Electrodeposited CoFeNi Medium-Entropy Alloy Coating on a Copper Substrate from Chlorides Solution with Enhanced Corrosion Resistance
Abstract
1. Introduction
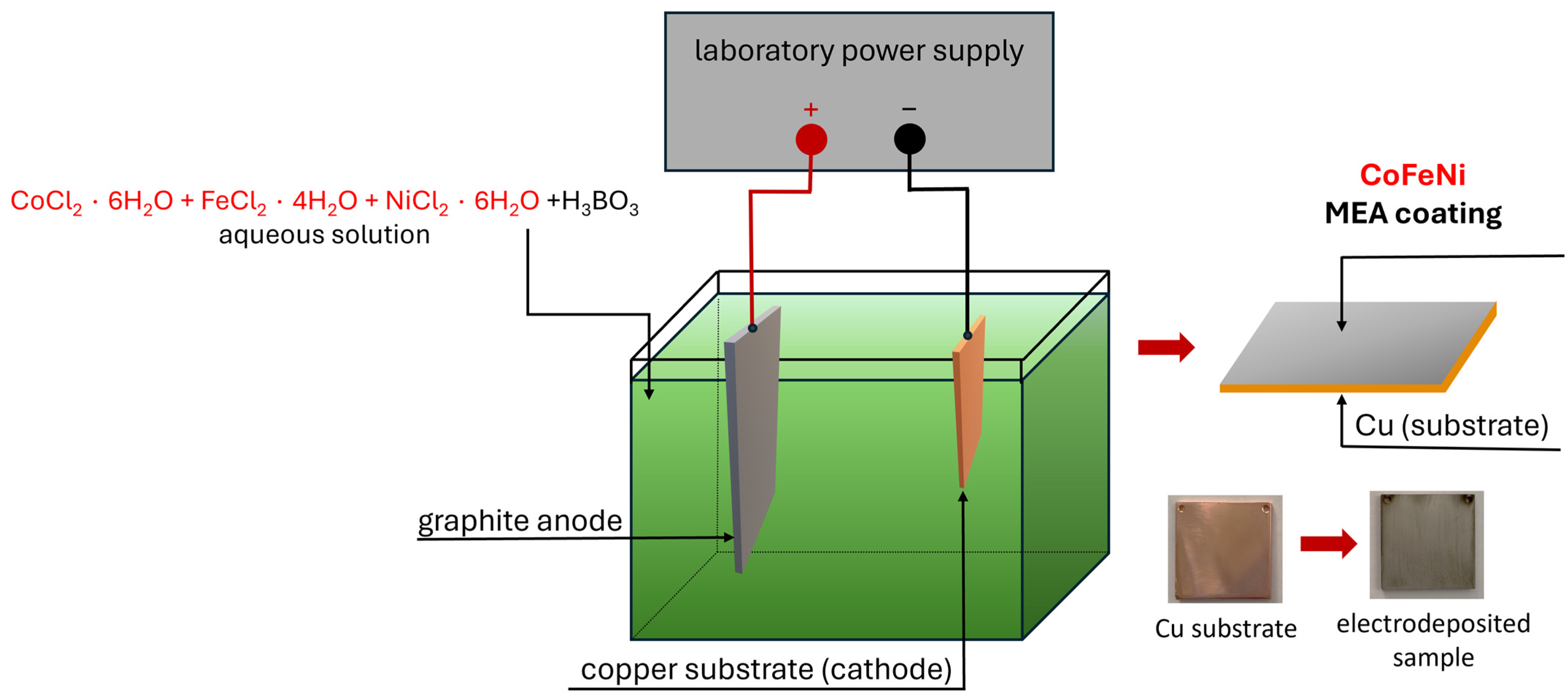
2. Materials and Methods
3. Results
3.1. X-Ray Diffraction
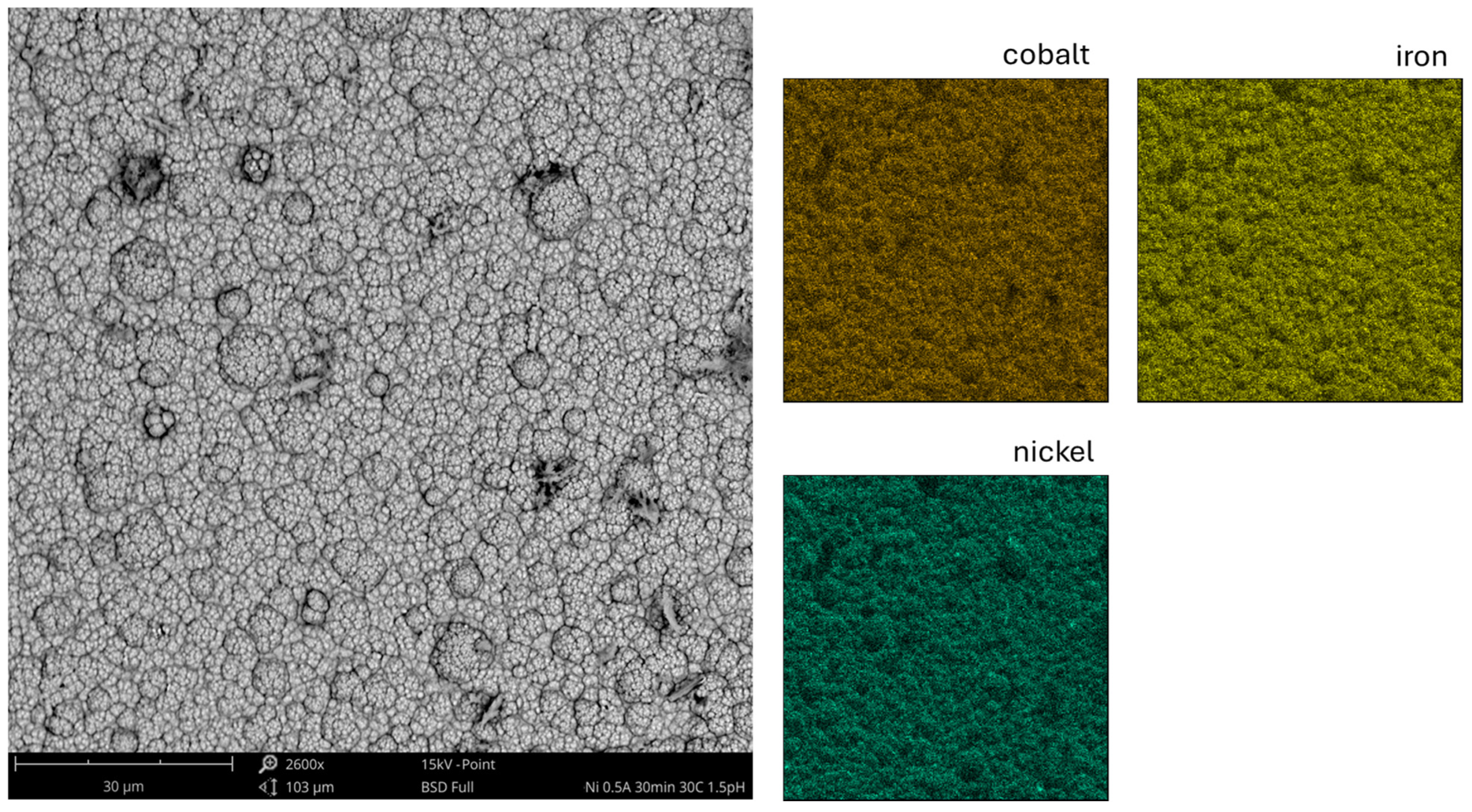
3.2. Scanning Electron and Atomic Force Microscopy
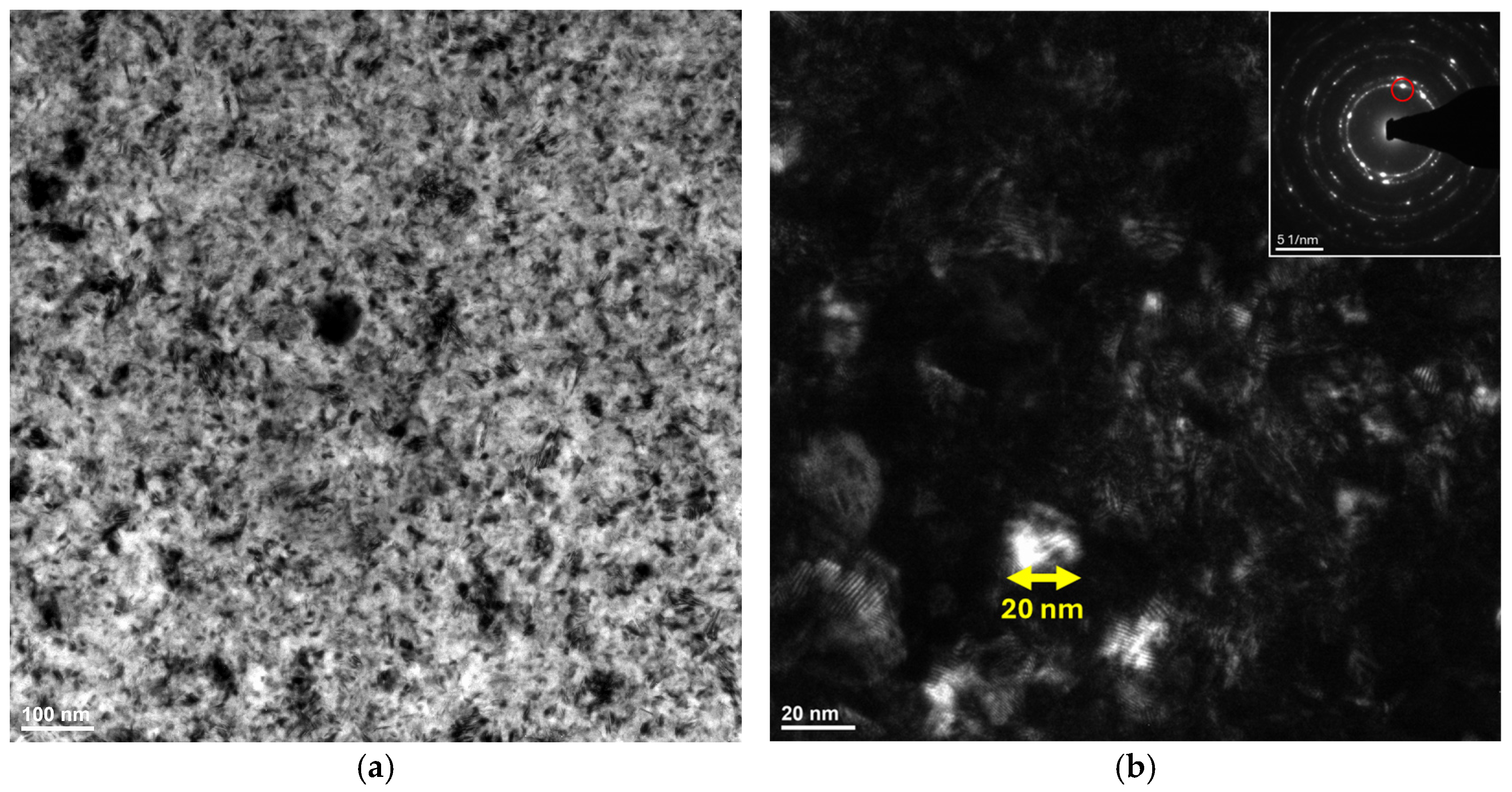
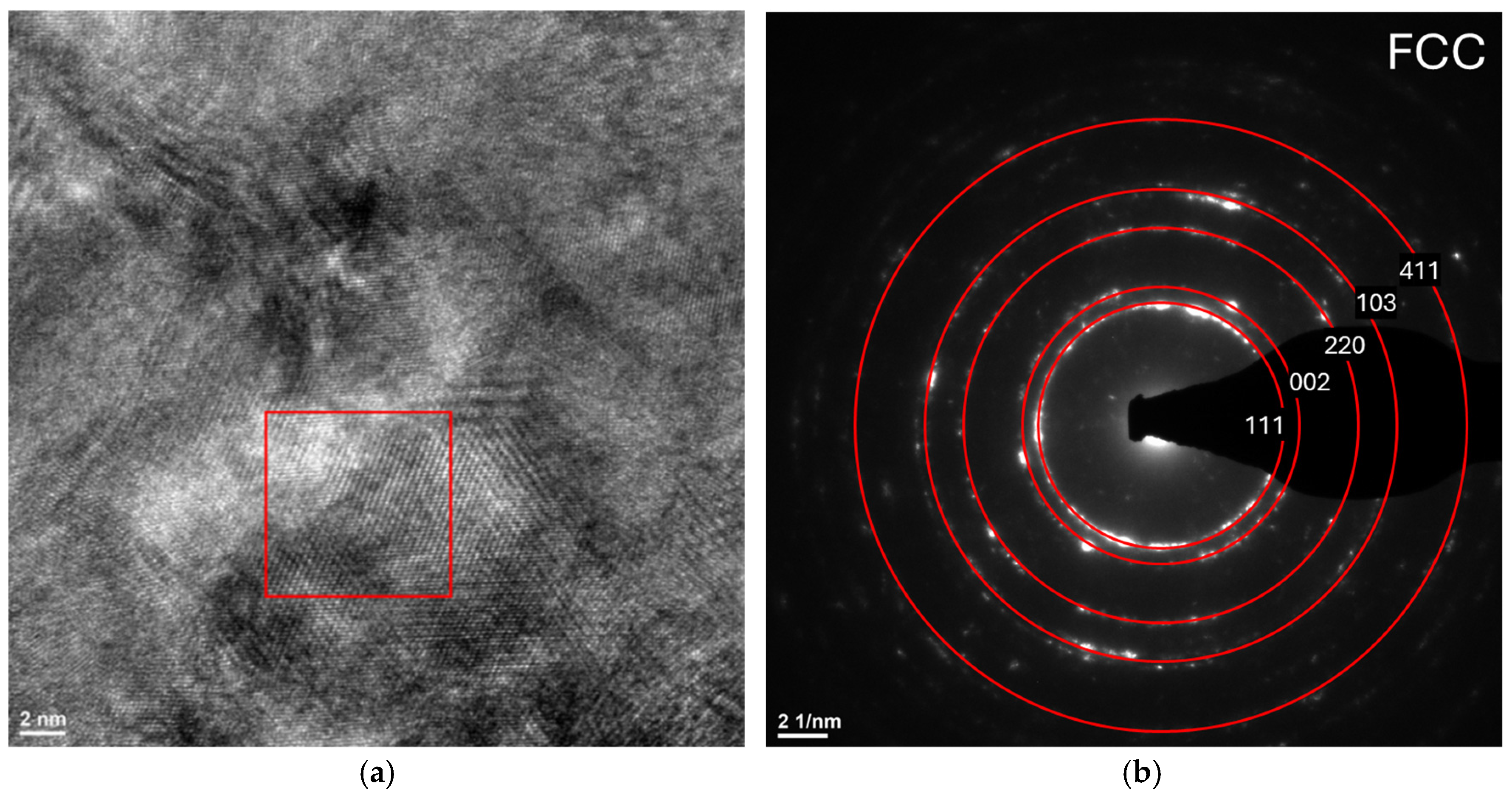
3.3. Transmission Electron Microscopy
3.4. Corrosion Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia Filho, F.D.C.; Ritchie, R.O.; Meyers, M.A.; Monteiro, S.N. Cantor-derived medium-entropy alloys: Bridging the gap between traditional metallic and high entropy alloys. J. Mater. Res. Technol. 2022, 17, 1868–1895. [Google Scholar] [CrossRef]
- Mishra, R.K.; Kumari, P.; Gupta, A.K.; Shahi, R.R. Comparison on structural and magnetic properties of FeCoNi medium entropy alloy, FeCoNiAl and FeCoNiAlTi high entropy alloys. Proc. Indian Natl. Sci. Acad. 2023, 89, 347–354. [Google Scholar] [CrossRef]
- Nartita, R.; Ionita, D.; Demetrescu, I.; Enachescu, M. A fresh perspective on medium entropy alloys applications as coating and coating substrate. Ann. Ser. Phys. Chem. 2022, 2, 34–46. [Google Scholar] [CrossRef]
- Wetzel, A.; von der Au, M.; Dietrich, P.M.; Radnik, J.; Ozcan, O.; Witt, J. The comparison of the corrosion behavior of the CrCoNi medium entropy alloy and CrMnFeCoNi high entropy alloy. Appl. Surf. Sci. 2022, 601, 154171. [Google Scholar] [CrossRef]
