Integrated Utilization Strategies for Red Mud: Iron Extraction, Sintered Brick Production, and Non-Calcined Cementitious Binder Development for Environmental Sustainability
Abstract
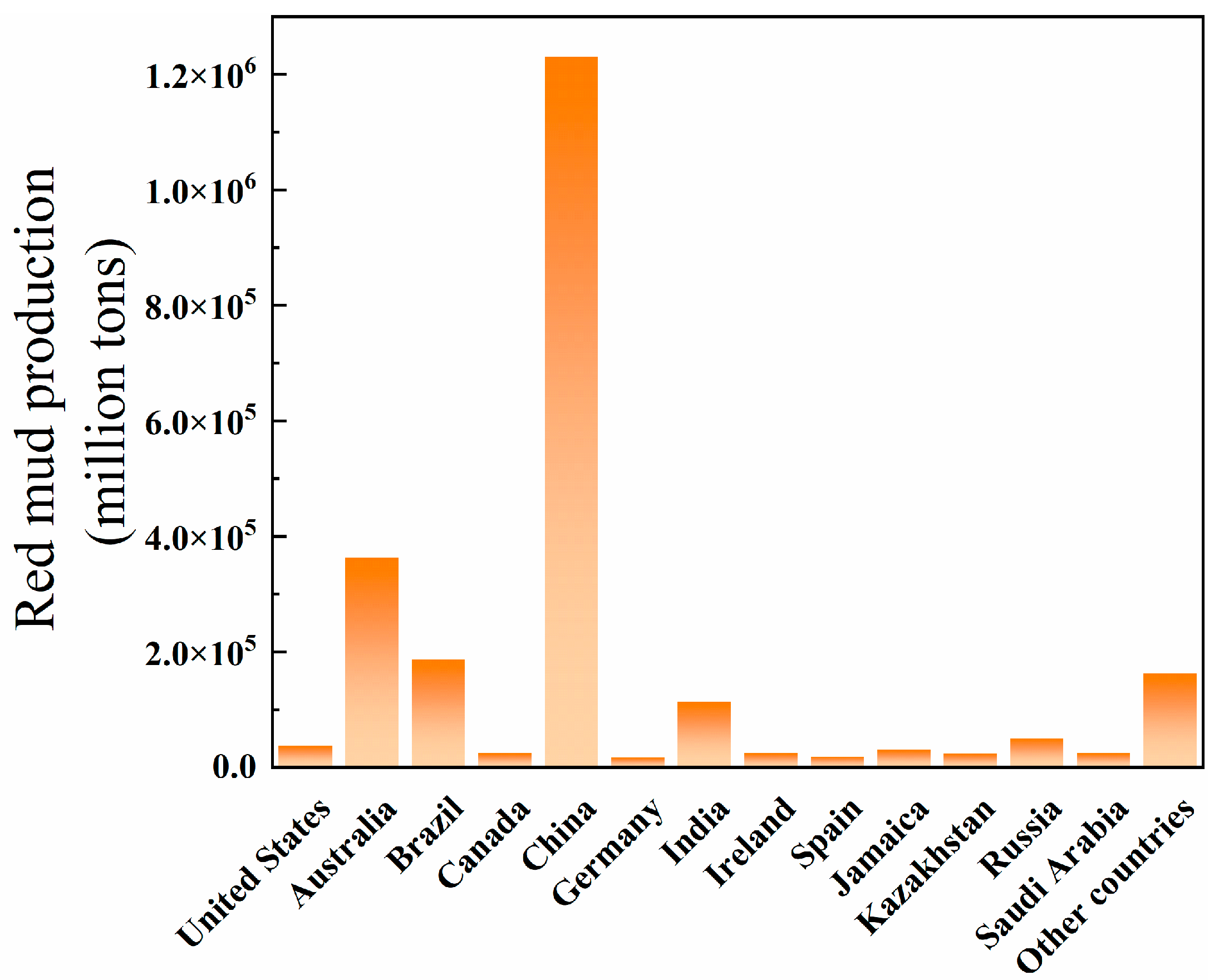
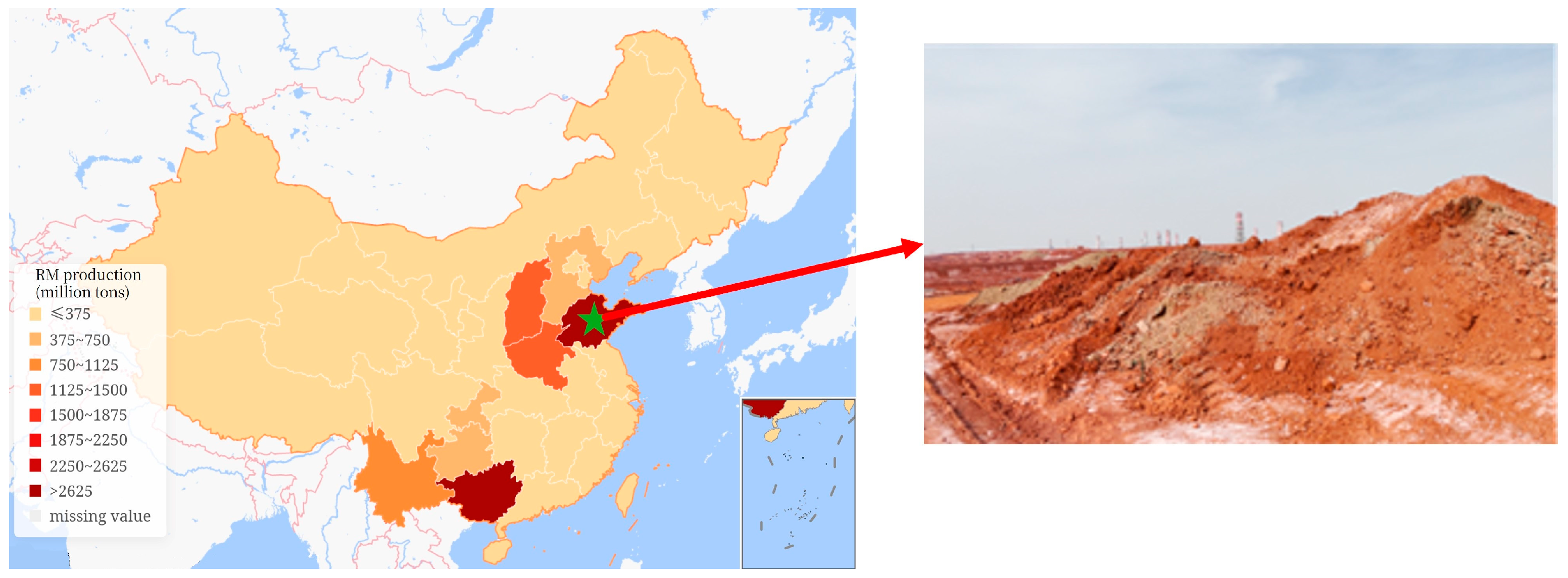
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Mineralogic Characterization
2.2.2. Microstructural Characterization
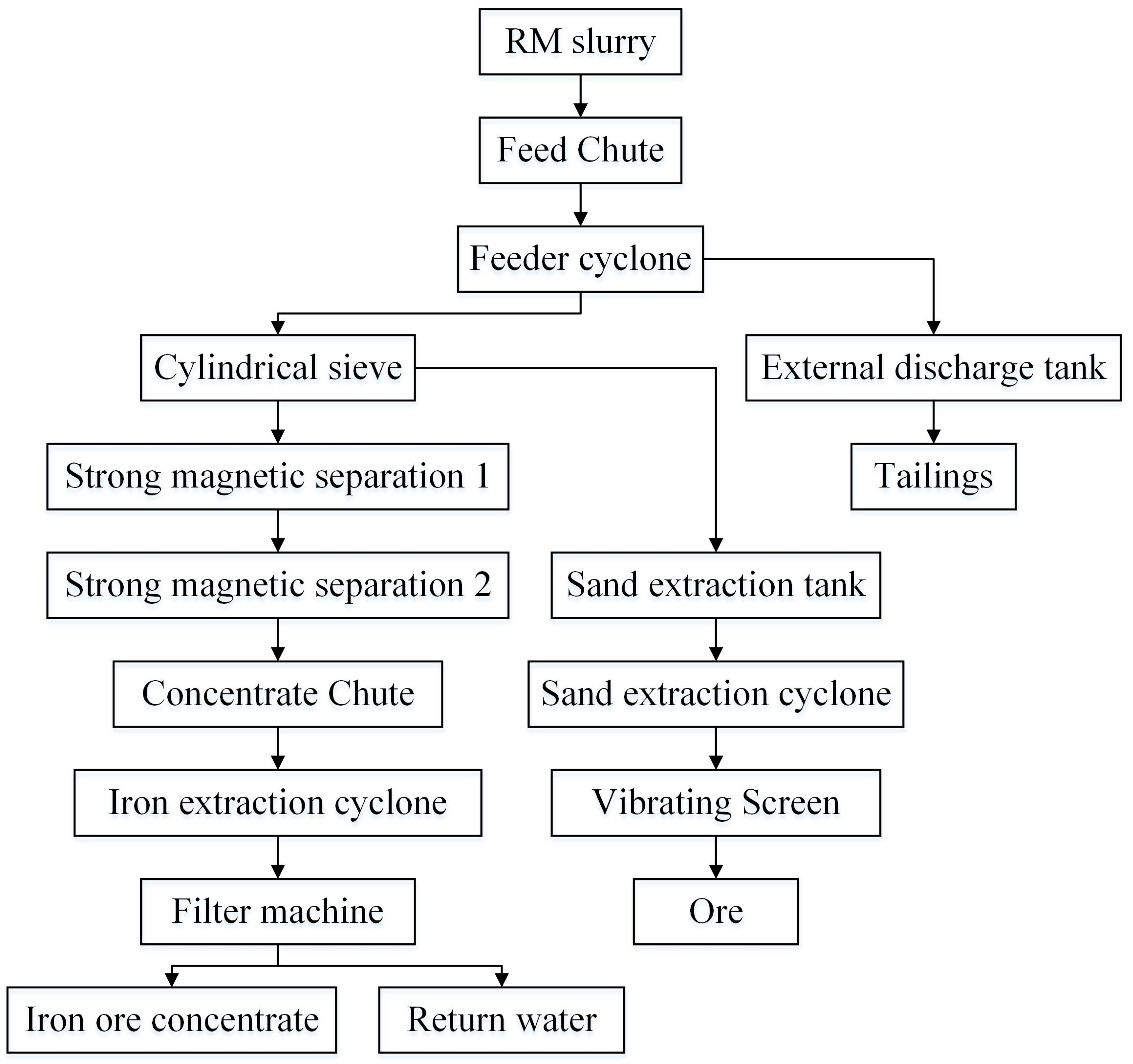
2.2.3. Iron Recovery via Magnetic Separation
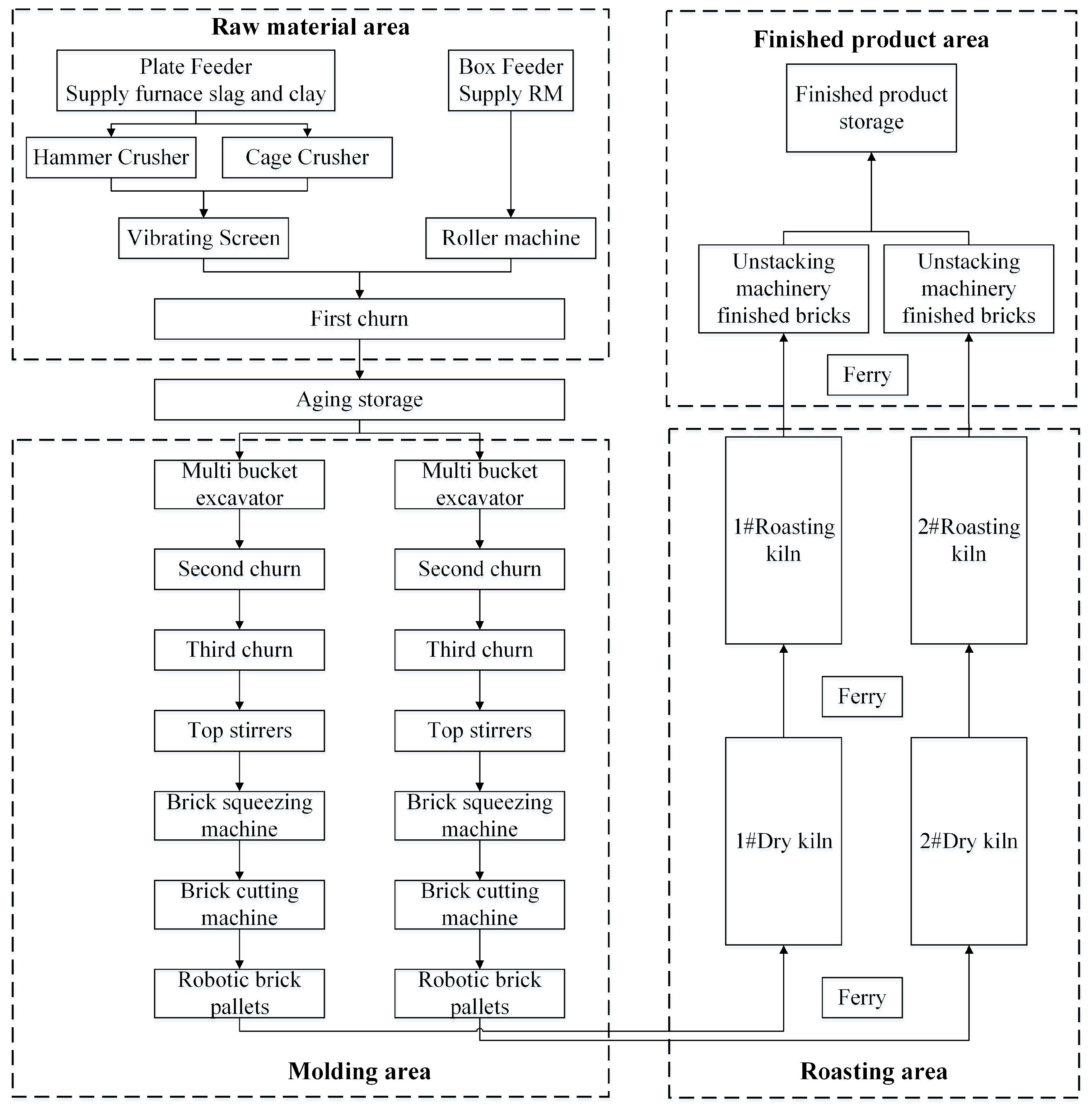
2.2.4. Sintered Brick Preparation
2.2.5. Non-Calcined Cementitious Binder Preparation
3. Results and Discussion
3.1. Iron Extraction from RM
3.2. RM-Based Sintered Bricks
3.2.1. Characteristics of Raw Materials
