [Google Scholar] [CrossRef]
- Davis, L.E.; MacDonald, N.C.; Palmberg, P.W.; Riach, G.E.; Weber, R.E. Handbook of Auger Electron Spectroscopy; Perkin-Elmer Corporation: Minneapolis, MN, USA, 1976. [Google Scholar]
- Valeri, S.; Borghi, A.; Gazzadi, G.C.; di Bona, A. Growth and Structure of Cobalt Oxide on (001) Bct Cobalt Film. Surf. Sci. 1999, 423, 346–356. [Google Scholar] [CrossRef]
- Thimsen, E.; Martinson, A.B.F.; Elam, J.W.; Pellin, M.J. Energy Levels, Electronic Properties, and Rectification in Ultrathin p-NiO Films Synthesized by Atomic Layer Deposition. J. Phys. Chem. C 2012, 116, 16830–16840. [Google Scholar] [CrossRef]
- Teply, C.; Tyler, B.A.; Berger, R.F. Tuning the Band Gaps of Oxide and Halide Perovskite Compounds via Biaxial Strain in All Directions. J. Phys. Chem. C 2021, 125, 25951–25958. [Google Scholar] [CrossRef]
- Dissanayake, D.M.C.U.; Wickramasinghe, J.S.T.; Samarasekara, P. Effect of Annealing Temperature on Photovoltaic Properties of Spin Coated CdS Films. arXiv 2018, arXiv:1804.04493. [Google Scholar]
- Altieri, S.; Finazzi, M.; Hsieh, H.H.; Haverkort, M.W.; Lin, H.-J.; Chen, C.T.; Frabboni, S.; Gazzadi, G.C.; Rota, A.; Valeri, S.; et al. Image Charge Screening: A New Approach to Enhance Magnetic Ordering Temperatures in Ultrathin Correlated Oxide Films. Phys. Rev. B 2009, 79, 174431. [Google Scholar] [CrossRef]


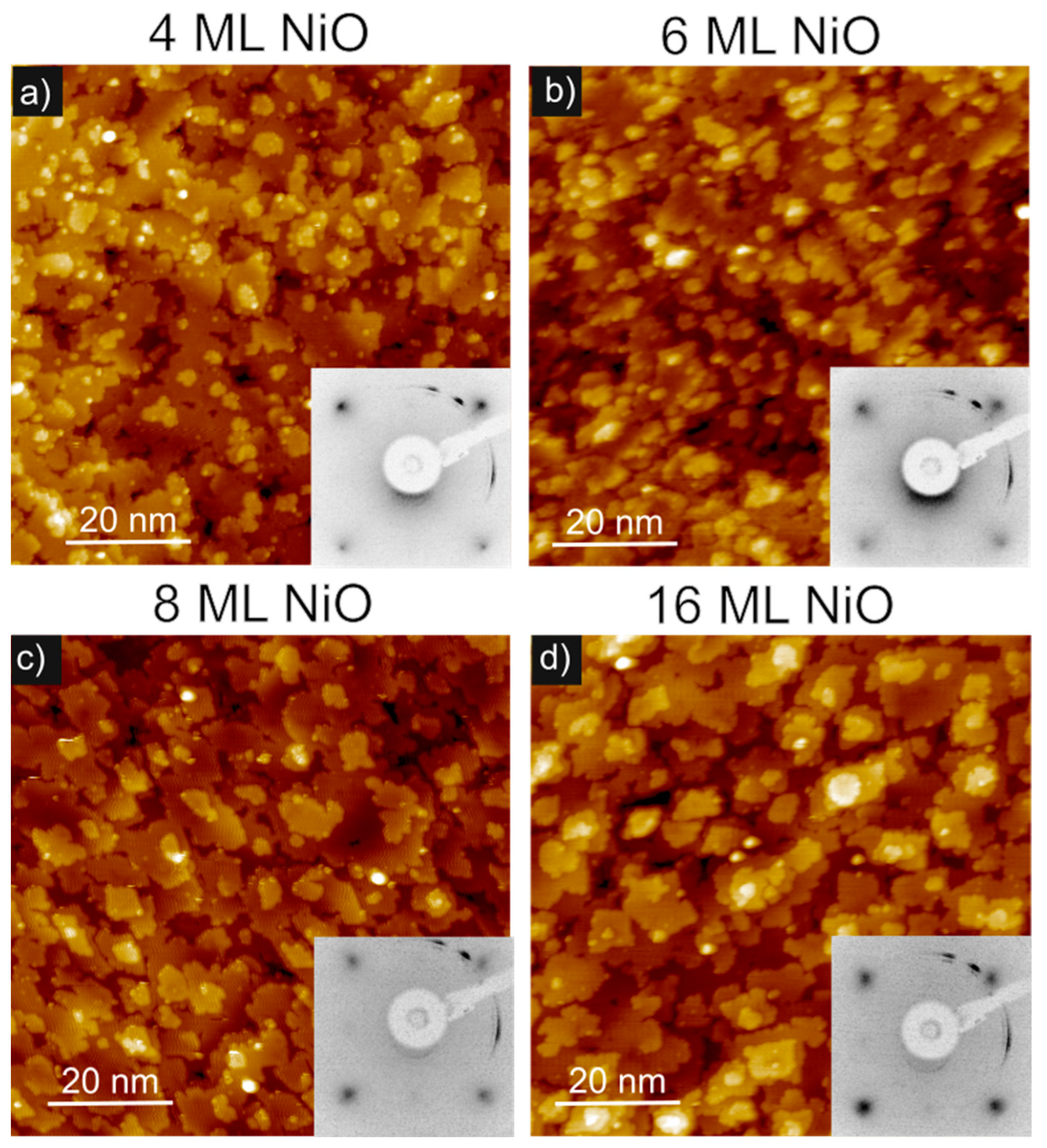
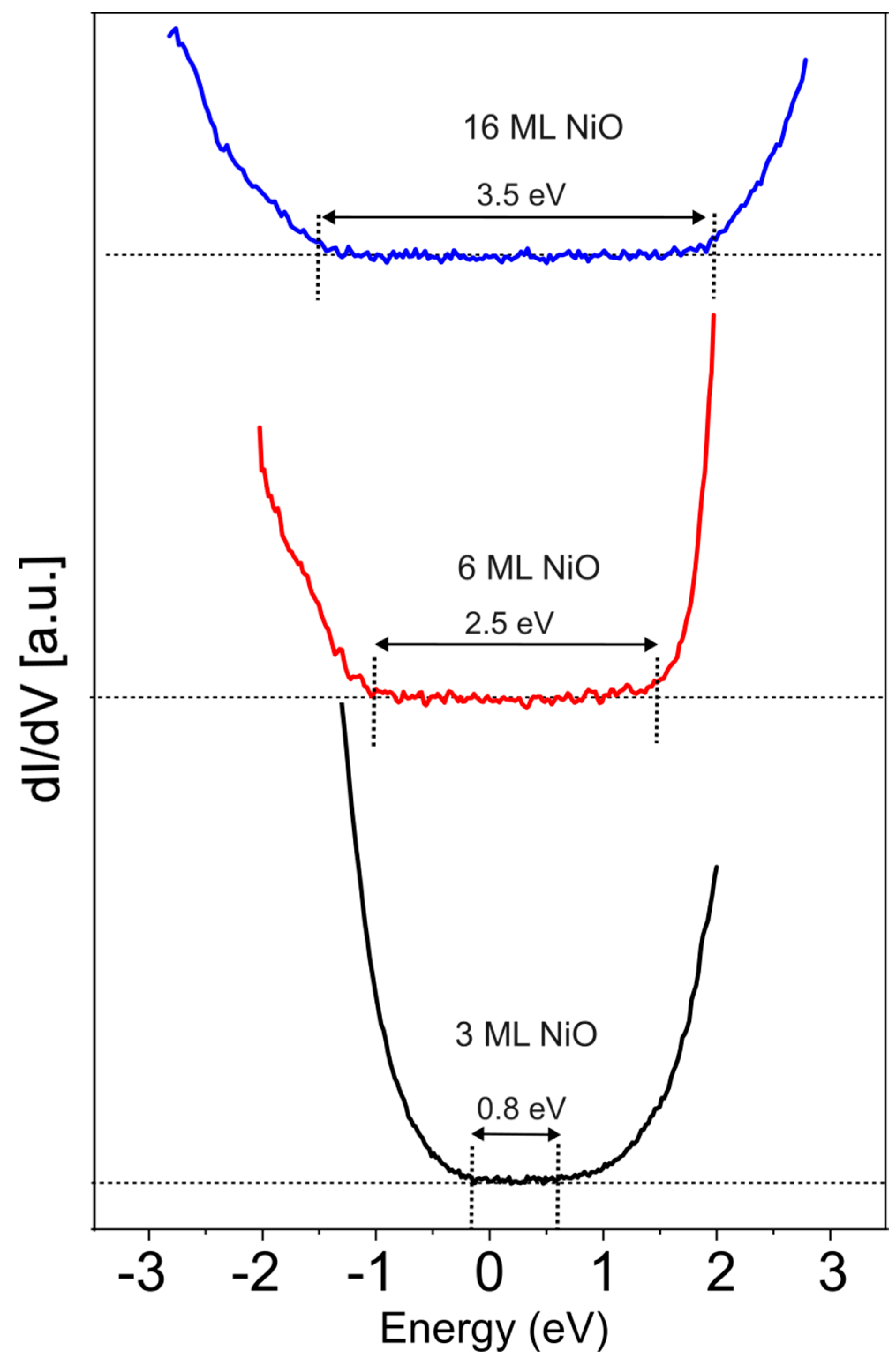
| NiO (ML) | OKLL/Ni850 |
|---|---|
| 3 | 3.16 |
| 4 | 3.10 |
| 6 | 3.45 |
| 8 | 3.39 |
| 16 | 3.20 |
| NiO (ML) | RMS (nm) |
|---|---|
| 3 | 0.12 |
| 4 | 0.15 |
| 6 | 0.13 |
| 8 | 0.14 |
| 16 | 0.17 |
| NiO (ML) | VB (eV) | CB (eV) | Energy Gap (eV) |
|---|---|---|---|
| 3 | −0.2 | 0.6 | 0.8 |
| 4 | −1.2 | 0.8 | 2 |
| 6 | −1 | 1.5 | 2.5 |
| 8 | −1.2 | 1.8 | 3 |
