Simple Summary
The fall armyworm (FAW), Spodoptera frugiperda (J.E. Smith), is a voracious insect pest native to the Western Hemisphere, particularly in South America. The polyphagous fall armyworm feeds on more than 350 plants in such families, including Poaceae, Asteraceae, and Fabaceae. Transgenic plants that express Bacillus thuringiensis proteins (Bt plants) and synthetic insecticides are the main tactics to control Spodoptera frugiperda, although widespread usage of synthetic chemicals has resulted in the emergence of resistance. We assessed cross-resistance, resistance mechanism, and fitness costs based on the life history traits of Spodoptera frugiperda. Results indicated that after 24 generations of selection, the resistance to indoxacarb was increased by 472.67-fold as compared to the Ind-UNSEL. Significantly longer developmental time of larvae extended pupal duration, shorter adult longevity, and lower fecundity were observed in Ind-SEL as compared with the Ind-UNSEL population. Butoxide synergist increased susceptibility to indoxacarb, indicating that P450 enzymes may be involved in indoxacarb resistance. Therefore, it is crucial that we comprehend how insecticides work and how resistance develops in order to develop techniques for managing resistance. These data are valuable to understand the indoxacarb resistance mechanism and provides vital information for scientific-based guidance of pest management decisions.
Abstract
The fall armyworm, Spodoptera frugiperda (J.E. Smith), is a voracious insect pest that is difficult to control due to resistance to insecticides and Bt proteins. We assessed cross-resistance, resistance mechanism, and fitness costs based on the life history traits of S. frugiperda. We established an S. frugiperda strain selected for resistance to indoxacarb (Ind-SEL) from a field-collected population and an unselected strain, Ind-UNSEL. Results indicated that after 24 generations of selection, the resistance to indoxacarb was increased by 472.67-fold as compared to the Ind-UNSEL. There was high cross-resistance to deltamethrin (31.23-fold) with very low or negligible cross-resistance to chlorantraniliprole, emamectin benzoate, and/or methoxyfenozide in the Ind-SEL population. Butoxide synergist increased susceptibility to indoxacarb, indicating that P450 enzymes may be involved in indoxacarb resistance. Significantly longer developmental time of larvae extended pupal duration, shorter adult longevity, and lower fecundity were observed in Ind-SEL as compared with the Ind-UNSEL population. The Net reproductive rate (R0) was the only growth parameter that differs between crosses of Ind-SEL♂ × Ind-UNSEL♀ (176 ± 46) and Ind-SEL♀ × Ind-UNSEL♂ (328 ± 57). On the other hand, all population growth parameters differ between Ind-SEL and Ind-UNSEL strains. Our work contributes to the growing body of research that demonstrates the importance of strain genetics in fitness cost experiments and helps resistance management programs make decisions.
1. Introduction
The fall armyworm (FAW), Spodoptera frugiperda (J.E. Smith), is a voracious insect pest native to the Western Hemisphere, particularly in South America [1]. It is one of the most rapidly spreading and highly invasive pests of maize across Africa and Asia [2,3,4,5]. S. frugiperda has become a pest species because of its biological characteristics such as polyphagy, concealed larval feeding habits, high reproductive capacity, adult dispersion, and multiple generations per year [3,4,5]. The polyphagous fall armyworm feeds on more than 350 plants in such families, including Poaceae, Asteraceae, and Fabaceae [6]. The FAW has two haplotypes that have been recognized for a long time: the “rice strain” (R strain), which prefers to eat rice and grasses, and the “corn strain” (C strain), which prefers to eat maize and sorghum [7,8,9]. It is very important to control S. frugiperda infestations and spread because it causes severe economic loss to several economically important crop plants and threatens global food security and the livelihoods of many households [2,5]. Currently, S. frugiperda represents a serious problem to maize crops in China and elsewhere in South Asia [4,10].
Transgenic plants that express Bacillus thuringiensis proteins (Bt plants) and synthetic insecticides are the main tactics to control FAW, although widespread usage of synthetic chemicals has resulted in the emergence of resistance [11,12,13,14]. Unfortunately, the foliar application of chemical insecticides against the S. frugiperda population in Bt and non-Bt maize crops has low control efficacy [13]. This may be due to the feeding behavior of S. frugiperda larvae which stay inside the maize whorl, thus reducing insecticide contact. In addition, Long-term use of chemical pesticides in the field poses serious risks to the environment due to contamination and causes the death of natural enemies, which often leads to pest resurgence, and inevitably has led to insecticide-resistant in different insects population [11,15,16,17,18,19,20]. Previously, some studies have shown that multiple field populations of S. frugiperda have developed high-level resistance as well as broad cross-resistance to diverse groups of synthetic insecticides, including pyrethroids, organophosphate, carbamate, chlorantraniliprole, abamectin, emamectin benzoate, lufenuron and spinosad [11,12,13,14,18,19]. In addition, widespread areas of Bt-crops without growing refuge in some mainly tropical countries have increased the evolution of resistance problems to Bt proteins in S. frugiperda populations [21,22,23]. The effort to control this pest is becoming exceedingly challenging all over the world.
Indoxacarb is a new oxadiazine insecticide with significant toxicity against a variety of lepidopteran, coleopteran, and sucking insect pests in agricultural as well as urban contexts [24]. Insect esterases or amidases can convert indoxacarb to an N-decarbomethoxylated metabolite (DCJW), a more potent sodium channel blocker than indoxacarb, which causes the target pest species to become paralyzed and die [25,26]. Indoxacarb is highly active when ingested, but there have been few reports of contact activity when applied topically [24,26,27,28]. Indoxacarb is a potent novel insecticide for crop protection because to its safety for humans and non-target organisms, superior environmental and residual qualities, broad spectrum, and quick reduction in insect feeding [24]. However, numerous studies have indicated that a number of insects have recently evolved resistance to indoxacarb due to its widespread use, including S. litura [29], such as C. rosaceana [30], M. domestica [31], S. exigua [32], P. xylostella [33] and H. armigera [34] have developed a significant level of resistance. Furthermore, resistance to several Bt maize products expressing Cry1F and Cry1Ab proteins has been reported in the field, increasing the use of chemical insecticides against S. frugiperda in maize [13,35]. The detoxification enzymes P450, esterase, and glutathione S-transferase (GST) are involved in the resistance to indoxacarb [33,34,36]. Additionally, novel sodium channel mutations (F1845Y and V1848I) have been reported to be associated with resistance to indoxacarb in P. xylostella and T. absoluta [33,37]. However, indoxacarb resistance is currently at a relatively low level in S. frugiperda populations, although the pest has been subjected to indoxacarb selection pressure. Insecticide resistance management in insect pests is a global challenge for entomologists. However, if resistance to a novel insecticide can be monitored and predicted, a proactive resistance management program can be established to reduce the risk of resistance [23,38,39]. Laboratory selection experiments can provide important insect resistance data [14,40]. Therefore, it is crucial that we comprehend how insecticides work and how resistance develops in order to develop techniques for managing resistance. Additionally, figuring out the molecular basis behind pesticide resistance may open up new possibilities for the creation of cutting-edge tactics for controlling insect pests. However, indoxacarb resistance mechanism, inheritance, and resistance-associated fitness costs in S. frugiperda have not been documented to date.
Understanding the resistance mechanism and resistance-associated fitness costs is essential as they directly affect the rate of resistance evolution in the field population and play significant roles in an insect resistance management (IRM) strategy [41,42]. Moreover, evaluating the fitness costs associated with resistance can aid in determining if vulnerability can be regained in the context of selection pressure [43,44,45]. In this study, to understand the potential mechanism of the fast-evolved resistance to indoxacarb, the inheritance of resistance and resistance-associated fitness costs in the S. frugiperda strain from a field population was evaluated. These data are valuable to understand the indoxacarb resistance mechanism and provides vital information for scientific-based guidance of pest management decisions.
2. Materials and Methods
2.1. Collection and Breeding of Insects
The larvae of the field population of FAW were originally collected from two different corn fields in Ping Hu County (Latitude: 30.705° N, Longitude: 121.118° E) Zhejiang Province in 2019, denoted as (PHZ19) and maintained on an artificial diet under control conditions at 26 ± 2 °C, 65 ± 5% relative humidity (RH), 14:10 h (light:dark, L:D) photoperiod until adults emerged. The newly emerged adults were paced in mating cages, according to Hafeez et al. (2021) [4], and fed a 10% sugar solution as a food source.
2.2. Toxicological Bioassays
The tested insecticide, indoxacarb (15% commercial formulation), was purchased from Mesa Tech Co., Ltd. (Beijing China). Using the diet-incorporation technique described by [46], a preliminary bioassay was carried out using second-instar larvae from the G2 generation with various concentrations of indoxacarb. The field-collected population (PHZ19) was reared on an artificial diet under laboratory conditions for one generation before bioassays. Insecticide concentrations were obtained from freshly prepared stock solution following serial dilutions for tested insecticide. The surfactant, Triton X–100, was used at 0.1% to each serial concentration to achieve a uniform mixture of the insecticide solution in the diet. Six concentrations of tested insecticide were used to determine the LC50 concentration with three replications per concentration. Each serial concentration was thoroughly mixed into the artificial diet before the agar solidified (40–45 °C) using the method developed [17]. After cooling, the diet was cut into small cubes and transferred into new sterile transparent plastic cups (3 cm diameter, 3.5 cm height). The artificial diet without insecticide was used as a standard control treatment. A total of 90 two-day-old second-instar larvae were used per concentration, with three replicates established for each concentration (30 larvae per replicate). Similarly, the control was prepared using 0.1% Triton X–100 in distilled water. Six concentrations of indoxacarb (0.125–400 μg mL−1 of diet) were used for the Ind-UNSEL and Ind-SEL strains. All were kept in a climate control chamber, as described above. Mortality was evaluated 72 h after exposure to indoxacarb. Larvae that did not move after being touched with a fine paintbrush were deemed dead.
2.3. Protocol for Indoxacarb Resistance Selection
A preliminary bioassay of indoxacarb was performed with the field population (PHZ19) to determine the lethal concentration (LC50) required for the selection of S. frugiperda with indoxacarb so that sufficient survivors were left for the next generation. Second instar larvae (one day old) were selected to be exposed continuously to lethal concentrations (LC50) of indoxacarb insecticide from G1 to G24 (Table 1). The diet incorporation method was used for the selection of bioassays. For each selection, an indoxacarb-treated diet was cut into small pieces (1 g) and kept in Petri dishes. The treated population in Petri dishes was placed in the laboratory under the conditions described above. Mortality data were taken after 72 h exposure to indoxacarb in each selection. After 72 h post-exposure, larvae that presented a survival rate of more than 50% were considered positive for resistance. Survivors of each selection were reared on a diet without exposure to indoxacarb to obtain the next progeny for subsequent indoxacarb selection.