3.2.2. Properties of an RM–FA–GBFS Mixture and Production of Sintered Bricks from the Mixture
3.3. Non-Calcined Cementitious Binders
3.3.1. Preparation Methods
3.3.2. The Properties of Non-Calcined Cementitious Binders
4. Conclusions
- (1)
- Iron Extraction from RM: The iron beneficiation process achieved a recovery rate of 23.85% for iron ore concentrate (grade: 58%) and 3.06% for ironsand (grade: 45%). Industrial-scale implementation demonstrated a consumption capacity of 3.6 million tons of RM annually, yielding 400,000 tons of iron powder and 100,000 tons of ironsand, with total annual profits exceeding CNY 31 million. However, optimization of alkalinity reduction prior to extraction is critical to enhance iron recovery efficiency and purity, thereby maximizing economic returns and minimizing residual waste.
- (2)
- RM-Based Sintered Bricks: The RM–FA–GBFS mixture (6:1:3 ratio) exhibited favorable plasticity (plasticity index: 7.1) and mechanical properties, with sintered bricks achieving compressive strengths of 10–15 MPa. The optimized production process, utilizing high-pressure extrusion and controlled sintering (950–1030 °C), demonstrated compatibility with industrial brickmaking standards. This approach not only substitutes traditional raw materials (e.g., shale) but also reduces production costs, offering an annual profit potential of CNY 4 million for a medium-scale facility.
