Influence of Sputtering Power of ZrB2 Target on Structure and Properties of Nanocomposite Zr-B-O Films
Abstract
:1. Introduction
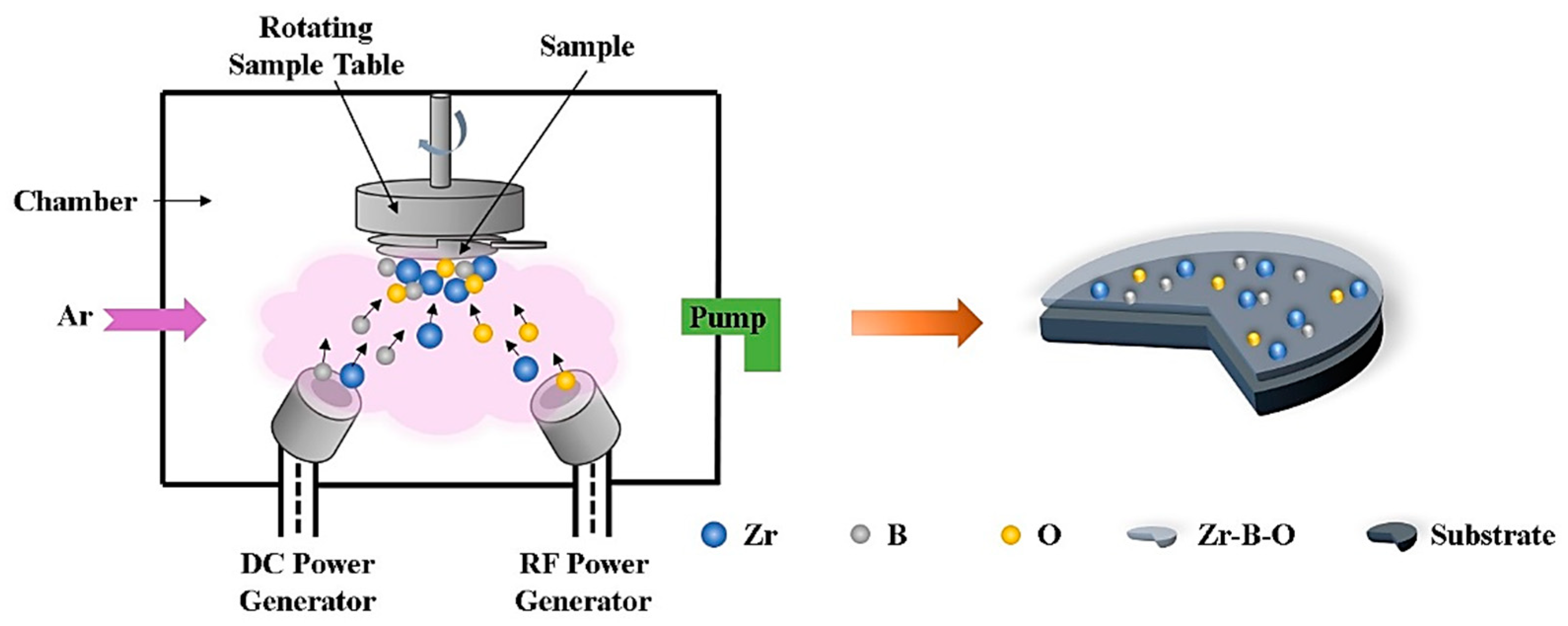
2. Materials and Methods
3. Results and Discussion
3.1. Microstructures and Mechanical Properties
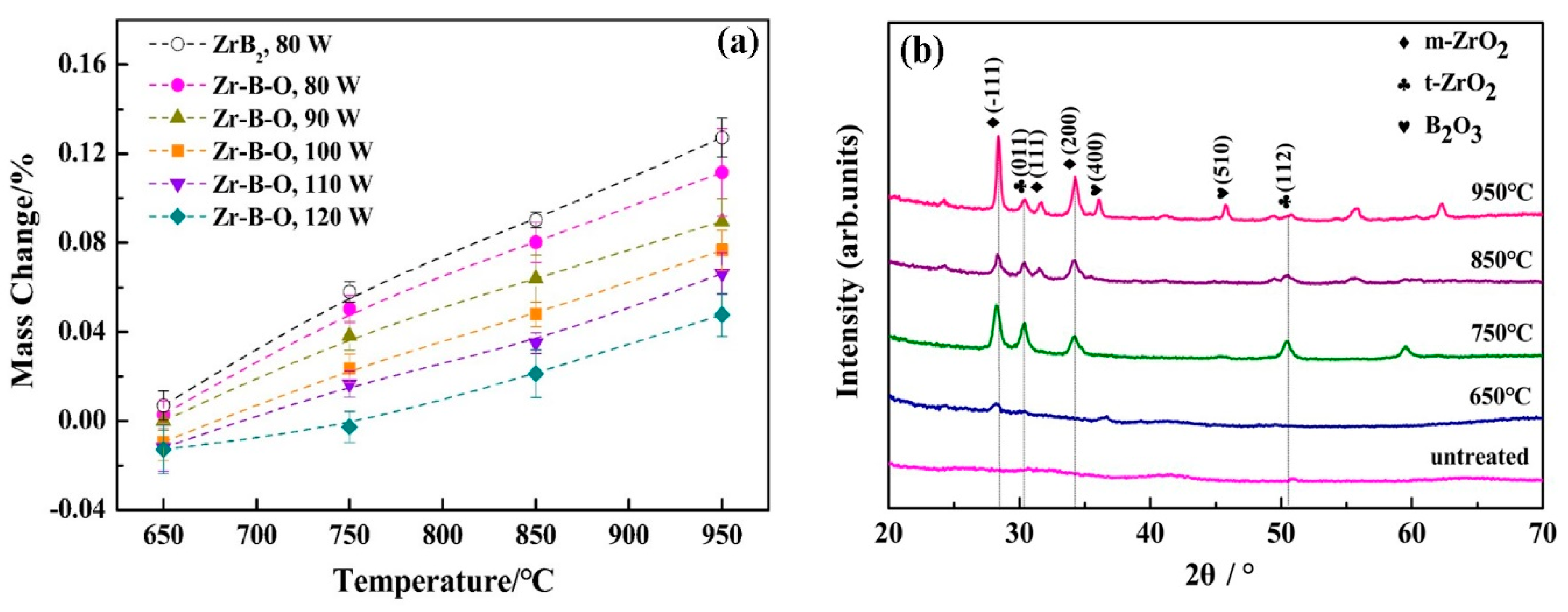
3.2. Oxidation Resistance Properties
3.3. Corrosion Resistance Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Veprek, S.; Veprek-Heijman, M.J.G. Industrial applications of superhard nanocomposite coatings. Surf. Coat. Technol. 2008, 202, 5063–5073. [Google Scholar] [CrossRef]
- Holubar, P.; Jilek, M.; Sima, M. Present and possible application of superhard nanocomposite coatings. Surf. Coat. Technol. 2000, 133, 145–151. [Google Scholar] [CrossRef]
- Sung, C.M.; Sung, M. Carbon nitride and other speculative superhard materials. Mater. Chem. Phys. 1996, 43, 1–18. [Google Scholar]
- Settineri, L.; Faga, M.G.; Gautier, G.; Perucca, M. Evaluation of wear resistance of AlSiTiN and AlSiCrN nanocomposite coatings for cutting tools. CIRP Ann. Manuf. Technol. 2008, 57, 575–578. [Google Scholar]
- Keunecke, M.; Bewilogua, K.; Wiemann, E.; Weigel, K.; Wittorf, R.; Thomsen, H. Boron containing combination tool coatings-characterization and application tests. Thin Solid Films 2006, 494, 58–62. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Sun, X.; Qiao, J. Mechanical and corrosion-resistance performance of electrodeposited titania-nickel nanocomposite coatings. Surf. Coat. Technol. 2005, 192, 331–335. [Google Scholar]
- Zhang, S.; Sun, D.; Fu, Y.Q.; Du, H.J. Recent advances of superhard nanocomposite coatings: A review. Surf. Coat. Technol. 2003, 167, 113–119. [Google Scholar] [CrossRef]
- Dubey, P.; Arya, V.; Srivastava, S.; Singh, D.; Chandra, R. Study on thermal stability and mechanical properties of nanocomposite Zr-W-B-N thin films. Surf. Coat. Technol. 2015, 284, 173–182. [Google Scholar]
- Kiryukhantsev-Korneev, F.V.; Sheveiko, A.N.; Komarov, V.A.; Blanter, M.S.; Skryleva, E.A.; Shirmanov, N.A.; Levashov, E.A.; Shtansky, D.V. Nanostructured Ti-Cr-B-N and Ti-Cr-Si-C-N coatings for hard-alloy cutting tools. Russ. J. Nonferrous Met. 2011, 52, 311–318. [Google Scholar]
- Yang, J.; Wang, M.X.; Kang, Y.B.; Li, D.J. Influence of bilayer periods on structural and mechanical properties of ZrC/ZrB2 superlattice coatings. Appl. Surf. Sci. 2007, 253, 5302–5305. [Google Scholar] [CrossRef]