- Xu, D.; Wang, M.; Li, T.; Wei, X.; Lu, Y. A critical review of the mechanical properties of CoCrNi-based medium-entropy alloys. Microstructures 2022, 2, 2022001. [Google Scholar] [CrossRef]
- Li, A.; Meng, L.; Yuan, Z.; Zhang, X. Research on microstructures and properties of CoNiCr and CoNiCu medium-entropy alloys. J. Phys. Conf. Ser. 2021, 2044, 012063. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, D.; Jin, X.; Zhang, L.; Du, X.; Li, B. Design of non-equiatomic medium entropy alloys. Sci. Rep. 2018, 8, 1236. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiao, Y.; Liu, R.; Wang, D.; Yu, Z.; Xi, Y.; Zhang, K.; Xu, S.; Liu, H.; Wen, L.; et al. A Review of mechanical properties and improvement methods of medium entropy alloys at high temperature. JOM 2024, 76, 353–361. [Google Scholar] [CrossRef]
- He, H.; Wang, Y.; Qi, Y.; Xu, Z.; Li, Y. Review on the preparation methods and strengthening mechanisms of medium-entropy alloys with CoCrNi as the main focus. J. Mater. Res. Technol. 2023, 27, 6275–6307. [Google Scholar] [CrossRef]
- Varcholová, D.; Kušnírová, K.; Oroszová, L.; Möllmer, J.; Lange, M.; Gáborová, K.; Bul’ko, B.; Demeter, P.; Saksl, K. New-generation materials for hydrogen storage in medium-entropy aloys. Materials 2024, 17, 2897. [Google Scholar] [CrossRef]