Table 1.
LC50 values with a resistance ratio of laboratory selection of S. frugiperda with indoxacarb in field-collected strains after selection in various generations (G).
2.4. Inheritance of Resistance
In order to assess the inheritance of resistance to indoxacarb in S. frugiperda, one-day-old second instar larvae from Ind-UNSEL and reciprocal crosses were used in a diet-incorporation method in small transparent Petri-dishes. Six to seven serial concentrations of indoxacarb were prepared in distilled water and thoroughly mixed in a freshly prepared artificial diet as described above. The surfactant, Triton X–100 (www.biofroxx.com) accessed on 14 March 2021, was used at 0.1% for each serial concentration to achieve a uniform mixture of the insecticide solution in the diet. The control was prepared using 0.1% Triton X–100 in distilled water. After cooling the diet, 90 s-instar larvae (one day old) per concentration with triplicate (30 larvae in each replicate) were transferred into each Petri Plate and shifted in a climatic chamber at standard conditions. 72 h after exposure to insecticide, the larval mortality was assessed. When stroked with a delicate paintbrush, larvae that did not move were assumed to be dead. Resistance ratios were calculated by dividing the LC50 values of the Ind-SEL or reciprocal cross by the corresponding parameter for the Ind-UNSEL strain, as described by [47]
2.5. The Degree of Dominance (D)
In order to determine the dominance of resistance, individual pupa from the Ind-SEL and Ind-UNSEL population was placed in transparent cups (50 mL). A reciprocal cross between Ind-SEL♂ × Ind-UNSEL ♀ and Ind-SEL♀ × Ind-UNSEL♂ populations were made (20 couples per cross) after adult emergence. The F1 offspring from each reciprocal cross was maintained on an artificial diet [48]. Second-instar larvae (one day old) larvae from Ind-SEL, Ind-UNSEL, and reciprocal crosses were then exposed to the concentration-response bioassays as described above. The degree of dominance (D) of indoxacarb resistance was estimated using the equation defined by [49]
where MRR, MSS, and MRS are the mortalities level of Ind-UNSEL, Ind-SEL, and reciprocal crosses, respectively, in different concentrations of indoxacarb. DML levels near 0 indicate effective recessive resistance, whereas values close to 1 indicate effective dominant resistance, and intermediate values imply effectively incomplete resistance.
DML = (MLRS-MLSS)/(MLRR-MLSS)
2.6. Cross-Resistance of Indoxacarb to Insecticides in S. frugiperda
Late second-instar larvae from the Ind-SEL and Ind-UNSEL strains were treated with several groups of pesticides using the diet-incorporation technique, as previously mentioned, to assess patterns of cross-resistance. The different groups of insecticides indoxacarb 15%, deltamethrin 25EC, chlorfenapyr 10%, cholorantraniliprole 5%, methoxyfenozide 240 SC, spinosad 5%, and emamectin benzoate 2% were tested. Concentration–mortality data were submitted to the same procedures described above in toxicity bioassays.
2.7. Synergism Bioassay
Bioassays with piperonyl butoxide (PBO), triphenyl phosphate (TPP), and diethyl maleate (DEM) as synergists (Aladdin Bio-chem Technology Co., Ltd., Shanghai, China) were carried out to assess the possible metabolic resistance to indoxacarb in S. frugiperda using the method described [17]. The stock solutions at the concentration of PBO; 50 mg/L, TPP; 50 mg/L, and DEM; 100 mg/L, were diluted in acetone (99.5% purity), respectively. Larvae were then treated with 1 μL of acetone/PBO solution as above; PBO (50 μg μL−1) using a hand applicator. The larvae were put into a transparent plastic cup with an indoxacarb-treated diet at various concentrations and a control diet that was subjected to 1 μL acetone alone for 72 h after exposure with synergists and without synergists for 2 h. All treatments were kept at a constant condition, as explained above. After 72 h exposure to the test insecticide, the larval mortality was evaluated.
2.8. Fitness Cost
In order to evaluate the fitness costs associated with indoxacarb resistance in S. frugiperda, 50 eggs were selected from Ind-SEL, Ind-UNSEL, and reciprocal crosses (Indo-SE♂ × Ind-UNSEL♀ and Ind-SEL♀ × Ind-UNSEL♂) and hatched neonates were transferred separately into small transparent plastic cups containing an artificial diet. All life history parameters such as developmental period of eggs, larval and pupae, adults (male and female) life span, and daily observations were used to calculate total fecundity (eggs per female), oviposition period, total developmental duration and survival (egg to adult), oviposition duration. There were 20 couples per treatment maintained in plastic mating cages that were 23 cm height by 10 cm wide, internally ornamented with the white paper that served as the oviposition substrate and covered at the top with a lid to measure the female fecundity. All Adults pairs were provided with a 10% honey solution as food.
The life-history data of Ind-SEL, Ind-UNSEL, and reciprocal crosses (Indo-SE♂ × Ind-UNSEL♀ and Ind-SEL♀ × Ind-UNSEL♂) were subjected to the computer-based program software (TWOSEX-MSChart) [50] and analyzed using age-stage two-sex life table theory [51,52]. The life table parameters such as age-stage survival rate (sxj), age-specific survival rate (lx), age-specific fecundity (mx), age-stage life expectancy (exj), and age-stage reproductive value (vxj), respectively, were estimated (where x is the age and j are the stages of insect). The population parameters, including the intrinsic rate of increase (r), finite rate of increase (λ), net reproductive rate (R0), and mean generation time (T), was also estimated. The life expectancy (exj) and the reproductive value (vxj) were determined according to [53,54,55]. The standard bootstrap method was used with 100,000 resampling to calculate the variance as well as standard errors for biological and population growth parameters [56,57] Paired-bootstrap-test at a 5% significance level based on the confidence interval of differences was applied to analyze differences among treatments using TWOSEX-MSChart computer program.
2.9. Statistical Analysis
Toxicity bioassay data were used to calculate the lethal (LC50) concentrations of indoxacarb by using Probit analysis [58] in PoloPlus 2.0 [59]. Data related to the life table was analyzed using the TWOSEX-MSChart computer program. All life table-related graphs were created using Sigma plot 12.5.
3. Results
3.1. Selection of S. frugiperda Resistant to Indoxacarb
The LC50 value of indoxacarb against second-instar larvae of the field-collected population of S. frugiperda was 0.674 μg mL−1 (Table 1), and with selection, the LC50 value of Ind-SEL from generation G2 to G24 increased from 0.787 to 201.83 μg mL−1. Our Ind-UNSEL population had an LC50 of 0.427 μg mL−1 indicating S. frugiperda showed a maximum resistance ratio (RR) to indoxacarb of 299.45-fold after 24 generations (Table 1).
3.2. Genetic Inheritance of Resistance to Indoxacarb
In resistance characterization studies, the indoxacarb concentrations needed for the assessment of LC50 values varied among Ind-SEL, Ind-UNSEL, and reciprocal strain crosses (Table 2). The resistance ratio of indoxacarb in the reciprocal cross, Indo-SE♂ × Ind-UNSEL♀ and Ind-SEL♀ × Ind-UNSEL♂ were 7.072- and 6.42-fold as compared with Ind-UNSEL. LC50 of reciprocal crosses were significantly different due to the overlapping of 95% CIs, demonstrating that indoxacarb resistance was not autosomal. Rather sex linkage or maternal effects were present in the tested S. frugiperda populations.

Table 2.
Concentration mortality (LC; μg mL−1) response of S. frugiperda strains and crosses to the insecticide indoxacarb after 24 generations of selection.
3.3. Dominance of Resistance
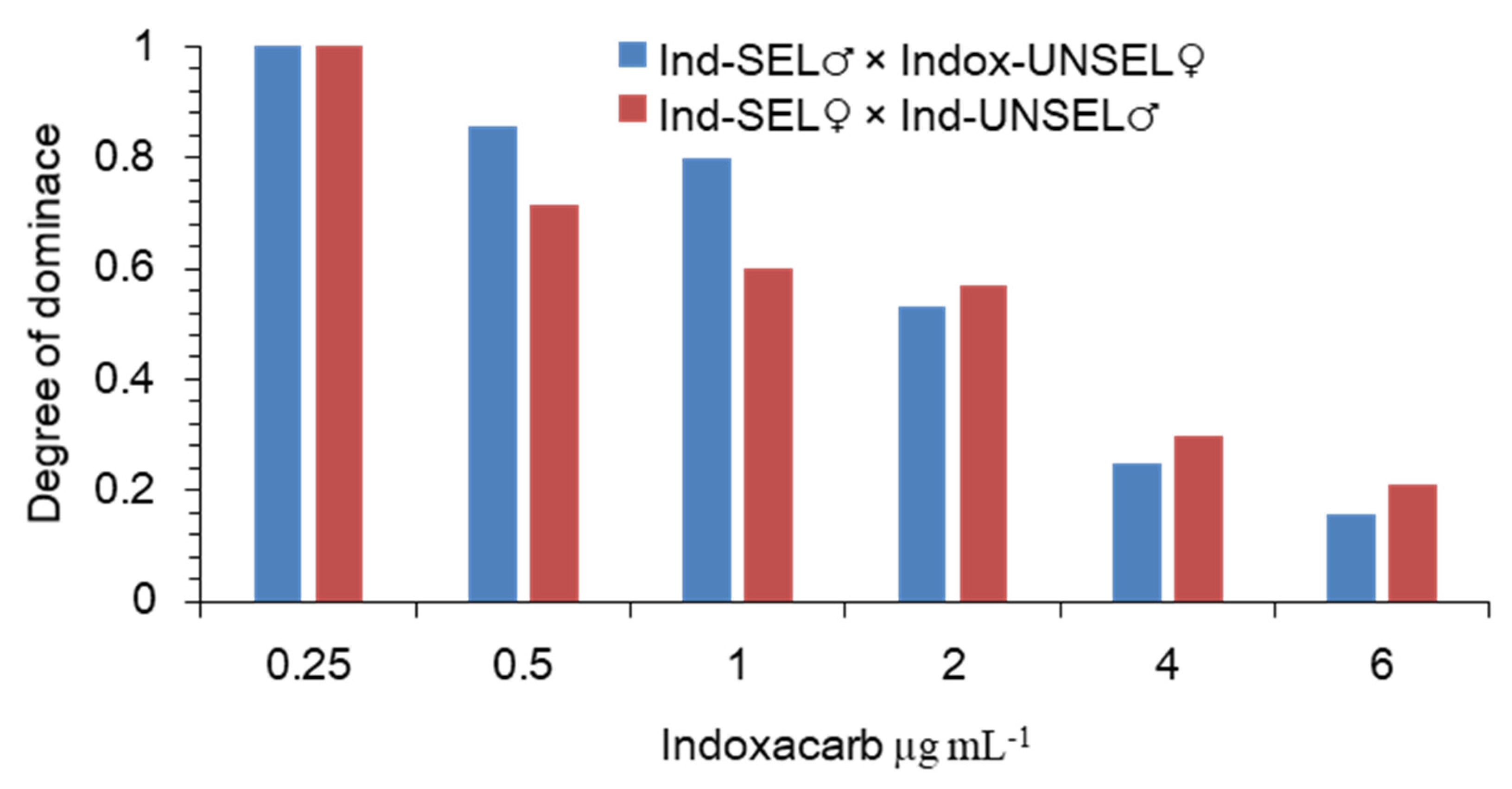
The degree of the dominance was calculated following the method of Bourguet et al. (2000) showed that the dominance values decreased as the indoxacarb concentrations increased. However, higher levels of dominance occurred at lower concentrations. The level of dominance was lower than 0.5 at the concentration of 3 μg-mL−1, which indicated an incompletely recessive dominance when the S. frugiperda larvae were exposed to this insecticide (Figure 1). Resistance can be defined as a dominant trait when (DML = 1). Using the method explained by the degree of dominance decreased with increasing concentration of indoxacarb, supporting an incompletely recessive inheritance at the higher concentrations (Figure 1).
