Recent Advances in Bacterial Amelioration of Plant Drought and Salt Stress
Simple Summary
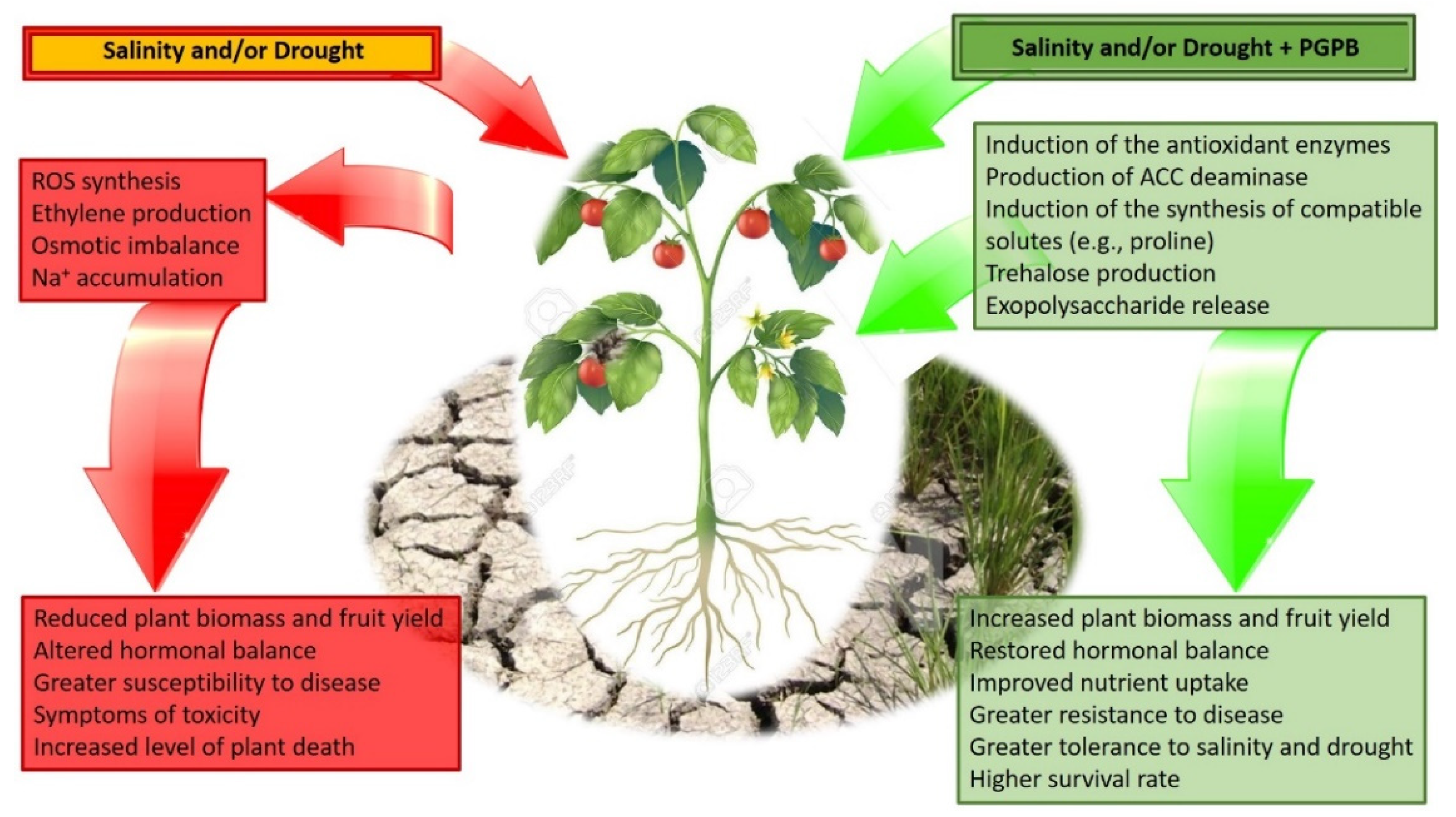
Abstract
1. Drought and Salt Stress
2. Plant Growth-Promoting Bacteria
2.1. How PGPB Mechanisms Deal with Plant Drought and Salt Stress
2.1.1. Mechanisms: ACC Deaminase, IAA, Cytokinin, and Metabolites Such as Proline and Trehalose
2.1.2. Details of How Each Mechanism Functions
| Plant | Bacteria | Comments | Reference |
|---|---|---|---|
| Arabidopsis thaliana (thale cress) | Flavobacterium crocinum HYN0056T | Inoculation with HYN0056T enhanced tolerance against drought and salt stress, possibly via induction of stomatal closure. Treatment with strain HYN0056T followed by drought or salt stress caused the upregulation of several drought- and salt-inducible Arabidopsis genes. | [55] |
| Arabidopsis thaliana (thale cress) | Kosakonia cowanii GG1 isolated as an endophyte of seeds of the xerophytic invasive plant Lactuca serriola | Inoculation of A. thaliana with K. cowanii GG1 stimulated plant growth under drought conditions. The bacterial strain reduced soil water loss, indicating that the synthesis of exopolysaccharides contributes to maintaining the soil water content. | [56] |
| C. arietinum L. (chickpea) | Mesorhizobium ciceri Ca181 | Mutants of M. ciceri defective in phosphorous solubilization and drought stress tolerance were selected. The results indicated that the otsA (Trehalose-6-phosphate synthase), Auc (Acetoin utilisation protein), and Usp (Universal stress protein) genes contributed to the mechanism of drought stress tolerance. | [57] |
| Eleusine coracana (L.) Gaertn. (finger millet) | Variovorax paradoxus RAA3 and ACC deaminase-producing bacteria (Ochrobactrum anthropi DPC9, Pseudomonas palleroniana DPB13 and DPB16, and Pseudomonas fluorescens DPB15) | Inoculation of plants with V. paradoxus RAA3 and the consortium of O. anthropi DPC9, P. palleroniana DPB13 and DPB16, and P. fluorescens DPB15 increased plant growth and nutrient levels in leaves. High amounts of ROS-scavenging enzymes, including superoxide dismutase, guaiacol peroxidase, catalase, and ascorbate peroxidase, as well as the cellular osmolytes proline and phenol, leaf chlorophyll, and a reduced level of hydrogen peroxide and malondialdehyde, were observed after inoculation with RAA3 and the consortium of the other four bacterial strains compared to untreated plants. | [58] |
| Eucalyptus grandis (rose gum) | Pseudomonas sp. M25 and N33 | Plants inoculated with strain M25 exposed to gradual water deficit showed a significant increase in plant water content and cell wall elasticity. Rapid water deficit conditions caused partial defoliation in the absence of added bacteria. Both PGPB strains stimulated the formation of new leaves; inoculation with strain M25 reduced the transpiration rate; and co-inoculation with both strains increased both growth and photosynthetic activity. | [59] |
| Glycine max L. Merrill (soybean) | Bradyrhizobium japonicum and Azospirillum brasilense | The inoculation of soybean plants with B. japonicum and/or A. brasilense and then subjected to drought stress yielded increased leaf membrane stability. Co-inoculation with these strains followed by drought stress improved nodulation. Treatment with one or both of these strains reduced the pod abortion rate under moderate drought stress but not severe drought stress. | [60] |
| Juglans regia L. (walnut.) | AM fungi (Funnelliformis mosseae, Claroideoglomus etunicatum), and Azotobacter chroococcum, Azospirillium lipoferum | Drought stress caused a reduction in plant growth and leaf nutrient content, while increased proline, soluble sugar, starch peroxidase enzyme activity, and phenolic content was seen in leaves. Inoculation with consortia alleviated the negative effects of drought stress on seedlings by increasing the phenol, proline, peroxidase activity, soluble sugar, and starch content. C. etunicatum was the most effective AM fungi. | [61] |
| Solanum lycopersicum Mill cv. F144 (tomato) and Capsicum annuum L. cv. Maor (pepper) | Achromobacter piechaudii ARV8 (isolated from the Arava region of the Negev desert, Israel) | Tomato and pepper seedlings exposed to transient water stress and inoculated with strain A. piechaudii ARV8 showed increased biomass. Moreover, ARV8 lowered ethylene synthesis in tomato seedlings during drought stress, favoring the recovery of ARV8-treated plants when watering was resumed. | [62] |
| Solanum lycopersicum L. (tomato) | Various rhizobacteria (12 Bacillus spp. strains, 6 Pseudomonas, 4 Brevibacillus and 1 Paenibacillus strain isolated from Cistanthe longiscapa (a native flowering desert plant from the Atacama desert, Chile) | The Bacillus strains were used to formulate three consortia and to inoculate tomato seeds that were subsequently exposed to different degrees of water limitation. Inoculated seedlings showed higher biomass and recovery rates compared to uninoculated ones. | [63] |
| Solanum tuberosum (cultivars Swift and Nevsky) (potato) | Achromobacter xylosoxidans Cm4, Pseudomonas oryzihabitans Ep4, and Variovorax paradoxus 5C-2 | PGPB inoculation increased tuber yield in field experiments in plants cultivated under water-limited conditions. However, the leaf water concentration both in inoculated and uninoculated plants was similar, suggesting that other mechanisms (such as the modulation of phytohormone levels) might be responsible for plant growth promotion. | [64] |
| Triticum aestivum L. (wheat) | Azospirillum brasilense and Herbaspirillum seropedicae | Inoculation with the bacterial strains induced drought resistance in the wheat cultivar CD-120. The grain index was improved with H. seropedicae under water stress conditions. | [65] |
| Triticum aestivum L. (wheat) | Variovorax paradoxus RAA3; Pseudomonas spp. DPC12, DPB13, DPB15, and DPB16; Achromobacter spp. PSA7 and PSB8; and Ochrobactrum anthropi DPC9 | In drought conditions, inoculation with strain RAA3 and a consortium of DPC9 + DPB13 + DPB15 + DPB16 improved wheat plant growth and foliar nutrient levels and positively modulated antioxidant properties compared to uninoculated plants. | [66] |
| Triticum aestivum L. (wheat) | Azospirillum brasilense NO40 and Stenotrophomonas maltophilia B11 | Seedling inoculation with the two bacterial strains overcame the negative effects of drought stress, including changes to the relative water content of roots, shoots, and leaves; the area of leaves; the contents of chlorophyll a and b and ascorbic acid; and the protein patterns of root extracts. Bacterial inoculation reduced the drought-induced negative changes (i.e., the leakage of electrolytes and accumulation of malondialdehyde and hydrogen peroxide, the production of proline, and the activities of catalase and peroxidase compared to their uninoculated counterparts). | [67] |
