Phosphotungstic Acid Intercalated MgAlLa Ternary Layered Double Hydroxides as High-Efficiency Additives for Epoxy Resin: Synergistic Enhancement of Flame Retardancy and Smoke Suppression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MgAlLa-PWA
2.3. Preparation of LDHs/EP Composite Materials
3. Results and Discussion
3.1. Characterization of LDHs
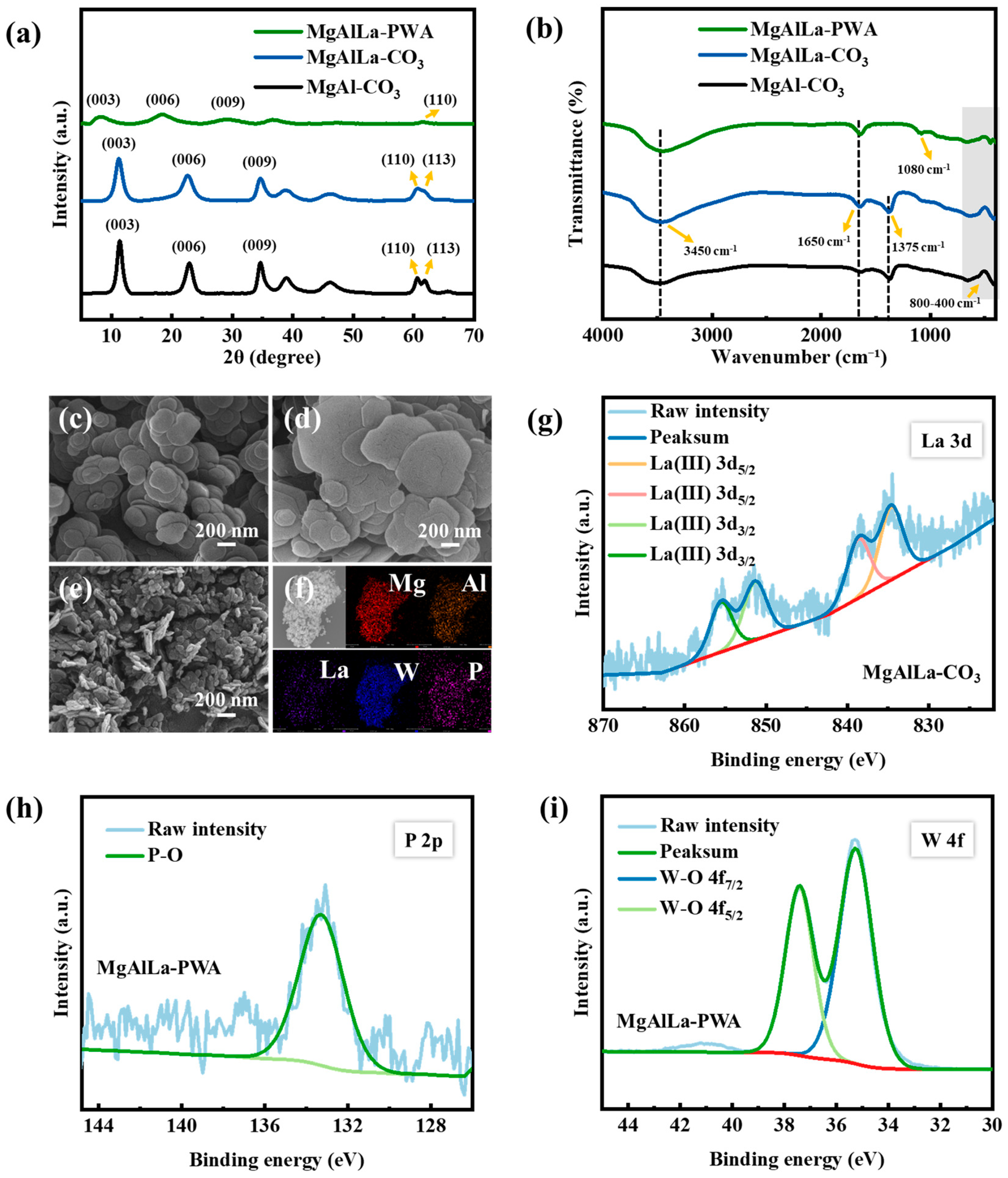
3.1.1. XRD Analysis
3.1.2. FT-IR Analysis
3.1.3. SEM-EDS Analysis
3.1.4. XPS
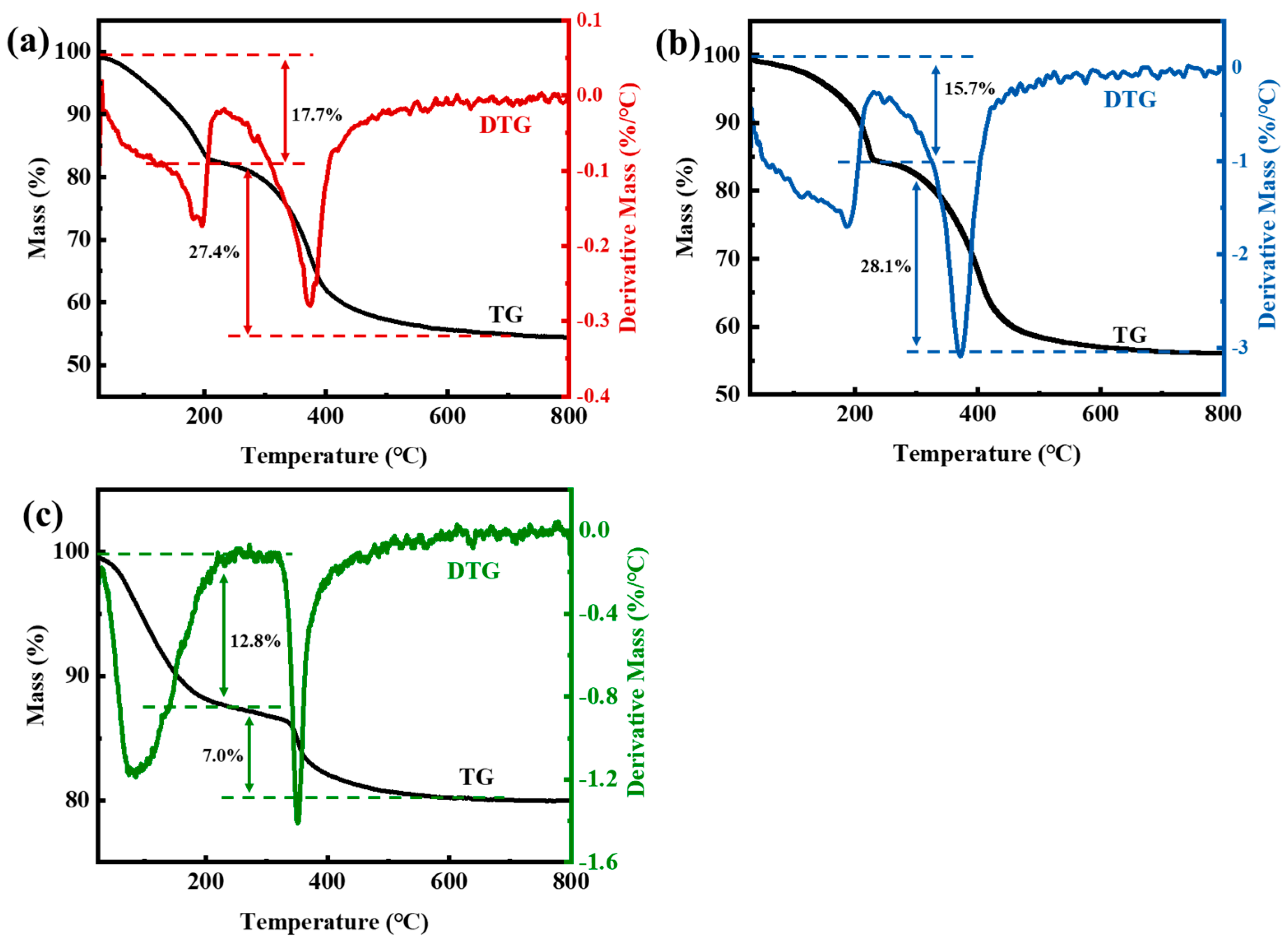
3.1.5. TGA of LDHs
3.2. Flame Retardancy of Composite EP
3.2.1. TGA of Composite EP
3.2.2. Burning Behaviors (LOI and UL-94 Tests)
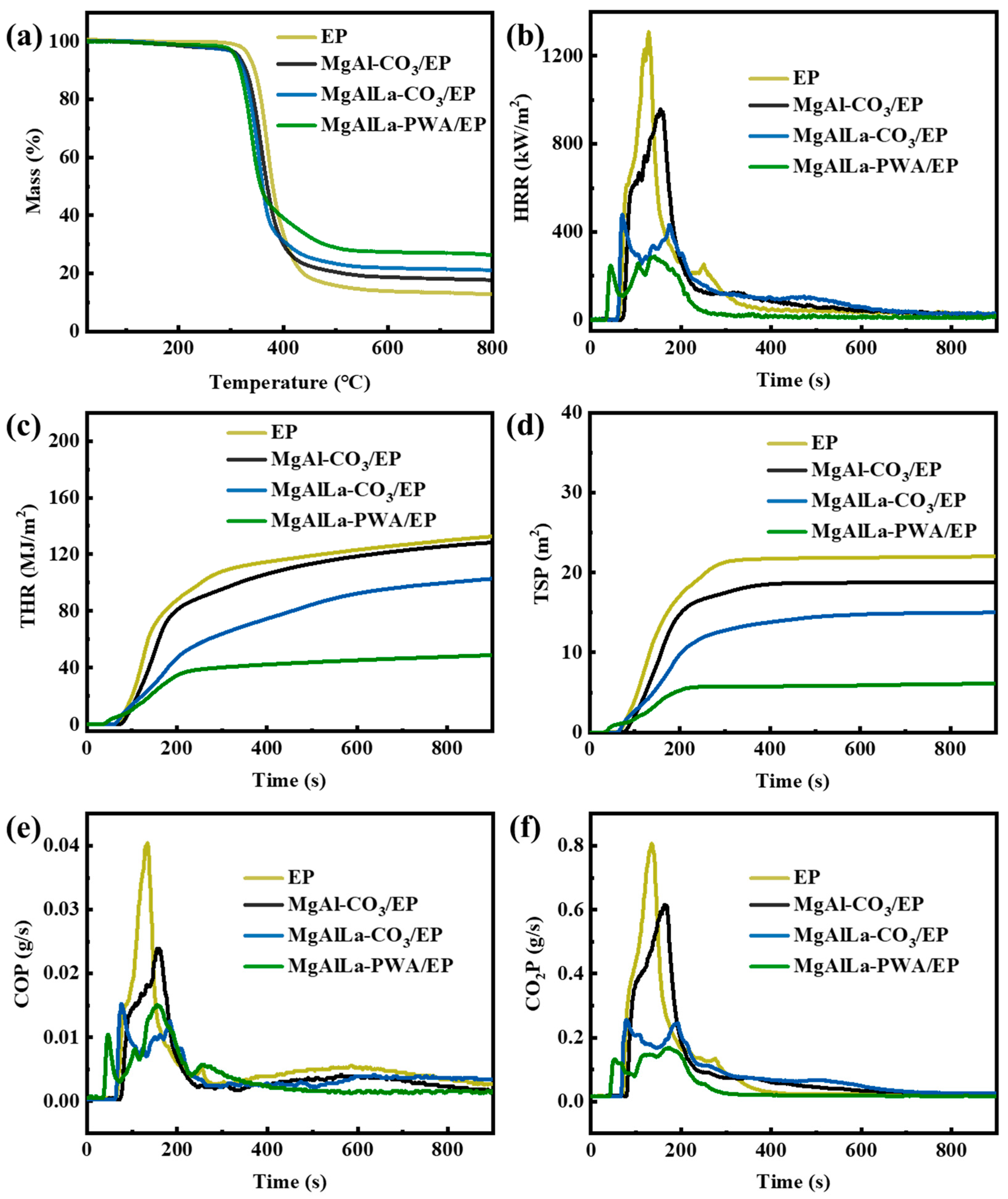
3.2.3. Cone Calorimeter Test
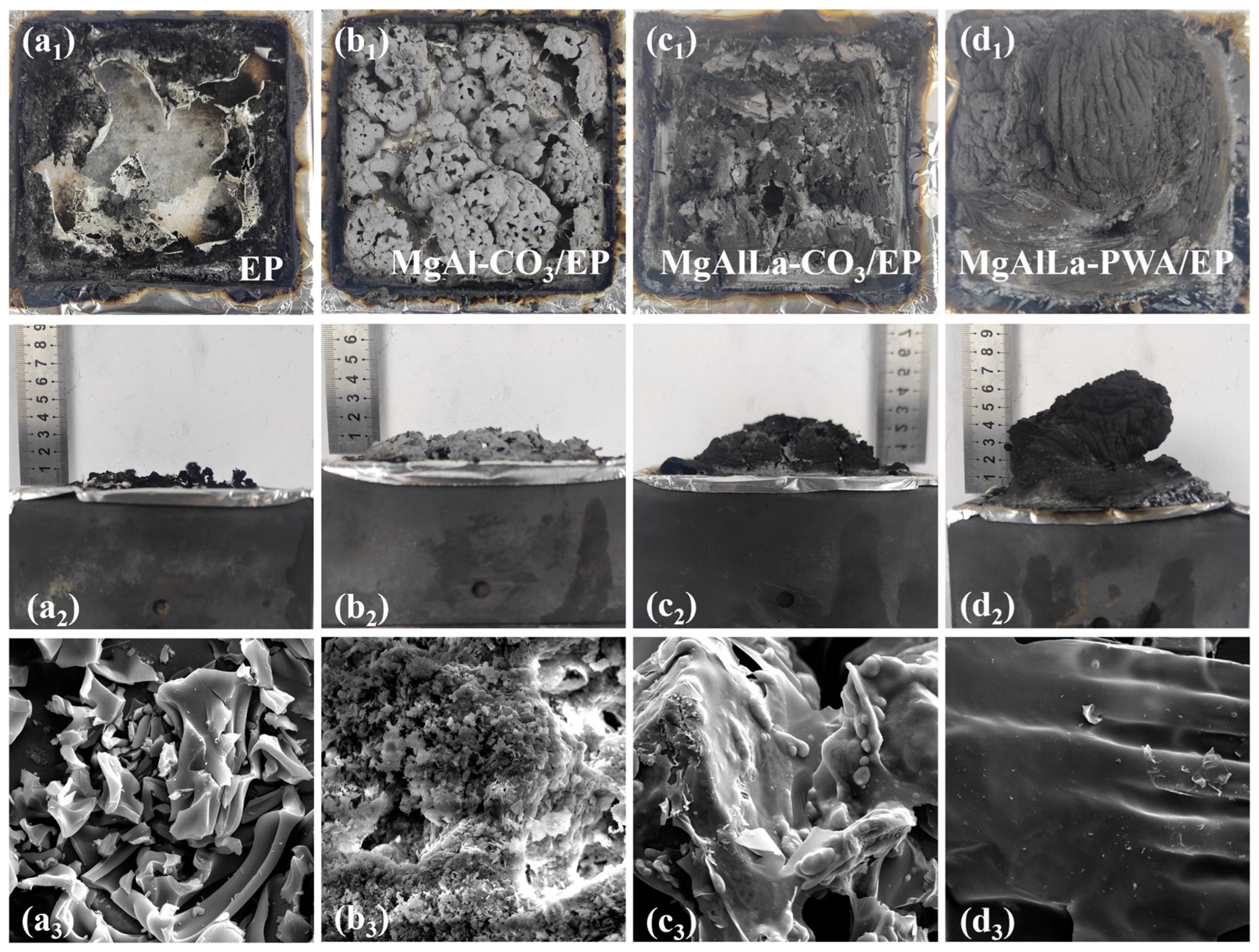
3.2.4. Charring Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Afzal, A.; Tariq, A.; Shakir, F.; Satti, A.N.; Taimoor, M.; Ghani, U.; Jaffer, U.; Rashid, I.A.; Khaliq, Z. Development and characterization of multifunctional carbon fabric-reinforced polymer composites incorporated with inorganic flame retardants. Polym. Compos. 2020, 41, 3043–3051. [Google Scholar] [CrossRef]
- Yang, Z.; Xiao, G.; Chen, C.; Chen, C.; Zhong, F.; Cao, M.; Wang, M.; Zou, R.; Li, R.; Li, Y. Bio-inspired adenosine triphosphate-modified h-BN-based coral-like CuAl-LDH nanosheets as a functional green flame retardant to improve the fire safety of epoxy resins via catalyzing intumescent char formation. Prog. Org. Coat. 2023, 182, 107648. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Zhang, S.; Guo, J.; Tang, W.; Li, H.; Gu, X. Effects of carboxymethyl chitosan microencapsulated melamine polyphosphate on the flame retardancy and water resistance of thermoplastic polyurethane. Polym. Degrad. Stab. 2019, 160, 168–176. [Google Scholar] [CrossRef]