- Yan, Y.J.; Huang, Z.R.; Dong, S.M.; Jiang, D.L. Pressureless sintering of high-density ZrB2-SiC ceramic composites. J. Am. Ceram. Soc. 2006, 89, 3589–3592. [Google Scholar] [CrossRef]
- Telle, R.; Sigl, L.S.; Takagi, K. Boride-based hard materials. In Handbook of Ceramic Hard Materials; Riedel, R., Ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2000; pp. 802–945. [Google Scholar]
- Monteverde, F.; Guicciardi, S.; Bellosi, A. Advances in microstructure and mechanical properties of Zirconium diboride based ceramics. Mater. Sci. Eng. A 2003, 346, 310–319. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, G.J.; Kan, Y.M.; Wang, P.L. Pressureless densification of ZrB2-SiC composites with vanadium carbide. Scr. Mater. 2008, 59, 309–312. [Google Scholar] [CrossRef]
- Li, C.; Dong, L.; Wan, R.X.; Gu, H.Q.; Li, D.J. Cotrollable phase structure and mechanical properties of ZrB2-NbN nanocomposite films by magnetron co-sputtering. Mater. Express 2016, 6, 493–500. [Google Scholar] [CrossRef]
- Shi, J.Y.; Verweij, H. Preparation and characterization of nanostructured ZrO2 coatings on dense and porous substrates. Thin Solid Films 2008, 516, 3919–3923. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, L.; Ren, C.; Wang, K.; Dai, G.Z.; Zheng, X.B.; He, Y.D. Preparation of Al2O3-ZrO2-Y2O3 composite coatings by a modified sol-gel technique for thermal barrier application. Oxid. Met. 2013, 80, 323–339. [Google Scholar] [CrossRef]
- Qin, W.; Nam, C.; Li, H.L.; Szpunar, J.A. Tetragonal phase stability in ZrO2 film formed on zirconium alloys and its effects on corrosion resistance. Acta Mater. 2007, 55, 1695–1701. [Google Scholar] [CrossRef]
- Li, W.J.; Zhang, X.H.; Hong, C.Q.; Han, W.B.; Han, J.C. Preparation, microstructure and mechanical properties of ZrB2-ZrO2 ceramics. J. Eur. Ceram. Soc. 2009, 29, 779–786. [Google Scholar] [CrossRef]
- Zhang, X.H.; Li, W.J.; Hong, C.Q.; Han, W.B. Microstructure and mechanical properties of ZrB2-based composites reinforced and toughened by zirconia. Int. J. Appl. Ceram. Technol. 2008, 5, 499–504. [Google Scholar] [CrossRef]
- Basu, B.; Vleugels, J.; Biest, O.V.D. Development of ZrO2-ZrB2 composites. J. Alloys Compd. 2002, 334, 200–204. [Google Scholar] [CrossRef]
- Basu, B.; Venkateswaran, T.; Kim, D.Y. Microstructure and properties of spark plasma-sintered ZrO2-ZrB2 nanoceramic composites. J. Am. Ceram. Soc. 2006, 89, 2405–2412. [Google Scholar] [CrossRef]
