Physiological and Psychological Effects of Treadmill Overtraining Implementation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
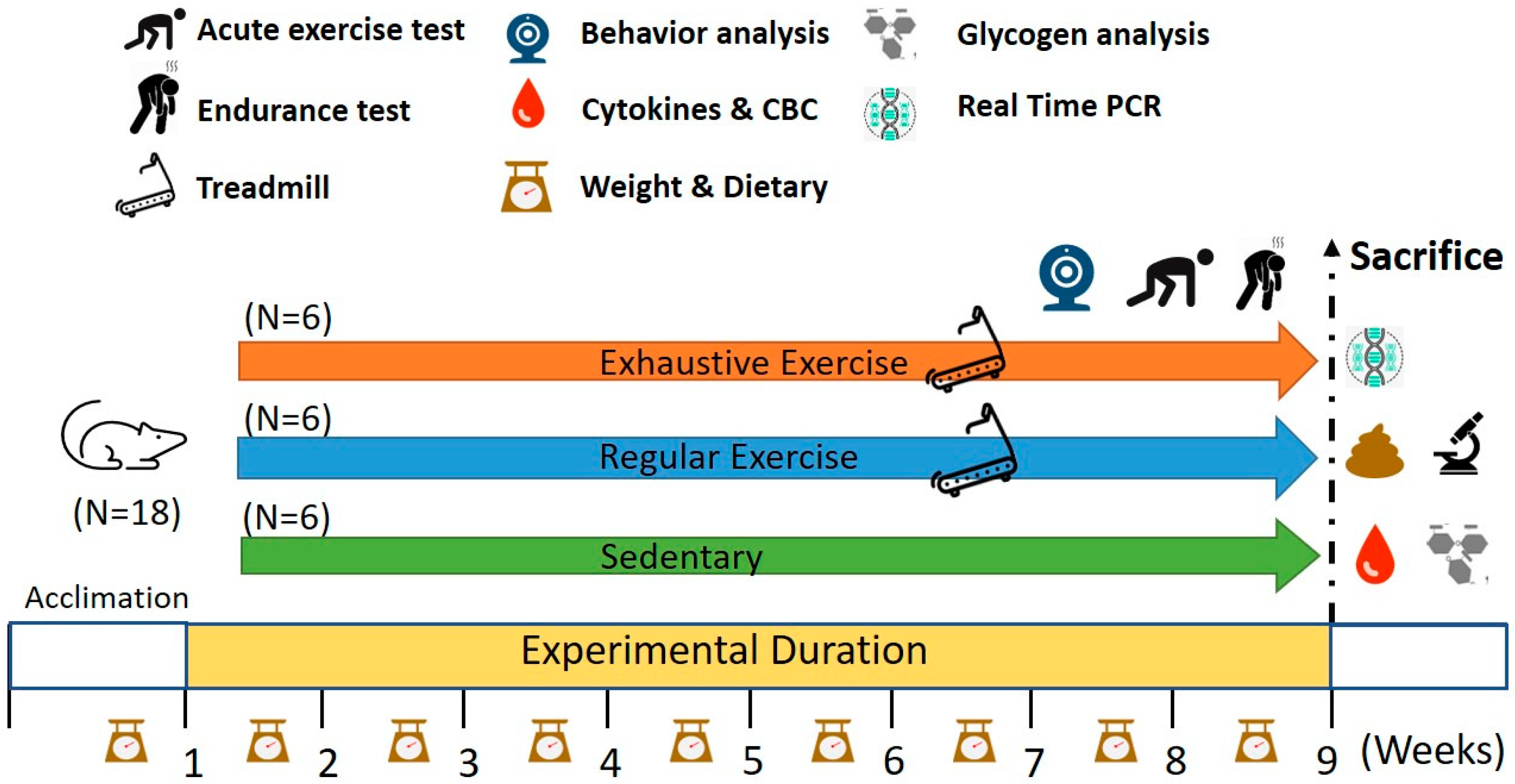
2.1. Experimental Design
2.2. Aerobic Treadmill Exercise Training
2.3. Exhaustive Exercise Protocol and Endurance Capacities
2.4. Peripheral Fatigue Biochemical Variables after Acute Exercise and Routine Blood Examination
2.5. Cytokine Analysis
2.6. Behavioral Assessment Using Open Field and Elevated Plus Maze Tests
2.7. Glucose Tolerance Test
2.8. Body Composition, Histology, and Glycogen Analysis
2.9. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction
2.10. Statistical Analysis
3. Results
3.1. Effects of Exercise Training Intervention on Growth Curve and Body Composition
3.2. Effects of Exercise Training Intervention on the Endurance Capacity Profile
3.3. Effects of Exercise Training Intervention on Glucose Tolerance
3.4. Effects of Exercise Training Intervention on Fatigue-Associated Biochemistry
3.5. Effects of the Exercise Training Intervention on Cytokines and Glycogen
3.6. Effects of Exercise Training Intervention on CBC
3.7. Effects of Exercise Training Intervention on Behavior Analysis
3.8. Effects of Exercise Training Intervention on Colon Tight Junction Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grandou, C.; Wallace, L.; Impellizzeri, F.M.; Allen, N.G.; Coutts, A.J. Overtraining in Resistance Exercise: An Exploratory Systematic Review and Methodological Appraisal of the Literature. Sports Med. 2020, 50, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Cadegiani, F.A.; da Silva, P.H.L.; Abrao, T.C.P.; Kater, C.E. Diagnosis of Overtraining Syndrome: Results of the Endocrine and Metabolic Responses on Overtraining Syndrome Study: EROS-DIAGNOSIS. J. Sports Med. 2020, 2020, 3937819. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, R.; Duclos, M.; Foster, C.; Fry, A.; Gleeson, M.; Nieman, D.; Raglin, J.; Rietjens, G.; Steinacker, J.; Urhausen, A. Prevention, diagnosis, and treatment of the overtraining syndrome: Joint consensus statement of the European College of Sport Science and the American College of Sports Medicine. Med. Sci. Sports Exerc. 2013, 45, 186–205. [Google Scholar] [CrossRef] [PubMed]
- Matos, N.F.; Winsley, R.J.; Williams, C.A. Prevalence of nonfunctional overreaching/overtraining in young English athletes. Med. Sci. Sports Exerc. 2011, 43, 1287–1294. [Google Scholar] [CrossRef]
- Vrijkotte, S.; Roelands, B.; Pattyn, N.; Meeusen, R. The Overtraining Syndrome in Soldiers: Insights from the Sports Domain. Mil. Med. 2019, 184, e192–e200. [Google Scholar] [CrossRef]