| Triticum aestivum (wheat) | Curtobacterium flaccumfaciens Cf D3-2 and Arthrobacter sp. Ar sp. D4-1 | The two bacterial strains, when used separately to inoculate wheat plants, showed the ability to promote growth under drought conditions. | [68] |
| Vigna mungo L. (black gam) and Pisum sativum L. (pea) | Ochrobactrum pseudogrignonense RJ12, Pseudomonas sp. RJ15, Bacillus subtilis RJ46, alone and in consortia | Consortium treatment increased seed germination, root and shoot length, and plant biomass. Under drought conditions, treated plants exhibited elevated ROS and cellular osmolyte synthesis, higher leaf chlorophyll content, and increased relative water content compared to uninoculated plants. Bacterial inoculation reduced ACC accumulation in plants and down-regulated ACC-oxidase gene expression. | [69] |
| Vigna unguiculata (cowpea) | Bacillus aryabhattai strain MoB09 (able to degrade the herbicide paraquat) | B. aryabhattai MoB09 promoted the growth of cowpea plants following drought stress. | [70] |
| Zea mays L. (maize) | 12 drought-tolerant bacterial strains producing ACC deaminase and/or exopolysaccharides | Strains that synthesize both ACC deaminase and exopolysaccharides induced increased photosynthesis rate, stomatal conductance, vapor pressure, water-use efficiency, and transpiration rate. The strain B. velezensis D3 was the best PGPB. | [71] |
| Zea mays L. (maize) | Commercial biostimulant BACSTIMR 100 composed of a consortium of two Bacillus licheniformis strains, two Brevibacillus laterosporus strains, and one Bacillus amyloliquefaciens strain | Plant inoculation conferred increaseed drought resistance in maize by altering several plant metabolic pathways, including pathways encoding redox homeostasis and strengthening of the plant cell wall, osmoregulation, energy production, and membrane remodelling. | [72] |
| Plant | Bacteria | Comments | Reference |
|---|---|---|---|
| Arabidopsis thaliana (thale cress) | Paraburkholderia phytofirmans PsJN | A. thaliana plants inoculated with strain PsJN showed higher survival rate when exposed to long-term salinity and reduced Na+ accumulation within leaf tissues compared to uninoculated plants. Mutants defective in ACC deaminase, auxin catabolism, N-acyl-homoserine-lactone production, and flagellin synthesis showed a low relevance of these functions to salinity tolerance. Bacterial release of volatile organic compounds (mainly 2-undecanone, 7-hexanol, 3-methylbutanol and dimethyl disulphide) reproduced the effects of direct bacterial inoculation of roots, increasing plant growth rate and tolerance to salt stress conditions. Exposure of A. thaliana to different amounts of these molecules demonstrated their capability to affect growth, while exposure to a mixture of the first three compounds mimicked the effects of the bacterial strain on plant growth stimulation and salinity tolerance. | [73] |
| Arachis hypogaea L. (peanut) | Klebsiella sp., Pseudomonas sp., Agrobacterium sp., and Ochrobactrum sp. isolated from the halophyte Arthrocnemum indicum | Five diazotrophic salt-resistant strains of Klebsiella, Pseudomonas, Agrobacterium, and Ochrobactrum produced IAA and ACC deaminase, fixed N2, and solubilized phosphate. All of the isolates promoted peanut growth under non-stressful conditions and increased the N content in plants. In plants that were previously inoculated with these bacterial strains and then exposed to salt stress, accumulation of ROS-modulating enzymes and increased biomass was recorded compared to uninoculated ones. | [74] |
| Brassica campestris L. (canola) | Brevibacterium epidermidis RS15 and Bacillus aryabhattai RS341 | 120 mM NaCl reduced the rate of seed germination by 50%. Inoculation with B. epidermidis RS15 and B. aryabhattai RS34, both halotolerant and able to synthesize ACC deaminase, enhanced seed germination under salt stress and reduced the ACC content in seeds. Inoculation with both bacterial strains increased hydrolytic enzyme activities (amylase, invertase, and protease) and decreased ethylene levels compared to uninoculated seeds exposed to salt stress. | [75] |
| Camelina sativa (camelina or false flax) | Pseudomonas putida UW4, two root endophytes Pseudomonas migulae 8R6 and Pseudomonas fluoresces YsS6 (both ACC deaminase producing strains), and the acdS minus mutants 8R6M and YsS6M | Soil inoculation with wild-type strains increased shoot length without salt, and seed yield under moderate salinity. Transgenic plants that expressed the acdS gene, encoding the enzyme ACC deaminase, showed reduced inhibition of root lengthening and biomass development, and increased seed production, better seed quality, and higher levels of seed oil production under salt stress. | [76] |
| Camelina sativa (camelina or false flax) | Pseudomonas migulae 8R6 | Both of the C. sativa plants treated with the ACC deaminase producing endophyte P. migulae 8R6 and transgenic plants expressing acdS demonstrated increased tolerance to salt. Inoculation with strain 8R6 positively impacted ethylene- and abscisic acid-dependent signalling. The expression of acdS in transgenic plants altered auxin, jasmonic acid, and brassinosteroid signalling and/biosynthesis. Expression of genes involved in carbohydrate metabolism were up-regulated, as was the expression of genes modulating the level of ROS released. The expression of the acdS gene also positively effected the expression of photosynthesis genes. | [77] |
| Camelina sativa (camelina or false flax) | Pseudomonas migulae 8R6 | Treatment of C. sativa, grown under salt stress, with the endophyte P. migulae 8R6, able to synthesize ACC deaminase, induced a negative modulation of ethylene signaling as well as auxin and jasmonic acid biosynthesis and signaling, while genes involved in regulation of gibberellin signaling were positively affected. In plants cultivated with salt and inoculated with 8R6, a moderate expression of the acdS gene under the control of the rolD promoter occurred, which was highly efficient in lowering the expression of the genes involved in the synthesis of ethylene and its signaling. | [78] |
| Capsicum annuum (pepper) | Brevibacterium iodinum RS16, Bacillus licheniformis RS656, and Zhihengliuela alba RS111 | Brevibacterium iodinum RS16, Bacillus licheniformis RS656, and Zhihengliuela alba RS111 were identified as both halotolerant and ACC deaminase producers. Single inoculation with the three bacterial strains in red pepper plants grown at three salinity levels induced lower ethylene production. Plant biomass and salt tolerance index (the ratio of the biomass of salt stressed to non-stressed plants) in inoculated plants was higher compared to non-inoculated plants. | [79] |
