Anterior Cruciate Ligament Reconstructed Patients Who Recovered Normal Postural Control Have Dissimilar Brain Activation Patterns Compared to Healthy Controls
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
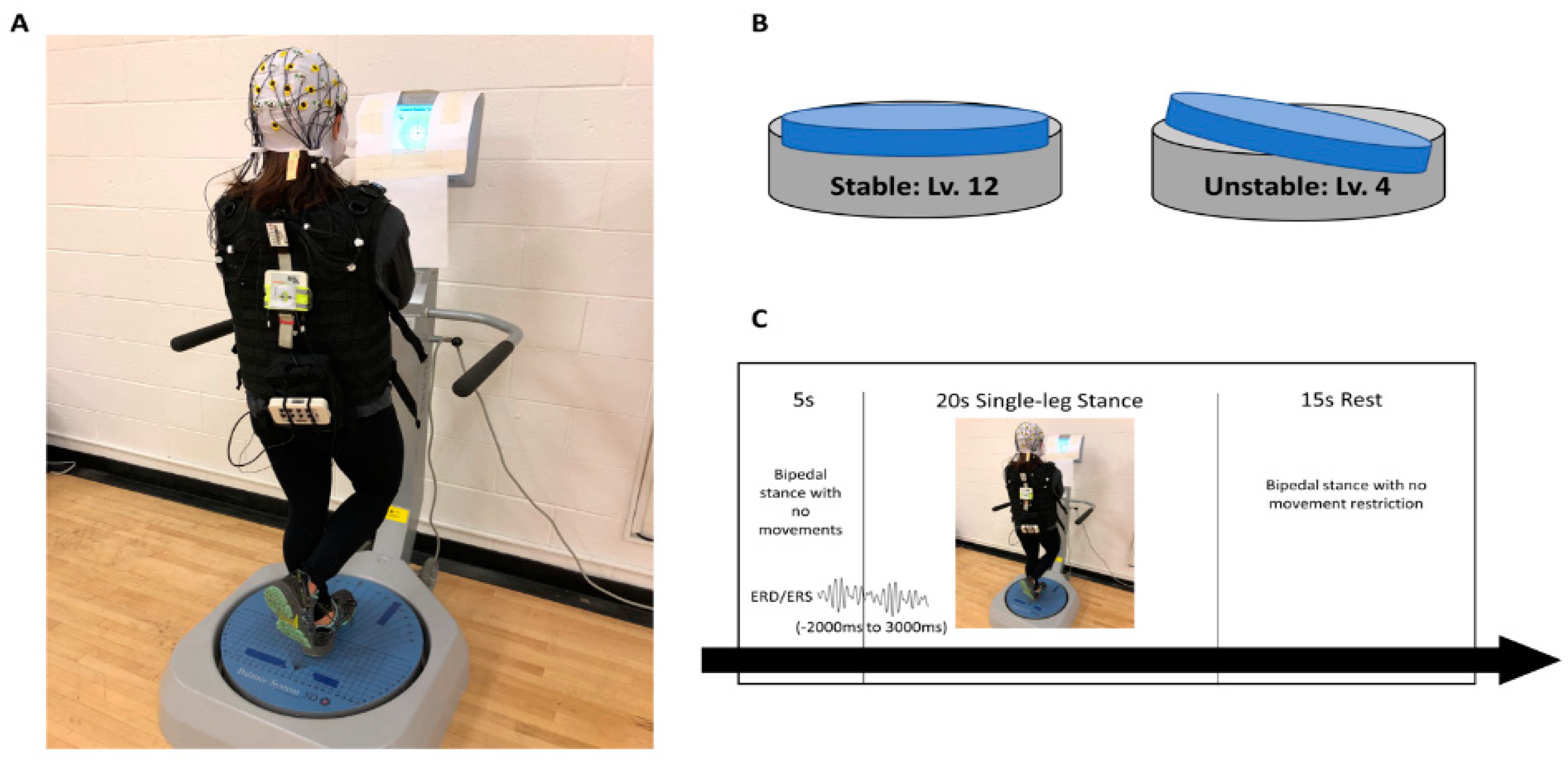
2.1. Experimental Design
2.2. Participants
2.3. Postural Control Assessment
2.4. Electrocortical Activity
2.5. Statistical Analysis
3. Results
3.1. Postural Control
3.2. Electrocorticla Activation
4. Discussion
4.1. ACLR Patients Had Similar Postural Control Patterns to Healthy Individuals
4.2. More Challenging Task Exhibited Altered Brain Activity during Balance Task
4.3. ACLR Patients Had Dissimilar Brain Activity Patterns to Healthy Individuals during Early Single-Leg Stance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosso, A.L.; Cenciarini, M.; Sparto, P.J.; Loughlin, P.J.; Furman, J.M.; Huppert, T.J. Neuroimaging of an attention demanding dual-task during dynamic postural control. Gait Posture 2017, 57, 193–198. [Google Scholar] [CrossRef]
- Echang, C.-J.; Eyang, T.-F.; Eyang, S.W.; Echern, J.-S. Cortical Modulation of Motor Control Biofeedback among the Elderly with High Fall Risk during a Posture Perturbation Task with Augmented Reality. Front. Aging Neurosci. 2016, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Limanowski, J.; Blankenburg, F. Integration of Visual and Proprioceptive Limb Position Information in Human Posterior Parietal, Premotor, and Extrastriate Cortex. J. Neurosci. 2016, 36, 2582–2589. [Google Scholar] [CrossRef] [Green Version]
- Rozzi, S.L.; Lephart, S.M.; Gear, W.S.; Fu, F.H. Knee Joint Laxity and Neuromuscular Characteristics of Male and Female Soccer and Basketball Players. Am. J. Sports Med. 1999, 27, 312–319. [Google Scholar] [CrossRef]
- Mohapatra, S.; Krishnan, V.; Aruin, A.S. Postural control in response to an external perturbation: Effect of altered proprioceptive information. Exp. Brain Res. 2012, 217, 197–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riemann, B.L.; Lephart, S.M. The sensorimotor system, part II: The role of proprioception in motor control and functional joint stability. J. Athl. Train. 2002, 37, 80–84. [Google Scholar] [PubMed]
- Peterson, S.M.; Ferris, D.P. Differentiation in theta and beta electrocortical activity between visual and physical perturbations to walking and standing balance. eNeuro 2018, 5, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Hülsdünker, T.; Mierau, A.; Neeb, C.; Kleinöder, H.; Strüder, H. Cortical processes associated with continuous balance control as revealed by EEG spectral power. Neurosci. Lett. 2015, 592, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bonfim, T.R.; Paccola, C.A.J.; Barela, J.A. Proprioceptive and behavior impairments in individuals with anterior cruciate ligament reconstructed knees. Arch. Phys. Med. Rehabil. 2003, 84, 1217–1223. [Google Scholar] [CrossRef]