- Kreher, J.B.; Schwartz, J.B. Overtraining syndrome: A practical guide. Sports Health 2012, 4, 128–138. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, S.; Huang, H.; Liang, J.; Wu, Y.; Li, C.; Yuan, H.; Zhao, X.; Lai, X.; Hou, S. Influence of excessive exercise on immunity, metabolism, and gut microbial diversity in an overtraining mice model. Scand. J. Med. Sci. Sports 2018, 28, 1541–1551. [Google Scholar] [CrossRef]
- Smith, L.L. Cytokine hypothesis of overtraining: A physiological adaptation to excessive stress? Med. Sci. Sports Exerc. 2000, 32, 317–331. [Google Scholar] [CrossRef]
- da Rocha, A.L.; Pinto, A.P.; Kohama, E.B.; Pauli, J.R.; de Moura, L.P.; Cintra, D.E.; Ropelle, E.R.; da Silva, A.S.R. The proinflammatory effects of chronic excessive exercise. Cytokine 2019, 119, 57–61. [Google Scholar] [CrossRef]
- Fry, A.C.; Kraemer, W.J.; Ramsey, L.T. Pituitary-adrenal-gonadal responses to high-intensity resistance exercise overtraining. J. Appl. Physiol. 1998, 85, 2352–2359. [Google Scholar] [CrossRef]
- Fry, A.C.; Kraemer, W.J.; Van Borselen, F.; Lynch, J.M.; Triplett, N.T.; Koziris, L.P.; Fleck, S.J. Catecholamine responses to short-term high-intensity resistance exercise overtraining. J. Appl. Physiol. 1994, 77, 941–946. [Google Scholar] [CrossRef]
- Sterczala, A.J.; Fry, A.C.; Chiu, L.Z.F.; Schilling, B.K.; Weiss, L.W.; Nicoll, J.X. β2-adrenergic receptor maladaptations to high power resistance exercise overreaching. Hum. Physiol. 2017, 43, 446–454. [Google Scholar] [CrossRef]
- Fry, A.C.; Kraemer, W.J.; van Borselen, F.; Lynch, J.M.; Marsit, J.L.; Roy, E.P.; Triplett, N.T.; Knuttgen, H.G. Performance decrements with high-intensity resistance exercise overtraining. Med. Sci. Sports Exerc. 1994, 26, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.; Leavey, G. Setting the bar: Athletes and vulnerability to mental illness. Br. J. Psychiatry 2012, 200, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Karhu, E.; Forsgard, R.A.; Alanko, L.; Alfthan, H.; Pussinen, P.; Hamalainen, E.; Korpela, R. Exercise and gastrointestinal symptoms: Running-induced changes in intestinal permeability and markers of gastrointestinal function in asymptomatic and symptomatic runners. Eur. J. Appl. Physiol. 2017, 117, 2519–2526. [Google Scholar] [CrossRef] [PubMed]
- Chantler, S.; Griffiths, A.; Matu, J.; Davison, G.; Jones, B.; Deighton, K. The Effects of Exercise on Indirect Markers of Gut Damage and Permeability: A Systematic Review and Meta-analysis. Sports Med. 2021, 51, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Keirns, B.H.; Koemel, N.A.; Sciarrillo, C.M.; Anderson, K.L.; Emerson, S.R. Exercise and intestinal permeability: Another form of exercise-induced hormesis? Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G512–G518. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Pereira, B.C.; da Rocha, A.L.; Pinto, A.P.; Pauli, J.R.; de Souza, C.T.; Cintra, D.E.; Ropelle, E.R.; de Freitas, E.C.; Zagatto, A.M.; da Silva, A.S. Excessive eccentric exercise-induced overtraining model leads to endoplasmic reticulum stress in mice skeletal muscles. Life Sci. 2016, 145, 144–151. [Google Scholar] [CrossRef]
