Regulation of Microstructure and Mechanical Properties of DC Electrodeposited Copper Foils by Electrolyte Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization of Structure and Properties
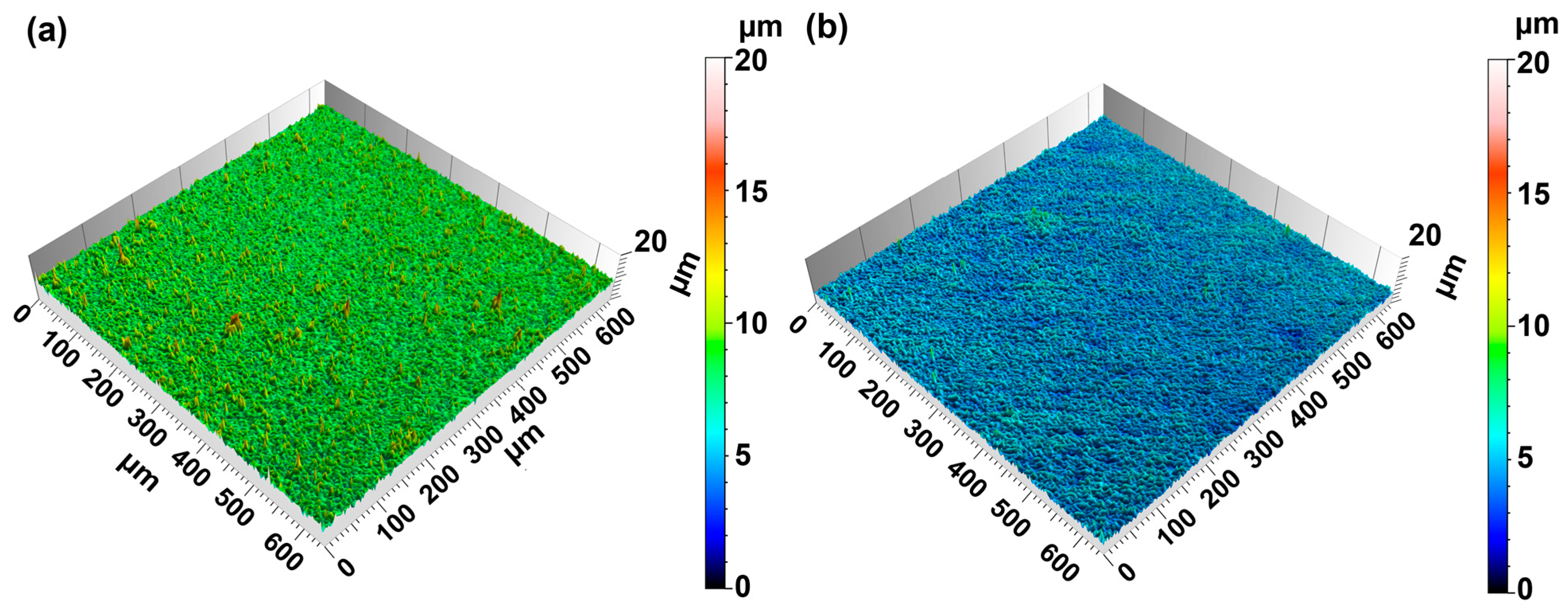
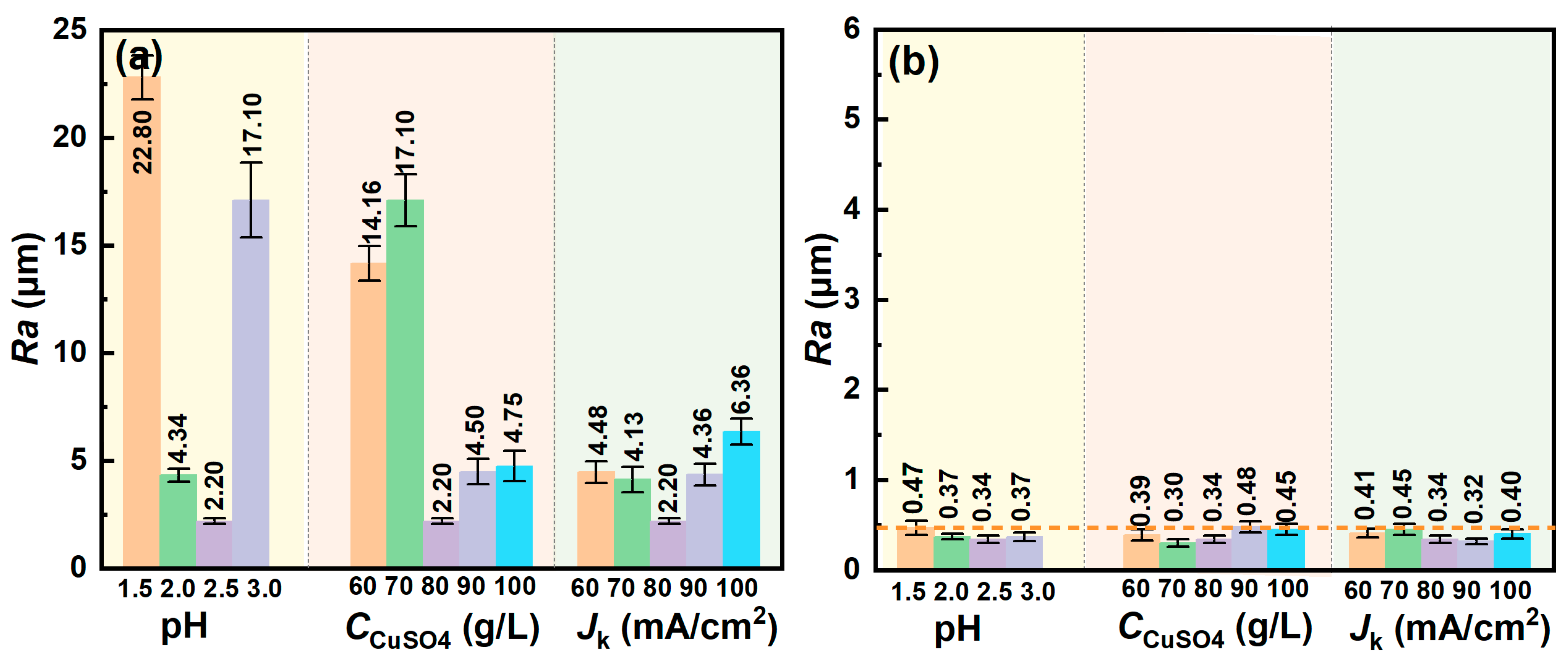
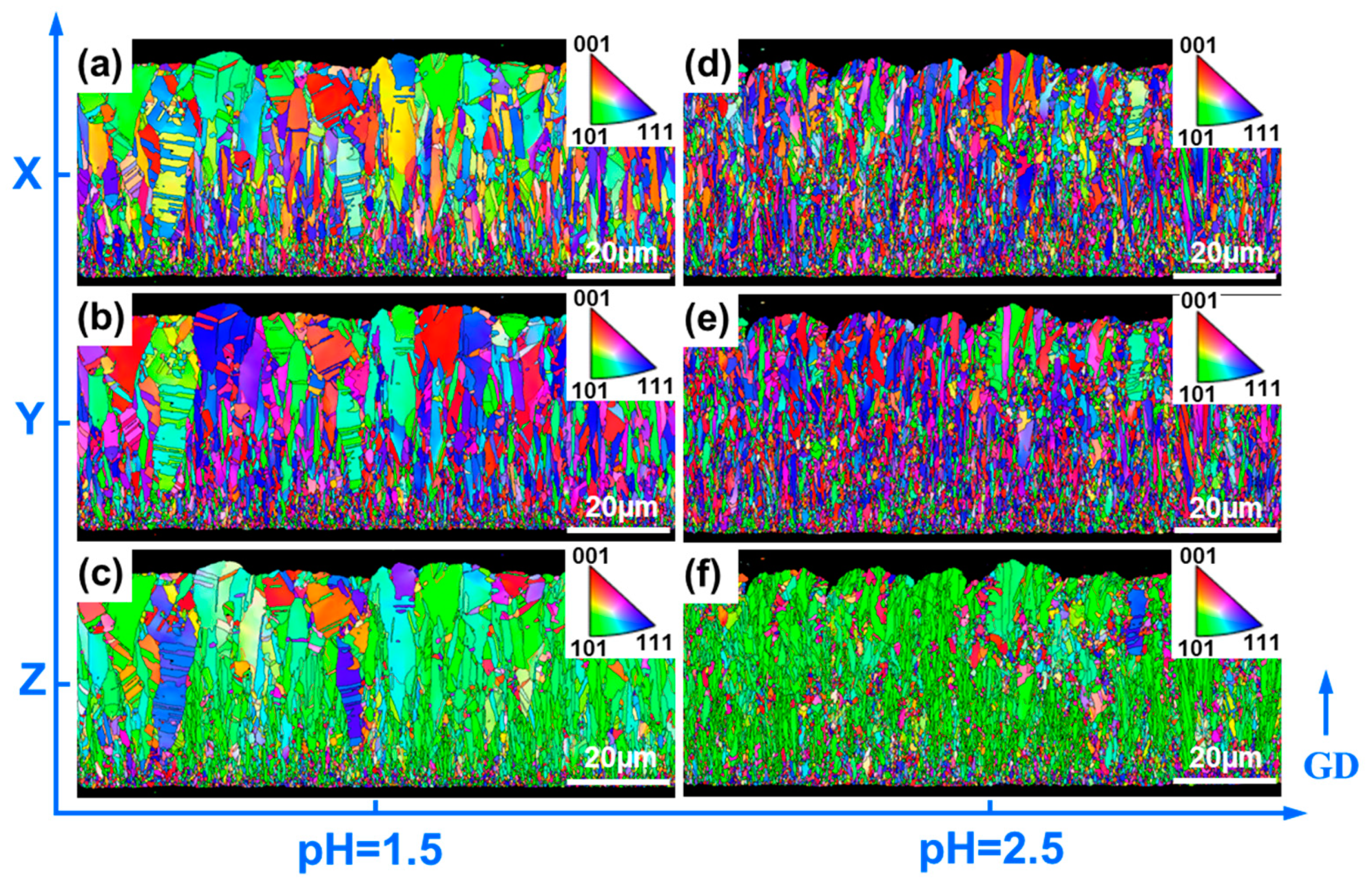
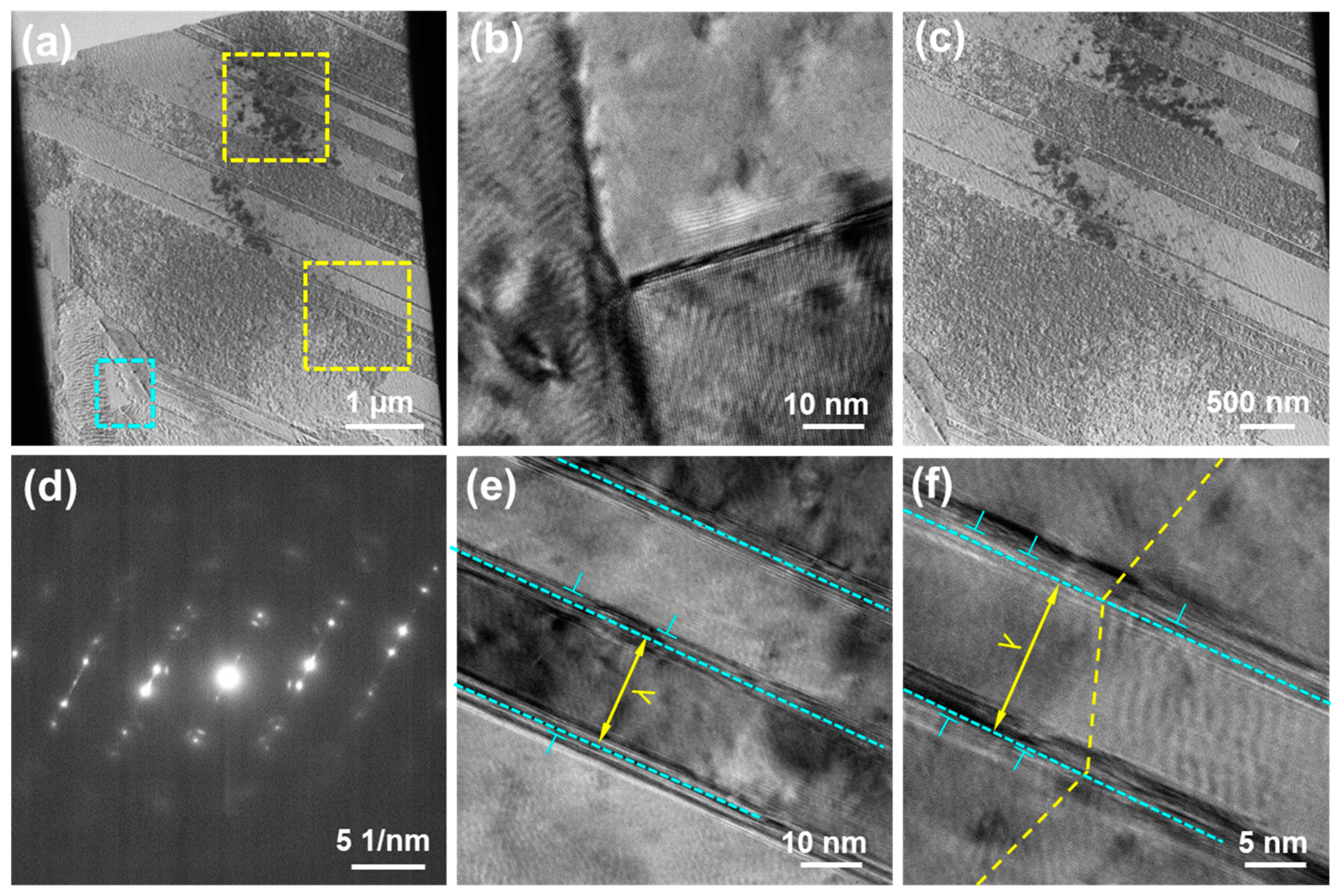
3. Results
4. Discussion
4.1. Influence of Different Electrolyte Parameters on the Microstructure of Copper Foil
4.2. Influence of Copper Foil Structure on Mechanical Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, S.; Wang, W.C.; Zhang, R.; Qin, S.P.; Wu, M.X.; Mitsuzaki, N.; Chen, Z.D. Effect of sodium alcohol thiyl propane sulfonate on electrolysis of high performance copper foil for lithium ion batteries. J. Electrochem. 2022, 28, 2104501. [Google Scholar] [CrossRef]
- Han, W.; Shen, C.; Zhu, D. High-density nanotwinned copper foils electrodeposited under low temperatures for lithium-ion batteries. Energy 2025, 320, 135241. [Google Scholar] [CrossRef]
- Kitada, A. Electrodeposition of metal foils for battery current collectors: Status and challenges. Energy Storage Mater. 2025, 75, 104073. [Google Scholar] [CrossRef]