- Fu, A.; Hui-Chan, C.W.Y. Ankle Joint Proprioception and Postural Control in Basketball Players with Bilateral Ankle Sprains. Am. J. Sports Med. 2005, 33, 1174–1182. [Google Scholar] [CrossRef]
- Swanik, C.B.; Lephart, S.M.; Giannantonio, F.P.; Fu, F.H. Reestablishing Proprioception and Neuromuscular Control in the ACL-Injured Athlete. J. Sport Rehabil. 1997, 6, 182–206. [Google Scholar] [CrossRef]
- Riemann, B.L.; Lephart, S.M. The sensorimotor system, part I: The physiologic basis of functional joint stability. J. Athl. Train. 2002, 37, 71–79. [Google Scholar]
- Baumeister, J.; Reinecke, K.; Weiss, M. Changed cortical activity after anterior cruciate ligament reconstruction in a joint position paradigm: An EEG study. Scand. J. Med. Sci. Sports 2007, 18, 473–484. [Google Scholar] [CrossRef]
- Baumeister, J.; Reinecke, K.; Schubert, M.; Weiß, M. Altered electrocortical brain activity after ACL reconstruction during force control. J. Orthop. Res. 2011, 29, 1383–1389. [Google Scholar] [CrossRef]
- An, Y.W.; Lobacz, A.D.; Lehmann, T.; Baumeister, J.; Rose, W.C.; Higginson, J.S.; Rosen, J.; Swanik, C.B. Neuroplastic changes in anterior cruciate ligament reconstruction patients from neuromechanical decoupling. Scand. J. Med. 2019, 29, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Hülsdünker, T.; Mierau, A.; Strüder, H.K. Higher Balance Task Demands are Associated with an Increase in Individual Alpha Peak Frequency. Front. Hum. Neurosci. 2015, 9, 695. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.; Lee, J.Y.; Lee, K.I.; Park, K.M. Are there differences in brain morphology according to handedness? Brain Behav. 2017, 7, e00730. [Google Scholar] [CrossRef] [PubMed]
- Dozolme, D.; Prigent, E.; Yang, Y.-F.; Amorim, M.-A. The neuroelectric dynamics of the emotional anticipation of other people’s pain. PLoS ONE 2018, 13, e0200535. [Google Scholar] [CrossRef]
- Anders, P.; Lehmann, T.; Müller, H.; Grønvik, K.B.; Skjæret-Maroni, N.; Baumeister, J.; Vereijken, B. Exergames Inherently Contain Cognitive Elements as Indicated by Cortical Processing. Front. Behav. Neurosci. 2018, 12, 102. [Google Scholar] [CrossRef]
- Wheaton, L.A.; Carpenter, M.; Mizelle, J.C.; Forrester, L. Preparatory band specific premotor cortical activity differentiates upper and lower extremity movement. Exp. Brain Res. 2008, 184, 121–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrosimone, B.G.; Lepley, A.S.; Ericksen, H.M.; Clements, A.; Sohn, D.H.; Gribble, P.A. Neural Excitability Alterations After Anterior Cruciate Ligament Reconstruction. J. Athl. Train. 2015, 50, 665–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Rajagovindan, R.; Han, S.