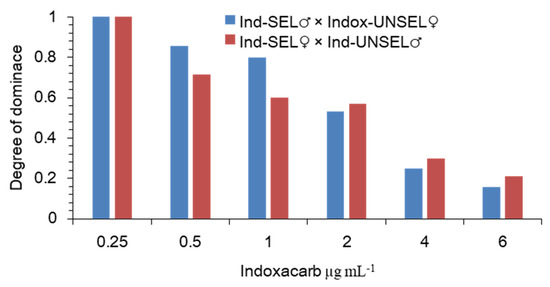
Figure 1.
Degree of the dominance of resistance to indoxacarb in S. frugiperda as a function of indoxacarb concentration.
3.4. Susceptibility of and Cross-Resistance of Ind-SEL and Ind-UNSEL Genotypes of S. frugiperda to Different Insecticides
The bioassays with deltamethrin, chlorfenapyr, cholorantraniliprole, methoxyfenozide, spinosad, and emamectin benzoate performed on the Ind-SEL population (G24) showed that the selection of S. frugiperda with indoxacarb induced very low cross-resistance to chlorfenapyr (RR = 3.24-fold; LC50 = 3.92 µg mL−1) and cholorantraniliprole (RR = 1.89-fold; LC50 = 1.09 µg mL−1), methoxyfenozide (RR = 1.59-fold; LC50 = 2.32 µg mL−1), spinosad (RR = 2.65-fold; LC50 = 1.27 µg mL−1) and emamectin benzoate (RR = 1.98-fold; LC50 = 0.89 µg mL−1) but a high level of cross-resistance to deltamethrin (RR = 31.23-fold; LC50 = 25.3 µg mL−1) when compared to Ind-UNSEL population (Table 3).

Table 3.
Susceptibility and cross-resistance of Ind-SEL and Ind-SEL genotypes of S. frugiperda to different insecticides.
3.5. Synergism of PBO, TPP and DEM
The synergism of PBO, TPP, and DEM was tested on indoxacarb in Ind-SEL (G24) and Ind-UNSEL (G24) (Table 4). Two synergists, PBO, significantly synergized the toxicity of indoxacarb in Ind-SEL (95% CI did not overlap) with resistance ratios of 3.22-fold, respectively. In contrast to this, DEM and TPP did not show any significant synergistic effect in Ind-SEL (95% CI overlap) (Table 4). These results suggest the two detoxification enzymes, mono-oxygenases, might play an important role in detoxifying indoxacarb and the development of resistance in S. frugiperda.

Table 4.
Concentration–mortality response of S. frugiperda larvae exposed to indoxacarb and synergists.
3.6. Fitness Costs with Distant-Related Genetic Backgrounds of S. frugiperda Strains
The development time for the egg stage (3.0 ± 0.0 d) did not differ significantly among strains, but there were significant differences among different larval stages with extended developmental time observed in the Ind-SEL strain. The total larval developmental time of the Ind-SEL strain was approximately (23.34 ± 0.33 d) as compared to the Ind-SEL♂ × Ind-UNSEL♀ (21.81 ± 0.18 d), Ind-SEL♀ × Ind-UNSEL♂ (22.32 ± 0.13 d) and the Ind-UNSEL (19.77 ± 0.13 d) (Table 5). The pupal developmental duration of the Ind-SEL strain (9.26 ± 0.66 d) was significantly higher as compared to the Ind-SEL♀ × Ind-UNSEL♂ (8.19 ± 0.66 d) and Ind-UNSEL strain (7.5 ± 0.08 d) respectively (Table 5).

Table 5.
Pre-Adults developmental time (Mean ± SE) of S. frugiperda
Longevity differed for female and male adults, with shorter longevity of female adults in the Ind-SEL (11.58 ± 0.58 d) and Ind-SEL♂ × Ind-UNSEL♀ (11.25 ± 0.3 d) as compared to Ind-SEL♀ + Ind-UNSEL♂ (12.6 ± 0.17 d) and Ind-UNSEL (12.91 ± 0.28 d) while no difference was noted between and Ind-SEL and Ind-SEL♂ × Ind-UNSEL♀. Whereas male adult longevity was significantly shorter in Ind-SEL♀ × Ind-UNSEL♂ (7.63 ± 0.99 d) as compared to other strains (Table 6). Similarly, significantly shorter longevity of adults was noted for Ind-SEL (9.58 ± 0.7 d) and the Ind-SEL♂ × Ind-UNSEL♀ (9.51 ± 0.54 d) as compared to the Ind-SEL♀ × Ind-UNSEL♂ and Ind-UNSEL while no significant difference was observed between Ind-SEL and Ind-SEL♂ × Ind-UNSEL♀ respectively (Table 6). The total pre-oviposition period (TPOP) was comparatively extended in the Ind-SEL strain (37.45 ± 0.56 d) followed by Ind-SEL♀ × Ind-UNSEL♂ (35.45 ± 0.37 d) and the Ind-SEL♂ × Ind-UNSEL♀ (33.75 ± 0.57 d) as compared to Ind-UNSEL (30.74 ± 0.18 d) (Table 6). A similar trend was noted for mean generation time in all strains. The total number of eggs produced per female was significant differences among strains. A significantly lower number of eggs per female was observed in the Ind-SEL (612.92 ± 68.02) and the Ind-SEL♂ × Ind-UNSEL♀ (732.0 ± 53.42) strain as compared to Ind-SEL♀ × Ind-UNSEL♂ and Ind-UNSEL (Table 6). The population parameters indicated significant differences among strains. The intrinsic rate of increase (r), net reproductive rate (R0), and Finite rate of population increase were significantly lowered in the Ind-SEL strain, followed by Ind-SEL♂ × Ind-UNSEL♀ and Ind-SEL♀ × Ind-UNSEL♂ as compared to Ind-UNSEL. The mean length of a generation (T) was significantly higher for the Ind-SEL strain (T = 40.65 ± 0.57 d) followed by Ind-SEL♀ × Ind-UNSEL♂ (38.61 ± 0.32 d) as compared to Ind-UNSEL (34.1 ± 0.23 d) and there was no statistical difference between Ind-SEL and Ind-SEL♀ × Ind-UNSEL♂ strains (Table 7).

Table 6.
Adult longevity (d), APOP, TPOP, Ovi-day, Fecundity, and MGT (Mean ± SE) of S. frugiperda.

Table 7.
Mean generation time, Net reproductive rate, intrinsic rate of increase, and finite rate of increase in S. frugiperda.