- Xu, Q.; Karimi, K.; Naghdi, A.H.; Huo, W.; Wei, C.; Papanikolaou, S. Nanoindentation responses of NiCoFe medium-entropy alloys from cryogenic to elevated temperatures. J. Iron Steel Res. Int. 2024, 31, 2068–2077. [Google Scholar] [CrossRef]
- An, X.L.; Chu, C.L.; Zhou, L.; Ji, J.; Shen, B.L.; Ch, P.K. Controlling the corrosion behavior of CoNiFe medium entropy alloy by grain boundary engineering. Mater Charact. 2020, 164, 110323. [Google Scholar] [CrossRef]
- Watanabe, A.; Yamamoto, T.; Takigawa, Y. Tensile strength of nanocrystalline FeCoNi medium-entropy alloy fabricated using electrodeposition. Sci. Rep. 2022, 12, 12076. [Google Scholar] [CrossRef]
- Watanabe, A.; Yamamoto, T.; Miyamoto, R.; Takigawa, Y. Microstructure and tensile strenght of electrodeposited Fe-rich bcc FeCoNi medium-entropy alloys. J. Mater. Sci. Technol. 2023, 39, 2028–2034. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Wu, T.; Huang, G. An all-around way to analyze the corrosion behavior and the potential applications of high-entropy alloys coating. Ceram. Int. 2024, 50, 5893–5913. [Google Scholar] [CrossRef]
- Kumar, S. Comprehensive review on high entropy alloy-based coating. Surf. Coat. Technol. 2024, 477, 130327. [Google Scholar] [CrossRef]
- Sharma, A. High entropy alloy coatings and technology. Coatings 2021, 11, 372. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Y.; Gao, X.; Peng, L.; Qiao, Q.; Xiao, J.; Guo, F.; Wang, R.; Yu, J. Electrochemical deposition and corrosion resistance characterization of FeCoNiCr high-entropy alloy coatings. Coatings 2023, 13, 1167. [Google Scholar] [CrossRef]