| Capsicum annuum L. (red pepper) | Pseudomonas frederiksbergensis OS261 | Plants were inoculated with strain OS261 and grown with three levels of salt. Growth parameters (height and plant biomass) of plants were increased by the presence of the bacterial strain compared to uninoculated controls. The amount of ethylene synthesized by plants grown under salinity stress was high, but inoculation with strain OS261 reduced the release of this hormone. The level of antioxidant enzyme activity in leaves of inoculated plants grown in salinity was increased, while the H+ concentration was reduced. | [80] |
| Capsicum annuum L. cv. Bulmat (red pepper) | Pseudomonas frederiksbergensis OB139, Pseudomonas vancouverensis OB155 | Plants were cultivated under four levels of salt concentration and inoculated or not with one or both strains. Salt stress inhibited plant growth through increased ethylene synthesis and the disruption of photosynthetic parameters compared to uninoculated plants. The combination of the two bacterial strains, both able to synthesize ACC deaminase, lowered ethylene levels in plants and increased catalase activity, leading to increased plant growth compared to a single bacterium or the uninoculated control. | [81] |
| Cicer arietinum L. (chickpea) | Mesorhizobium ciceri EE-7 (salt-sensitive) and Mesorhizobium ciceri G-55 (salt-tolerant) | Two isolates of M. ciceri, one that was salt sensitive and another that was salt tolerant, were transformed with an isolated acdS gene encoding ACC deaminase. Salt stress reduced the biomass of plants inoculated with the wild-type strains. The salt-tolerant bacterial strain induced a higher nodulation rate in chickpeas compared to the salt-sensitive strain. The shoot dry weight was increased in plants inoculated with the salt-sensitive transformant strain. In plants inoculated with the salt-sensitive transformant strain, nodulation was found to be comparable to that induced by the salt-tolerant strain. | [82] |
| Coriandrum sativum L. (coriander) | Azospirillum brasiliense and Azotobacter chroococcum | Inoculation of coriander seeds, exposed to four levels of salt stress, with a mixture of A. brasiliense and A. chroococcum enhanced chlorophyll content and increased grain yield and plant biomass compared to uninoculated plants. Combined inoculation and salt stress increased catalase and decreased the level of ascorbate peroxidase and guaiacol peroxidase compared to untreated plants. Inoculation with both PGPB lowered Na and increased the K concentration in coriander leaves compared to untreated plants. The presence of PGPB improved plant growth in both the absence and presence of salt stress conditions. | [83] |
| Cucumis sativus (cucumber) | Pseudomonas fluorescens, Bacillus megaterium, and Variovorax paradoxus | The ability to solubilize phosphates and synthesize ACC deaminase, siderophores, and IAA was assessed in the three PGPB strains grown at two salt concentrations (2 and 5% NaCl w/v). While B. megaterium was the least affected by high salinity, ACC deaminase activity as well as siderophore and IAA production in P. fluorescens remained unaffected under salt stress. On the contrary, V. paradoxus was not tolerant to salt, and its expression of plant beneficial traits was reduced by salinity. When inoculated onto cucumber plants grown at three different salinity levels, P. fluorescens was the most effective of the three strains at decreasing the inhibitory effects of salinity. | [84] |
| Hordeum vulgare L. (barley), Trifolium repens L. (clover), and Pennisetum glaucum L.R. Br. (pearl millet) | Pseudomonas putida UW3 and UW4 | Barley, clover, and pearl millet plants grown in the presence of salt and inoculated with P. putida UW3 and UW4. P. putida UW4 increased barley biomass compared to uninoculated plants. Strain UW3 increased the biomass of the three crops. Shoot and root length and weight were increased in inoculated plants, suggesting a more efficient photosynthetic activity in the presence of the bacterial strains. Data from pulse amplitude modulation fluorometry showed that the reduction of plant photosynthetic activity induced by salt stress was recovered once the strains were applied. | [85] |
| Medicago sativa L. (alfalfa) | Bacillus megaterium NRCB001, Bacillus subtilis subsp. subtilis NRCB002, and Bacillus subtilis NRCB003 | Thirteen bacterial strains were isolated from the rice rhizosphere and characterized for their plant beneficial traits. B. megaterium NRCB001, B. subtilis subsp. subtilis NRCB002, and B. subtilis NRCB003 synthesized auxin, siderophores, NH3, and ACC deaminase and solubilized phosphate and potassium. Strains NRCB001 and NRCB002 tolerated 1750 mM NaCl. The three strains were inoculated onto M. sativa grown under normal conditions and salinity stress. Strains NRCB002 and NRCB003 increased the dry weight of alfalfa compared with non-inoculated seedlings treated with 130 mM NaCl. | [86] |
| Oryza sativa L. (rice) | Streptomyces sp. GMKU 336 and its ACC deaminase-deficient mutant | Plants of Thai jasmine rice cultivar Khao Dok Mali 105 grown under salt stress were inoculated with the endophyte Streptomyces sp. GMKU 336 or with its mutant lacking ACC deaminase activity. Strain GMKU 336 increased plant growth and chlorophyll, proline, K+, Ca+, and water content. The amount of released ethylene was reduced, as was the content of ROS and Na+, and the Na+/K+ ratio, compared to uninoculated plants or to those inoculated with the mutant. Plants treated with the wild type showed down-regulation of genes involved in the ethylene synthesis pathway, ACO1 and EREBP1, while acdS was up-regulated. Genes involved in osmotic balance, Na+ transport, calmodulin, and antioxidant enzymes were upregulated. | [87] |
| Oryza sativa (rice) | Bacillus tequilensis 10b (UPMRB9) | The effect of strains 10b UPMRB9′ on the growth of rice that was grown in the presence of salt was assessed. Strain 10b UPMRB9′ improved osmoprotectant properties such as proline, the soluble sugar concentration, and the levels of the antioxidant enzymes uperoxide dismutase, peroxidase, and catalase. Rice inoculated with strain UPMRB9 accumulated a greater amount of N and Ca in plant tissues, suggesting that this strain could behave as a bio-augmenter to improve biochemical and nutritional features in rice plants under salinity stress. | [88] |
| Panax ginseng (ginseng) | Paenibacillus yonginensis DCY84T | The impact of strain DCY84T, able to synthesize IAA and siderophore and solubilize phosphate, was assessed under short- and long-term salinity stress. Ginseng seedlings inoculated with the bacterial strain, following exposure to salt stress, were protected by the induction of plant defense-related systems (ion transport, ROS scavenging enzymes, proline content, total sugars, and ABA biosynthetic genes), as well as genes involved in root hair formation. The metabolome of the seedlings treated with DCY84T and exposed to salt stress overlapped with that of control plants. | [89] |