3.7. Survival Rate of S. frugiperda Calculated by Two-Sex Life Table Analysis
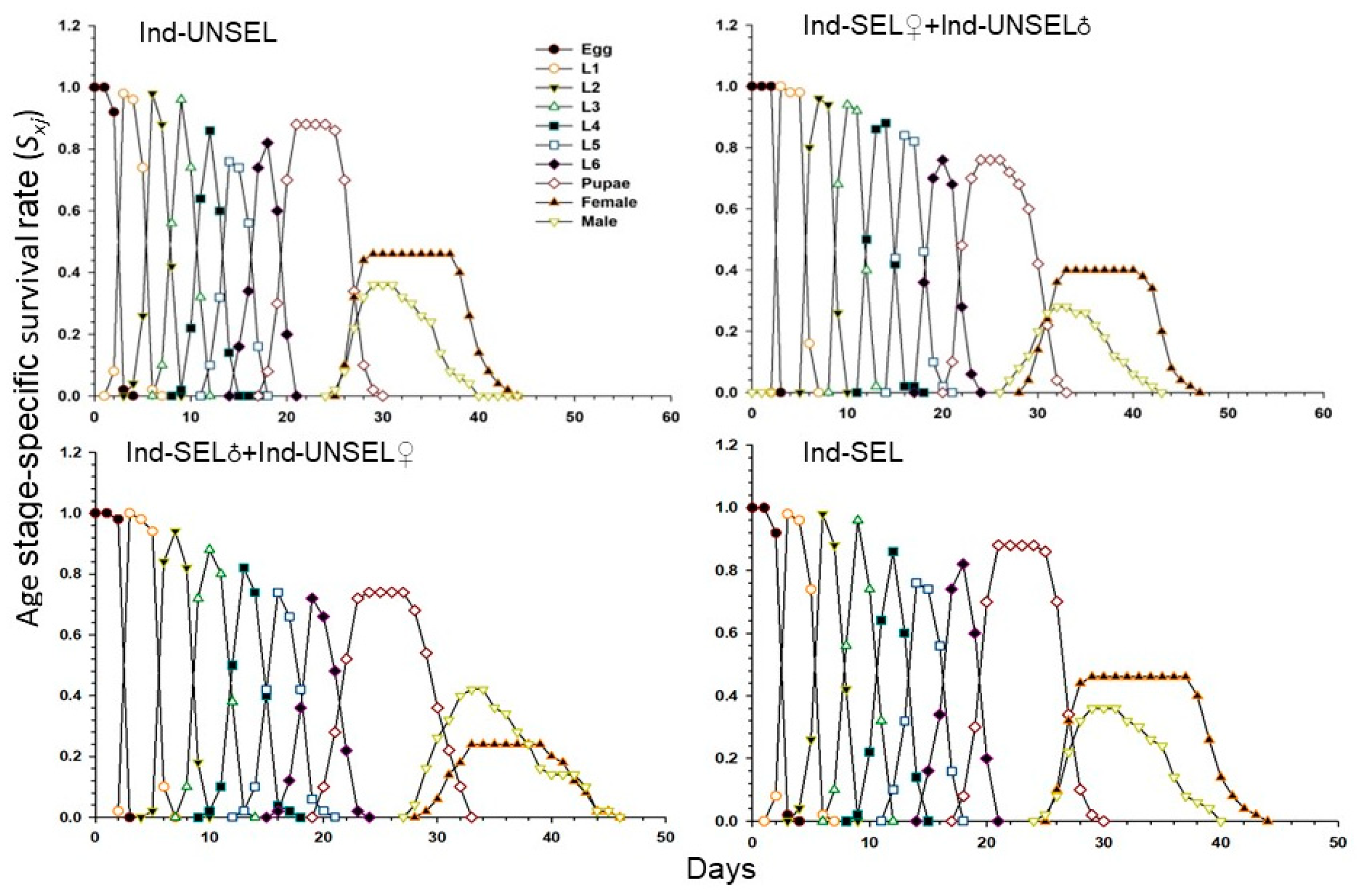
Survival rate (Sxj) of the Ind-UNSEL, Ind-SEL♀ × Ind-UNSEL♂, Ind-SEL♂ × Ind-UNSEL♀, and Ind-SEL are shown in (Figure 2). The values differed significantly across the different developmental stages, suggesting that the growth rates differed among the individuals. The survival curves of different age stages of S. frugiperda larvae overlap, and larvae completed development at 21 days in Ind-SEL and Ind-UNSEL, compared with the Ind-SEL♀ × Ind-UNSEL♂ (24 days) and Ind-SEL♂ × Ind-UNSEL♀ (23 days) strains (Figure 2). However, there was shorter survival of adults in the Ind-UNSEL and Ind-SEL♀ x Ind-UNSEL♂ (20 days for both) as compared to the Ind-SEL♂ × Ind-UNSEL♀ and Ind-SEL. Similarly, the age stage-survival rate of males and females of S. frugiperda from egg to adult were in Ind-UNSEL (0.27 and 0.45), Ind-SEL♀ × Ind-UNSEL♂ (0.24 and 0.4), Ind-SEL♂ × Ind-UNSEL♀ (0.4 and 0.22) and Ind-SEL (0.42 and 0.28) respectively, (Figure 2).
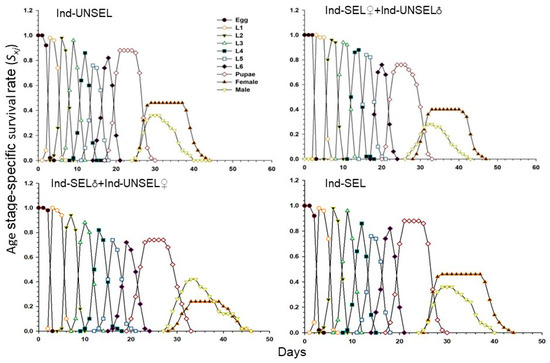
Figure 2.
Age-stage specific survival rate (sxj) of Ind-UNSEL, Ind-SEL♀ × Ind-UNSEL♂, Ind-SEL♂ × Ind-UNSEL♀ and Ind-SEL population of S. frugiperda.
3.8. Population Survival Rate and Fecundity of S. frugiperda
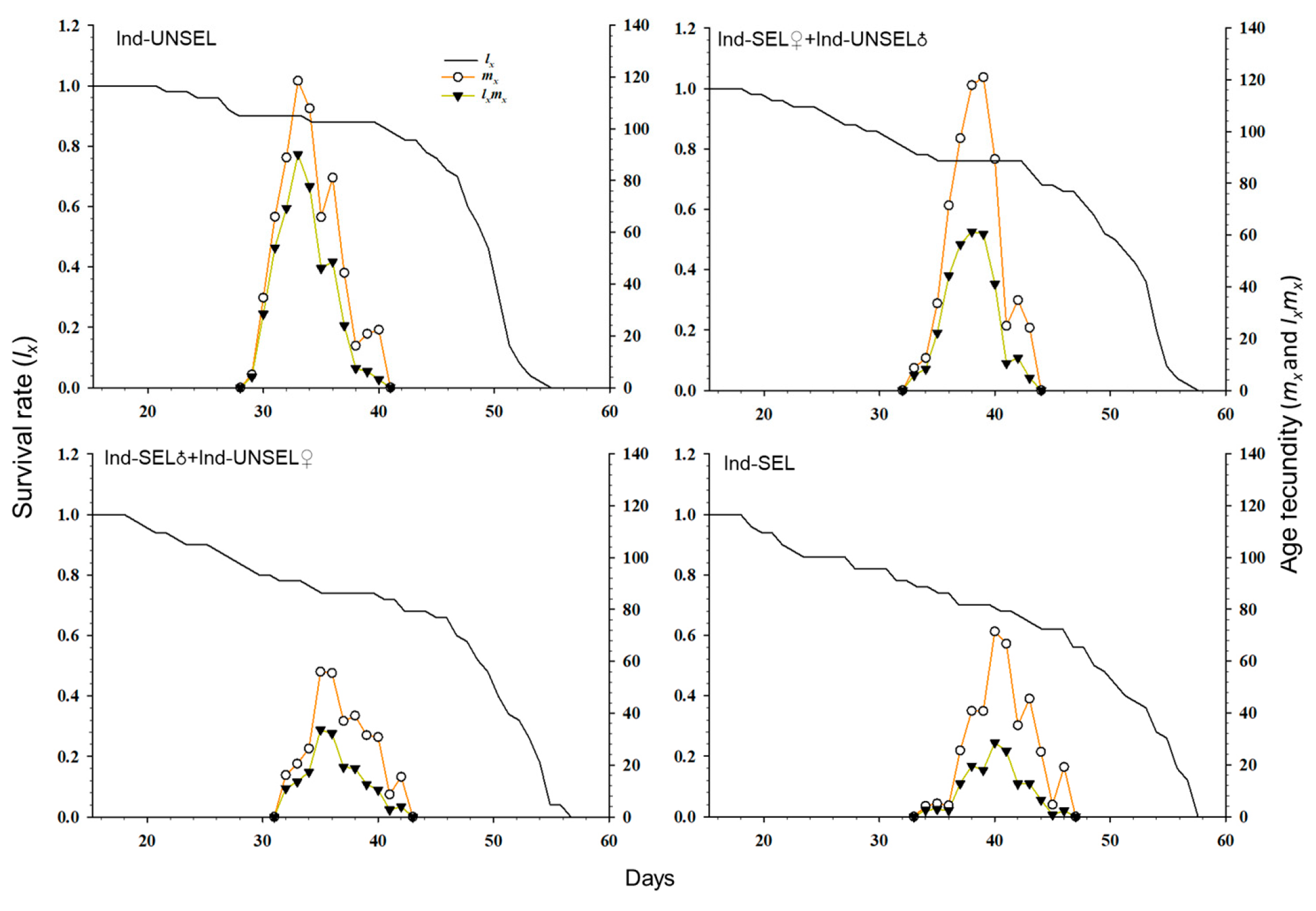
The age-specific survival rate (sxj) and fecundity of S. frugiperda (Figure 3): lx and lxmx on Ind-SEL (56 days 125 days) showed a downward trend as compared to Ind-UNSEL (55 days, 200 days). Thus, the results indicated that the selection pressure of insecticide was not in favor of the development and reproduction of S. frugiperda. Furthermore, the deviations in the fecundity curve of S. frugiperda were advocated that the emergence and oviposition did not happen at specific ages and times, respectively (Figure 3).
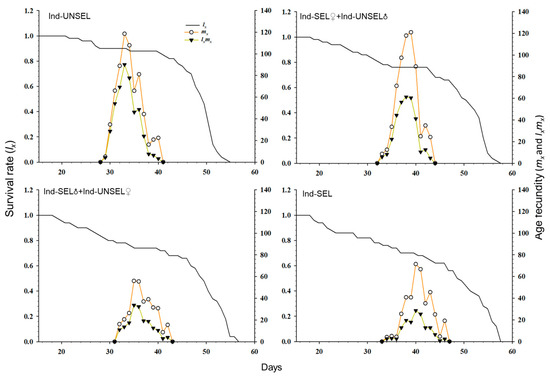
Figure 3.
Age-specific survival rate (lx), age-specific fecundity (mx), and net maternity (lxmx) of Ind-UNSEL, Ind-SEL♀ × Ind-UNSEL♂, Ind-SEL♂ × Ind-UNSEL♀ and Ind-SEL population of S. frugiperda.