- Huang, J.; Liu, W.; Chen, M.; Tang, Y.; Fan, X. Electrodeposition of 15 μm nanotwinned Cu foils with low warpage and excellent mechanical properties. J. Alloys Compd. 2025, 1010, 178156. [Google Scholar] [CrossRef]
- Lu, L.; Chen, X.; Huang, X.; Lu, K. Revealing the maximum strength in nanotwinned copper. Science 2009, 323, 607–610. [Google Scholar] [CrossRef]
- Zhan, X.; Lian, J.; Li, H.; Wang, X.; Zhou, J.; Trieu, K.; Zhang, X. Preparation of highly (111) textured nanotwinned copper by medium-frequency pulsed electrodeposition in an additive-free electrolyte. Electrochim. Acta 2021, 365, 137391. [Google Scholar] [CrossRef]
- Cheng, Z.; Jin, S.; Lu, L. Effect of Electrolyte Temperature on Microstructures of Direct-Current Electrodeposited Nanotwinned Cu. Acta Metall. 2018, 54, 428–434. [Google Scholar] [CrossRef]
- Jin, S.; Pan, Q.S.; Lu, L. Microstructure dependence of directcurrent electrodeposited bulk cu with preferentially oriented nanotwins on the current densities. Acta Metall. Sin. 2013, 49, 635–640. [Google Scholar] [CrossRef]
- Fang, T.H.; Li, W.L.; Tao, N.R.; Lu, K. Revealing Extraordinary intrinsic tensile plasticity in gradient nano-grained copper. Science 2011, 331, 1587–1590. [Google Scholar] [CrossRef]