| Pisum sativum (pea) | Bacillus marisflavi (CHR JH 203) and Bacillus cereus (BST YS1_42) | Inoculation of pea plants with B. marisflavi CHR JH 203 and B. cereus BST YS1_42, both synthesizing a high amount of ACC deaminase, grown under salinity, improved plant biomass as well as the amount of plant carbohydrates, reducing sugars, proteins, chlorophylls, phenol, flavonoids, and antioxidant enzymes levels. In addition, plant ROS scavenging genes, defense genes, and cell rescue genes were all overexpressed in inoculated plants in the presence of 1% NaCl. | [90] |
| Seidlitzia rosmarinus Ehrenb. ex Boiss (perennial-green desert species of saltwort) | Rothia terrae, Kocuria palustris, Pseudomonas baetica, Pseudomonas fluorescens Staphylococcus warneri, Staphylococcus epidermidis, Staphylococcus succinus, Paenibacillus amylolyticus, Brevibacterium frigoritolerans, Stenotrophomonas pavanii, Halomonas sulfidaeris, Planococcus salinarum, Planomicrobium koreense, Planococcus halocryophilus, Planomicrobium soli | Culturable endophytic bacteria from the halophytic plant Seidlitzia rosmarinus Ehrenb. ex Boiss. were isolated and characterized to evaluate their plant beneficial traits under salt stress. Root endophytes belonged to genera Rothia, Kocuria, Pseudomonas, Staphylococcus, Paenibacillus, and Brevibacterium; shoot isolates belonged to Staphylococcus, Rothia, Stenotrophomonas, Brevibacterium, Halomonas, Planococcus, Planomicrobium, and Pseudomonas genera; Staphylococcus, Rothia, and Brevibacterium occurred in both roots and shoots. Synthesis of IAA and ACC deaminase was higher in bacteria from roots than from shoots. Finally, S. pavanii JST3 and P. fluorescens JST2 improved both shoot and root growth of Lepidium sativuum under salinity conditions. | [91] |
| Solanum lycopersicum (tomato) | Pseudomonas azotoformans CHB 1107 | Strain CHB 1107 wild-type (producing ACC deaminase) lowered ethylene and proline levels in tomato plants exposed to high salt levels, increasing the dry weights of shoots and roots compared with uninoculated plants. Plants that were inoculated with a mutant that lacked ACC deaminase activity showed reduced K, Ca, and Mn uptake compared with plants inoculated with the wild-type strain. The wild-type strain CHB 1107 reduced the uptake of Na by tomato plants compared with the mutant strain under salt stress. Tomato plants inoculated with the wild-type strain yielded a higher K/Na ratio than those that were inoculated with the mutant. | [92] |
| Sorghum vulgare (sorghum) | Pseudomonas migulae SVB3R2, SVB3R3, SVB3R4, Pseudomonas sp. SVB3R5, Pseudomonas brassicacearum SVB6R1 | Sorghum, tomato, and cucumber bacterial endophytes were characterized by 16S rRNA sequence determination and tested for plant beneficial traits. The activity of five endophytes was tested on plants grown with salinity stress. Strains SVB3R3 and SVB3R4 increased plant biomass, and strains SVB3R3 and SVB3R4 and SVB6R1 decreased the symptoms of plant salinity stress. Only strain SVB6R1 could produce ACC deaminase. | [93] |
| Triticum aestivum (wheat) | Bacillus pumilus SU3, Bacillus aquimaris SU8, Bacillus pumilus SU10, Bacillus arsinicus SU13, Arthrobacter sp. SU18, Bacillus cereus SU24, Pseudomonas mendocina SU40, Bacillus aquimaris SU44, and Bacillus subtilis SU47 | Salt-tolerant (ST) PGPB positively influenced the growth and yield of wheat in saline soil. All nine tested strains improved plant growth in saline soil under greenhouse conditions, with strain DU18 being the most efficient. Under field conditions, strains SU44 and SU8 were the best in increasing plant biomass. Plant inoculation with strain SU8 led to higher proline content and total soluble sugar accumulation in wheat, while strain SU44 resulted in a higher accumulation of reducing sugars. The amounts of N, K, and P in wheat leaves increased significantly after inoculation with all the strains; B. subtilis SU47 lowered the sodium (Na) content in wheat leaves. | [94] |
| Triticum aestivum (wheat) | Serratia marcescens CDP-13 | Serratia marcescens CDP-13 is halotolerant, produces ACC deaminase, solubilizes phosphate, synthesizes siderophores and IAA, and fixes N2. Wheat inoculation with strain CDP-13 increased plant biomass under salinity stress, reducing inhibition of plant growth caused by salt and lowering the amount of osmoprotectants (such as proline, malondialdehyde, soluble sugar), protein, and IAA content in plants. | [95] |
| Triticum aestivum L. (wheat). | Bacillus megaterium PN89 | B. megaterium PN89, able to synthesize IAA, induced increased germination rate and root and shoot length in wheat plants exposed to salt stress, compared to non-inoculated controls. | [96] |
| Triticum aestivum (wheat) | Brevibacterium frigoritolerans, Bacillus velezensis, and Bacillus thuringiensis | The expression of plant-beneficial traits of Br. frigoritolerans alone or in combination with B. velezensis and B. thuringiensis under six salinity levels was characterized, and the effects on wheat of these strains alone or in combination under salt stress were assessed. B. frigoritolerans was the most effective, both for physiological trait expression and wheat plant growth promotion. Under salinity stress, the mixed inoculation of the three bacterial strains was more efficient than any single inoculation. | [97] |
| Triticum durum (wheat) | Fourteen strains of the genera Streptomyces and Nocardiopsis | Fourteen Actinomycetes strains were tested for expression of plant-beneficial activities under salinity conditions. The isolates could solubilize inorganic phosphate and synthesize IAA, HCN, and ammonia when grown in the presence of different salt concentrations. The majority of the strains produced ACC deaminase. Plant inoculation with these strains improved biomass and yielded an increased amount of chlorophyll and proline compared to uninoculated plants, both with and without salt. | [98] |
| Vigna radiata L. (mung bean) | Enterobacter cloacae KBPD | Strain KBPD is an ACC deaminase producer, able to solubilize phosphates and synthesize IAA, siderophore, ammonia, hydrogen cyanide, and exopolysaccharide. V. radiata plants exposed to salinity and inoculated with this bacterial strain showed increased shoot length, root length, and fresh and dry weights. Inoculation with strain KBPD also reduced proline content in plants grown with salt stress. | [99] |
| Vigna radiata (L.) R. Wilczekspring (mung bean) | Rhizobium sp. LSMR-32 and Enterococcus mundtii LSMRS-3 | Under salt stress conditions, separate inoculation with the two bacterial strains induced increased seed germination, grain yield, plant height, biomass, chlorophyll content, and nutrient uptake compared to uninoculated plants. Inoculation with both strains increased both symbiotic parameters (nodulation rate, nodule biomass, and leghaemoglobin amount) and soil phosphatase and dehydrogenase levels. The microbial consortium enhanced the level of proline and anti-oxidative enzymes. | [100] |