- Hong, J.; Wu, T.; Wu, H.; Zeng, B.; Zeng, S.; Chen, T.; Wang, X.; Lu, Z.; Yuan, C.; Balaji, K.; et al. Nanohybrid silver nanoparticles@halloysite nanotubes coated with polyphosphazene for effectively enhancing the fire safety of epoxy resin. Chem. Eng. J. 2021, 407, 127087. [Google Scholar] [CrossRef]
- Sun, R.; Pan, C.; Peng, F.; Wu, Y.; Chen, X.; Mai, B. Alternative halogenated flame retardants (AHFRs) in green mussels from the south China sea. Environ. Res. 2020, 182, 109082. [Google Scholar] [CrossRef]
- He, S.; Deng, C.; Zhao, Z.-Y.; Chen, Z.-X.; Wang, Y.-Z. Hyperbranched polyamide-amine based phosphorous-containing flame retardant for simultaneous flame retardancy and high performance of polypropylene. Compos. Part B Eng. 2023, 250, 110431. [Google Scholar] [CrossRef]
- Zielonka, P.; Duda, S.; Lesiuk, G.; Błażejewski, W.; Wiśniewska, M.; Warycha, J.; Stabla, P.; Smolnicki, M.; Babiarczuk, B. The effect of flame retardant—Aluminum trihydroxide on mixed mode I/II fracture toughness of epoxy resin. Polymers 2022, 14, 4386. [Google Scholar] [CrossRef]
- Hu, J.; Hu, J.-Y.; Chen, Y.-J.; Tan, L.; Luo, Q.; Yan, W.-J.; Tang, Z.-H.; Zhang, J.-J.; Wang, L.; Wang, N.-L.; et al. Construction of hydrophobic core-shell flame retardant: A novel strategy towards reducing fire hazards and reinforcing mechanical properties of polypropylene. Appl. Clay Sci. 2024, 251, 107297. [Google Scholar] [CrossRef]
- Deng, C.; Liu, Y.; Jian, H.; Liang, Y.; Wen, M.; Shi, J.; Park, H. Study on the preparation of flame retardant plywood by intercalation of phosphorus and nitrogen flame retardants modified with Mg/Al-LDH. Constr. Build. Mater. 2023, 374, 130939. [Google Scholar] [CrossRef]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Wang, M.; Wu, B.; He, X.; Qian, Y.; Wang, S. Anti-metastatic and anti-angiogenic activities of core–shell SiO2@LDH loaded with etoposide in non-small cell lung cancer. Adv. Sci. 2016, 3, 1600229. [Google Scholar] [CrossRef] [PubMed]