- Cao, F.; Munroe, P.; Zhou, Z.; Xie, Z. Medium entropy alloy CoCrNi coatings: Enhancing hardness and damage tolerance through a nanotwinned structuring. Surf. Coat. Technol. 2018, 335, 257–264. [Google Scholar] [CrossRef]
- Li, S.; Li, H.; Zhai, Z.; Cao, X.; Liu, D.; Jiang, J. Corrosion resistance and tribological behavior of FeCoCrNi@GO/Ni high entropy alloy-based composite coatings prepared by electrodeposition. Surf. Coat. Technol. 2024, 477, 130379. [Google Scholar] [CrossRef]
- Shojaei, Z.; Khayati, G.R.; Darezereshki, E. Review of electrodeposition methods for the preparation of high-entropy alloys. Int. J. Miner. Metall. Mater. 2022, 29, 1683. [Google Scholar] [CrossRef]
- Zhu, Z.; Meng, H.; Ren, P. CoNiWReP high entropy alloy coatings prepared by pulse current electrodeposition from aqueous solution. Colloid. Surf. A 2022, 648, 129404. [Google Scholar] [CrossRef]
- Li, Y.; Wang, P.; Zhai, Y.; Lv, J.; Sun, Y. Preparation and high-temperature microplastic forming performance of nano-FeCoNi medium-entropy alloy foils. Adv. Eng. Mater. 2025, 27, 2402502. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, B.; Li, W.; Liaw, P.K.; Yang, S.; Zhong, N. Effect of additive and current density on microstructures and corrosion behavior of a multi-component NiFeCoCu alloy prepared by electrodeposition. Crystals 2024, 14, 171. [Google Scholar] [CrossRef]