| Zea mays (maize) | Azospirillum lipoferum or Azotobacter chroococcum | Maize (corn) plants that were exposed to salt stress had reduced growth parameters, pigments, soluble proteins, K+, and a K+/Na+ ratio. Salinity led to increased levels of soluble sugars, proline, Na+, malondialdehyde, and peroxidase and catalase activity, while the activity of plant ascorbate peroxidase remained unaffected. Plants inoculated with A. lipoferum or A. chroococcum increased growth parameters, pigments, K+, osmolytes, K+/Na+ ratio, and the antioxidative enzymes in salt-affected maize. Both bacterial strains also lowered malondialdehyde and Na+ in maize plants. | [101] |
3. Groups of Microorganisms to Lower Plant Drought and Salt Stress
3.1. Bacterial Consortia
3.2. Bacteria Plus Fungi (Including AMF)
4. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Glick, B.R. Beneficial Plant-Bacterial Interactions; Springer: Berlin/Heidelberg, Germany, 2020; 383p. [Google Scholar]
- Forni, C.; Duca, D.; Glick, B.R. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 2017, 410, 335–356. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.N.; Mostofa, M.G.; Akter, M.M.; Srivastava, A.K.; Sayed, M.A.; Hasan, M.S.; Tran, L.S.P. Impact of salt-induced toxicity on growth and yield-potential of local wheat cultivars: Oxidative stress and ion toxicity are among the major determinants of salt-tolerant capacity. Chemosphere 2017, 187, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Gepstein, S.; Sabehi, G.; Carp, M.-J.; Hajouj, T.; Nesher, M.F.O.; Yariv, I.; Dor, C.; Bassani, M. Large-scale identification of leaf senescence-associated genes. Plant J. 2003, 36, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005, 24, 23–28. [Google Scholar] [CrossRef]
- Umezawa, T.; Fujita, M.; Fujita, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Engineering drought tolerance in plants: Discovering and tailoring genes to unlock the future. Curr. Opin. Biotechnol. 2006, 17, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Dodd, I.C.; Zinovkina, N.Y.; Safronova, V.I.; Belimov, A.A. Rhizobacterial mediation of plant hormone status. Ann. Appl. Biol. 2010, 157, 361–379. [Google Scholar] [CrossRef]
- Dodd, I.C.; Pérez-Alfocea, F. Microbial amelioration of crop salinity stress. J. Exp. Bot. 2012, 63, 3415–3428. [Google Scholar] [CrossRef] [PubMed]
- Gepstein, S.; Glick, B.R. Strategies to ameliorate abiotic stress-induced plant senescence. Plant Mol. Biol. 2013, 82, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and salinity stress responses and microbe-induced tolerance in plants. Front. Plant Sci. 2020, 11, 591911. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Mosqueda, M.C.; Glick, B.R.; Santoyo, G. ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt in crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.; Cho, Y.-C. Plant hormones in salt stress tolerance. J. Plant Biol. 2015, 58, 147–155. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef]
- Christakis, C.A.; Daskalogiannis, G.; Chatzaki, A.; Markakis, E.A.; Mermigka, G.; Sagia, A.; Rizzo, G.F.; Catara, V.; Lagkouvardos, I.; Studholme, D.J.; et al. Endophytic bacterial isolates from haplophytes demonstrate phytopathogen biocontrol and plant growth promotion under high salinity. Front. Microbiol. 2021, 12, 681567. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Jabborova, D.; Hashem, A. Pseudomonas induces salinity tolerance in cotton (Gossypium hirsutum) and resistance to Fusarium root rot through the modulation of indole-3-acetic acid. Saudi J. Biol. Sci. 2015, 22, 773–779. [Google Scholar] [CrossRef]
- Mavrodi, D.V.; Mavrodi, O.V.; Parejko, J.A.; Bonsall, R.F.; Kwak, Y.-S.; Paulitz, T.C.; Thomashow, L.S.; Weller, D.M. Accumulation of the antibiotic phenazine-1-carboxylic acid in the rhizosphere of dryland cereals. Appl. Environ. Microbiol. 2012, 78, 804–812. [Google Scholar] [CrossRef]
- Timmusk, S.; Paalme, V.; Pavlicek, T.; Bergquist, J.; Vangala, A.; Danilas, T.; Nevo, E. Bacterial distribution in the rhizosphere of wild barley under contrasting microclimates. PLoS ONE 2011, 6, e17968. [Google Scholar] [CrossRef] [PubMed]
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Gamalero, E.; Glick, B.R. Mechanisms used by plant growth-promoting bacteria. In Bacteria in Agrobiology: Plant Nutrient Management; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 17–46. [Google Scholar]
- Gamalero, E.; Glick, B.R. Plant growth-promoting bacteria in agriculture and stressed environments. In Modern Soil Microbiology, 3rd ed.; van Elsas, J.D., Trevors, J.T., Soares Rosado, A., Nannipieri, P., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 361–380. [Google Scholar]
- Glick, B.R. The enhancement of plant growth by free-living bacteria. Can. J. Microbiol. 1995, 41, 109–117. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Park, E.; Glick, B.R. 1-Aminocyclopropane-1-carboxylate (ACC) deaminase from Pseudomonas putida UW4 facilitates the growth of canola in the presence of salt. Can. J. Microbiol. 2007, 53, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Gamalero, E.; Berta, G.; Glick, B.R. The use of microorganisms to facilitate the growth of plants in saline soils. In Microbial Strategies for Crop Improvement; Khan, M.S., Zaidi, A., Musarrat, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–22. [Google Scholar]
- Kim, Y.-C.; Glick, B.R.; Bashan, Y.; Ryu, C.-M. Enhancement of plant drought tolerance by microbes. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 383–413. [Google Scholar]
- Sergeeva, E.; Shah, S.; Glick, B.R. Tolerance of transgenic canola expressing a bacterial ACC deaminase gene to high concentrations of salt. World J. Microbiol. Biotechnol. 2006, 22, 277–282. [Google Scholar] [CrossRef]
- Santoyo, G.; Gamalero, E.; Glick, B.R. Mycorrhizal-bacterial amelioration of plant abiotic and biotic stress. Front. Sustain. Food Syst. 2021, 5, 672881. [Google Scholar] [CrossRef]
- Reed, M.L.E.; Glick, B.R. Applications of plant growth-promoting bacteria for plant and soil systems. In Applications of Microbial Engineering; Gupta, V.K., Schmoll, M., Maki, M., Tuohy, M., Mazutti, M.A., Eds.; Taylor and Francis: Enfield, CT, USA, 2013; pp. 181–229. [Google Scholar]
- Pattyn, J.; Vaughan-Hirsh, J.; Van de Poel, B. The regulation of ethylene biosynthesis: A complex control circuitry. New Phytol. 2021, 229, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of plant growth by ACC deaminase-containing soil bacteria. Eur. J. Plant Pathol. 2007, 119, 329–339. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Ali, S.; Charles, T.C.; Glick, B.R. Amelioration of damages caused by high salinity stress by plant growth-promoting bacterial endophytes. Plant Physiol. Biochem. 2014, 80, 160–167. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.C.; Duan, J.; DiBernardo, M.; Zetter, E.; Campos-García, J.; Glick, B.R.; Santoyo, G. The production of ACC deaminase and trehalose by the plant growth promoting bacterium Pseudomonas sp. UW4 synergistically protect tomato plants against salt stress. Front. Microbiol. 2019, 10, 1392. [Google Scholar] [CrossRef]