- Li, B.; Deng, J.X.; Wu, Z. Effect of cutting atmosphere on dry machining performance with Al2O3/ZrB2/ZrO2 ceramic tool. Int. J. Adv. Manuf. Technol. 2010, 49, 459–467. [Google Scholar] [CrossRef]
- Chen, L.; Huang, Y.H.; Wang, Y.J.; Shen, H.F.; Rao, J.C.; Zhou, Y. Effect of ZrO2 content on microstructure, mechanical properties and thermal shock resistance of (ZrB2+3Y-ZrO2)/BN composites. Mater. Sci. Eng. A 2013, 573, 106–110. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Mitterer, C. Borides in thin film technology. J. Solid State Chem. 1997, 133, 279–291. [Google Scholar] [CrossRef]
- Zhuang, C.Q.; Schlemper, C.; Fuchs, R.; Zhang, L.; Huang, N.; Vogel, M.; Staedler, T.; Jiang, X. Mechanical behavior related to various bonding states in amorphous Si-C-N hard films. Surf. Coat. Technol. 2014, 258, 353–358. [Google Scholar] [CrossRef]
- Zhao, B.; Li, D.M.; Zeng, F.; Pan, F. Ion beam induced formation of metastable alloy phases in Cu-Mo system during ion beam assisted deposition. Appl. Surf. Sci. 2003, 207, 334–340. [Google Scholar] [CrossRef]
- Wang, T.G.; Liu, Y.M.; Zhang, T.F.; Kim, D.; Kim, K.H. Influence of nitrogen flow ratio on the microstructure, composition, and mechanical properties of DC magnetron sputtered Zr–B–O–N films. J. Mater. Sci. Technol. 2012, 28, 981–991. [Google Scholar] [CrossRef]
- Pierson, J.F.; Billard, A.; Belmonte, T.; Michel, H.; Frantz, C. Influence of oxygen flow rate on the structural and mechanical properties of reactively magnetron sputter-deposited Zr-B-O coatings. Thin Solid Films 1999, 347, 78–84. [Google Scholar] [CrossRef]
- Zhang, G.J.; Ni, D.W.; Zou, J.; Liu, H.T.; Wu, W.W.; Liu, J.X.; Suzuki, T.S.; Sakka, Y. Inherent anisotrotropy in transition metal diborides and microstructure/property tailoring in ultra-high temperature ceramics—A review. J. Eur. Ceram. Soc. 2018, 38, 371–389. [Google Scholar] [CrossRef]
- Otani, S.; Korsukova, M.M.; Aizawa, T. High-temperature hardness of ReB2 single crystals. J. Alloys Compd. 2009, 477, L28–L29. [Google Scholar] [CrossRef]
- Feng, T.; Li, H.J.; Shi, X.H.; Yang, X.; Wang, S.L. Oxidation and ablation resistance of ZrB2-SiC-Si/B-modified SiC coating for carbon/carbon composites. Corros. Sci. 2013, 67, 292–297. [Google Scholar] [CrossRef]
- Lian, J.; Zhang, J.M.; Namavar, F.; Zhang, Y.W.; Lu, F.Y.; Haider, H.; Garvin, K.; Weber, W.J.; Ewing, R.C. Ion beam-induced amorphous-to-tetragonal phase transformation and grain growth of nanocrystalline zirconia. Nanotechnology 2009, 20, 245303. [Google Scholar] [CrossRef] [PubMed]