- (3)
- Non-Calcined Cementitious Binders: Geopolymer pastes formulated with RM, PG, and GBFS exhibited remarkable 28-day compressive strengths of 27.3–41.1 MPa, comparable to conventional cement-based materials. The synergistic activation of RM (alkalinity source) and PG (sulfate source) facilitated hydration without high-temperature calcination, significantly reducing energy consumption and carbon emissions. The absence of cement in the binder formulation highlights its potential for large-scale co-utilization of industrial wastes, addressing both RM and PG stockpiling challenges.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RM | Red mud |
| PG | Phosphogypsum |
| MASB | MgO-activated slag and bentonite |
| GBFS | Granulated blast furnace slag |
| AFT | Aluminum fluoride trihydrate |
| XRF | X-ray fluorescence spectroscopy |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscope |
| FA | Fly ash |
| P | Soluble phosphorus |
| F | Fluorine |
| SF | Silica fume |
References
- Hind, A.R.; Bhargava, S.K.; Grocott, S.C. The Surface Chemistry of Bayer Process Solids: A Review. Colloids Surf. Physicochem. Eng. Asp. 1999, 146, 359–374. [Google Scholar] [CrossRef]
- Agrawal, S.; Rayapudi, V.; Dhawan, N. Extraction of Iron Values from Red Mud. Mater. Today Proc. 2018, 5, 17064–17072. [Google Scholar] [CrossRef]
- Xue, S.; Kong, X.; Zhu, F.; Hartley, W.; Li, X.; Li, Y. Proposal for Management and Alkalinity Transformation of Bauxite Residue in China. Environ. Sci. Pollut. Res. 2016, 23, 12822–12834. [Google Scholar] [CrossRef]
- Yalçın, N.; Sevinç, V. Utilization of Bauxite Waste in Ceramic Glazes. Ceram. Int. 2000, 26, 485–493. [Google Scholar] [CrossRef]
- Liu, X.; Han, Y.; He, F.; Gao, P.; Yuan, S. Characteristic, Hazard and Iron Recovery Technology of Red Mud—A Critical Review. J. Hazard. Mater. 2021, 420, 126542. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Liu, X.; Gao, P.; Han, Y. A Semi-Industrial Experiment of Suspension Magnetization Roasting Technology for Separation of Iron Minerals from Red Mud. J. Hazard. Mater. 2020, 394, 122579. [Google Scholar] [CrossRef]
- Wang, S.; Ang, H.M.; Tadé, M.O. Novel Applications of Red Mud as Coagulant, Adsorbent and Catalyst for Environmentally Benign Processes. Chemosphere 2008, 72, 1621–1635. [Google Scholar] [CrossRef]
- Xue, S.; Zhu, F.; Kong, X.; Wu, C.; Huang, L.; Huang, N.; Hartley, W. A Review of the Characterization and Revegetation of Bauxite Residues (Red Mud). Environ. Sci. Pollut. Res. 2016, 23, 1120–1132. [Google Scholar] [CrossRef]
- Alam, S.; Das, S.K.; Rao, B.H. Characterization of Coarse Fraction of Red Mud as a Civil Engineering Construction Material. J. Clean. Prod. 2017, 168, 679–691. [Google Scholar] [CrossRef]
- Xie, W.; Zhou, F.; Liu, J.; Bi, X.; Huang, Z.; Li, Y.; Chen, D.; Zou, H.; Sun, S. Synergistic Reutilization of Red Mud and Spent Pot Lining for Recovering Valuable Components and Stabilizing Harmful Element. J. Clean. Prod. 2020, 243, 118624. [Google Scholar] [CrossRef]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The Composition, Recycling and Utilisation of Bayer Red Mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Li, Y.-C.; Min, X.-B.; Ke, Y.; Chai, L.-Y.; Shi, M.-Q.; Tang, C.-J.; Wang, Q.-W.; Liang, Y.-J.; Lei, J.; Liu, D.-G. Utilization of Red Mud and Pb/Zn Smelter Waste for the Synthesis of a Red Mud-Based Cementitious Material. J. Hazard. Mater. 2018, 344, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Krishnan, G.S.; Babu, L.G.; Singaravelu, D.L. Synergistic Effect of Red Mud-Iron Sulfide Particles on Fade-Recovery Characteristics of Non-Asbestos Organic Brake Friction Composites. Mater. Res. Express 2019, 6, 105311. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Lyu, G.; Guo, F.; Zhang, W.; Zhang, Y. Recovery of Alkali and Alumina from Bauxite Residue (Red Mud) and Complete Reuse of the Treated Residue. J. Clean. Prod. 2018, 188, 456–465. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, K.; Chen, W.; Lei, Q.; Huang, Y.; Peng, C. Recovery of Iron and Rare Earth Elements from Red Mud through an Acid Leaching-Stepwise Extraction Approach. J. Cent. South Univ. 2019, 26, 458–466. [Google Scholar] [CrossRef]
- Mudgal, M.; Singh, A.; Chouhan, R.K.; Acharya, A.; Srivastava, A.K. Fly Ash Red Mud Geopolymer with Improved Mechanical Strength. Clean. Eng. Technol. 2021, 4, 100215. [Google Scholar] [CrossRef]
- Wang, S.; Jin, H.; Deng, Y.; Xiao, Y. Comprehensive Utilization Status of Red Mud in China: A Critical Review. J. Clean. Prod. 2021, 289, 125136. [Google Scholar] [CrossRef]
- Bonomi, C.; Cardenia, C.; Yin, P.T.W.; Panias, D. Review of Technologies in the Recovery of Iron, Aluminium, Titanium and Rare Earth Elements from Bauxite Residue (Red Mud). In Proceedings of the International Symposium on Enhanced Landfill Mining, Lisboa, Portugal, 8–10 February 2016. [Google Scholar]
