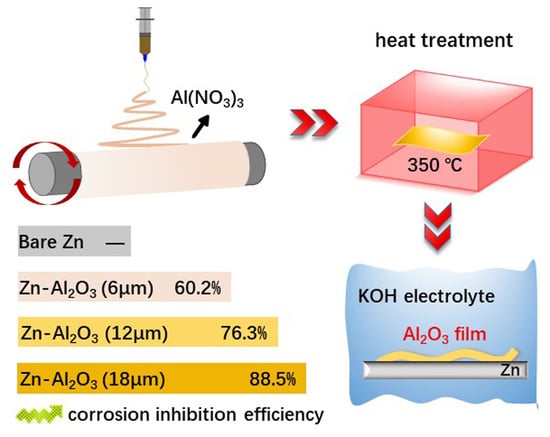
Al2O3 Coatings on Zinc for Anti-Corrosion in Alkaline Solution by Electrospinning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
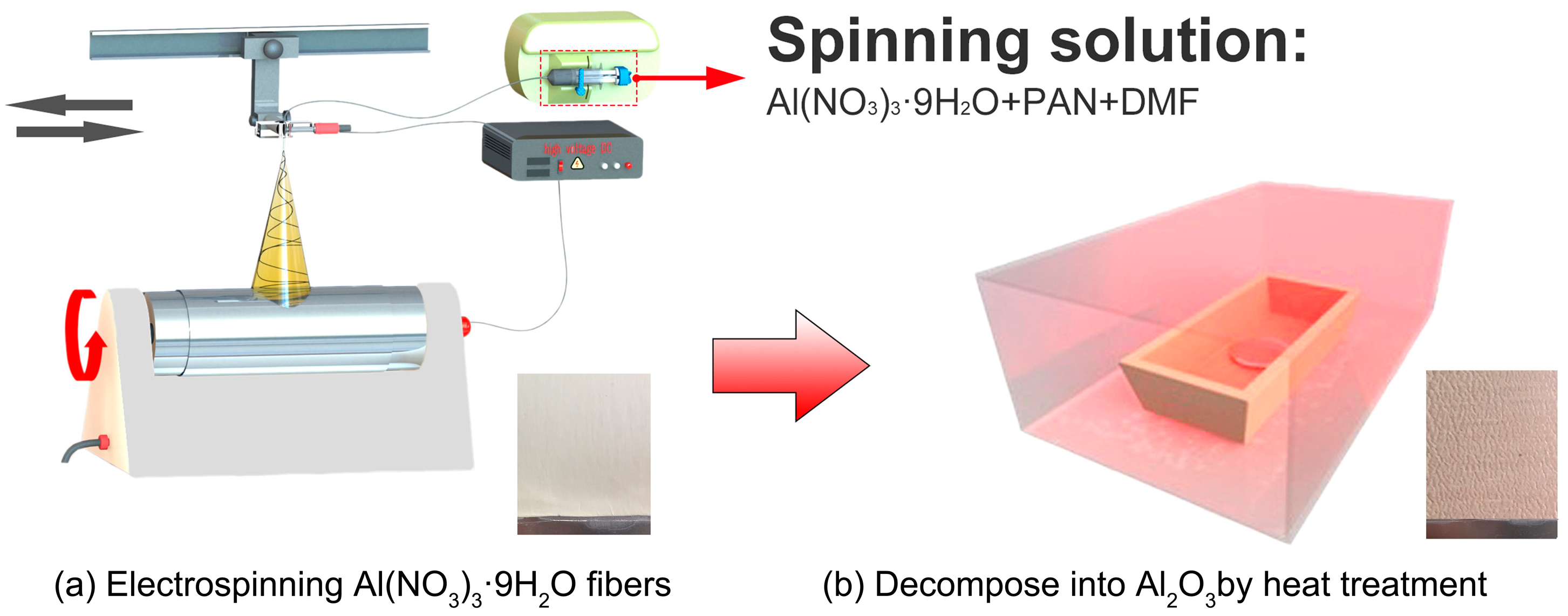
2.2. Preparation of Al2O3 Coatings
2.3. Characterization
2.4. Hydrogen Evolution Tests and Electrochemical Measurementsc
3. Results and Discussion
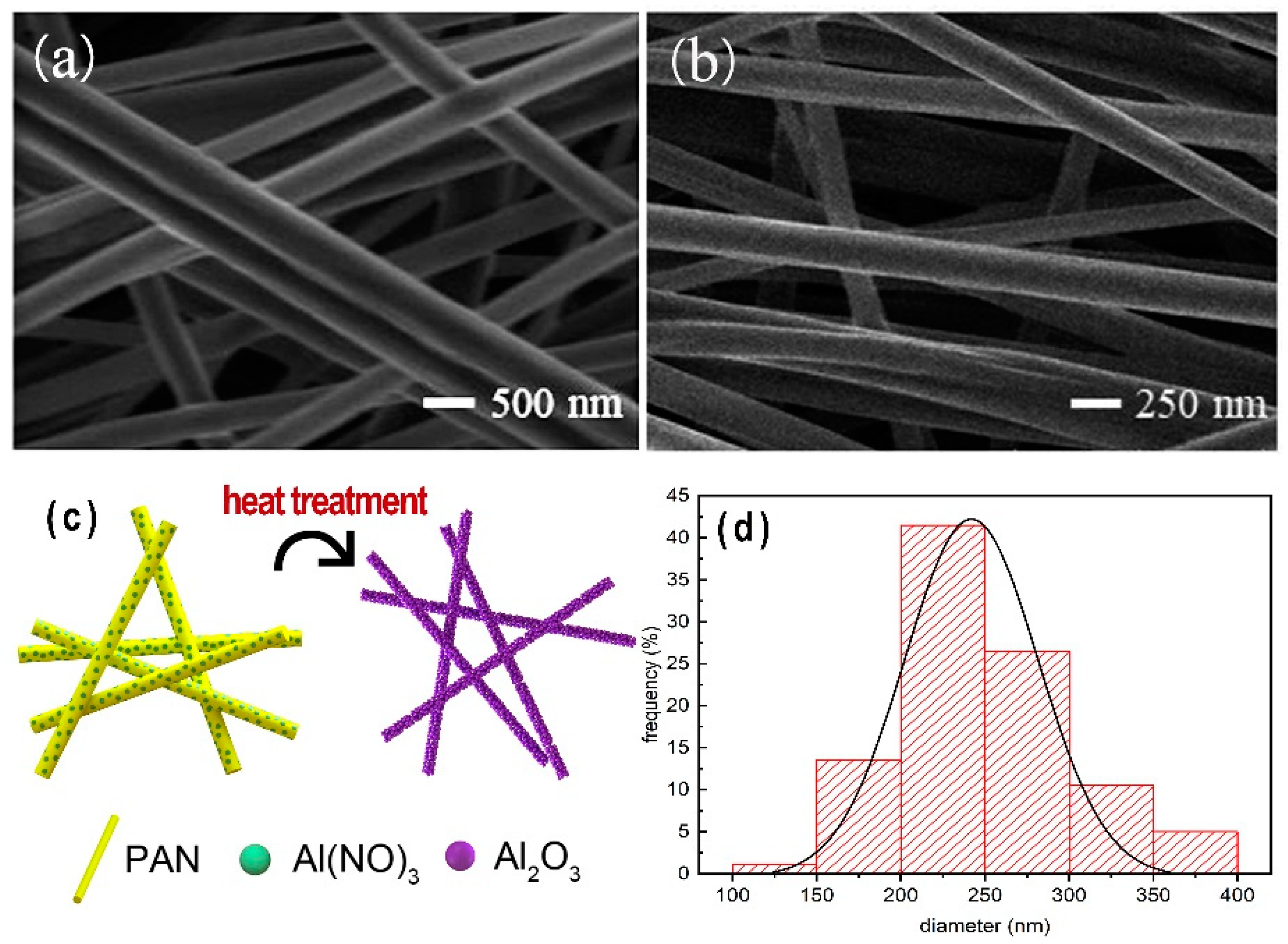
3.1. Morphology Characterization of Coatings
3.2. Hydrogen Gas Evolution Tests
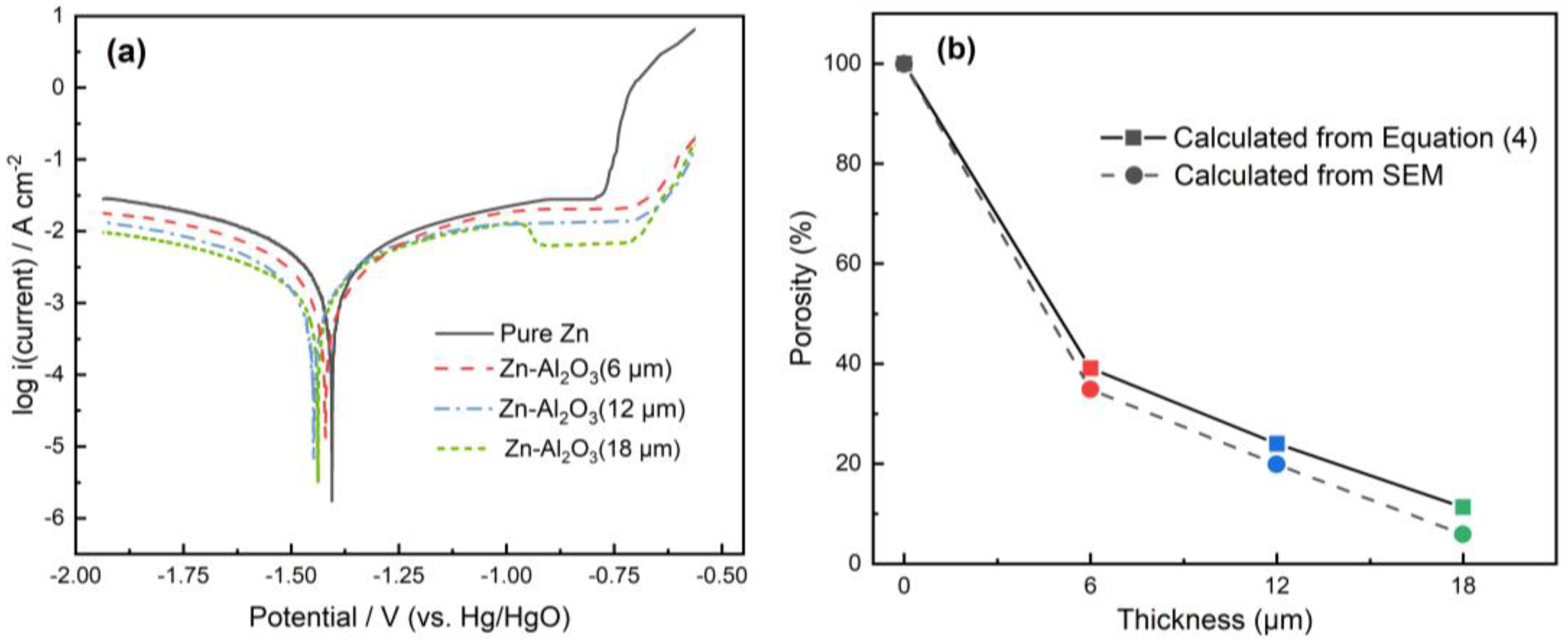
3.3. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sun, Y.; Liu, X.; Jiang, Y.; Li, J.; Ding, J.; Hu, W.; Zhong, C. Recent advances and challenges in divalent and multivalent metal electrodes for metal-air batteries. J. Mater. Chem. A 2019, 7, 18183–18208. [Google Scholar] [CrossRef]
- Li, H.; Ma, L.; Han, C.; Wang, Z.; Liu, Z.; Tang, Z.; Zhi, C. Advanced rechargeable zinc-based batteries: Recent progress and future perspectives. Nano Energy 2019, 62, 550–587. [Google Scholar] [CrossRef]
- Ryu, J.; Park, M.; Cho, J. Advanced technologies for high-energy aluminum-air batteries. Adv. Mater. 2019, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Sun, W.; Shen, J.; Lu, C.; Wang, Y.; Wang, Z.; Sun, K. Improved structural design of single- and double-wall MnCo2O4 nanotube cathodes for long-life Li-O2 batteries. Nanoscale 2018, 10, 13149–13158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, X.; Chen, J.; Wang, Q. Polyoxometalate on rice paper derived 3D mesoporous carbon paper: An electrocatalyst as cathode for asymmetric Zn-air battery. J. Power Sources 2019, 430, 201–209. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, W.; Yin, C.; Wei, L.; Wu, W.; Hu, Z.; Wu, M. Synergistic effects between doped nitrogen and phosphorus in metal-free cathode for zinc-air battery from covalent organic frameworks coated CNT. ACS Appl. Mater. Interfaces 2017, 9, 44519–44528. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Hong, Y.; Ci, S.; Wen, Z. In situ integration of CoFe alloy nanoparticles with nitrogen-doped carbon nanotubes as advanced bifunctional cathode catalysts for Zn-air batteries. Nanoscale 2016, 8, 20048–20055. [Google Scholar] [CrossRef]
- Meng, F.L.; Liu, K.H.; Zhang, Y.; Shi, M.M.; Zhang, X.B.; Yan, J.M.; Jiang, Q. Recent advances toward the rational design of efficient bifunctional air electrodes for rechargeable Zn-Air batteries. Small 2018, 14, 1–20. [Google Scholar] [CrossRef]
- Han, S.; Hu, X.; Wang, J.; Fang, X.; Zhu, Y. Novel Route to Fe-based cathode as an efficient bifunctional catalysts for rechargeable Zn-air battery. Adv. Energy Mater. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Lee, J.S.; Nam, G.; Sun, J.; Higashi, S.; Lee, H.W.; Lee, S.; Chen, W.; Cui, Y.; Cho, J. Composites of a prussian blue analogue and gelatin-derived nitrogen-doped carbon-supported porous spinel oxides as electrocatalysts for a Zn-air battery. Adv. Energy Mater. 2016, 6, 1601052. [Google Scholar] [CrossRef]
- Amiinu, I.S.; Pu, Z.; Liu, X.; Owusu, K.A.; Monestel, H.G.R.; Boakye, F.O.; Zhang, H.; Mu, S. Multifunctional Mo-N/C@MoS2 electrocatalysts for HER, OER, ORR, and Zn–air batteries. Adv. Funct. Mater. 2017, 27, 1–11. [Google Scholar] [CrossRef]
- Liu, K.; Zhong, H.; Meng, F.; Zhang, X.; Yan, J.; Jiang, Q. Recent advances in metal-nitrogen-carbon catalysts for electrochemical water splitting. Mater. Chem. Front. 2017, 1, 2155–2173. [Google Scholar] [CrossRef]
- Zhao, Z.; Fan, X.; Ding, J.; Hu, W.; Zhong, C.; Lu, J. The challenges in zinc electrodes for rechargeable alkaline zinc-air batteries: Obstacles to commercialization. ACS Energy Lett. 2019, 4, 2259–2270. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Ionic liquids as green and sustainable corrosion inhibitors for metals and alloys: An overview. J. Mol. Liq. 2017, 233, 403–414. [Google Scholar] [CrossRef]