- Matusinovic, Z.; Wilkie, C.A. Fire retardancy and morphology of layered double hydroxide nanocomposites: A review. J. Mater. Chem. 2012, 22, 18701–18704. [Google Scholar] [CrossRef]
- Evans, D.G.; Duan, X. Preparation of layered double hydroxides and their applications as additives in polymers, as precursors to magnetic materials and in biology and medicine. Chem. Commun. 2006, 2006, 485–496. [Google Scholar] [CrossRef]
- Wang, J.; Yu, S.; Wang, Z.; Zhang, Y.; Pan, W. Strongly-coupled magnetic nanospheres@trimetallic LDH hooding with thin MXenes veil: Sandwiched ternary nanostructure towards forging fire-safe EP with low toxicity. Appl. Surf. Sci. 2023, 612, 155904. [Google Scholar] [CrossRef]
- Zhu, J.; Lan, S.; Zhu, D. Functionalized LDH via intercalation of gluconate anion and surface assembly of ultrafine copper hydroxide for improving mechanical properties and the fire safety of epoxy resin. Appl. Surf. Sci. 2025, 680, 161458. [Google Scholar] [CrossRef]
- Li, Z.; Huang, G.-B.; Li, H.; Zhang, L.; Liu, Z.; De La Vega, J.; Díaz, R.S.; Zeng, Q.; Wang, D.-Y. Fire-safe and multifunctional epoxy/layered double hydroxide composites via an interfacial catalysis. Appl. Clay Sci. 2024, 260, 107545. [Google Scholar] [CrossRef]
- Antoniadis, A.; Takavakoglou, V.; Zalidis, G.; Darakas, E.; Poulios, I. Municipal wastewater treatment by sequential combination of photocatalytic oxidation with constructed wetlands. Catal. Today 2010, 151, 114–118. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, Y.; Wang, W.; Gu, X.; Li, H.; Li, J.; Sun, J. Intercalation of phosphotungstic acid into layered double hydroxides by reconstruction method and its application in intumescent flame retardant poly (lactic acid) composites. Polym. Degrad. Stab. 2018, 147, 142–150. [Google Scholar] [CrossRef]