- Geng, C.; Sun, T.; Ma, Y.; Xu, C.; Yang, H. Effects of Embedding Direct Reduction Followed by Magnetic Separation on Recovering Titanium and Iron of Beach Titanomagnetite Concentrate. J. Iron Steel Res. Int. 2017, 24, 156–164. [Google Scholar] [CrossRef]
- Guo, Z.; Zhu, D.; Pan, J.; Zhang, F. Innovative Methodology for Comprehensive and Harmless Utilization of Waste Copper Slag via Selective Reduction-Magnetic Separation Process. J. Clean. Prod. 2018, 187, 910–922. [Google Scholar] [CrossRef]
- Zhu, D.; Yang, C.; Pan, J.; Lu, L.; Guo, Z.; Liu, X. An Integrated Approach for Production of Stainless Steel Master Alloy from a Low Grade Chromite Concentrate. Powder Technol. 2018, 335, 103–113. [Google Scholar] [CrossRef]
- Evans, K. The History, Challenges, and New Developments in the Management and Use of Bauxite Residue. J. Sustain. Metall. 2016, 2, 316–331. [Google Scholar] [CrossRef]
- Kumar, R.; Srivastava, J.P. Utilization of Iron Values of Red Mud for Metallurgical Applications; National Metallurgical Laboratory: Jamshedpur, India, 1998. [Google Scholar]
- Liu, S.; Guan, X.; Zhang, S.; Dou, Z.; Feng, C.; Zhang, H.; Luo, S. Sintered Bayer Red Mud Based Ceramic Bricks: Microstructure Evolution and Alkalis Immobilization Mechanism. Ceram. Int. 2017, 43, 13004–13008. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, N. Utilization of Red Mud in Cement Production: A Review. Waste Manag. Res. J. Sustain. Circ. Econ. 2011, 29, 1053–1063. [Google Scholar] [CrossRef]
- Mukiza, E.; Zhang, L.; Liu, X.; Zhang, N. Utilization of Red Mud in Road Base and Subgrade Materials: A Review. Resour. Conserv. Recycl. 2019, 141, 187–199. [Google Scholar] [CrossRef]
- Jones, B.E.H.; Haynes, R.J.; Phillips, I.R. Addition of an Organic Amendment and/or Residue Mud to Bauxite Residue Sand in Order to Improve Its Properties as a Growth Medium. J. Environ. Manage. 2012, 95, 29–38. [Google Scholar] [CrossRef]
- Sushil, S.; Batra, V.S. Catalytic Applications of Red Mud, an Aluminium Industry Waste: A Review. Appl. Catal. B Environ. 2008, 81, 64–77. [Google Scholar] [CrossRef]
- Vangelatos, I.; Angelopoulos, G.N.; Boufounos, D. Utilization of Ferroalumina as Raw Material in the Production of Ordinary Portland Cement. J. Hazard. Mater. 2009, 168, 473–478. [Google Scholar] [CrossRef]
- Deo, N.; Vasan, S.S.; Modak, J.M.; Natarajan, K.A. Selective Biodissolution of Calcium and Iron from Bauxite in the Presence of Bacillus polymyxa. In Process Metallurgy; Elsevier: Amsterdam, The Netherlands, 1999; Volume 9, pp. 463–472. ISBN 978-0-444-50193-6. [Google Scholar]
- Eisele, T.C.; Gabby, K.L. Review of Reductive Leaching of Iron by Anaerobic Bacteria. Miner. Process. Extr. Metall. Rev. 2014, 35, 75–105. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, J.; Xu, H.; Zhao, K.; Shi, X. Nuggets Production by Direct Reduction of High Iron Red Mud. J. Iron Steel Res. Int. 2013, 20, 24–27. [Google Scholar] [CrossRef]
- Jamieson, E.; Jones, A.; Cooling, D.; Stockton, N. Magnetic Separation of Red Sand to Produce Value. Miner. Eng. 2006, 19, 1603–1605. [Google Scholar] [CrossRef]
- Jayasankar, K.; Ray, P.K.; Chaubey, A.K.; Padhi, A.; Satapathy, B.K.; Mukherjee, P.S. Production of Pig Iron from Red Mud Waste Fines Using Thermal Plasma Technology. Int. J. Miner. Metall. Mater. 2012, 19, 679–684. [Google Scholar] [CrossRef]
- Lu, G.; Zhang, T.; Ma, L.; Wang, Y.; Zhang, W.; Zhang, Z.; Wang, L. Utilization of Bayer Red Mud by a Calcification–Carbonation Method Using Calcium Aluminate Hydrate as a Calcium Source. Hydrometallurgy 2019, 188, 248–255. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Wang, X.; Wang, B.; Luan, Z. Feasibility Study of Iron Mineral Separation from Red Mud by High Gradient Superconducting Magnetic Separation. Phys. C Supercond. 2011, 471, 91–96. [Google Scholar] [CrossRef]
- Yu, J.; Li, Y.; Lv, Y.; Han, Y.; Gao, P. Recovery of Iron from High-Iron Red Mud Using Suspension Magnetization Roasting and Magnetic Separation. Miner. Eng. 2022, 178, 107394. [Google Scholar] [CrossRef]
- Singh, S.; Aswath, M.U.; Ranganath, R.V. Performance Assessment of Bricks and Prisms: Red Mud Based Geopolymer Composite. J. Build. Eng. 2020, 32, 101462. [Google Scholar] [CrossRef]
- Zhao, H.; Gou, H. Unfired Bricks Prepared with Red Mud and Calcium Sulfoaluminate Cement: Properties and Environmental Impact. J. Build. Eng. 2021, 38, 102238. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Wang, Y.; Li, Z.; Li, Y.; Ren, Y. Binary Reaction Behaviors of Red Mud Based Cementitious material: Hydration Characteristics and Na+ Utilization. J. Hazard. Mater. 2021, 410, 124592. [Google Scholar] [CrossRef]
- Primary Aluminium Production. Available online: https://international-aluminium.org/statistics/primary-aluminium-production/ (accessed on 18 April 2025).