- You, Z.S.; Lu, L. Effect of strain rate on tensile ductility and fracture behavior of bulk nanotwinned copper. Adv. Funct. Mater. 2015, 17, 1754–1759. [Google Scholar] [CrossRef]
- You, Z.S.; Lu, L.; Lu, K. Tensile behavior of columnar grained Cu with preferentially oriented nanoscale twins. Acta Mater. 2011, 59, 6927–6937. [Google Scholar] [CrossRef]
- Li, Y.J.; Tu, K.N.; Chen, C. Tensile Properties of <111>-Oriented Nanotwinned Cu with Different Columnar Grain Structures. Materials 2020, 13, 1310. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.Y.; Liu, C.M.; Lin, H.W.; Liu, T.C.; Lu, C.L.; Huang, Y.S.; Chen, C.; Tu, K.N. Unidirectional growth of microbumps on (111)-oriented and nanotwinned copper. Science 2012, 336, 1007–1010. [Google Scholar] [CrossRef]
- Liu, C.M.; Lin, H.W.; Lu, C.L.; Chen, C. Effect of grain orientations of Cu seed layers on the growth of <111>-oriented nanotwinned Cu. Sci. Rep. 2014, 4, 6123. [Google Scholar] [CrossRef]
- Lin, H.W.; Lu, C.L.; Liu, C.M.; Chen, C.; Chen, D.; Kuo, J.C.; Tu, K.N. Microstructure control of unidirectional growth of η-Cu6Sn5 in microbumps on <111> oriented and nanotwinned Cu. Acta Mater. 2013, 61, 4910–4919. [Google Scholar] [CrossRef]
- Liu, T.C.; Liu, C.M.; Huang, Y.S.; Chen, C.; Tu, K.N. Eliminate Kirkendall voids in solder reactions on nanotwinned copper. Scr. Mater. 2013, 68, 241–244. [Google Scholar] [CrossRef]