- Zuo, Y.; Yu, Y.; Zuo, C.; Ning, C.; Liu, H.; Gu, Z.; Cao, Q.; Shen, C. Low-temperature performance of Al-air batteries. Energies 2019, 12, 612. [Google Scholar] [CrossRef]
- Mainar, A.R.; Colmenares, L.C.; Blázquez, J.A.; Urdampilleta, I. A brief overview of secondary zinc anode development: The key of improving zinc-based energy storage systems. Int. J. Energy Res. 2018, 42, 903–918. [Google Scholar] [CrossRef]
- Thomas, S.; Birbilis, N.; Venkatraman, M.S.; Cole, I.S. Self-repairing oxides to protect zinc: Review, discussion and prospects. Corros. Sci. 2013, 69, 11–22. [Google Scholar] [CrossRef]
- Zeng, R.C.; Hu, Y.; Zhang, F.; Huang, Y.D.; Wang, Z.L.; Li, S.Q.; Han, E.H. Corrosion resistance of cerium-doped zinc calcium phosphate chemical conversion coatings on AZ31 magnesium alloy. Trans. Nonferr. Met. Soc. China 2016, 26, 472–483. [Google Scholar] [CrossRef] [Green Version]
- Bahmani, A.; Arthanari, S.; Shin, K.S. Corrosion behavior of Mg-Mn-Ca alloy: Influences of Al, Sn and Zn. J. Magnes. Alloy 2019, 7, 38–46. [Google Scholar] [CrossRef]
- Nam Jo, Y.; Santhoshkumar, P.; Prasanna, K.; Vediappan, K.; Woo Lee, C. Improving self-discharge and anti-corrosion performance of Zn-air batteries using conductive polymer-coated Zn active materials. J. Ind. Eng. Chem. 2019, 76, 396–402. [Google Scholar] [CrossRef]
- Lu, X.; Wang, S.; Xiong, T.; Wen, D.; Wang, G.; Du, H. Anticorrosion properties of Zn-Al composite coating prepared by cold spraying. Coatings 2019, 9, 210. [Google Scholar] [CrossRef]
- Boshkova, N.; Tabakova, N.; Atanassova, G.; Boshkov, N. Electrochemical obtaining and corrosion behavior of zinc-polyaniline (Zn-PANI) hybrid coatings. Coatings 2019, 9, 487. [Google Scholar] [CrossRef]