- Çoruh, S.; Ergun, O.N. Use of Fly Ash, Phosphogypsum and Red Mud as a Liner Material for the Disposal of Hazardous Zinc Leach Residue Waste. J. Hazard. Mater. 2010, 173, 468–473. [Google Scholar] [CrossRef]
- Huang, X.; Li, J.; Jiang, W.; Chen, Z.; Wan, Y.; Xue, Q.; Liu, L.; Poon, C.S. Recycling of Phosphogypsum and Red Mud in Low Carbon and Green Cementitious Materials for Vertical Barrier. Sci. Total Environ. 2022, 838, 155925. [Google Scholar] [CrossRef]
- Gijbels, K.; Pontikes, Y.; Samyn, P.; Schreurs, S.; Schroeyers, W. Effect of NaOH Content on Hydration, Mineralogy, Porosity and Strength in Alkali/Sulfate-Activated Binders from Ground Granulated Blast Furnace Slag and Phosphogypsum. Cem. Concr. Res. 2020, 132, 106054. [Google Scholar] [CrossRef]
- ASTM C62-23; Standard Specification for Building Brick (Solid Masonry Units Made from Clay or Shale). ASTM International: West Conshohocken, PA, USA, 2023.
- GB/T 8074-2008; Testing Method for Specific Surface of Cement-Blaine Method. Bureau of Quality and Technical Supervision of China: Beijing, China, 2008. (In Chinese)
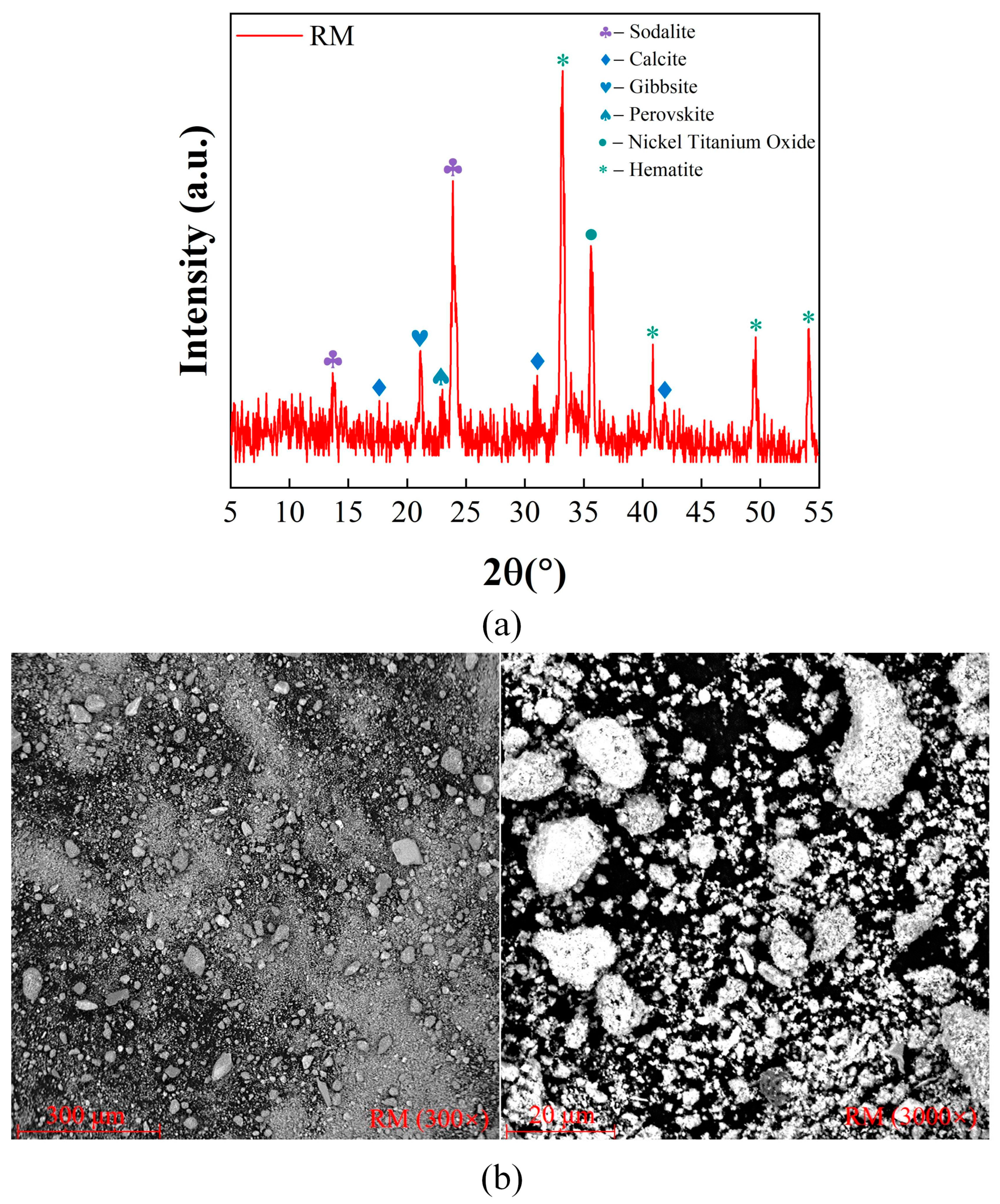
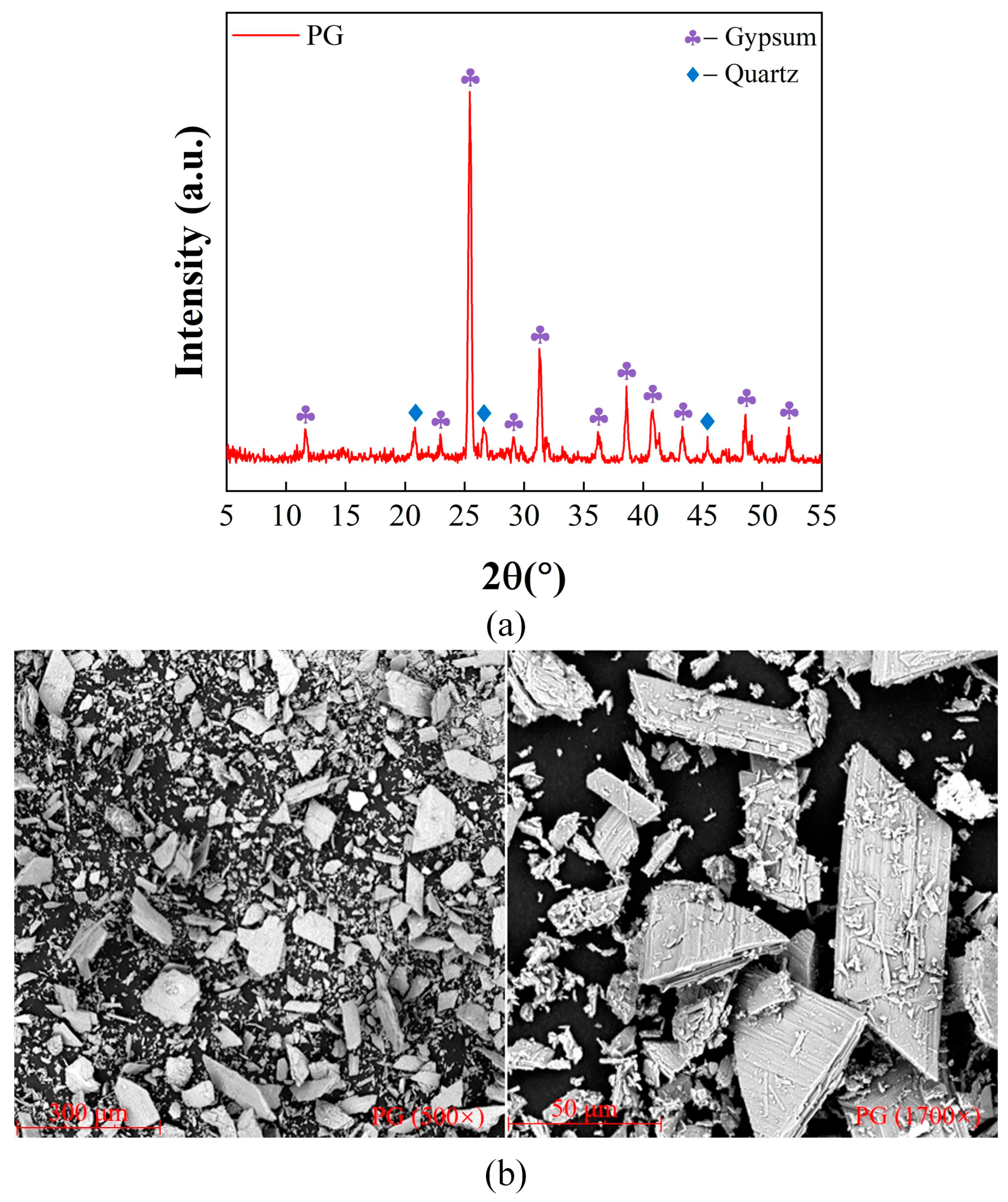


| Sample | Fe2O3 | Na2O | Al2O3 | SiO2 | TiO2 | CaO | SO3 | P2O5 | MgO | MnO | K2O | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RM | 33.79 | 21.95 | 20.54 | 11.54 | 5.44 | 5.31 | 2.95 | 0.52 | 0.22 | 0.10 | 0.05 | 4.94 |
| PG | 3.29 | 0.50 | 0.96 | 8.34 | 0.66 | 49.53 | 39.40 | 2.01 | 0.21 | 0.02 | 0.15 | 4.21 |
| Appearance | Modulus | Baume Degree | Na2O | SiO2 |
|---|---|---|---|---|
| Clear and Colorless Liquid | 3.24 | 39.5 | 9.25 | 29 |
| Type | Yield | Grade | Recycling Rate |
|---|---|---|---|
| Raw material | 100 | 49.30 | 100 |
| Iron ore concentrate | 20.07 | 58.24 | 23.85 |
| Ore | 3.31 | 45.32 | 3.06 |
| Tailings | 76.72 | 47.02 | 73.08 |
| Group | wt.% | Water-Binder Ratio | ||||
|---|---|---|---|---|---|---|
| RM | PG | GBFS | SF | Activator | ||
| Sample 1 | 20 | 10 | 30 | / | 10.3 | 0.44 |
| Sample 2 | 15 | 15 | 30 | / | 7.0 | 0.46 |
| Sample 3 | 20 | 10 | 25 | 5 | 10.3 | 0.46 |