- Tsukanova, K.A.; Chebotar, V.K.; Meyer, J.J.M.; Bibikova, T.N. Effect of plant growth-promoting rhizobacteria on plant hormone homeostasis. S. Afr. J. Bot. 2017, 113, 91–102. [Google Scholar] [CrossRef]
- Duca, D.; Lorv, J.; Patten, C.L.; Rose, D.; Glick, B.R. Indole-3-acetic acid in plant-microbe interactions. Antonie Van Leeuwenhoek 2014, 106, 85–125. [Google Scholar] [CrossRef]
- Duca, D.R.; Glick, B.R. Indole-3-acetic acid biosynthesis and its regulation in plant-associated bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 8607–8619. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Ha-Tran, D.M.; Nguyen, T.T.M.; Hung, S.-H.; Huang, E.; Huang, C.-C. Roles of Plant Growth-Promoting Rhizobacteria (PGPR) in stimulating salinity stress defense in plants: A review. Int. J. Mol. Sci. 2021, 22, 3154. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.-L.; Luo, L. Effects of engineered Sinorhizobium meliloti on cytokinin synthesis and tolerance of alfalfa to extreme drought stress. Appl. Environ. Microbiol. 2012, 78, 8056–8061. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Jiang, W.; Cheng, Z.; Heikkila, J.J.; Glick, B.R. The complete genome sequence of the plant growth-promoting bacterium Pseudomonas putida UW4. PLoS ONE 2013, 8, e58640. [Google Scholar]
- Chaplin, M. Do we underestimate the importance of water in cell biology? Nat. Rev. Mol. Cell. Biol. 2006, 7, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Julca, I.; Alaminos, M.; Gonzalez-Lopez, J.; Manzanera, M. Xeroprotectants for the stabilization of biomaterials. Biotechnol. Adv. 2012, 30, 1641–1654. [Google Scholar] [CrossRef] [PubMed]
- Iturriaga, G.; Suárez, R.; Nova-Franco, B. Trehalose metabolism: From osmoprotection to signaling. Int. J. Mol. Sci. 2009, 10, 3793–3810. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.M.S.; Abdelgawad, Z.A.; El-Bassiouny, H.M.S. Alleviation of the adverse effects of salinity stress using trehalose in two rice varieties. S. Afr. J. Bot. 2016, 103, 275–282. [Google Scholar] [CrossRef]
- Lau, J.A.; Lennon, J.T. Microbe-mediated adaptation to novel environments. Proc. Natl. Acad. Sci. USA 2012, 109, 14058–14062. [Google Scholar] [CrossRef] [PubMed]
- Vílchez, J.I.; García-Fontana, C.; Román-Naranjo, D.; González-López, J.; Manzanera, M. Plant drought tolerance enhancement by trehalose production of desiccation-tolerant microorganisms. Front. Microbiol. 2016, 7, 1577. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.P.; Grover, M.; Chourasiya, D.; Bharti, A.; Agnihotri, R.; Maheshwari, H.S.; Pareek, A.; Buyer, J.S.; Sharma, S.K.; Schütz, L.; et al. Deciphering the role of trehalose in tripartite symbiosis among rhizobia, arbuscular mycorrhizal fungi, and legumes for enhancing abiotic stress tolerance in crop plants. Front. Microbiol. 2020, 17, 509919. [Google Scholar] [CrossRef] [PubMed]
- Onwe, R.O.; Onwosi, C.O.; Ezugworie, F.N.; Ekwealor, C.C.; Okonkwo, C.C. Microbial trehalose boosts the ecological fitness of biocontrol agents, the viability of probiotics during long-term storage and plants tolerance to environmental-driven abiotic stress. Sci. Total Environ. 2022, 1, 150432. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, S.; Song, L.; Yan, H.; Liu, K.; Meng, W.; Wang, T. Trehalose production using recombinant trehalose synthase in Bacillus subtilis by integrating fermentation and biocatalysis. J. Agric. Food Chem. 2019, 67, 9314–9324. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Patel, M.K.; Kumar, N.; Bajpai, A.B.; Siddique, K.H.M. Metabolomics and molecular approaches reveal drought stress tolerance in plants. Int. J. Mol. Sci. 2021, 22, 9108. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Sen, S.; Mohapatra, S. Modulation of proline metabolic gene expression in Arabidopsis thaliana under water-stressed conditions by a drought-mitigating Pseudomonas putida strain. Ann. Microbiol. 2017, 67, 655–668. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, F.; Mickan, B.S.; Wang, D.; Wang, W. Physiological, proteomic, and metabolomic analysis provide insights into Bacillus sp.-mediated salt tolerance in wheat. Plant Cell Rep. 2021, 41, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-E.; Woo, O.-G.; Bae, Y.; Keum, H.L.; Chung, S.; Sul, W.J.; Lee, J.-H. Enhanced drought and salt stress tolerance in Arabidopsis by Flavobacterium crocinum HYN0056T. J. Plant Biol. 2020, 63, 63–71. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, T.M.; Choi, B.; Kim, Y.; Kim, E. Invasive Lactuca serriola seeds contain endophytic bacteria that contribute to drought tolerance. Sci. Rep. 2021, 11, 13307. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Singh, R.P.; Singh, A.L.; Singh, M. Identification of genes involved in phosphate solubilization and drought stress tolerance in chickpea symbiont Mesorhizobium ciceri Ca181. Arch. Microbiol. 2021, 203, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Chandra, D.; Srivastava, R.; Glick, B.R.; Sharma, A.K. Rhizobacteria producing ACC deaminase mitigate water-stress response in finger millet (Eleusine coracana (L.) Gaertn.). 3 Biotech 2020, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Chaín, J.M.; Tubert, E.; Graciano, C.; Castagno, L.N.; Recchi, M.; Pieckenstain, F.L.; Estrella, M.J.; Gudesblat, G.; Amodeo, G.; Baroli, I. Growth promotion and protection from drought in Eucalyptus grandis seedlings inoculated with beneficial bacteria embedded in a superabsorbent polymer. Sci. Rep. 2020, 26, 18221. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.R.; Zoz, J.; Oliveira, C.E.S.; Zuffo, A.M.; Steiner, F.; Zoz, T.; Vendruscolo, E.P. Can co-inoculation of Bradyrhizobium and Azospirillum alleviate adverse effects of drought stress on soybean (Glycine max L. Merrill.). Arch. Microbiol. 2019, 201, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Behrooz, A.; Vahdati, K.; Rejali, F.; Lotfi, M.; Sarikhani, S.; Leslie, C. Arbuscular mycorrhiza and plant growth-promoting bacteria alleviate drought stress in walnut. Hort. Sci. 2019, 54, 1087–1092. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria that confer resistance in tomato to salt stress. Plant Physiol. Biochem. 2004, 42, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Astorga-Eló, M.; Gonzalez, S.; Acuña, J.J.; Sadowski, M.J.; Jorquera, M.A. Rhizobacteria from ‘flowering desert’ events contribute to the mitigation of water scarcity stress during tomato seedling germination and growth. Sci. Rep. 2021, 11, 13745. [Google Scholar] [CrossRef]