- Shivkumar, R.; Kalaignan, G.P.; Vasudevan, T. Studies with porous zinc electrodes with additives for secondary alkaline batteries. J. Power Sources 1998, 75, 90–100. [Google Scholar] [CrossRef]
- Chakarova, V.; Boiadjieva-Scherzer, T.; Kovacheva, D.; Kronberger, H.; Monev, M. Corrosion behaviour of electrodeposited Zn-Cr alloy coatings. Corros. Sci. 2018, 140, 73–78. [Google Scholar] [CrossRef]
- Ehsani, A.; Mahjani, M.G.; Nasseri, M.; Jafarian, M. Influence of electrosynthesis conditions and Al2O3 nanoparticles on corrosion protection effect of polypyrrole films. Anti-Corros. Methods Mater. 2014, 61, 146–152. [Google Scholar] [CrossRef]
- Huai, X.; Zhao, S.; Li, W. Corrosion resistance of Al2O3 coating on a steel substrate. J. Ceram. Process. Res. 2009, 10, 618–620. [Google Scholar]
- Lee, S.M.; Kim, Y.J.; Eom, S.W.; Choi, N.S.; Kim, K.W.; Cho, S.B. Improvement in self-discharge of Zn anode by applying surface modification for Zn-air batteries with high energy density. J. Power Sources 2013, 227, 177–184. [Google Scholar] [CrossRef]
- Jung, Y.S.; Cavanagh, A.S.; Dillon, A.C.; Groner, M.D.; George, S.M.; Lee, S.H. Enhanced stability of LiCoO2 cathodes in lithium-ion batteries using surface modification by atomic layer deposition. J. Korean Ceram. Soc. 2010, 47, 61–65. [Google Scholar] [CrossRef]
- Díaz, B.; Härkönen, E.; Światowska, J.; Maurice, V.; Seyeux, A.; Marcus, P.; Ritala, M. Low-temperature atomic layer deposition of Al2O3 thin coatings for corrosion protection of steel: Surface and electrochemical analysis. Corros. Sci. 2011, 53, 2168–2175. [Google Scholar] [CrossRef]
- Wongrujipairoj, K.; Poolnapol, L.; Arpornwichanop, A.; Suren, S.; Kheawhom, S. Suppression of zinc anode corrosion for printed flexible zinc-air battery. Phys. Status Solidi B 2017, 254, 1600442. [Google Scholar] [CrossRef]