- Yang, D.F.; Shen, Y.L.; Wu, C.; Huang, Y.S.; Lee, P.Y.; Er, N.X.; Huang, W.C.; Tung, Y.T. Sleep deprivation reduces the recovery of muscle injury induced by high-intensity exercise in a mouse model. Life Sci. 2019, 235, 116835. [Google Scholar] [CrossRef]
- Huang, W.C.; Hsu, Y.J.; Huang, C.C.; Liu, H.C.; Lee, M.C. Exercise Training Combined with Bifidobacterium longum OLP-01 Supplementation Improves Exercise Physiological Adaption and Performance. Nutrients 2020, 12, 1145. [Google Scholar] [CrossRef] [PubMed]
- Chamberland, V.; Rioux, P. Not only students can express alcohol dehydrogenase: Goldfish can too! Adv. Physiol. Educ. 2010, 34, 222–227. [Google Scholar] [CrossRef]
- da Rocha, A.L.; Pereira, B.C.; Pauli, J.R.; Cintra, D.E.; de Souza, C.T.; Ropelle, E.R.; da Silva, A.S. Downhill Running-Based Overtraining Protocol Improves Hepatic Insulin Signaling Pathway without Concomitant Decrease of Inflammatory Proteins. PLoS ONE 2015, 10, e0140020. [Google Scholar] [CrossRef]
- Pereira, B.C.; Lucas, G.; da Rocha, A.L.; Pauli, J.R.; Ropelle, E.R.; Cintra, D.; de Souza, C.T.; Bueno, C.R.; da Silva, A.S. Eccentric Exercise Leads to Glial Activation but not Apoptosis in Mice Spinal Cords. Int. J. Sports Med. 2015, 36, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.C.; da Rocha, A.L.; Pauli, J.R.; Ropelle, E.R.; de Souza, C.T.; Cintra, D.E.; Sant’Ana, M.R.; da Silva, A.S. Excessive eccentric exercise leads to transitory hypothalamic inflammation, which may contribute to the low body weight gain and food intake in overtrained mice. Neuroscience 2015, 311, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Hohl, R.; Ferraresso, R.L.; De Oliveira, R.B.; Lucco, R.; Brenzikofer, R.; De Macedo, D.V. Development and characterization of an overtraining animal model. Med. Sci. Sports Exerc. 2009, 41, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J. Exercise Physiology in Special Populations; Churchill Livingstone: London, UK; Elsevier: New York, NY, USA; British Association of Sport and Exercise Sciences: Edinburgh, UK, 2008. [Google Scholar]
- Holloszy, J.O.; Schultz, J.; Kusnierkiewicz, J.; Hagberg, J.M.; Ehsani, A.A. Effects of exercise on glucose tolerance and insulin resistance. Brief review and some preliminary results. Acta Med. Scand. Suppl. 1986, 711, 55–65. [Google Scholar] [CrossRef]
- Gholamnezhad, Z.; Boskabady, M.H.; Hosseini, M.; Sankian, M.; Khajavi Rad, A. Evaluation of immune response after moderate and overtraining exercise in wistar rat. Iran. J. Basic Med. Sci. 2014, 17, 1–8. [Google Scholar]