- Sun, F.L.; Gao, L.Y.; Liu, Z.Q.; Zhang, H.; Sugahara, T.; Nagao, S.; Suganuma, K. Electrodeposition and growth mechanism of preferentially orientated nanotwinned Cu on silicon wafer substrate. J. Mater. Sci. Technol. 2018, 34, 1885–1890. [Google Scholar] [CrossRef]
- Sun, F.L.; Liu, Z.Q.; Li, C.F.; Zhu, Q.S.; Zhang, H.; Suganuma, K. Bottom-up electrodeposition of large-scale nanotwinned copper within 3D through silicon via. Materials 2018, 11, 319. [Google Scholar] [CrossRef]
- Wen, S.; Wang, B.; Zhao, C. Study on microstructure and hardness of direct-current electrodeposited nanotwinned Cu. Hot Work. Technol. 2017, 46, 107–109. [Google Scholar] [CrossRef]
- Lu, Q.; You, Z.; Huang, X.; Hansen, N.; Lu, L. Dependence of dislocation structure on orientation and slip systems in highly oriented nanotwinned Cu. Acta Mater. 2017, 127, 85–97. [Google Scholar] [CrossRef]
- Liu, L.; Bu, Y.; Sun, Y.; Pan, J.; Liu, J.; Ma, J.; Qiu, L.; Fang, Y. Trace bis-(3-sulfopropyl)-disulfide enhanced electrodeposited copper foils. J. Mater. Sci. Technol. 2021, 74, 237–245. [Google Scholar] [CrossRef]
- GB/T 5230–1995; Electrodeposited Copper Foil. Standardization Administration of China: Beijing, China, 1995.