- Abdelrahman, A.A.; El Naggar, A.M.A.; Zahran, A.I. Ultrasound oxidative desulfurization of petroleum gas oil using phosphotungstic acid/ZnAl calcined LDH as efficient and recoverable nanocomposite. Inorg. Chem. Commun. 2023, 158, 111600. [Google Scholar] [CrossRef]
- Royan, F.; Abbasi, A.; Hosseini, M.-S.; Ghorbani, P. Enhanced cationic dye removal from water media via electrostatic interaction-assisted incorporation of phosphotungstic acid into NH2-MIL-88B(Fe) through the breathing effect. Colloids Surf. A Physicochem. Eng. Asp. 2025, 708, 135977. [Google Scholar] [CrossRef]
- Feng, Z.; Guo, J.; Yan, Y.; Sun, J.; Zhang, S.; Wang, W.; Gu, X.; Li, H. Modification of mesoporous silica with phosphotungstic acid and its effects on the combustion and thermal behavior of polylactic acid composites. Polym. Degrad. Stab. 2019, 160, 24–34. [Google Scholar] [CrossRef]
- Yu, J.; Lu, K.; Wang, C.; Wang, Z.; Fan, C.; Bai, G.; Wang, G.; Yu, F. Modification of NiFe layered double hydroxide by lanthanum doping for boosting water splitting. Electrochim. Acta 2021, 390, 138824. [Google Scholar] [CrossRef]
- Xu, R.; Wang, X.; Yang, Z.; Chang, Y.; Chen, X.; Wang, J.; Li, H. Electrodeposition fabrication of La-doped NiFe layered double hydroxide to improve conductivity for efficient overall water splitting. ACS Appl. Energy Mater. 2024, 7, 3866–3875. [Google Scholar] [CrossRef]
- Guo, R.; Ding, C.; Liu, Y.; Cheng, X.; Zhang, L.; Zhao, W.; Sheng, X. In situ growth of layered double hydroxides on zirconium phosphate for reinforcing anti-corrosion and wear resistance of waterborne epoxy coatings. Polymer 2025, 319, 128048. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Krivovichev, S.V.; Pekov, I.V.; Yakovenchuk, V.N.; Pakhomovsky, Y.A. Correlation between the d-value and the M2+:M3+ cation ratio in Mg–Al–CO3 layered double hydroxides. Appl. Clay Sci. 2016, 130, 2–11. [Google Scholar] [CrossRef]