- Shah, A.; Chauhan, B.; Rai, R.K.; Mundotiya, B.M. Electrodeposition of high entropy alloy coating: A brief of the deposition parameters. In Futuristic Trends in Chemical Material Sciences & Nano Technology; IIP Series; Selfypage Developers Pvt Ltd.: Karnataka, India, 2024; Volume 3, pp. 61–74. [Google Scholar] [CrossRef]
- Ghaferi, Z.; Sharafi, S.; Bahrololoom, M.E. Effect of current density and bath composition on crystalline structure and magnetic properties of electrodeposited FeCoW alloy. Appl. Surf. Sci. 2015, 355, 766–773. [Google Scholar] [CrossRef]
- Huo, W.; Wang, S.; Fang, F.; Tan, S.; Kurpaska, Ł.; Xie, Z.; Kim, H.S.; Jiang, J. Microstructure and corrosion resistance of highly <111> oriented electrodeposited CoNiFe medium entropy alloy films. J. Mater. Res. Technol. 2022, 20, 1677–1684. [Google Scholar] [CrossRef]
- Watanabe, A.; Yamamoto, T.; Miyamoto, R.; Takigawa, Y. High-strength bcc FeCoNi medium-entropy alloy with fine grains fabricated using electrodeposition. SSRN 2021. [Google Scholar] [CrossRef]
- Haché, M.J.R.; Tam, J.; Erb, U.; Zou, Y. Electrodeposited nanocrystalline medium-entropy alloys—An effective strategy of producing stronger and more stable nanomaterials. J. Alloys Compd. 2022, 899, 163233. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical behavior of emerging materials. Mater. Today 2012, 15, 486–498. [Google Scholar] [CrossRef]