- Li, Z.G.; Gao, L.Y.; Li, Z.; Sun, R.; Liu, Z.Q. Regulating the orientation and distribution of nanotwins by trace of gelatin during direct current electroplating copper on titanium substrate. J. Mater. Sci. 2022, 57, 17797–17811. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Fan, B.; Shan, H.; Chen, Q.; Jiang, C.; Hou, G.; Tang, Y. Study on the relationship between crystal plane orientation and strength of electrolytic copper foil. J. Alloys Compd. 2021, 884, 161044. [Google Scholar] [CrossRef]
- Yu, W.; Lin, C.; Li, Q.; Zhang, J.; Yang, P.; An, M. A novel strategy to electrodeposit high-quality copper foils using composite additive and pulse superimposed on direct current. J. Appl. Electrochem. 2021, 51, 489–501. [Google Scholar] [CrossRef]
- Xiao, Q.; Lin, G.; Wang, J.; Zheng, K.; Feng, X. Effect of pH Value on Electroless Deposition of Copper Graphite Powders. Prot. Met. Phys. Chem. Surf. 2021, 57, 132–138. [Google Scholar] [CrossRef]
- Li, Y.; Huang, G.; Yin, X.; Chen, X.; Ma, X.; Li, Y.; Yao, E. Effect of copper ion concentration on microstructure and mechanical properties of electrolytic copper foil. IOP Conf. Ser. Mater. Sci. Eng. 2018, 381, 012166. [Google Scholar] [CrossRef]
- Lin, C.C.; Yen, C.H.; Lin, S.C.; Hu, C.C.; Dow, W.P. Interactive effects of additives and electrolyte flow rate on the microstructure of electrodeposited copper foils. J. Electrochem. Soc. 2017, 164, D810–D817. [Google Scholar] [CrossRef]