- Belimov, A.A.; Dodd, I.C.; Safronova, V.I.; Shaposhnikov, A.I.; Azarova, T.S.; Makarova, N.M.; Davies, W.J.; Tikhonovich, I.A. Rhizobacteria that produce auxins and contain ACC deaminase decrease amino acid concentrations in the rhizosphere and improve growth and yield of well-watered and water-limited potato (Solanum tuberosum). Ann. Appl. Biol. 2015, 167, 11–25. [Google Scholar] [CrossRef]
- Furlan, F.; Saatkamp, K.; Gazolla Volpiano, C.; de Assis Franco, F.; Fonseca dos Santos, M.; Gruszka Vendruscolo, E.C.; Guimarães, V.F.; Torres da Costa, A.C. Plant growth-promoting bacteria effect in withstanding drought in wheat cultivars. Sci. Agrar. 2017, 18, 104–113. [Google Scholar] [CrossRef]
- Chandra, D.; Srivastava, R.; Gupta, V.V.S.R.; Franco, C.M.M.; Sharma, A.K. Evaluation of ACC-deaminase-producing rhizobacteria to alleviate water-stress impacts in wheat (Triticum aestivum L.) plants. Can. J. Microbiol. 2019, 65, 387–403. [Google Scholar] [CrossRef]
- Kasim, W.A.; Osman, M.E.H.; Omar, M.N.; Salama, S. Enhancement of drought tolerance in Triticum aestivum L. seedlings using Azospirillum brasilense NO40 and Stenotrophomonas maltophilia B11. Bull. Natl. Res. Cent. 2021, 45, 95. [Google Scholar] [CrossRef]
- Hone, H.; Mann, R.; Yang, G.; Kaur, J.; Tannenbaum, I.; Li, T.; Spangenberg, G.; Sawbridge, T. Profiling, isolation and characterisation of beneficial microbes from the seed microbiomes of drought tolerant wheat. Sci. Rep. 2021, 7, 11916. [Google Scholar] [CrossRef] [PubMed]
- Saikia, J.; Sarma, R.K.; Dhandia, R.; Yadav, A.; Bharali, R.; Gupta, V.K.; Saikia, R. Alleviation of drought stress in pulse crops with ACC deaminase producing rhizobacteria isolated from acidic soil of Northeast India. Sci. Rep. 2018, 23, 3560. [Google Scholar] [CrossRef] [PubMed]
- Inthama, P.; Pumas, P.; Pekkoh, J.; Pathom-Aree, W.; Pumas, C. Plant growth and drought tolerance-promoting bacterium for bioremediation of paraquat pesticide residues in agriculture soils. Front. Microbiol. 2021, 12, 604662. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Tufail, M.A.; Asghar, H.N.; Nazli, F.; Zahir, Z.A. Appraising the potential of EPS-producing rhizobacteria with ACC-deaminase activity to improve growth and physiology of maize under drought stress. Physiol. Plant. 2021, 172, 463–476. [Google Scholar] [CrossRef]
- Nephali, L.; Moodley, V.; Piater, L.; Steenkamp, P.; Buthelezi, N.; Dubery, I.; Burgess, K.; Huyser, J.; Tugizimana, F. A metabolomic landscape of maize plants treated with a microbial biostimulant under well-watered and drought conditions. Front. Plant Sci. 2021, 12, 676632. [Google Scholar] [CrossRef] [PubMed]
- Ledger, T.; Rojas, S.; Timmermann, T.; Pinedo, I.; Poupin, M.J.; Garrido, T.; Richter, P.; Tamayo, J.; Donoso, R. Volatile-mediated effects predominate in Paraburkholderia phytofirmans growth promotion and salt stress tolerance of Arabidopsis thaliana. Front. Microbiol. 2016, 7, 1838. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kulkarni, J.; Jha, B. Halotolerant rhizobacteria promote growth and enhance salinity tolerance in peanut. Front. Microbiol. 2016, 7, 1600. [Google Scholar] [CrossRef] [PubMed]
- Siddikee, M.A.; Sundaram, S.; Chandrasekaran, M.; Kim, K.; Selvakumar, G.; Sa, T. Halotolerant bacteria with ACC deaminase activity alleviate salt stress effect in canola seed germination. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 237–241. [Google Scholar] [CrossRef]
- Heydarian, Z.; Yu, M.; Gruber, M.; Glick, B.R.; Zhou, R.; Hegedus, D.D. Inoculation of soil with plant growth promoting bacteria producing 1-aminocyclopropane-1-carboxylate deaminase or expression of the corresponding acdS gene in transgenic plants increases salinity tolerance in Camelina sativa. Front. Microbiol. 2016, 7, 1966. [Google Scholar] [CrossRef]
- Heydarian, Z.; Gruber, M.; Glick, B.R.; Hegedus, D.D. Gene expression patterns in roots of Camelina sativa with enhanced salinity tolerance arising from inoculation of soil with Plant Growth Promoting Bacteria producing 1-aminocyclopropane-1-carboxylate deaminase or expression the corresponding acdS gene. Front. Microbiol. 2018, 9, 1297. [Google Scholar] [CrossRef] [PubMed]
- Heydarian, Z.; Gruber, M.; Coutu, C.; Glick, B.R.; Hegedus, D.D. Gene expression patterns in shoots of Camelina sativa with enhanced salinity tolerance provided by plant growth promoting bacteria producing 1-aminocyclopropane-1-carboxylate deaminase or expression of the corresponding acdS gene. Sci. Rep. 2021, 11, 4260. [Google Scholar] [CrossRef] [PubMed]
- Siddikee, M.A.; Glick, B.R.; Chauhan, P.S.; Yim, W.J.; Sa, T. Enhancement of growth and salt tolerance of red pepper seedlings (Capsicum annuum L.) by regulating stress ethylene synthesis with halotolerant bacteria containing 1-aminocyclopropane-1-carboxylic acid deaminase activity. Plant Physiol. Biochem. 2011, 49, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Samaddar, S.; Anandham, R.; Kang, Y.; Kim, K.; Selvakumar, G.; Sa, T. Beneficial soil bacterium Pseudomonas frederiksbergensis OS261 augments salt tolerance and promotes red pepper plant growth. Front. Plant Sci. 2017, 8, 705. [Google Scholar] [CrossRef] [PubMed]
- Samaddar, S.; Chatterjee, P.; Choudhury, A.R.; Ahmed, S.; Sa, T. Interactions between Pseudomonas spp. and their role in improving the red pepper plant growth under salinity stress. Microbiol. Res. 2019, 219, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Brígido, C.; Nascimento, F.X.; Duan, J.; Glick, B.R.; Oliveira, S. Expression of an exogenous 1-aminocyclopropane-1-carboxylate deaminase gene in Mesorhizobium spp. reduces the negative effects of salt stress in chickpea. FEMS Microbiol. Lett. 2013, 349, 46–53. [Google Scholar] [PubMed]
- Rabiei, Z.; Hosseini, S.J.; Pirdashti, H.; Hazrati, S. Physiological and biochemical traits in coriander affected by plant growth-promoting rhizobacteria under salt stress. Heliyon 2020, 6, e05321. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.M.; Ahmad, M.; Naveed, M.; Imran, M.; Zahir, Z.A.; Crowley, D.E. Relationship between in vitro characterization and comparative efficacy of plant growth-promoting rhizobacteria for improving cucumber salt tolerance. Arch. Microbiol. 2016, 198, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Alkowni, R.; Jodeh, S.; Hamed, R.; Samhan, S.; Daraghmeh, H. The impact of Pseudomonas putida UW3 and UW4 strains on photosynthetic activities of selected field crops under saline conditions. Int. J. Phytoremediat. 2019, 21, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, H.; Leng, J.; Niu, H.; Chen, X.; Liu, D.; Chen, Y.; Gao, N.; Ying, H. Isolation and characterization of plant growth-promoting rhizobacteria and their effects on the growth of Medicago sativa L. under salinity conditions. Antonie Van Leeuwenhoek 2020, 113, 1263–1278. [Google Scholar] [CrossRef] [PubMed]