-M.; Ding, M. Top-Down Control of Visual Alpha Oscillations: Sources of Control Signals and Their Mechanisms of Action. Front. Hum. Neurosci. 2016, 10, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paterno, M.V.; Schmitt, L.C.; Ford, K.; Rauh, M.J.; Myer, G.D.; Huang, B.; Hewett, T.E. Biomechanical Measures during Landing and Postural Stability Predict Second Anterior Cruciate Ligament Injury after Anterior Cruciate Ligament Reconstruction and Return to Sport. Am. J. Sports Med. 2010, 38, 1968–1978. [Google Scholar] [CrossRef]
- Slobounov, S.; Cao, C.; Jaiswal, N.; Newell, K.M. Neural basis of postural instability identified by VTC and EEG. Exp. Brain Res. 2009, 199, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Van Veen, V.; Carter, C.S. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol. Behav. 2002, 77, 477–482. [Google Scholar] [CrossRef]
- Sauseng, P.; Klimesch, W.; Schabus, M.; Doppelmayr, M. Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. Int. J. Psychophysiol. 2005, 57, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, J.; Reinecke, K.; Schubert, M.; Schade, J.; Weiss, M. Effects of induced fatigue on brain activity during sensorimotor control. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 112, 2475–2482. [Google Scholar] [CrossRef]
- Popivanov, D.; Mineva, A.; Krekule, I. EEG patterns in theta and gamma frequency range and their probable relation to human voluntary movement organization. Neurosci. Lett. 1999, 267, 5–8. [Google Scholar] [CrossRef]
- Klimesch, W.; Doppelmayr, M.; Hanslmayr, S. Upper alpha ERD and absolute power: Their meaning for memory performance. Neuper Klimesch 2006, 159, 151–165. [Google Scholar] [CrossRef]
- Mierau, A.; Hülsdünker, T.; Strüder, H.K. Changes in cortical activity associated with adaptive behavior during repeated balance perturbation of unpredictable timing. Front. Behav. Neurosci. 2015, 9, 272. [Google Scholar] [CrossRef] [Green Version]
- Jiganti, M.R.; Meyer, B.C.; Chang, E.; Romanelli, D.A.; An, Y.W. Altered cortical activation after anterior cruciate ligament reconstruction during single-leg balance task. Transl. Sports Med. 2020, 3, 496–503. [Google Scholar] [CrossRef]
- Edwards, A.; Guven, O.; Furman, M.D.; Arshad, Q.; Bronstein, A.M. Electroencephalographic Correlates of Continuous Postural Tasks of Increasing Difficulty. Neuroscience 2018, 395, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Kalaska, J.; Rizzolatti, G. Voluntary movement: The primary motor cortex. In Principles of Neural Science, 5th ed.; McGraw-Hill: New York, NY, USA, 2013; pp. 835–864. [Google Scholar]
- MacKay, W.A. Wheels of motion: Oscillatory potentials in the motor cortex. In Motor Cortex in Voluntary Movements: A Distributed System for Distributed Functions, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 181–211. [Google Scholar]