- Hu, J.; Fan, B.; Wu, Z.; Zuo, D.; Zhang, J.; Chen, Q.; Hou, G.; Tang, Y. Research progress on the texture of electrolytic copper foils. J. Electron. Mater. 2024, 53, 3460–3473. [Google Scholar] [CrossRef]
- Lu, L.; Schwaiger, R.; Shan, Z.W.; Dao, M.; Lu, K.; Suresh, S. Nano-sized twins induce high rate sensitivity of flow stress in pure copper. Acta Mater. 2005, 53, 2169–2179. [Google Scholar] [CrossRef]
- Shen, Y.F.; Lu, L.; Dao, M.; Suresh, S. Strain rate sensitivity of Cu with nanoscale twins. Scr. Mater. 2006, 55, 319–322. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, T.; Shen, Y.F.; Dao, M.; Lu, K.; Suresh, S. Stress relaxation and the structure size-dependence of plastic deformation in nanotwinned copper. Acta Mater. 2009, 57, 5165–5173. [Google Scholar] [CrossRef]
- Lu, L.; Dao, M.; Zhu, T.; Li, J. Size dependence of rate-controlling deformation mechanisms in nanotwinned copper. Scr. Mater. 2009, 60, 1062–1066. [Google Scholar] [CrossRef]
- You, Z.S.; Lu, L.; Lu, K. Temperature effect on rolling behavior of nano-twinned copper. Scr. Mater. 2010, 62, 415–418. [Google Scholar] [CrossRef]
- Tamakawa, K.; Sakutani, K.; Miura, H. Effect of micro texture of electroplated copper thin films on their mechanical properties. Zair. J. Soc. Mater. Sci. 2007, 56, 907–912. [Google Scholar] [CrossRef]
- Wei, K.X.; Zheng, X.C.; Wei, W.; Alexandrov, I.V. High tensile properties and low surface roughness of Gr/Cu foils. J. Mater. Eng. Perform. 2022, 31, 9362–9369. [Google Scholar] [CrossRef]