| Sample 4 | 15 | 15 | 30 | / | 10.3 | 0.44 |
| Sample 5 | 12 | 18 | 30 | / | 10.3 | 0.44 |
| Before Iron Extraction | After Iron Extraction | ||
|---|---|---|---|
| Time | Consumption (tons) | Time | Consumption (tons) |
| 2018/01 | 429,335.28 | 2020/01 | 410,516.10 |
| 2018/02 | 462,054.6 | 2020/09 | 450,505.26 |
| 2018/03 | 402,305.58 | 2020/11 | 434,541.96 |
| 2018/04 | 386,835.3 | 2020/12 | 417,683.34 |
| 2018/05 | 308,954.88 | 2021/01 | 388,748.88 |
| 2018/06 | 408,262.86 | 2021/02 | 422,603.64 |
| 2018/07 | 352,341.18 | 2021/03 | 382,105.08 |
| 2018/08 | 370,591.56 | 2021/04 | 379,579.86 |
| 2018/09 | 403,959.06 | 2021/05 | 384,717.42 |
| Total amount of dry RM (ton) | 3,524,640.30 | 3,671,001.54 | |
| Materials | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | S | LOI |
|---|---|---|---|---|---|---|---|---|---|
| RM | 25.65 | 21.27 | 23.44 | 2.12 | 0.85 | 0.45 | 7.91 | 0.96 | 10.87 |
| FA | 73.43 | 12.54 | 2.77 | 0.79 | 0.56 | 0.87 | 0.53 | 0.57 | 5.55 |
| slag | 53.5 | 14.29 | 0.53 | 0.95 | 0.78 | 0.29 | 0.35 | 0.78 | 15.26 |
| Materials | Dry Sensitivity Factor | Line Shrinkage (%) | Liquid Limit (%) | Plastic Limit (%) | Plasticity Index |
|---|---|---|---|---|---|
| RM | 0.82 | 3.24 | 35.77 | 27.98 | 7.79 |
| FA | 0.87 | 4.02 | 25.9 | 20.7 | 5.2 |
| slag | 0.72 | 2.63 | 25.03 | 18.53 | 6.5 |
| Material | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | S | LOI |
|---|---|---|---|---|---|---|---|---|---|
| Mixtures | 38.78 | 18.31 | 14.5 | 1.63 | 0.8 | - | - | 0.87 | 11.66 |
| Material | Dry Sensitivity Factor | Total Shrinkage Rate (%) | Liquid Limit (%) | Plastic Limit (%) | Plasticity Index |
|---|---|---|---|---|---|
| Mixtures | 0.82 | 3.15 | 22.9 | 15.7 | 7.1 |
| Aperture mm | Specific Gravimetric Method | |||||
|---|---|---|---|---|---|---|
| >2.0 mm | 2–1.5 mm | 1.5–0.5 mm | 0.5–0.1 mm | <0.1 mm | ||
| Mixtures | Percentage content | 3.1 | 18.1 | 15.4 | 10.0 | 53.4 |
| Samples | Length (mm) | Width (mm) | Maximum Load (kN) | Compression Strength (MPa) |
|---|---|---|---|---|
| RMB-1 | 100 | 108 | 123.3 | 11.4 |
| RMB-2 | 102 | 112 | 149.8 | 13.1 |