- Rizzolatti, G.; Kalaska, J.F. Voluntary movement: The parietal and premotor cortex. In Principles of Neural Science, 5th ed.; McGraw-Hill: New York, NY, USA, 2012; pp. 865–893. [Google Scholar] [CrossRef]
- Pineda, J.A. The functional significance of mu rhythms: Translating “seeing” and “hearing” into “doing”. Brain Res. Brain Res. Rev. 2005, 50, 57–68. [Google Scholar] [CrossRef]
- Grooms, D.R.; Page, S.J.; Nichols-Larsen, D.S.; Chaudhari, A.; White, S.E.; Onate, J.A. Neuroplasticity Associated With Anterior Cruciate Ligament Reconstruction. J. Orthop. Sports Phys. Ther. 2017, 47, 180–189. [Google Scholar] [CrossRef]
- Kapreli, E.; Athanasopoulos, S.; Gliatis, J.; Papathanasiou, M.; Peeters, R.; Strimpakos, N.; Van Hecke, P.; Gouliamos, A.; Sunaert, S. Anterior cruciate ligament deficiency causes brain plasticity: A functional MRI study. Am. J. Sports Med. 2009, 37, 2419–2426. [Google Scholar] [CrossRef] [PubMed]
- Kapreli, E.; Athanasopoulos, S. The anterior cruciate ligament deficiency as a model of brain plasticity. Med. Hypotheses 2006, 67, 645–650. [Google Scholar] [CrossRef]
- Hurd, W.J.; Axe, M.J.; Snyder-Mackler, L. Influence of Age, Gender, and Injury Mechanism on the Development of Dynamic Knee Stability after Acute ACL Rupture. J. Orthop. Sports Phys. Ther. 2008, 38, 36–41. [Google Scholar] [CrossRef]
- Culvenor, A.G.; Alexander, B.C.; Clark, R.; Collins, N.; Ageberg, E.; Morris, H.G.; Whitehead, T.S.; Crossley, K.M. Dynamic Single-Leg Postural Control Is Impaired Bilaterally Following Anterior Cruciate Ligament Reconstruction: Implications for Reinjury Risk. J. Orthop. Sports Phys. Ther. 2016, 46, 357–364. [Google Scholar] [CrossRef] [PubMed]



| Demographic Data (Mean ± SD) | ||||
|---|---|---|---|---|
| CONT (N = 15) | ACLR (N = 15) | p-Value * | ||
| Sex, N | Male | 10 | 10 | |
| Female | 5 | 5 | ||
| Age, years | 23.07 ± 3.45 | 23.13 ± 3.20 | 0.957 | |
| Height, cm | 175.68 ± 11.58 | 172.55 ± 9.95 | 0.433 | |
| Weight, kg | 71.09 ± 11.31 | 76.02 ± 17.22 | 0.362 | |
| Time from surgery, years | 2.97 ± 2.28 | |||
| CONT | ACLR | Group-by-Condition Effect | |||||
|---|---|---|---|---|---|---|---|
| Hz | Cerebral Region | SP | UP | SP | UP | F | p |
| Theta | Central prefrontal c | 36.59 ± 28.47 | 44.33 ± 35.92 | 12.54 ± 20.74 | 36.67 ± 38.16 | 2.206 | 0.149 |
| IL premotor | 12.09 ± 13.78 | 6.65 ± 15.57 | 2.56 ± 32.93 | 24.56 ± 31.47 *a*b | 4.718 | 0.039 * | |
| NIL premotor c | −2.31 ± 13.31 | 8.84 ± 16.23 | −2.66 ± 7.42 | 12.59 ± 26.69 | 0.230 | 0.636 | |
| Central primary motor c | 22.35 ± 28.35 | 30.40 ± 34.47 | 10.29 ± 21.19 | 32.98 ± 23.88 | 1.852 | 0.186 | |
| IL primary motor | 3.44 ± 14.97 | 5.34 ± 20.97 | 12.32 ± 19.50 | 21.38 ± 29.72 | 0.511 | 0.481 | |