- Dao, M.; Lu, L.; Shen, Y.F.; Suresh, S. Strength, strain-rate sensitivity and ductility of copper with nanoscale twins. Acta Mater. 2006, 54, 5421–5432. [Google Scholar] [CrossRef]
- Jérusalem, A.; Dao, M.; Suresh, S.; Radovitzky, R. Three-dimensional model of strength and ductility of polycrystalline copper containing nanoscale twins. Acta Mater. 2008, 56, 4647–4657. [Google Scholar] [CrossRef]
- Li, X.; Wei, Y.; Lu, L.; Lu, K.; Gao, H. Dislocation nucleation governed softening and maximum strength in nano-twinned metals. Nature 2010, 464, 877–880. [Google Scholar] [CrossRef]
- Shabib, I.; Miller, R.E. Deformation characteristics and stress–strain response of nanotwinned copper via molecular dynamics simulation. Acta Mater. 2009, 57, 4364–4373. [Google Scholar] [CrossRef]
- Jang, D.C.; Li, X.Y.; Gao, H.J.; Greer, J.R. Deformation mechanisms in nanotwinned metal nanopillars. Nat. Nanotech. 2012, 7, 594–601. [Google Scholar] [CrossRef]
- Wang, Y.M.; Sansoz, F.; LaGrange, T.; Ott, R.Y.; Marian, J.; Barbee, T.W., Jr.; Hamza, A.V. Defective twin boundaries in nanotwinned metals. Nat. Mater. 2013, 12, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.C.; Cai, C.; Greer, J.R. Influence of homogeneous interfaces on the strength of 500 nm diameter Cu nanopillars. Nano Lett. 2011, 11, 1743–1746. [Google Scholar] [CrossRef] [PubMed]
- Afanasyev, K.A.; Sansoz, F. Strengthening in gold nanopillars with nanoscale twins. Nano Lett. 2007, 7, 2056–2062. [Google Scholar] [CrossRef]
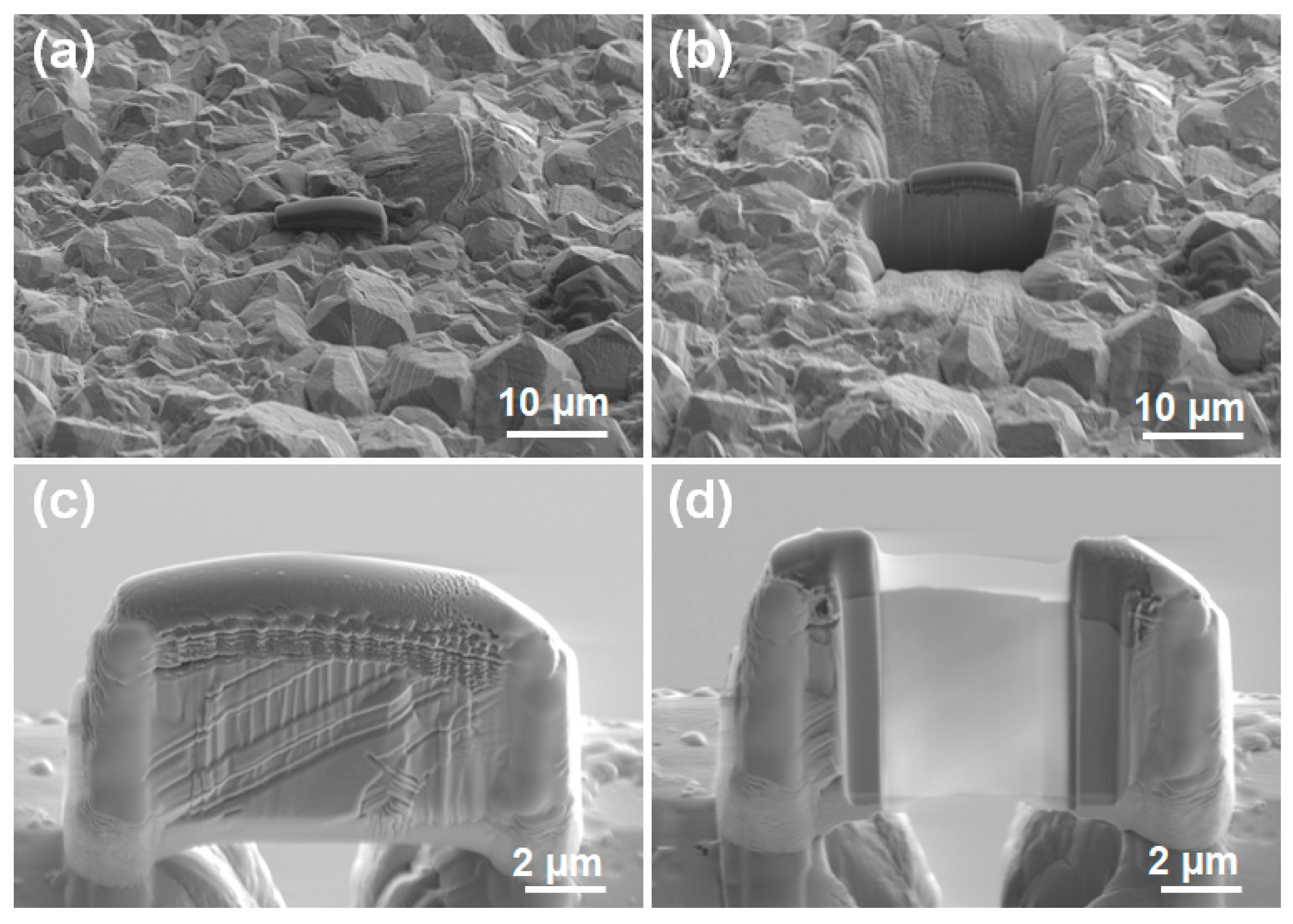
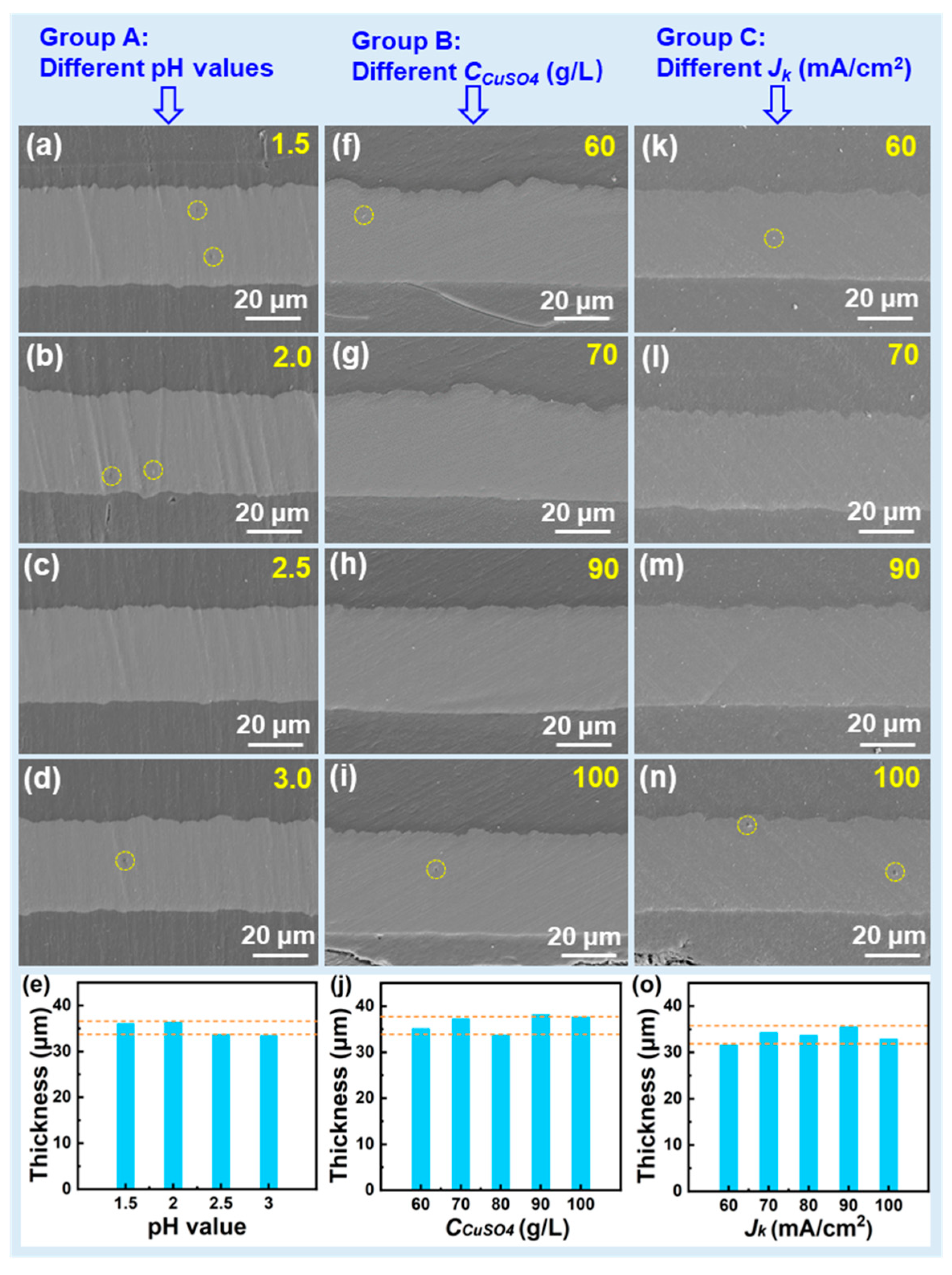