- Wu, J.; Wu, L.; Yao, W.; Zhou, Y.; Wu, M.; Yuan, Y.; Xie, Z.; Atrens, A.; Wang, J.; Pan, F. Self-healing PEO/MgAlLa LDHs-MXene composite coating loaded with 4-aminophenol for corrosion protection of Mg-Gd-Y-Zn LPSO Mg alloy. Electrochim. Acta 2024, 491, 144358. [Google Scholar] [CrossRef]
- Pavel, O.D.; Zăvoianu, R.; Bîrjega, R.; Angelescu, E.; Pârvulescu, V.I. Mechanochemical versus co-precipitated synthesized lanthanum-doped layered materials for olefin oxidation. Appl. Catal. A Gen. 2017, 542, 10–20. [Google Scholar] [CrossRef]
- Qin, Q.; Xu, H.; Sun, S.; Zhao, Z.; Ren, X.; Li, M.; Song, B.; Shao, G.; Wang, H.; Lu, H. Facile synthesis of MgAl-LDH/g-C3N4 composites for the photocatalytic degradation toward ciprofloxacin. J. Environ. Chem. Eng. 2025, 13, 116299. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, N.; Wen, C.; Lou, G.; Zhong, B. Corrosion inhibition effect of Mg-Al-pAB-LDH coating for steel in the marine environment. Coatings 2024, 14, 1307. [Google Scholar] [CrossRef]
- Hsiao, W.-L.; Chu, P.-W. Fabrication of vanadate-exchanged electrodeposited Zn-Al layered double hydroxide (LDH) coating on a ZX21 Mg alloy to improve the corrosion resistance. Coatings 2024, 14, 1047. [Google Scholar] [CrossRef]
- Ding, C.; Wu, J.; Liu, Y.; Sheng, X.; Cheng, X.; Xiong, X.; Zhao, W. A waterborne epoxy composite coating with smart corrosion resistance based on 2-phenylbenzimidazole-5-sulfonic acid/layered double hydroxide composite. Molecules 2023, 28, 5199. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xing, G.; Yang, Q.; Zhou, J.; Yao, G.; Qi, L. The synergistic effect of phosphotungstic acid as catalyst and Cu-based metal-organic framework in dry desulfurization enhances the ability to capture SO2: Synergistic effect of catalytic action and CO2 selectivity performance. J. Environ. Chem. Eng. 2025, 13, 115385. [Google Scholar]