3.9. Reproduction Value and Life Expectancy of S. frugiperda
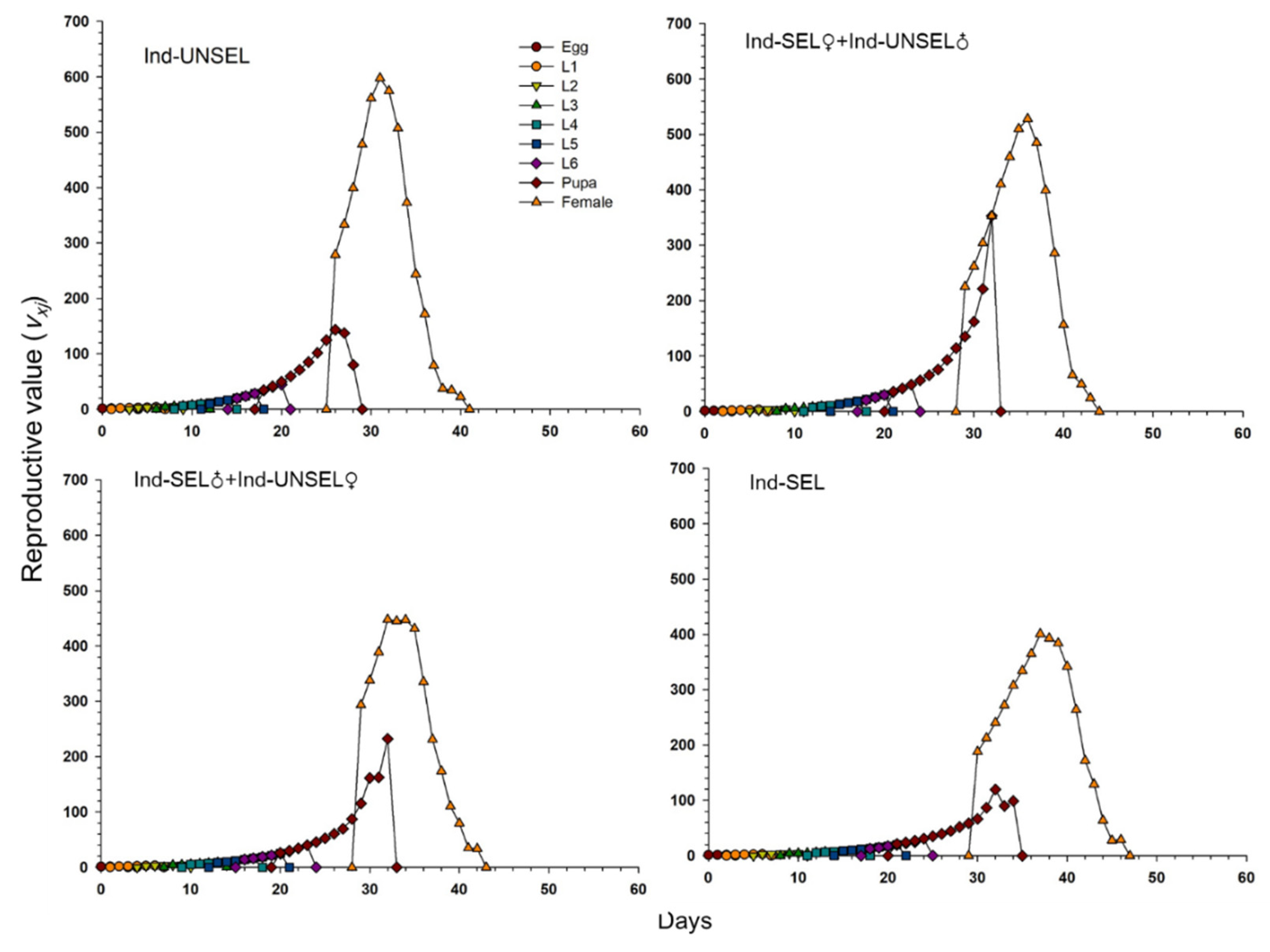
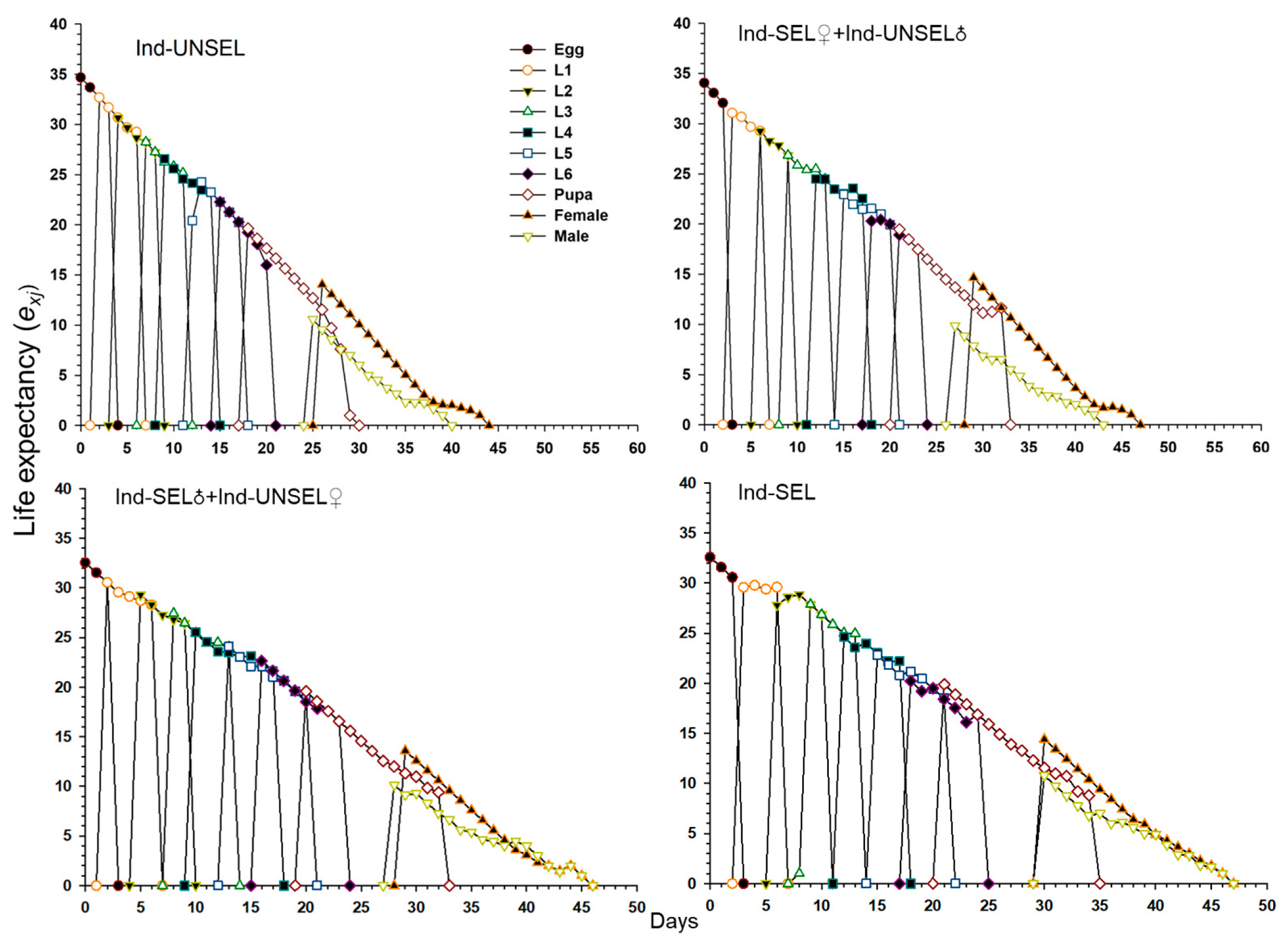
Significantly lower reproductive value (vxj) of S. frugiperda on Ind-UNSEL (1.197), Ind-SEL♀ × Ind-UNSEL♂ (1.162), Ind-SEL♂ × Ind-UNSEL♀ (1.151) and Ind-SEL (51.13) at age zero (v0, 1), respectively, which were close to λ (Figure 4). The peak value of the vxj curve of all strains exhibited an upward with increasing trend age and developmental stage, with the maximum value at 31 days on Ind-SEL (609.7) and at 36 days on Ind-SEL♀ × Ind-UNSEL♂ (539.769) as compared to other strains (Figure 4). Furthermore, the life expectancy value (exj) of S. frugiperda on all strains indicated a decreasing trend, with significantly highest average longevity values on Ind-UNSEL (35 days), Ind-SEL♀ × Ind-UNSEL♂ (34 days), Ind-SEL♂ × Ind-UNSEL♀ (33 days) and Ind-SEL of (32 days), respectively (Figure 5). The exj value of S. frugiperda was lower on Ind-SEL and Ind-SEL♂ × Ind-UNSEL♀ than on Ind-SEL♀ × Ind-UNSEL♂ and Ind-UNSEL in the first 9 days, but the trend was reversed afterward, representing that S. frugiperda developed more slowly on Ind-SEL and Ind-SEL♂ × Ind-UNSEL♀ (Figure 5).
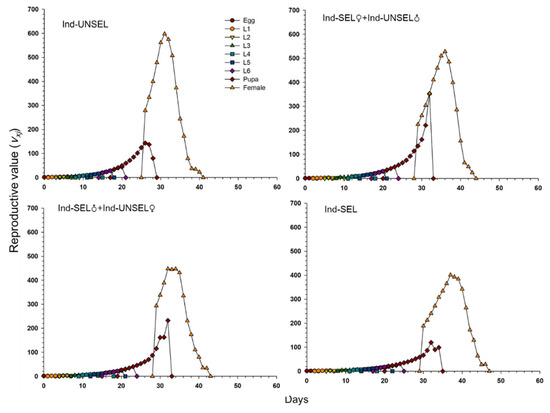
Figure 4.
Age-stage specific life expectancy (exj) of Ind-UNSEL, Ind-SEL♀ × Ind-UNSEL♂, Ind-SEL♂ × Ind-UNSEL♀ and Ind-SEL population of S. frugiperda.
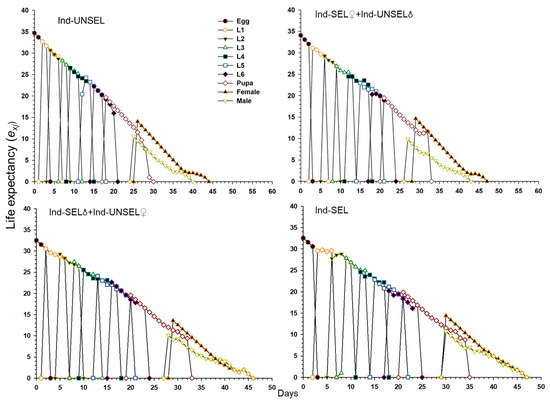
Figure 5.
Age-Stage specific reproductive value (vxj) of Ind-UNSEL, Ind-SEL♀ × Ind-UNSEL♂, Ind-SEL♂ × Ind-UNSEL♀ and Ind-SEL population of S. frugiperda.