- Park, J.H.; Fathipour, S.; Kwak, I.; Sardashti, K.; Ahles, C.F.; Wolf, S.F.; Edmonds, M.; Vishwanath, S.; Xing, H.G.; Fullerton-Shirey, S.K.; et al. Atomic layer deposition of Al2O3 on WSe2 functionalized by titanyl phthalocyanine. ACS Nano 2016, 10, 6888–6896. [Google Scholar] [CrossRef] [PubMed]
- Parvinzadeh Gashti, M.; Pakdel, E.; Alimohammadi, F. Nanotechnology-Based Coating Techniques for Smart Textiles; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; ISBN 9780081002650. [Google Scholar]
- Castro-Mayorga, J.L.; Fabra, M.J.; Cabedo, L.; Lagaron, J.M. On the use of the electrospinning coating technique to produce antimicrobial polyhydroxyalkanoate materials containing in situ-stabilized silver nanoparticles. Nanomaterials 2017, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Bessaire, B.; Mathieu, M.; Salles, V.; Yeghoyan, T.; Celle, C.; Simonato, J.P.; Brioude, A. Synthesis of continuous conductive PEDOT: PSS nanofibers by electrospinning: A conformal coating for optoelectronics. ACS Appl. Mater. Interfaces 2017, 9, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Deyab, M.A. Hydrogen generation by tin corrosion in lactic acid solution promoted by sodium perchlorate. J. Power Sources 2014, 268, 765–770. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Zhang, J.; Hall, M.E. Flammability of polyacrylonitrile and its copolymers II. Thermal behaviour and mechanism of degradation. Polym. Int. 1994, 33, 303–314. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Wang, S.; Chen, W.; Lei, Y. A convenient route to polyacrylonitrile/silver nanoparticle composite by simultaneous polymerization-reduction approach. Polymer 2001, 42, 8315–8318. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A. Electrochemical polarization I. A theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Wang, P.; Liang, C.; Wu, B.; Huang, N.; Li, J. Protection of copper corrosion by modification of dodecanethiol self-assembled monolayers prepared in aqueous micellar solution. Electrochim. Acta 2010, 55, 878–883. [Google Scholar] [CrossRef]