- Radziuk, J.; Pye, S. Hepatic glucose uptake, gluconeogenesis and the regulation of glycogen synthesis. Diabetes Metab. Res. Rev. 2001, 17, 250–272. [Google Scholar] [CrossRef]
- Kotani, K.; Peroni, O.D.; Minokoshi, Y.; Boss, O.; Kahn, B.B. GLUT4 glucose transporter deficiency increases hepatic lipid production and peripheral lipid utilization. J. Clin. Investig. 2004, 114, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Zisman, A.; Peroni, O.D.; Abel, E.D.; Michael, M.D.; Mauvais-Jarvis, F.; Lowell, B.B.; Wojtaszewski, J.F.; Hirshman, M.F.; Virkamaki, A.; Goodyear, L.J.; et al. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat. Med. 2000, 6, 924–928. [Google Scholar] [CrossRef]
- Pereira, B.C.; Pauli, J.R.; De Souza, C.T.; Ropelle, E.R.; Cintra, D.E.; Freitas, E.C.; da Silva, A.S. Eccentric exercise leads to performance decrease and insulin signaling impairment. Med. Sci. Sports Exerc. 2014, 46, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Ørtenblad, N.; Nielsen, J. Muscle glycogen and cell function—Location, location, location. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. 4), 34–40. [Google Scholar] [CrossRef]
- Bergström, J.; Hermansen, L.; Hultman, E.; Saltin, B. Diet, muscle glycogen and physical performance. Acta Physiol. Scand. 1967, 71, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.H.; Browning, K.S.; Ivy, J.L. Regulation of GLUT4 protein expression and glycogen storage after prolonged exercise. Acta Physiol. Scand. 1999, 165, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Costill, D.L.; Flynn, M.G.; Kirwan, J.P.; Houmard, J.A.; Mitchell, J.B.; Thomas, R.; Park, S.H. Effects of repeated days of intensified training on muscle glycogen and swimming performance. Med. Sci. Sports Exerc. 1988, 20, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.P.; Costill, D.L.; Mitchell, J.B.; Houmard, J.A.; Flynn, M.G.; Fink, W.J.; Beltz, J.D. Carbohydrate balance in competitive runners during successive days of intense training. J. Appl. Physiol. 1988, 65, 2601–2606. [Google Scholar] [CrossRef]
- Gleeson, M. Biochemical and immunological markers of over-training. J. Sports Sci. Med. 2002, 1, 31–41. [Google Scholar] [PubMed]
- Kim, N.-I.; Kim, S.-J.; Jang, J.-H.; Shin, W.-s.; Eum, H.-j.; Kim, B.; Choi, A.; Lee, S.-S. Changes in Fatigue Recovery and Muscle Damage Enzymes after Deep-Sea Water Thalassotherapy. Appl. Sci. 2020, 10, 8383. [Google Scholar] [CrossRef]
- Cairns, S.P. Lactic acid and exercise performance: Culprit or friend? Sports Med. 2006, 36, 279–291. [Google Scholar] [CrossRef]