- Agustianingrum, M.P.; Yoshida, S.; Tsuji, N.; Park, N. Effect of aluminum addition on solid solution strengthening in CoCrNi medium-entropy alloy. J. Alloys Compd. 2019, 781, 866–872. [Google Scholar] [CrossRef]
- Meng, A.; Liang, F.; Gu, L.; Mao, Q.; Zhang, Y.; Chen, X.; Zhao, Y. An exceptionally wear-resistant CoFeNi2 medium entropy alloy via tribo-induced nanocrystallites with amorphous boundaries. Appl. Surf. Sci. 2023, 614, 156102. [Google Scholar] [CrossRef]
- Milyaev, M.A.; Bannikova, N.S.; Naumova, L.I.; Proglyado, V.V.; Patrakov, E.I.; Glazunov, N.P.; Ustinov, V.V. Effective Co-rich ternary CoFeNi alloys for spintronics application. J. Alloys Compd. 2021, 854, 157171. [Google Scholar] [CrossRef]
- Murakami, Y.; Maeda, Y.; Kitada, A.; Murase, K.; Fukami, K. Electrodeposition of a CoNiCu medium-entropy alloy in a water-in-oil emulsion. Electrochem. Commun. 2021, 128, 107057. [Google Scholar] [CrossRef]
- Kong, A.; Gong, B.K.; Wang, G.; Cui, H.W. Influence of surface roughness of substrate on the properties of Ni-Co-Fe electrodeposition coating on copper. Surf. Rev. Lett. 2018, 25, 1850120. [Google Scholar] [CrossRef]
- Takadoum, J.; Bennani, H.H. Influence of substrate roughness and coating thickness on adhesion, friction and wear of TiN films. Surf. Coat. Technol. 1997, 96, 272–282. [Google Scholar] [CrossRef]