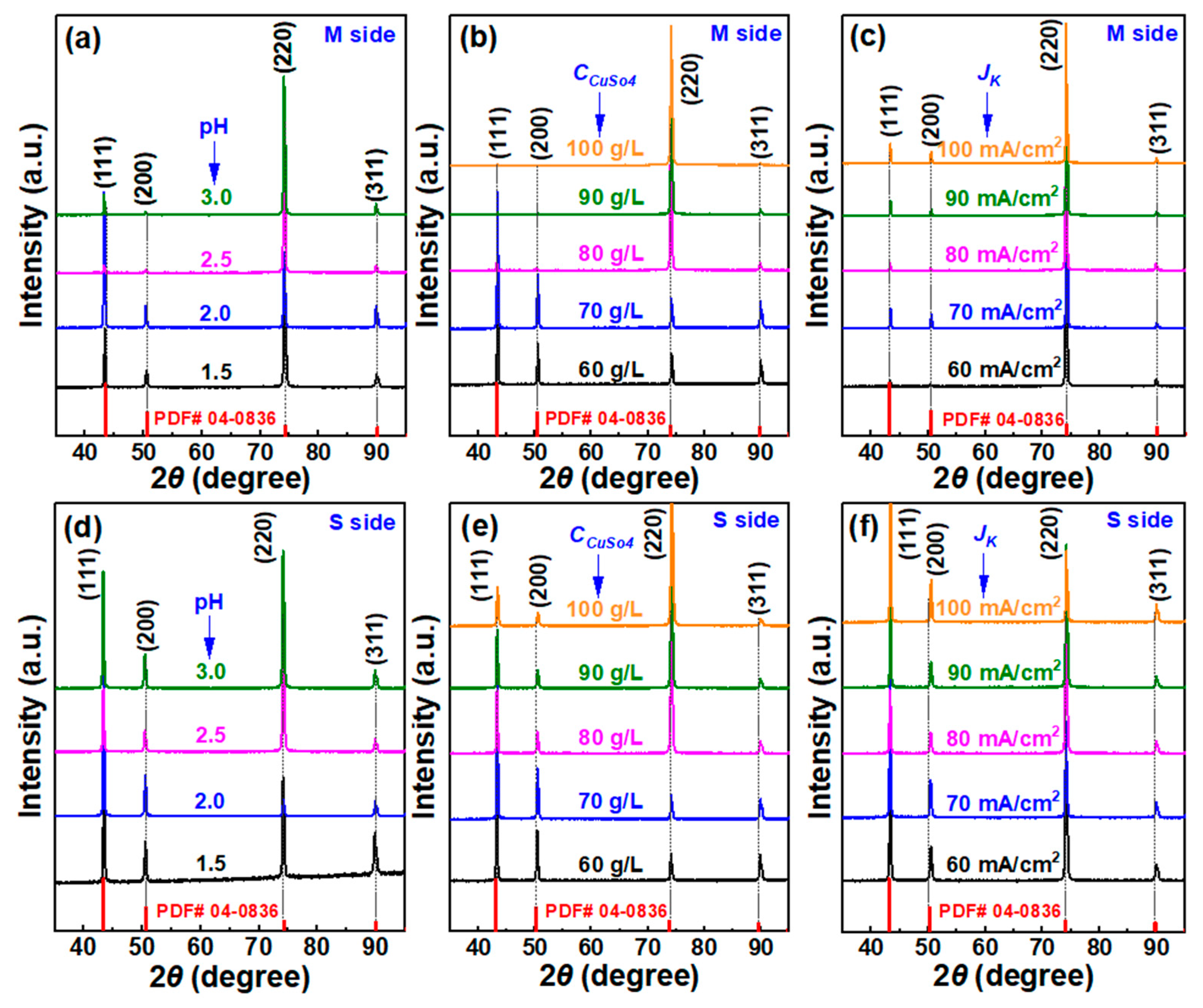
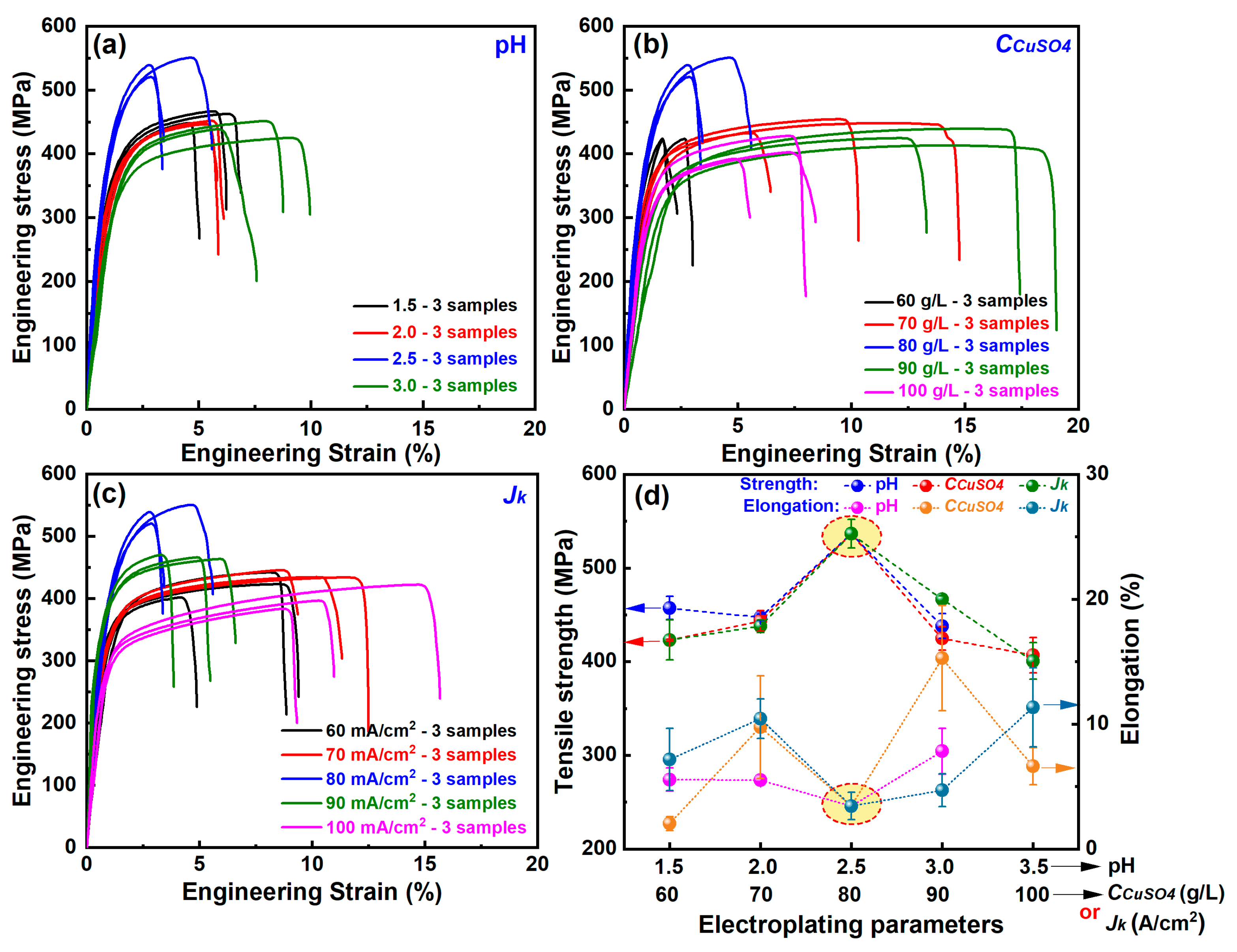

| Group No. | pH Value | CCuSO4 (g/L) | Jk (mA/cm2) | CSPS (ppm) | CGelatin (ppm) | CCl− (ppm) |
|---|---|---|---|---|---|---|
| A | 1.5 | 80 | 80 | 2 | 2 | 60 |
| 2.0 | ||||||
| 2.5 | ||||||
| 3.0 | ||||||
| B | 2.5 | 60 | 80 | 2 | 2 | 60 |
| 70 | ||||||
| 90 | ||||||
| 100 | ||||||
| C | 2.5 | 80 | 60 | 2 | 2 | 60 |
| 70 | ||||||
| 90 | ||||||
| 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, W.; Zheng, Y.; Luo, C.; Feng, T.; Dong, G.; Gao, H.; La, P. Regulation of Microstructure and Mechanical Properties of DC Electrodeposited Copper Foils by Electrolyte Parameters. Coatings 2025, 15, 521. https://doi.org/10.3390/coatings15050521
Ma W, Zheng Y, Luo C, Feng T, Dong G, Gao H, La P. Regulation of Microstructure and Mechanical Properties of DC Electrodeposited Copper Foils by Electrolyte Parameters. Coatings. 2025; 15(5):521. https://doi.org/10.3390/coatings15050521
Chicago/Turabian StyleMa, Wenwen, Yuehong Zheng, Chong Luo, Tao Feng, Gang Dong, Haoyang Gao, and Peiqing La. 2025. "Regulation of Microstructure and Mechanical Properties of DC Electrodeposited Copper Foils by Electrolyte Parameters" Coatings 15, no. 5: 521. https://doi.org/10.3390/coatings15050521
APA StyleMa, W., Zheng, Y., Luo, C., Feng, T., Dong, G., Gao, H., & La, P. (2025). Regulation of Microstructure and Mechanical Properties of DC Electrodeposited Copper Foils by Electrolyte Parameters. Coatings, 15(5), 521. https://doi.org/10.3390/coatings15050521