- Tato, W.; Landolt, D. Electrochemical determination of the porosity of single and duplex PVD coatings of titanium and titanium nitride on brass. J. Electrochem. Soc. 1998, 145, 4173–4181. [Google Scholar] [CrossRef]
- Ahn, S.H.; Choi, Y.S.; Kim, J.G.; Han, J.G. A study on corrosion resistance characteristics of PVD Cr-N coated steels by electrochemical method. Surf. Coat. Technol. 2002, 150, 319–326. [Google Scholar] [CrossRef]



| Samples | Polarization | Impedance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| −Ecorr | Icorr | ba | −bc | η% | Rp | PR% | Re | Rct | n | C | |
| mV | mA/cm2 | mV/dec | mV/dec | – | Ω·cm2 | – | Ω·cm2 | Ω·cm2 | – | ×10−5 F·cm2 | |
| Bare zinc | 1399 | 526.3 | 1160 | 840 | – | 577 | 100 | 25.7 | 620 | 0.83 | 1.69 |
| Al2O3 6 μm | 1416 | 209.4 | 1004 | 793 | 60.2 | 1476 | 39.1 | 26.5 | 1598 | 0.85 | 1.53 |
| Al2O3 12 μm | 1421 | 124.7 | 927 | 785 | 76.3 | 2475 | 23.3 | 26.9 | 2569 | 0.86 | 1.31 |
| Al2O3 18 μm | 1436 | 60.6 | 871 | 770 | 88.5 | 5106 | 11.3 | 29.3 | 5167 | 0.85 | 0.83 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Zuo, Y.; Zhang, Z.; Wu, L.; Ning, C.; Zuo, C. Al2O3 Coatings on Zinc for Anti-Corrosion in Alkaline Solution by Electrospinning. Coatings 2019, 9, 692. https://doi.org/10.3390/coatings9110692
Yu Y, Zuo Y, Zhang Z, Wu L, Ning C, Zuo C. Al2O3 Coatings on Zinc for Anti-Corrosion in Alkaline Solution by Electrospinning. Coatings. 2019; 9(11):692. https://doi.org/10.3390/coatings9110692
Chicago/Turabian StyleYu, Ying, Yuxin Zuo, Zhonghao Zhang, Lei Wu, Chuanlong Ning, and Chuncheng Zuo. 2019. "Al2O3 Coatings on Zinc for Anti-Corrosion in Alkaline Solution by Electrospinning" Coatings 9, no. 11: 692. https://doi.org/10.3390/coatings9110692