| NIL primary motor | 2.71 ± 1055 | 11.52 ± 19.56 | 8.00 ± 17.59 | 19.23 ± 27.78 | 0.054 | 0.818 | |
| Central somatosensory c | 1.67 ± 14.10 | 11.97 ± 30.24 | 5.83 ± 18.09 | 17.66 ± 26.29 | 0.027 | 0.871 | |
| IL somatosensory c | 2.71 ± 12.75 | 12.86 ± 27.63 | 11.62 ± 21.03 | 23.47 ± 29.34 | 0.037 | 0.848 | |
| NIL somatosensory c | 1.84 ± 11.97 | 11.83 ± 26.03 | 9.70 ± 22.48 | 23.91 ± 26.96 | 0.215 | 0.647 | |
| Primary visual c | −1.61 ± 14.55 | 8.73 ± 23.93 | 6.18 ± 21.00 | 16.68 ± 28.94 | 0.000 | 0.987 | |
| Alpha-2 | Central prefrontal | 5.57 ± 24.15 | −2.37 ± 18.29 | 2.51 ± 8.67 | 15.14 ± 14.44 *a*b | 8.317 | 0.008 * |
| IL premotor | −11.16 ± 13.72 | −12.33 ± 24.92 | −10.02 ± 18.78 | −3.02 ± 13.62 | 1.292 | 0.266 | |
| NIL premotor d | −7.92 ± 18.94 | −14.32 ± 11.23 | 5.68 ± 23.00 | −4.04 ± 9.40 | 0.169 | 0.684 | |
| Central primary motor | −17.69 ± 23.53 | −13.27 ± 26.00 | −10.09 ± 22.55 | −6.92 ± 23.23 | 0.026 | 0.873 | |
| IL primary motor c | −18.63 ± 23.36 | −29.73 ± 30.56 | −8.84 ± 28.84 | −22.15 ± 33.48 | 0.056 | 0.814 | |
| NIL primary motor | −22.47 ± 23.34 | −19.09 ± 34.62 | −5.67 ± 12.16 *a*b | −23.37 ± 25.23 | 7.535 | 0.011 * | |
| Central somatosensory | −25.65 ± 27.73 | −17.28 ± 28.21 | −13.23 ± 35.67 *a | −28.35 ± 21.69 | 6.015 | 0.021 * | |
| IL somatosensory | −23.08 ± 30.52 | −23.73 ± 28.74 | −11.58 ± 38.50 | −26.30 ± 28.17 | 2.084 | 0.160 | |
| NIL somatosensory | −26.49 ± 26.23 | −14.84 ± 34.99 | −8.75 ± 37.51 | −24.34 ± 28.52 | 7.114 | 0.013 * | |
| Primary visual | −20.58 ± 23.43 | −8.21 ± 25.43 *a | −6.49 ± 23.01 | −14.12 ± 19.04 | 5.883 | 0.023 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, Y.W.; Kang, Y.; Jun, H.-P.; Chang, E. Anterior Cruciate Ligament Reconstructed Patients Who Recovered Normal Postural Control Have Dissimilar Brain Activation Patterns Compared to Healthy Controls. Biology 2022, 11, 119. https://doi.org/10.3390/biology11010119
An YW, Kang Y, Jun H-P, Chang E. Anterior Cruciate Ligament Reconstructed Patients Who Recovered Normal Postural Control Have Dissimilar Brain Activation Patterns Compared to Healthy Controls. Biology. 2022; 11(1):119. https://doi.org/10.3390/biology11010119
Chicago/Turabian StyleAn, Yong Woo, Yangmi Kang, Hyung-Pil Jun, and Eunwook Chang. 2022. "Anterior Cruciate Ligament Reconstructed Patients Who Recovered Normal Postural Control Have Dissimilar Brain Activation Patterns Compared to Healthy Controls" Biology 11, no. 1: 119. https://doi.org/10.3390/biology11010119
APA StyleAn, Y. W., Kang, Y., Jun, H.-P., & Chang, E. (2022). Anterior Cruciate Ligament Reconstructed Patients Who Recovered Normal Postural Control Have Dissimilar Brain Activation Patterns Compared to Healthy Controls. Biology, 11(1), 119. https://doi.org/10.3390/biology11010119