- Holloszy, J.O.; Booth, F.W. Biochemical adaptations to endurance exercise in muscle. Annu. Rev. Physiol. 1976, 38, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Apple, F.S.; Rogers, M.A. Skeletal muscle lactate dehydrogenase isozyme alterations in men and women marathon runners. J. Appl. Physiol. 1986, 61, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J. Biomarkers of peripheral muscle fatigue during exercise. BMC Musculoskelet. Disord. 2012, 13, 218. [Google Scholar] [CrossRef]
- Wells, C.L.; Stern, J.R.; Hecht, L.H. Hematological changes following a marathon race in male and female runners. Eur. J. Appl. Physiol. Occup. Physiol. 1982, 48, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Kurihara, S.; Titchenal, C.A.; Ohtani, M. Suppression of exercise-induced neutrophilia and lymphopenia in athletes by cystine/theanine intake: A randomized, double-blind, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2010, 7, 23. [Google Scholar] [CrossRef]
- Peake, J.M.; Neubauer, O.; Walsh, N.P.; Simpson, R.J. Recovery of the immune system after exercise. J. Appl. Physiol. 2017, 122, 1077–1087. [Google Scholar] [CrossRef]
- Wahl, P.; Mathes, S.; Bloch, W.; Zimmer, P. Acute Impact of Recovery on the Restoration of Cellular Immunological Homeostasis. Int. J. Sports Med. 2020, 41, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Yıldız, A.; Türker Duyuler, P.; Duyuler, S.; Yılmaz, S.; Basyigit, F.; Elalmis, O.U.; Guray, U.; Ileri, M. Combination of change in hematological parameters with exercise stress test to predict coronary artery disease. J. Clin. Lab. Anal. 2018, 32. [Google Scholar] [CrossRef] [PubMed]
- Posthuma, J.J.; van der Meijden, P.E.; Ten Cate, H.; Spronk, H.M. Short- and Long-term exercise induced alterations in haemostasis: A review of the literature. Blood Rev. 2015, 29, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.M.; Chen, P.J.; Liu, Q.; Wang, R.; Xiao, W.H.; Zhang, Y.J. Reverse Effects of DPI Administration Combined with Glutamine Supplementation on Function of Rat Neutrophils Induced by Overtraining. Int. J. Sport Nutr. Exerc. Matab. 2013, 23, 137–149. [Google Scholar] [CrossRef]
- Khansari, D.N.; Murgo, A.J.; Faith, R.E. Effects of stress on the immune system. Immunol. Today 1990, 11, 170–175. [Google Scholar] [CrossRef]
- Mackinnon, L.T. Exercise and Immunology, 2nd ed.; Human Kinetics: Champaign, IL, USA, 1992. [Google Scholar]
- Ma, T.Y.; Iwamoto, G.K.; Hoa, N.T.; Akotia, V.; Pedram, A.; Boivin, M.A.; Said, H.M. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G367–G376. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Hata, M.; Furuse, K.; Yoshida, Y.; Haratake, A.; Sugitani, Y.; Noda, T.; Kubo, A.; Tsukita, S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J. Cell Biol. 2002, 156, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Podolsky, D.K. Mucosal immunity and inflammation. V. Innate mechanisms of mucosal defense and repair: The best offense is a good defense. Am. J. Physiol. 