- Croll, S.G. Surface roughness profile and its effect on coating adhesion and corrosion protection: A review. Prog. Org. Coat. 2020, 148, 105847. [Google Scholar] [CrossRef]
- Stevenson, J.O.; Hosking, F.M.; Guilinger, T.R.; Yost, F.G.; Sorensen, N.R. Inspection of chemically roughened copper surfaces using optical interferometry and scanning electron microscopy: Establishing a correlation between surface morphology and solderability. In Proceedings of the International Metallographic Society (IMS) Conference, Albuquerque, NM, USA, 25–26 July 1995; Available online: https://www.osti.gov/biblio/97211/ (accessed on 20 February 2025).
- Ledwig, P.; Kac, M.; Kopia, A.; Falkus, J.; Dubiel, B. Microstructure and Properties of Electrodeposited Nanocrystalline Ni-Co-Fe Coatings. Materials 2021, 14, 3886. [Google Scholar] [CrossRef]
- Aliyu, A.; Srivastava, C. Microstructure and electrochemical properties of FeNiCoCu medium entropy alloy-graphene oxide composite coatings. J. Alloys Compd. 2021, 864, 158851. [Google Scholar] [CrossRef]
- Aliyu, A.; Srivastava, C. Phase constitution, surface chemistry and corrosion behavior of electrodeposited MnFeCoNiCu high entropy alloy-graphene oxide composite coatings. Surf. Coat. Technol. 2022, 429, 127943. [Google Scholar] [CrossRef]

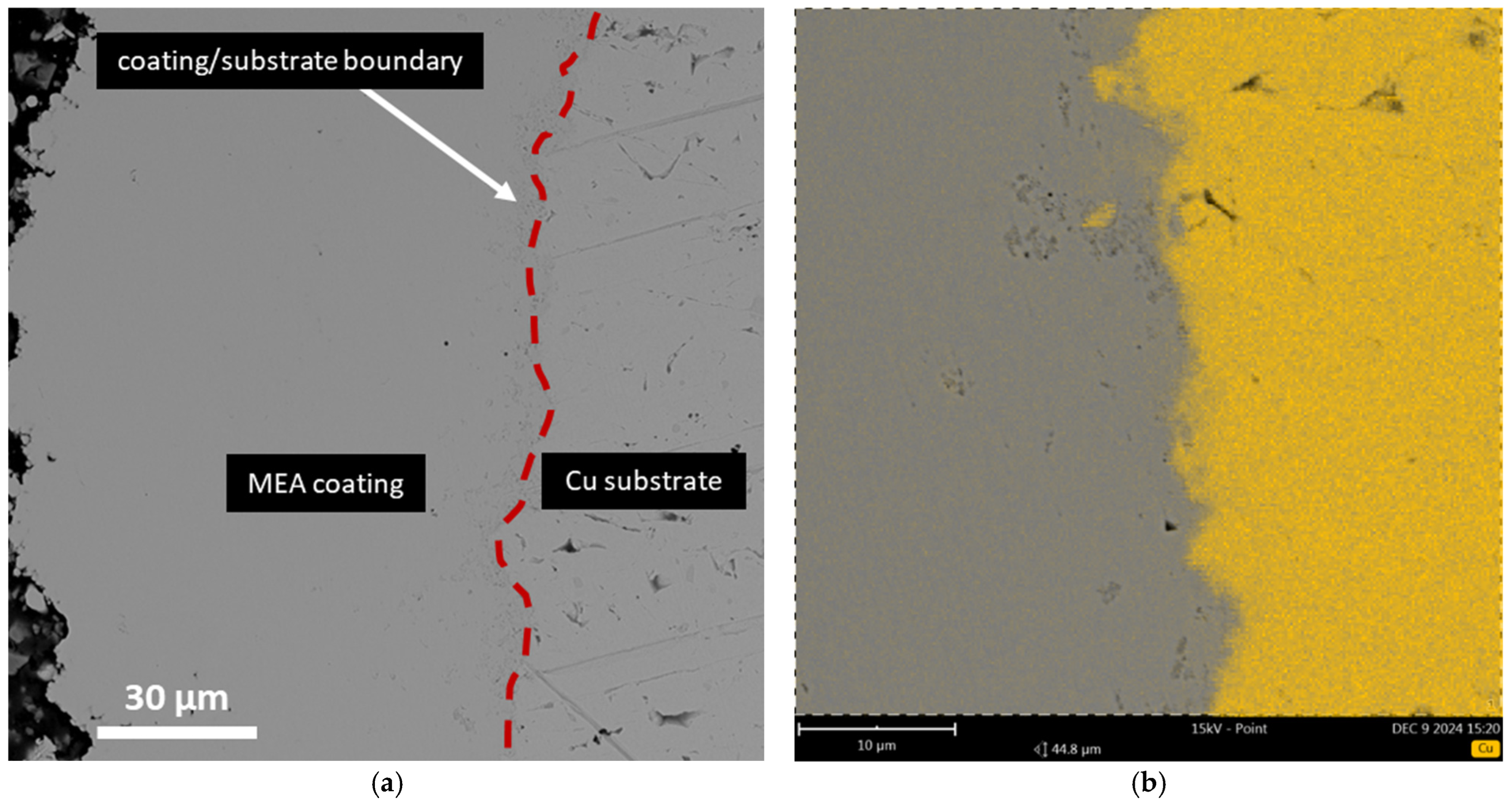

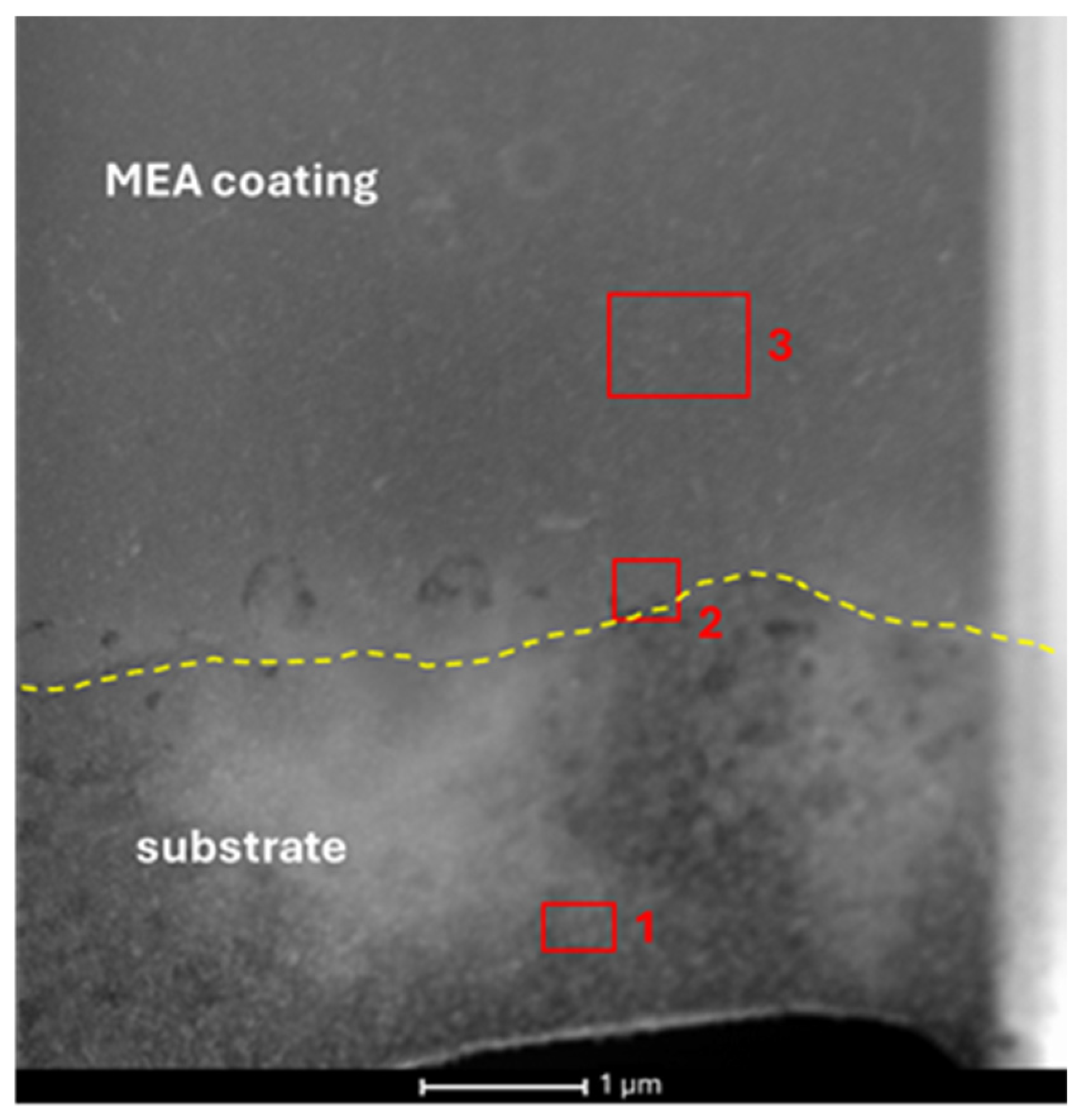

| Area Number | Zone | Co | Fe | Ni | Cu |
|---|---|---|---|---|---|
| 1 | Cu substrate | 0.5 | 1.3 | - | 98.2 |