| 16 | −1.5 | 2 | 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picone, A.; Ciccacci, F.; Duò, L.; Brambilla, A. Stabilization of Epitaxial NiO(001) Ultra-Thin Films on Body-Centered-Cubic Ni(001)-p(1x1)O. Coatings 2025, 15, 507. https://doi.org/10.3390/coatings15050507
Picone A, Ciccacci F, Duò L, Brambilla A. Stabilization of Epitaxial NiO(001) Ultra-Thin Films on Body-Centered-Cubic Ni(001)-p(1x1)O. Coatings. 2025; 15(5):507. https://doi.org/10.3390/coatings15050507
Chicago/Turabian StylePicone, Andrea, Franco Ciccacci, Lamberto Duò, and Alberto Brambilla. 2025. "Stabilization of Epitaxial NiO(001) Ultra-Thin Films on Body-Centered-Cubic Ni(001)-p(1x1)O" Coatings 15, no. 5: 507. https://doi.org/10.3390/coatings15050507
APA StylePicone, A., Ciccacci, F., Duò, L., & Brambilla, A. (2025). Stabilization of Epitaxial NiO(001) Ultra-Thin Films on Body-Centered-Cubic Ni(001)-p(1x1)O. Coatings, 15(5), 507. https://doi.org/10.3390/coatings15050507