1999, 277, G495–G499. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.P. Role of gastrointestinal permeability in exertional heatstroke. Exerc. Sport Sci. Rev. 2004, 32, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Dokladny, K.; Moseley, P.L.; Ma, T.Y. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G204–G212. [Google Scholar] [CrossRef] [PubMed]







| Characteristic | Sedentary | Exercise | Exhaustion |
|---|---|---|---|
| Liver (g) | 1.11 ± 0.12 | 1.10 ± 0.10 | 1.03 ± 0.07 |
| Muscle (g) | 0.31 ± 0.02 | 0.30 ± 0.02 | 0.28 ± 0.03 |
| Kidney (g) | 0.28 ± 0.01 | 0.30 ± 0.01 | 0.29 ± 0.03 |
| Heart (g) | 0.14 ± 0.02 | 0.15 ± 0.02 | 0.14 ± 0.03 |
| EFP (g) | 0.33± 0.06 b | 0.27± 0.03 a | 0.24 ± 0.01 a |
| Diet (g/mouse/day) | 4.49 ± 0.59 b | 4.32 ± 0.64 b | 3.89 ± 0.87 a |
| Water (mL/mouse/day) | 7.14 ± 1.02 | 7.00 ± 2.22 | 8.08 ± 3.37 |
| Time Point | Sedentary | Exercise | Exhaustion |
|---|---|---|---|
| Lactate (mmol/L) | |||
| Before running (A) | 2.48 ± 0.16 a | 2.45 ± 0.26 a | 2.93 ± 0.33 b |
| After running (B) | 3.78 ± 0.23 a | 3.77 ± 0.61 a | 5.83 ± 1.31 b |
| Lactate production rate | |||
| Production rate = B/A | 1.53 ± 0.09 a | 1.56 ± 0.32 a | 1.98 ± 0.32 b |
| Parameter | Sedentary | Exercise | Exhaustion |
|---|---|---|---|
| WBC (103/μL) | 4.4 ± 1.1 b | 3.6 ± 0.7 ab | 3.3 ± 0.8 a |
| Neu (%) | 15.0 ± 2.8 a | 12.5± 2.9 a | 28.0 ± 8.6 b |
| Lym (%) | 84.5 ± 3.0 b | 86.7 ± 2.8 b | 69.5 ± 8.7 a |
| Mono (%) | 0.5 ± 0.8 a | 0.9 ± 1.2 a | 2.7 ± 1.0 b |
| Eosi (%) | 0.10 ± 0.1 | 0.09± 0.1 | 0.09 ± 0.1 |
| Baso (%) | 0.11 ± 0.1 | 0.12 ± 0.1 | 0.11 ± 0.2 |
| RBC (million/μL) | 10.2 ± 0.4 | 10.0 ± 0.2 | 10.3 ± 0.3 |
| Hb (g/dL) | 15.6 ± 0.4 | 15.4 ± 0.4 | 15.5 ± 0.6 |
| Platelet (103/μL) | 1077 ± 65 ab | 1025 ± 77 a | 1149 ± 86 b |
| PLR | 0.31 ± 0.1 a | 0.34 ± 0.1 a | 0.53 ± 0.1 b |
| NLR | 0.18 ± 0.04 a | 0.14 ± 0.04 a | 0.42 ± 0.2 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, Y.; Hsiao, Y.-T.; Huang, W.-C. Physiological and Psychological Effects of Treadmill Overtraining Implementation. Biology 2021, 10, 515. https://doi.org/10.3390/biology10060515
Chung Y, Hsiao Y-T, Huang W-C. Physiological and Psychological Effects of Treadmill Overtraining Implementation. Biology. 2021; 10(6):515. https://doi.org/10.3390/biology10060515
Chicago/Turabian StyleChung, Yi, Yi-Ting Hsiao, and Wen-Ching Huang. 2021. "Physiological and Psychological Effects of Treadmill Overtraining Implementation" Biology 10, no. 6: 515. https://doi.org/10.3390/biology10060515
APA StyleChung, Y., Hsiao, Y.-T., & Huang, W.-C. (2021). Physiological and Psychological Effects of Treadmill Overtraining Implementation. Biology, 10(6), 515. https://doi.org/10.3390/biology10060515