| 2 | Cu/MEA boundary | 32.5 | 20.9 | 28.6 | 18.0 |
| 3 | MEA coating | 35.5 | 25.1 | 30.5 | 8.9 |
| Sample | Ecorr [mV] | jcorr [µA/cm2] | Rp [kΩcm2] |
|---|---|---|---|
| CoFeNi MEA coating | −0.182 | 0.03 | 278.8 |
| Cu substrate | −0.298 | 2.0 | 4.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Młynarek-Żak, K.; Spilka, M.; Matus, K.; Góral, A.; Babilas, R. Electrodeposited CoFeNi Medium-Entropy Alloy Coating on a Copper Substrate from Chlorides Solution with Enhanced Corrosion Resistance. Coatings 2025, 15, 509. https://doi.org/10.3390/coatings15050509
Młynarek-Żak K, Spilka M, Matus K, Góral A, Babilas R. Electrodeposited CoFeNi Medium-Entropy Alloy Coating on a Copper Substrate from Chlorides Solution with Enhanced Corrosion Resistance. Coatings. 2025; 15(5):509. https://doi.org/10.3390/coatings15050509
Chicago/Turabian StyleMłynarek-Żak, Katarzyna, Monika Spilka, Krzysztof Matus, Anna Góral, and Rafał Babilas. 2025. "Electrodeposited CoFeNi Medium-Entropy Alloy Coating on a Copper Substrate from Chlorides Solution with Enhanced Corrosion Resistance" Coatings 15, no. 5: 509. https://doi.org/10.3390/coatings15050509
APA StyleMłynarek-Żak, K., Spilka, M., Matus, K., Góral, A., & Babilas, R. (2025). Electrodeposited CoFeNi Medium-Entropy Alloy Coating on a Copper Substrate from Chlorides Solution with Enhanced Corrosion Resistance. Coatings, 15(5), 509. https://doi.org/10.3390/coatings15050509