- Sobol, A.A.; Voronko, Y.k. Stress-induced cubic-tetragonal transformation in partially stabilized ZrO2: Raman spectroscopy study. J. Phys. Chem. Solids 2004, 65, 1103–1112. [Google Scholar] [CrossRef]
- Li, W.; Liu, P.; Chen, P.C.; Zhang, K.; Ma, F.C.; Liu, X.K.; Feng, R.; Liaw, P.K. Microstructure and a coherent-interface strengthening mechanism of NbSiN nanocomposite film. Thin Solid Films 2017, 636, 1–7. [Google Scholar] [CrossRef]
- Li, W.; Liu, P.; Zhao, S.; Zhang, K.; Ma, F.C.; Liu, X.K.; Chen, X.H.; He, D.H. Microstructural evolution, mechanical properties and strengthening mechanism of TiN/Ni nanocomposite film. J. Alloys Compd. 2017, 691, 159–164. [Google Scholar] [CrossRef]
- Lee, N.L.; Fisher, G.B.; Schulz, R. Sputter deposition of a corrosion-resistance amorphous metallic coating. J. Mater. Res. 1988, 3, 862–871. [Google Scholar] [CrossRef]
- Liu, D.G.; Tu, J.P.; Chen, R.; Gu, C.D. Microstructure, corrosion resistance and biocompatibility of titanium incorporated amorphous carbon nitride films. Surf. Coat. Technol. 2011, 206, 165–171. [Google Scholar] [CrossRef]








| Samples | Untreated | ZrB2 | Zr-B-O (80 W) | Zr-B-O (100 W) | Zr-B-O (120 W) |
|---|---|---|---|---|---|
| icorr (A·cm−2) | 2.9057 × 10−6 | 2.8869 × 10−6 | 4.5310 × 10−7 | 4.7362 × 10−7 | 4.9919 × 10−7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Mao, D.; Dong, L.; Zhao, M.; Wu, J.; Li, D. Influence of Sputtering Power of ZrB2 Target on Structure and Properties of Nanocomposite Zr-B-O Films. Coatings 2019, 9, 611. https://doi.org/10.3390/coatings9100611
Xu Y, Mao D, Dong L, Zhao M, Wu J, Li D. Influence of Sputtering Power of ZrB2 Target on Structure and Properties of Nanocomposite Zr-B-O Films. Coatings. 2019; 9(10):611. https://doi.org/10.3390/coatings9100611
Chicago/Turabian StyleXu, Yang, Dong Mao, Lei Dong, Mengli Zhao, Jie Wu, and Dejun Li. 2019. "Influence of Sputtering Power of ZrB2 Target on Structure and Properties of Nanocomposite Zr-B-O Films" Coatings 9, no. 10: 611. https://doi.org/10.3390/coatings9100611
APA StyleXu, Y., Mao, D., Dong, L., Zhao, M., Wu, J., & Li, D. (2019). Influence of Sputtering Power of ZrB2 Target on Structure and Properties of Nanocomposite Zr-B-O Films. Coatings, 9(10), 611. https://doi.org/10.3390/coatings9100611