4. Discussion
The selection pressure induced by the indiscriminate use of pesticides has resulted in the dramatic evolution of insecticide resistance in insect pests. Indoxacarb belongs to the novel oxadiazine group with wide-spectrum insecticidal activity against several lepidopteran species as well as some other groups of insect pests such as homopteran and coleopteran and has a low toxicity profile for non-target organisms [24,60]. Numerous insect species have been used to investigate the mechanisms underlying indoxacarb resistance. Two mutations (F1845Y and V1848I) have been found in indoxacarb-resistant populations of two pest species, Plutella xylostella [61] and Tuta absoluta [38]. Gao et al. [32] identified one point mutation (L1014F) in indoxacarb selected strain of S. exigua, whether L1014F mutation in S. exigua is associated with indoxacarb resistance or not the functional verification is needed by gene editing or electrophysiology. Similarly, Samantsidis et al. (2019) [62] and Wang et al. (2022) [63] found that only F1845Y and V1848I mutations had been proven to confer resistance to indoxacarb in Plutella xylostella and Drosophila. In our study, we showed a significant selection response to indoxacarb in a field-collected population of S. frugiperda. Following continuous laboratory selection for 24 generations, a field-collected population Ind-SEL of S. frugiperda exhibited a very high level of resistance (472.67-fold) to indoxacarb as compared to the Ind-UNSEL population
It is possible that there was a high frequency of resistance allele in this field-collected population as it was collected from a region where insecticides were used extensively to manage various maize pests. Similarly, Muraro et al. (2021) [46] evaluated the evolution of resistance to emamectin benzoate in the field-collected population S. frugiperda, and after 10 generations of continuous selection to pesticide, the resistance ratio increased ∼2283.44-fold. In previous studies, high resistance levels to indoxacarb have been reported in various lepidopteran insects after continuous selection pressure. For example, P. xylostella (2594-fold after six generations of selection) [64], S. exigua (240-fold after 12 generations) [32], H. armigera (1239-fold after eight generations) [65], and S. litura (95-fold after three generations) [66]. In contrast, comparatively low level of indoxacarb resistance has been documented in H. virescens (55-fold after six generations) [67], H. armigera (4.43-fold after 11 selected generations) [68] and P. xylostella (31.3-fold after ten selected generations) [69]. These disparities may be due to differences in species’ geographical origin or to the effects of initial sampling.
The level of dominance resistance in the field depends on several factors, such as the concentration used, the stage of development of the insect, and the environment[42,49]. In the present study, the resistance was characterized as an incompletely recessive trait with polygenic effects when S. frugiperda was exposed to indoxacarb. A similar pattern of resistance was reported when S. litura [66], H. armigera [68], S. exigua [32], and P. solenopsis (Tinsley) were exposed to indoxacarb, respectively [70]. In contrast, dominant and polygenic resistance to indoxacarb was reported in S. litura [66]. Predictions of effective dominance based on laboratory data, however, must be carefully considered because the range of concentrations required to establish dominance may differ between laboratory and field populations, as well as the effect of inducible insecticidal concentration due to chemical degradation [71]. High levels of cross-resistance between insecticides with the same and different modes of action used in a rotation strategy are one of the key problems for the success of IRM programs. In our study, compared to the Ind-UNSEL strain, the Ind-SEL strain of S. frugiperda exhibited obvious cross-resistance to deltamethrin (31.23-fold), low and negligible levels of cross-resistance to chlorfenapyr (3.24-fold), spinosad (2.65-fold), respectively. In previous studies, a low and high level of cross-resistance between indoxacarb, spinosad, flubendiamide, fenvalerate, emamectin benzoate and chlorantraniliprole was found in S. frugiperda, S. exigua, P. xylostella and S. litura [72,73,74,75]. As we know, it has not been reported in other studies for this type of cross-resistance in indoxacarb. It might be that indoxacarb and deltamethrin have the same or cross-molecular targets based on a biochemical mechanism that needs to be investigated.
The oxidative metabolism mediated by cytochrome P450 monooxygenases and the hydrolysis and/or sequestration caused by carboxylesterases is the most frequent mechanisms linked to pesticide resistance in insect pests [76]. Based on the synergistic effects of metabolic inhibitors on indoxacarb toxicity, the involvement of metabolic mechanisms in indoxacarb resistance has been reported in several insect species [34,77]. Present results with synergists showed that the toxicity of indoxacarb against S. frugiperda was increased by PBO, indicating that mono-oxygenases P450 enzyme may be associated with indoxacarb resistance in the Ind-SEL population. In a Malaysian field-derived strain of P. xylostella, high-level (813-fold) resistance to indoxacarb was greatly reduced by PBO or a PBO analog specific for esterases, suggesting that indoxacarb resistance was attributable to improved metabolic detoxification by esterases [78]. Metabolic resistance associated with an increased level of detoxification enzymes, for example, cytochrome P450, carboxy/cholinesterase (CCE), and glutathione S-transferase (GST)) in insecticide-resistant populations have been reported worldwide [79]. In previous studies, it has been reported that P450, carboxylesterase, and GST were involved in the resistance to indoxacarb in M. domestica, P. xylostella but carboxylesterase and GST were the main factors in S. exigua leading to indoxacarb resistance [27,78]. Similar to our study, elevated activity of the metabolic enzyme P450 enzyme conferred indoxacarb resistance in H. armigera and S. litura [68,80]. In a previous study, it was reported that the metabolic inhibitor PBO reduced resistance in the indoxacarb-selected strain, suggesting that metabolic detoxification enzymes were probably involved in indoxacarb resistance in H. armigera [34]. These results represent a first step towards understanding the indoxacarb resistance mechanisms in a selected strain of S. frugiperda.
The decline in biological fitness among individuals in different insect populations during the development of resistance can influence their relative abundance and genetic impact on future generations. Traits such as insecticide resistance are advantageous when under selection, and genotypes conferring these phenotypes can rapidly increase in a population [81]. Resistance-related fitness costs must be assessed in homozygous resistant individuals and heterozygotes that act as carriers of resistant genes in the early stages of resistance [82]. We evaluated fitness costs in two hybrid populations (Ind-SEL♂ × Ind-UNSEL♀ and Ind-SEL♀ × Ind-UNSEL♂) of the Ind-SEL and the Ind-UNSEL Population. We found significantly longer developmental time of larvae, extended pupal duration, shorter adult longevity, and lower fecundity in the Ind-SEL as compared with the other strains and Ind-UNSEL population. The only parameter that differs between Ind-SEL♂ × Ind-UNSEL♀ (175.68 ± 45.88) and Ind-SEL♀ × Ind-UNSEL♂ (328.03 ± 57.22) was the Net reproductive rate (R0). On the other hand, all population growth parameters differ between Ind-SEL and Ind-UNSEL strains. Differences in fitness costs associated with insecticide resistance have been reported in many insect populations, including S. frugiperda, H. armigera, H. virescens, P. xylostella, and O. hyalinipennis [14,66,67,83]. Understanding the occurrence of fitness costs associated with insecticide resistance is essential in developing and implementing IRM programs.
5. Conclusions
S. frugiperda resistance to indoxacarb has been characterized for the first time in this research and provided data to support resistance management strategies. Results demonstrated that S. frugiperda has resistance to indoxacarb and that this can be minimized by rotating this insecticide with chlorantraniliprole, emamectin benzoate, and/or methoxyfenozide due to very low cross-resistance and avoiding rotation with deltamethrin, which has high cross-resistance. Overall, this study highlights the significance of genetics in resistance management strategies and the need for future fitness cost studies to take a more comprehensive approach, as experimental design and criteria may change the results, with significant ramifications for the management of resistant pests in the field.
Author Contributions
Conceptualization, M.H. X.L. and Y.L. (Yaobin Lu); methodology, M.H. and X.L.; software, M.H. F.U.; validation, M.H., Z.Z. and J.Z.; formal analysis, M.H. and J.A.S.; investigation, M.H.; resources, Y.L. (Yaobin Lu) and J.H.; data curation, Y.L.; writing—original draft preparation, M.H. L.C. X.R. and S.Z.; writing—review and editing, M.H. Y.L. (Yaobin Lu) Y.L. (Yonggen Lou) M.A.A. J.A.S. S.Z. and M.P.Z.; visu-alization, M.H. and M.I.; supervision, Y.L. (Yaobin Lu) and Y.L. (Yonggen Lou); project administration, Y.L. (Yaobin Lu); funding acquisition, Y.L. (Yaobin Lu) and Y.L. (Yonggen Lou). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Key R&D Program of Zhejiang Province (2020C02003), the Shanghai Innovation Project for Agricultural Promotion (2019N3- 9), and the Joint Agricultural Project between Pinghu County and Zhejiang Academy of Agricultural Sciences (PH20190002). M. A. A. expresses appreciation to the Deanship of Scientific Research at King Khalid University Saudi Arabia for funding through the research groups program under grant number R.G.P.2/170/43. Project funded by China National Postdoctoral Science Foundation (2020M681921) and the Primary Research & Development Plan of Lishui (No. 2021ZDYF10).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and analyzed during this study are available from the corresponding author on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pogue, M. A World Revision of the Genus Spodoptera Guenée (Lepidoptera: Noctuidae); American Entomological Society: Philadephia, PA, USA, 2002; Volume 43, pp. 1–102. [Google Scholar]
- Tambo, J.A.; Kansiime, M.K.; Rwomushana, I.; Mugambi, I.; Nunda, W.; Mloza Banda, C.; Nyamutukwa, S.; Makale, F.; Day, R. Impact of fall armyworm invasion on household income and food security in Zimbabwe. Food Energy Secur. 2021, 10, e281. [Google Scholar] [CrossRef]