- Zhou, E.; Liu, Y.; Yuan, H.; Cheng, X.; Zhong, Y.; He, J.; Lu, X. Mechanism of sodium dodecyl diphenyl ether disulfonate filled hydrotalcite inhibiting the photo-degradation of polyvinyl chloride under different ranges of ultraviolet wavelength irradiation. Coatings 2023, 13, 985. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, M.; Zhu, Y.; Chai, Y.; Liu, B. A band structure modulated 1D/2D CdS/MgAl-LDH S-scheme heterojunction toward simultaneous photocatalytic removal of tetracycline and hexavalent chromium. Appl. Surf. Sci. 2025, 693, 162789. [Google Scholar] [CrossRef]
- Yao, W.; Chen, Y.; Wu, L.; Jiang, B.; Pan, F. Preparation of slippery liquid-infused porous surface based on MgAlLa-layered double hydroxide for effective corrosion protection on AZ31 Mg alloy. J. Taiwan Inst. Chem. Eng. 2022, 131, 104176. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, Z.; Zhang, Y.; Shao, W.; Wu, D.; Peng, G.; Liu, Z. Introduction of bimetallic oxide-modified carbon nanotubes for boosting the energy storage performance of NiCo-LDH based in-plane micro-supercapacitors on paper. Chem. Eng. J. 2024, 494, 153242. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Greenwell, H.C.; Krzhizhanovskaya, M.G.; Apperley, D.C.; Pekov, I.V.; Yakovenchuk, V.N. Thermal evolution of natural layered double hydroxides: Insight from quintinite, hydrotalcite, stichtite, and iowaite as reference samples for CO3- and Cl-members of the hydrotalcite supergroup. Minerals 2020, 10, 961. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, Y.; Liu, S.; Chi, Z.; Xu, J. Synergistic effect of La2O3 on the flame retardant properties and the degradation mechanism of a novel PP/IFR system. Polym. Degrad. Stab. 2012, 97, 707–714. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Ren, J.; Wang, Y.; Li, M.; Jiao, C.; Chen, X. Flame retardant thermoplastic polyurethane based on a combination of chitosan and phosphotungstic acid. J. Therm. Anal. Calorim. 2022, 147, 12791–12803. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, Y.; Zhang, X.; Kong, Q.; Zhang, J.; Liu, H.; Zhang, F. Improving the flame-retardant efficiency of layered double hydroxide with disodium phenylphosphate for epoxy resin. J. Therm. Anal. Calorim. 2020, 140, 149–156. [Google Scholar] [CrossRef]
- Schartel, B.; Bartholmai, M.; Knoll, U. Some comments on the main fire retardancy mechanisms in polymer nanocomposites. Polym. Adv. Technol. 2006, 17, 772–777. [Google Scholar] [CrossRef]
- Shi, X.-H.; Li, X.-L.; Shi, H.; Liu, Q.-Y.; Xie, W.-M.; Wu, S.-J.; Zhao, N.; Wang, D.-Y. Insight into the flame-retardant mechanism of different organic-modified layered double hydroxide for epoxy resin. Appl. Clay Sci. 2024, 248, 107233. [Google Scholar] [CrossRef]

| Sample | Crystalline Lattice | 2θ (°) | d (nm) | c (nm) |
|---|---|---|---|---|
| MgAl-CO3 | (003) | 11.37 | 0.778 | 2.335 |
| MgAlLa-CO3 | (003) | 11.24 | 0.787 | 2.362 |
| MgAlLa-PWA | (003) | 8.20 | 1.078 | 3.234 |
| Composites | T5% (°C) | T50% (°C) | Tmax (°C) | Char Residues at 800 °C (%) |
|---|---|---|---|---|
| EP | 334.8 | 381.0 | 370.0 | 12.6 |
| MgAl-CO3/EP | 314.6 | 370.2 | 358.6 | 17.5 |
| MgAlLa-CO3/EP | 311.6 | 361.6 | 355.9 | 20.9 |
| MgAlLa-PWA/EP | 308.7 | 357.9 | 341.2 | 26.2 |
| Samples | Combustion Performance | |
|---|---|---|
| LOI (%) | UL-94 | |
| EP | 22.4 | NR |
| MgAl-CO3/EP | 24.4 | V-2 |
| MgAlLa-CO3/EP | 24.6 | V-2 |
| MgAlLa-PWA/EP | 26.8 | V-0 |
| Samples | TTI (s) | pHRR (kW/m2) | THR (MJ/m2) | TSR (m2) | COP (g/s) | CO2P (g/s) | Char Residue (%) |