| RMB-3 | 100 | 110 | 173.6 | 15.8 |
| RMB-4 | 100 | 110 | 134.5 | 12.2 |
| RMB-5 | 98 | 114 | 160.1 | 14.3 |
| RMB-6 | 100 | 111 | 137.1 | 12.4 |
| RMB-7 | 97 | 113 | 149.1 | 13.6 |
| RMB-8 | 102 | 110 | 144.5 | 12.9 |
| RMB-9 | 100 | 111 | 149.6 | 13.5 |
| RMB-10 | 100 | 111 | 159.3 | 14.4 |
| Performance Indicators | Initial Setting Time | Final Setting Time | Flow Degree (mm) | 3 d Strength (MPa) | 7 d Strength (MPa) | 14 d Strength (MPa) | 28 d Strength (MPa) |
|---|---|---|---|---|---|---|---|
| Sample 1 | 10 h 2 min | 14 h 34 min | 114 | 10.7 | 27.3 | 39.1 | 40.8 |
| Sample 2 | 11 h 25 min | 16 h 27 min | 135 | 7.1 | 26.5 | 38.3 | 41.1 |
| Sample 3 | 13 h 49 min | 19 h 56 min | 121 | / | 2.8 | 23.6 | 27.3 |
| Sample 4 | 10 h 54 min | 15 h 8 min | 109 | 2.1 | 12.1 | 32.2 | 37.5 |
| Sample 5 | 12 h 24 min | 17 h 43 min | 102 | / | 6.8 | 18.8 | 33.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Xu, F.; Ding, Y.; Zheng, F.; Zou, J. Integrated Utilization Strategies for Red Mud: Iron Extraction, Sintered Brick Production, and Non-Calcined Cementitious Binder Development for Environmental Sustainability. Coatings 2025, 15, 522. https://doi.org/10.3390/coatings15050522
Li B, Xu F, Ding Y, Zheng F, Zou J. Integrated Utilization Strategies for Red Mud: Iron Extraction, Sintered Brick Production, and Non-Calcined Cementitious Binder Development for Environmental Sustainability. Coatings. 2025; 15(5):522. https://doi.org/10.3390/coatings15050522
Chicago/Turabian StyleLi, Bin, Fang Xu, Yan Ding, Fei Zheng, and Junpeng Zou. 2025. "Integrated Utilization Strategies for Red Mud: Iron Extraction, Sintered Brick Production, and Non-Calcined Cementitious Binder Development for Environmental Sustainability" Coatings 15, no. 5: 522. https://doi.org/10.3390/coatings15050522
APA StyleLi, B., Xu, F., Ding, Y., Zheng, F., & Zou, J. (2025). Integrated Utilization Strategies for Red Mud: Iron Extraction, Sintered Brick Production, and Non-Calcined Cementitious Binder Development for Environmental Sustainability. Coatings, 15(5), 522. https://doi.org/10.3390/coatings15050522