- Li, X.J.; Wu, M.F.; Ma, J.; Gao, B.Y.; Wu, Q.L.; Chen, A.D.; Liu, J.; Jiang, Y.Y.; Zhai, B.P.; Early, R.; et al. Prediction of migratory routes of the invasive fall armyworm in eastern China using a trajectory analytical approach. Pest Manag. Sci. 2020, 76, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, M.; Li, X.; Ullah, F.; Zhang, Z.; Zhang, J.; Huang, J.; Khan, M.M.; Chen, L.; Ren, X.; Zhou, S.; et al. Behavioral and physiological plasticity provides insights into molecular based adaptation mechanism to strain shift in Spodoptera frugiperda. Int. J. Mol. Sci. 2021, 22, 10284. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.; Das, B.; Ramesh, R. Predicting climate change impacts on potential worldwide distribution of fall armyworm based on CMIP6 projections. J. Pest Sci. 2022, 95, 841–854. [Google Scholar] [CrossRef]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host Plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Pashley, D.P.; Hammond, A.M.; Hardy, T.N. Reproductive Isolating Mechanisms in Fall Armyworm Host Strains (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 1992, 85, 400–405. [Google Scholar] [CrossRef]
- Pashley, D.P. Host-associated Genetic Differentiation in Fall Armyworm (Lepidoptera: Noctuidae): A Sibling Species Complex? Ann. Entomol. Soc. Am. 1986, 79, 898–904. [Google Scholar] [CrossRef]
- Johnson, S.J. Migration and the life history strategy of the fall armyworm, Spodoptera frugiperda in the western hemisphere. Int. J. Trop. Insect Sci. 1987, 8, 543–549. [Google Scholar] [CrossRef]
- Roy, D.; Biswas, S.; Mondal, D.; Majumder, S.; Sarkar, P.K. Efficacy and safety-evaluation of insecticidal modules against Spodoptera frugiperda (Lepidoptera: Noctuidae) and the residues of the most effective schedule in maize. Int. J. Trop. Insect Sci. 2021, 41, 3155–3166. [Google Scholar] [CrossRef]
- Muraro, D.S.; Garlet, C.G.; Godoy, D.N.; Cossa, G.E.; dos Rodrigues, G.L., Jr.; Stacke, R.F.; Medeiros, S.L.P.; Guedes, J.V.C.; Bernardi, O. Laboratory and field survival of Spodoptera frugiperda (Lepidoptera: Noctuidae) on Bt and non-Bt maize and its susceptibility to insecticides. Pest Manag. Sci. 2019, 75, 2202–2210. [Google Scholar] [CrossRef] [PubMed]
- Huang, F. Resistance of the fall armyworm, Spodoptera frugiperda, to transgenic Bacillus thuringiensis Cry1F corn in the Americas: Lessons and implications for Bt corn IRM in China. Insect Sci. 2021, 28, 574–589. [Google Scholar] [CrossRef] [PubMed]
- Burtet, L.M.; Bernardi, O.; Melo, A.A.; Pes, M.P.; Strahl, T.T.; Guedes, J.V.C. Managing fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), with Bt maize and insecticides in southern Brazil. Pest Manag. Sci. 2017, 73, 2569–2577. [Google Scholar] [CrossRef]
- Okuma, D.M.; Bernardi, D.; Horikoshi, R.J.; Bernardi, O.; Silva, A.P.; Omoto, C. Inheritance and fitness costs of Spodoptera frugiperda (Lepidoptera: Noctuidae) resistance to spinosad in Brazil. Pest Manag. Sci. 2018, 74, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Bai-Zhong, Z.; Xu, S.; Cong-Ai, Z.; Liu-Yang, L.; Ya-She, L.; Xing, G.; Dong-Mei, C.; Zhang, P.; Ming-Wang, S.; Xi-Ling, C. Silencing of Cytochrome P450 in Spodoptera frugiperda (Lepidoptera: Noctuidae) by RNA Interference Enhances Susceptibility to Chlorantraniliprole. J. Insect Sci. 2020, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Xue, H.; Zhu, X.; Wang, L.; Zhang, K.; Li, D.; Ji, J.; Niu, L.; Gao, X.; Luo, J.; et al. Silencing of cytochrome P450 gene CYP321A1 effects tannin detoxification and metabolism in Spodoptera litura. Int. J. Biol. Macromol. 2022, 194, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; Huang, J.M.; Ni, H.; Guo, D.; Yang, F.X.; Wang, X.; Wu, S.F.; Gao, C.F. Susceptibility of fall armyworm, Spodoptera frugiperda (J.E.Smmith), to eight insecticides in China, with special reference to lambda-cyhalothrin. Pestic. Biochem. Physiol. 2020, 168, 104623. [Google Scholar] [CrossRef]
- Gutirrez-Moreno, R.; Mota-Sanchez, D.; Blanco, C.A.; Whalon, M.E.; Terán-Santofimio, H.; Rodriguez-Maciel, J.C.; Difonzo, C. Field-Evolved Resistance of the Fall Armyworm (Lepidoptera: Noctuidae) to Synthetic Insecticides in Puerto Rico and Mexico. J. Econ. Entomol. 2019, 112, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J. Insecticide resistance in the fall armyworm, Spodoptera frugiperda (J. E. Smith). Pestic. Biochem. Physiol. 1991, 39, 84–91. [Google Scholar] [CrossRef]
- Miranda, G.R.B.; Raetano, C.G.; Silva, E.; Daam, M.A.; Cerejeira, M.J. Environmental fate article: Environmental fate of neonicotinoids and classification of their potential risks to hypogean, epygean, and surface water ecosystems in Brazil. Hum. Ecol. Risk Assess. 2011, 17, 981–995. [Google Scholar] [CrossRef]
- Horikoshi, R.J.; Bernardi, D.; Bernardi, O.; Malaquias, J.B.; Okuma, D.M.; Miraldo, L.L.; De Amaral, F.S.A.E.; Omoto, C. Effective dominance of resistance of Spodoptera frugiperda to Bt maize and cotton varieties: Implications for resistance management. Sci. Rep. 2016, 6, 34864. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Qureshi, J.A.; Meagher, R.L.; Reisig, D.D.; Head, G.P.; Andow, D.A.; Ni, X.; Kerns, D.; Buntin, G.D.; Niu, Y.; et al. Cry1F resistance in fall armyworm Spodoptera frugiperda: Single gene versus pyramided Bt maize. PLoS ONE 2014, 9, e112958. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Kerns, D.L.; Brown, S.; Kurtz, R.; Dennehy, T.; Braxton, B.; Head, G.; Huang, F. Performance and cross-crop resistance of Cry1F-maize selected Spodoptera frugiperda on transgenic Bt cotton: Implications for resistance management. Sci. Rep. 2016, 6, 28059. [Google Scholar] [CrossRef] [PubMed]
- Wing, K.D.; Sacher, M.; Kagaya, Y.; Tsurubuchi, Y.; Mulderig, L.; Connair, M.; Schnee, M. Bioactivation and mode of action of the oxadiazine indoxacarb in insects. Crop Prot. 2000, 19, 537–545. [Google Scholar] [CrossRef]
- Shi, L.; Shi, Y.; Zhang, Y.; Liao, X. A systemic study of indoxacarb resistance in Spodoptera litura revealed complex expression profiles and regulatory mechanism. Sci. Rep. 2019, 9, 14997. [Google Scholar] [CrossRef]
- Wing, K.D.; Andaloro, J.T.; McCann, S.F.; Salgado, V.L. Indoxacarb and the Sodium Channel Blocker Insecticides: Chemistry, Physiology, and Biology in Insects. In Comprehensive Molecular Insect Science; Elsevier: Amsterdam, The Netherlands, 2005; pp. 31–53. ISBN 9780444519245. [Google Scholar]
- Nehare, S.; Moharil, M.P.; Ghodki, B.S.; Lande, G.K.; Bisane, K.D.; Thakare, A.S.; Barkhade, U.P. Biochemical analysis and synergistic suppression of indoxacarb resistance in Plutella xylostella L. J. Asia. Pac. Entomol. 2010, 13, 91–95. [Google Scholar] [CrossRef]
- Alves, A.P.; Allgeier, W.J.; Siegfried, B.D. Effects of the synergist S,S,S-tributyl phosphorotrithioate on indoxacarb toxicity and metabolism in the European corn borer, Ostrinia nubilalis (Hübner). Pestic. Biochem. Physiol. 2008, 90, 26–30. [Google Scholar] [CrossRef]
- Shi, Y.; Li, W.; Zhou, Y.; Liao, X.; Shi, L. Contribution of multiple overexpressed carboxylesterase genes to indoxacarb resistance in Spodoptera litura. Pest Manag. Sci. 2022, 78, 1903–1914. [Google Scholar] [CrossRef] [PubMed]
- Wing, K.D.; Schnee, M.E.; Sacher, M.; Connair, M. A Novel Oxadiazine Insecticide Is Bioactivated in Lepidopteran Larvae. Arch. Insect Biochem. Physiol. 1998, 73, 91–103. [Google Scholar] [CrossRef]
- Shono, T.; Zhang, L.; Scott, J.G. Indoxacarb resistance in the house fly, Musca domestica. Pestic. Biochem. Physiol. 2004, 80, 106–112. [Google Scholar] [CrossRef]
- Gao, M.; Mu, W.; Wang, W.; Zhou, C.; Li, X. Resistance mechanisms and risk assessment regarding indoxacarb in the beet armyworm, Spodoptera exigua. Phytoparasitica 2014, 42, 585–594. [Google Scholar] [CrossRef]
- Wang, X.L.; Su, W.; Zhang, J.H.; Yang, Y.H.; Dong, K.; Wu, Y.D. Two novel sodium channel mutations associated with resistance to indoxacarb and metaflumizone in the diamondback moth, Plutella xylostella. Insect Sci. 2016, 23, 50–58. [Google Scholar] [CrossRef]
- Bird, L.J. Genetics, cross-resistance and synergism of indoxacarb resistance in Helicoverpa armigera (Lepidoptera: Noctuidae). Pest Manag. Sci. 2017, 73, 575–581. [Google Scholar] [CrossRef]
- Omoto, C.; Bernardi, O.; Salmeron, E.; Sorgatto, R.J.; Dourado, P.M.; Crivellari, A.; Carvalho, R.A.; Willse, A.; Martinelli, S.; Head, G.P. Field-evolved resistance to Cry1Ab maize by Spodoptera frugiperda in Brazil. Pest Manag. Sci. 2016, 72, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, X.; Shen, J.; Li, D.; Wan, H.; You, H.; Li, J. Cross-resistance and biochemical mechanisms of resistance to indoxacarb in the diamondback moth, Plutella xylostella. Pestic. Biochem. Physiol. 2017, 140, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Roditakis, E.; Mavridis, K.; Riga, M.; Vasakis, E.; Morou, E.; Rison, J.L.; Vontas, J. Identification and detection of indoxacarb resistance mutations in the para sodium channel of the tomato leafminer, Tuta absoluta. Pest Manag. Sci. 2017, 73, 1679–1688. [Google Scholar] [CrossRef]
- Shah, R.M.; Abbas, N.; Shad, S.A.; Sial, A.A. Selection, resistance risk assessment, and reversion toward susceptibility of pyriproxyfen in Musca domestica L. Parasitol. Res. 2015, 114, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Sayyed, A.H.; Omar, D.; Wright, D.J. Genetics of spinosad resistance in a multi-resistant field-selected population of Plutella xylostella. Pest Manag. Sci. 2004, 60, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Firko, M.J.; Hayes, J.L. Quantitative genetic tools for insecticide resistance risk assessment: Estimating the heritability of resistance. J. Econ. Entomol. 1990, 83, 647–654. [Google Scholar] [CrossRef]
- Roush, R.T.; McKenzie, J.A. Ecological genetics of insecticide and acaricide resistance. Annu. Rev. Entomol. 1987, 32, 361–380. [Google Scholar] [CrossRef]
- Georghiou, G.P.; Taylor, C.E. Genetic and biological influences in the evolution of insecticide resistance. J. Econ. Entomol. 1977, 70, 319–323. [Google Scholar] [CrossRef]
- Carrière, Y.; Ellers-Kirk, C.; Biggs, R.; Higginson, D.M.; Dennehy, T.J.; Tabashnik, B.E. Effects of gossypol on fitness costs associated with resistance to Bt cotton in pink bollworm. J. Econ. Entomol. 2004, 97, 1710–1718. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, M.; Liu, S.; Jan, S.; Ali, B.; Shahid, M.; Fernández-Grandon, G.M.; Nawaz, M.; Ahmad, A.; Wang, M. Gossypol-induced fitness gain and increased resistance to deltamethrin in beet armyworm, Spodoptera exigua (Hübner). Pest Manag. Sci. 2019, 75, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Carrière, Y.; Tabashnik, B.E. Reversing insect adaptation to transgenic insecticidal plants. Proc. R. Soc. B Biol. Sci. 2001, 268, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Muraro, D.S.; de Oliveira Abbade Neto, D.; Kanno, R.H.; Kaiser, I.S.; Bernardi, O.; Omoto, C. Inheritance patterns, cross-resistance and synergism in Spodoptera frugiperda (Lepidoptera: Noctuidae) resistant to emamectin benzoate. Pest Manag. Sci. 2021, 77, 5049–5057. [Google Scholar] [CrossRef]
- Robertson, J.L.; Preisler, H.K. Pesticide Bioassays with Arthropods; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Lira, E.C.; Bolzan, A.; Nascimento, A.R.B.; Amaral, F.S.A.; Kanno, R.H.; Kaiser, I.S.; Omoto, C. Resistance of Spodoptera frugiperda (Lepidoptera: Noctuidae) to spinetoram: Inheritance and cross-resistance to spinosad. Pest Manag. Sci. 2020, 76, 2674–2680. [Google Scholar] [CrossRef]
- Bourguet, D.; Genissel, A.; Raymond, M. Insecticide resistance and dominance levels. J. Econ. Entomol. 2000, 93, 1588–1595. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis; National Chung Hsing University: Taichung, Taiwan, 2022; Available online: http://140.120.197.173/Ecology/Download/Twosex-MsChart.rar (accessed on 14 March 2021).