|---|---|---|---|---|---|---|---|
| EP | 64 | 1307.41 | 133.15 | 22.02 | 0.040 | 0.805 | 1.5 |
| MgAl-CO3/EP | 73 | 957.87 | 129.12 | 18.73 | 0.024 | 0.607 | 11.7 |
| MgAlLa-CO3/EP | 59 | 478.96 | 110.06 | 15.00 | 0.015 | 0.261 | 19.5 |
| MgAlLa-PWA/EP | 38 | 289.13 | 50.13 | 6.13 | 0.010 | 0.132 | 48.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Jin, J.; Guang, Z.; Chen, H.; Liu, Y.; Cheng, X.; Liu, Y.; Wei, X.; He, J.; Zhao, W. Phosphotungstic Acid Intercalated MgAlLa Ternary Layered Double Hydroxides as High-Efficiency Additives for Epoxy Resin: Synergistic Enhancement of Flame Retardancy and Smoke Suppression. Coatings 2025, 15, 523. https://doi.org/10.3390/coatings15050523
Zhao W, Jin J, Guang Z, Chen H, Liu Y, Cheng X, Liu Y, Wei X, He J, Zhao W. Phosphotungstic Acid Intercalated MgAlLa Ternary Layered Double Hydroxides as High-Efficiency Additives for Epoxy Resin: Synergistic Enhancement of Flame Retardancy and Smoke Suppression. Coatings. 2025; 15(5):523. https://doi.org/10.3390/coatings15050523
Chicago/Turabian StyleZhao, Wensheng, Jiao Jin, Zhengkai Guang, Haosen Chen, Yangu Liu, Xiaoling Cheng, Yuan Liu, Xing Wei, Jiebing He, and Wenlin Zhao. 2025. "Phosphotungstic Acid Intercalated MgAlLa Ternary Layered Double Hydroxides as High-Efficiency Additives for Epoxy Resin: Synergistic Enhancement of Flame Retardancy and Smoke Suppression" Coatings 15, no. 5: 523. https://doi.org/10.3390/coatings15050523
APA StyleZhao, W., Jin, J., Guang, Z., Chen, H., Liu, Y., Cheng, X., Liu, Y., Wei, X., He, J., & Zhao, W. (2025). Phosphotungstic Acid Intercalated MgAlLa Ternary Layered Double Hydroxides as High-Efficiency Additives for Epoxy Resin: Synergistic Enhancement of Flame Retardancy and Smoke Suppression. Coatings, 15(5), 523. https://doi.org/10.3390/coatings15050523