- Chi, H. Life-Table Analysis Incorporating Both Sexes and Variable Development Rates Among Individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H.; Su, H.Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Chi, H.; You, M.; Atlıhan, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.-J.; Fu, J.-W.; Xu, Y.-Y.; et al. Age-Stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 2, 103–124. [Google Scholar] [CrossRef]
- Tuan, S.J.; Lee, C.C.; Chi, H. Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Manag. Sci. 2014, 70, 805–813. [Google Scholar] [CrossRef]
- Akköprü, E.P.; Atlihan, R.; Okut, H.; Chi, H. Demographic assessment of plant cultivar resistance to insect pests: A case study of the dusky-veined walnut aphid (Hemiptera: Callaphididae) on five walnut cultivars. J. Econ. Entomol. 2015, 108, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap. In Monographs on Statistics and Applied Probability; Chapman and Hall: London, UK, 1993; Volume 57, p. 436. ISBN 0412042312. [Google Scholar]
- Finney, D.J. Probit Analysis; Cambridge University Press: New York, NY, USA, 1971; Volume 60. [Google Scholar]
- LeOra Software LLC. LeOra Software; LeOra Software: Petaluma, CA, USA, 2005. [Google Scholar]
- Monteiro, H.R.; Pestana, J.L.T.; Novais, S.C.; Soares, A.M.V.M.; Lemos, M.F.L. Toxicity of the insecticides spinosad and indoxacarb to the non-target aquatic midge Chironomus riparius. Sci. Total Environ. 2019, 666, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, Q.; Hao, Q.; Ran, S.; Wu, Y.; Cui, P.; Yang, J.; Jiang, C.; Yang, Q. Insecticide resistance and enhanced cytochrome P450 monooxygenase activity in field populations of Spodoptera litura from Sichuan, China. Crop Prot. 2018, 106, 110–116. [Google Scholar] [CrossRef]
- Samantsidis, G.R.; O’Reilly, A.O.; Douris, V.; Vontas, J. Functional validation of target-site resistance mutations against sodium channel blocker insecticides (SCBIs) via molecular modeling and genome engineering in Drosophila. Insect Biochem. Mol. Biol. 2019, 104, 73–81. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Yang, Y.; Wu, Y. Equivalent intensity but differential dominance of sodium channel blocker insecticide resistance conferred by F1845Y and V1848I mutations of the voltage-gated sodium channel in Plutella xylostella. Insect Sci. 2022, 1–10. [Google Scholar] [CrossRef]
- Sayyed, A.H.; Wright, D.J. Genetics and evidence for an esterase-associated mechanism of resistance to indoxacarb in a field population of diamondback moth (Lepidoptera: Plutellidae). Pest Manag. Sci. 2006, 62, 1045–1051. [Google Scholar] [CrossRef]
- Ghodki, B.S.; Thakare, S.M.; Moharil, M.P.; Rao, N.G.V. Genetics of Indoxacarb resistance in Helicoverpa armigera (Hubner). Entomol. Res. 2009, 39, 50–54. [Google Scholar] [CrossRef]
- Sayyed, A.H.; Ahmad, M.; Saleem, M.A. Cross-resistance and genetics of resistance to indoxacarb in Spodoptera litura (Lepidoptera: Noctuidae). J. Econ. Entomol. 2008, 101, 472–479. [Google Scholar] [CrossRef]
- Sayyed, A.H.; Ahmad, M.; Crickmore, N. Fitness costs limit the development of resistance to indoxacarb and deltamethrin in Heliothis virescens (Lepidoptera: Noctuidae). J. Econ. Entomol. 2008, 101, 1927–1933. [Google Scholar] [CrossRef]
- Cui, L.; Wang, Q.; Qi, H.; Wang, Q.; Yuan, H.; Rui, C. Resistance selection of indoxacarb in Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae): Cross-resistance, biochemical mechanisms and associated fitness costs. Pest Manag. Sci. 2018, 74, 2636–2644. [Google Scholar] [CrossRef] [PubMed]
- Nehare, S.; Ghodki, B.S.; Lande, G.K.; Pawade, V.; Thakare, A.S. Inheritance of resistance and cross resistance pattern in indoxacarb-Resistant diamondback moth Plutella xylostella L. Entomol. Res. 2010, 40, 18–25. [Google Scholar] [CrossRef]
- Afzal, M.B.S.; Shad, S.A.; Ejaz, M.; Serrao, J.E. Laboratory selection, cross-resistance, and estimations of realized heritability of indoxacarb resistance in Phenacoccus solenopsis (Homoptera: Pseudococcidae). Pest Manag. Sci. 2020, 76, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Roush, R.T.; Tabashnik, B.E. The Role of Population Genetics in Resistance Research and Management. In Pesticide Resistance in Arthropods; Chapman and Hall: London, UK, 1990. [Google Scholar]
- Che, W.; Huang, J.; Guan, F.; Wu, Y.; Yang, Y. Cross-Resistance and Inheritance of Resistance to Emamectin Benzoate in Spodoptera exigua (Lepidoptera: Noctuidae). J. Econ. Entomol. 2015, 108, 2015–2020. [Google Scholar] [CrossRef] [PubMed]
- Bolzan, A.; Padovez, F.E.O.; Nascimento, A.R.B.; Kaiser, I.S.; Lira, E.C.; Amaral, F.S.A.; Kanno, R.H.; Malaquias, J.B.; Omoto, C. Selection and characterization of the inheritance of resistance of Spodoptera frugiperda (Lepidoptera: Noctuidae) to chlorantraniliprole and cross-resistance to other diamide insecticides. Pest Manag. Sci. 2019, 75, 2682–2689. [Google Scholar] [CrossRef]
- Rehan, A.; Freed, S. Selection, mechanism, cross resistance and stability of spinosad resistance in Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). Crop Prot. 2014, 56, 10–15. [Google Scholar] [CrossRef]
- Liang, P.; Gao, X.W.; Zheng, B.Z. Genetic basis of resistance and studies on cross-resistance in a population of diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Pest Manag. Sci. 2003, 59, 1232–1236. [Google Scholar] [CrossRef]
- Nauen, R.; Bass, C.; Feyereisen, R.; Vontas, J. The Role of Cytochrome P450s in Insect Toxicology and Resistance. Annu. Rev. Entomol. 2022, 67, 105–124. [Google Scholar] [CrossRef]
- Li, Q.; Huang, J.; Yuan, J. Status and preliminary mechanism of resistance to insecticides in a field strain of housefly (Musca domestica, L). Rev. Bras. Entomol. 2018, 62, 311–314. [Google Scholar] [CrossRef]
- Ahmad, M.; Hollingworth, R.M. Synergism of insecticides provides evidence of metabolic mechanisms of resistance in the obliquebanded leafroller Choristoneura rosaceana (Lepidoptera: Tortricidae). Pest Manag. Sci. 2004, 60, 465–473. [Google Scholar] [CrossRef]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007, 269, 231–253. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Hussain, D.; Ghouse, G.; Abbas, M.; Fisher, S.W. Monitoring of insecticide resistance in Spodoptera litura (Lepidoptera: Noctuidae) from four districts of Punjab, Pakistan to conventional and new chemistry insecticides. Crop Prot. 2016, 79, 177–184. [Google Scholar] [CrossRef]
- Hawkins, N.J.; Bass, C.; Dixon, A.; Neve, P. The evolutionary origins of pesticide resistance. Biol. Rev. 2019, 94, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Liu, Y.; Zhu, Y.C.; Liu, X.; Gao, C.; Shen, J. Inheritance, fitness cost and mechanism of resistance to tebufenozide in Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae). Pest Manag. Sci. 2009, 65, 996–1002. [Google Scholar] [CrossRef]
- Banazeer, A.; Shad, S.A.; Shahzad Afzal, M.B. Laboratory induced bifenthrin resistance selection in Oxycarenus hyalinipennis (Costa) (Hemiptera: Lygaeidae): Stability, cross-resistance, dominance and effects on biological fitness. Crop Prot. 2020, 132, 105107. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).