- Jaemsaeng, R.; Jantasuriyarat, C.; Thamchaipenet, A. Molecular interaction of 1-aminocyclopropane-1-carboxylate deaminase (ACCD)-producing endophytic Streptomyces sp. GMKU 336 towards salt-stress resistance of Oryza sativa L. cv. KDML105. Sci. Rep. 2018, 8, 1950. [Google Scholar] [CrossRef] [PubMed]
- Shultana, R.; Kee Zuan, A.T.; Yusop, M.R.; Saud, H.M.; El-Shehawi, A.M. Bacillus tequilensis strain ‘UPMRB9′ improves biochemical attributes and nutrient accumulation in different rice varieties under salinity stress. PLoS ONE 2021, 13, e0260869. [Google Scholar] [CrossRef] [PubMed]
- Sukweenadhi, J.; Balusamy, S.R.; Kim, Y.J.; Lee, C.H.; Kim, Y.J.; Koh, S.C.; Yang, D.C. A growth-promoting bacteria, Paenibacillus yonginensis DCY84T enhanced salt stress tolerance by activating defense-related systems in Panax ginseng. Front. Plant Sci. 2018, 9, 813. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Bano, A.; Rai, S.; Kumar, M.; Ali, J.; Sharma, S.; Pathak, N. ACC deaminase producing plant growth promoting rhizobacteria enhance salinity stress tolerance in Pisum sativum. 3 Biotech 2021, 11, 514. [Google Scholar] [CrossRef] [PubMed]
- Shurigin, V.; Egamberdieva, D.; Li, L.; Davranov, K.; Panosyan, H.; Birkeland, N.K.; Wirth, S.; Bellingrath-Kimura, S.D. Endophytic bacteria associated with halophyte Seidlitzia rosmarinus Ehrenb. ex Boiss. from saline soil of Uzbekistan and their plant beneficial traits. J. Arid Land 2020, 12, 730–740. [Google Scholar] [CrossRef]
- Liu, C.H.; Siew, W.; Hung, Y.T.; Jiang, Y.T.; Huang, C.H. 1-Aminocyclopropane-1-carboxylate (ACC) deaminase gene in Pseudomonas azotoformans is associated with the amelioration of salinity stress in tomato. J. Agric. Food Chem. 2021, 69, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Gamalero, E.; Favale, N.; Bona, E.; Novello, G.; Cesaro, P.; Massa, N.; Glick, B.R.; Orozco-Mosqueda, M.d.C.; Berta, G.; Lingua, G. Screening of bacterial endophytes able to promote plant growth and increase salinity tolerance. Appl. Sci. 2020, 10, 5767. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, D.P. Effect of salt-tolerant plant growth-promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biol. 2015, 17, 288–293. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. The Multifarious PGPR Serratia marcescens CDP-13 Augments induced systemic resistance and enhanced salinity tolerance of wheat (Triticum aestivum L.). PLoS ONE 2016, 11, e0155026. [Google Scholar] [CrossRef]
- Lee, D.G.; Lee, J.M.; Choi, C.G.; Lee, H.; Moon, J.C.; Chung, N. Effect of plant growth-promoting rhizobacterial treatment on growth and physiological characteristics of Triticum aestivum L. under salt stress. Appl. Biol. Chem. 2021, 64, 89. [Google Scholar] [CrossRef]
- Wang, R.; Wang, C.; Feng, Q.; Liou, R.M.; Lin, Y.F. Biological inoculant of salt-tolerant bacteria for plant growth stimulation under different saline soil conditions. J. Microbiol. Biotechnol. 2021, 28, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Djebaili, R.; Pellegrini, M.; Rossi, M.; Forni, C.; Smati, M.; Del Gallo, M.; Kitouni, M. Characterization of Plant Growth-Promoting traits and inoculation effects on Triticum durum of Actinomycetes isolates under salt stress conditions. Soil Syst. 2021, 5, 26. [Google Scholar] [CrossRef]
- Bhise, K.K.; Bhagwat, P.K.; Dandge, P.B. Plant growth-promoting characteristics of salt tolerant Enterobacter cloacae strain KBPD and its efficacy in amelioration of salt stress in Vigna radiata L. J. Plant Growth Regul. 2017, 36, 215–226. [Google Scholar] [CrossRef]
- Kumawat, K.C.; Sharma, P.; Nagpal, S.; Gupta, R.K.; Sirari, A.; Nair, R.M.; Bindumadhava, H.; Singh, S. Dual microbial inoculation, a game changer—Bacterial biostimulants with multifunctional growth promoting traits to mitigate salinity stress in spring mungbean. Front. Microbiol. 2021, 11, 600576. [Google Scholar] [CrossRef] [PubMed]
- Latef, A.A.H.; Abu Alhmad, M.F.; Kordrostami, M.; Abo-Baker, A.B.A.E.; Zakir, A. Inoculation with Azospirillum lipoferum or Azotobacter chroococcum reinforces maize growth by improving physiological activities under saline conditions. J. Plant Growth Regul. 2020, 39, 1293–1306. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.-P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Martínez-Viveros, M.; Jorquera, M.A.; Crowley, D.E.; Gajardo, G.; Mora, M.L. Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J. Soil Sci. Plant Nutr. 2010, 10, 293–319. [Google Scholar] [CrossRef]
- Vassilev, N.; Vassileva, M.; Martos, V.; Garcia Del Moral, L.F.; Kowalska, J.; Tylkowski, B.; Malusá, E. Formulation of microbial inoculants by encapsulation in natural polysaccharides: Focus on beneficial properties of carrier additives and derivatives. Front. Plant Sci. 2020, 1, 270. [Google Scholar] [CrossRef] [PubMed]
- Rubiano-Labrador, C.; Bland, C.; Miotello, G.; Armengaud, J.; Baena, S. Salt stress induced changes in the exoproteome of the halotolerant bacterium Tistlia consotensis deciphered by proteogenomics. PLoS ONE 2015, 10, e0135065. [Google Scholar] [CrossRef] [PubMed]
- Bogati, K.; Walczak, M. The impact of drought stress on soil microbial community, enzyme activities and plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Louca, S.; Polz, M.F.; Mazel, F.; Albright, M.B.N.; Huber, J.A.; O’Connor, M.I.; Ackermann, M.; Hahn, A.S.; Srivastava, D.S.; Crowe, S.A.; et al. Function and functional redundancy in microbial systems. Nat. Ecol. Evol. 2018, 2, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Microbial inoculants: Reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express 2019, 21, 205. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Pepe, O. Microbial consortia: Promising probiotics as plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1801. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 23, 1473. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, Z. PGPR: Present role, mechanism of action and future prospects along bottlenecks in commercialization. EQA-Int. J. Environ. Qual. 2021, 41, 9–15. [Google Scholar]
- Choudhury, A.R.; Choi, J.; Walitang, D.I.; Trivedi, P.; Lee, Y.; Sa, T. ACC deaminase and indole acetic acid producing endophytic bacterial co-inoculation improves physiological traits of red pepper (Capsicum annum L.) under salt stress. J. Plant Physiol. 2021, 267, 153544. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Gómez, S.; Mesa-Marín, J.; Pérez-Romero, J.A.; López-Jurado, J.; García-López, J.V.; Mariscal, V.; Molina-Heredia, F.P.; Pajuelo, E.; Rodríguez-Llorente, I.D.; Flowers, T.J.; et al. Consortia of Plant-Growth-Promoting Rhizobacteria isolated from halophytes improve response of eight crops to soil salinization and climate change conditions. Agronomy 2021, 11, 1609. [Google Scholar] [CrossRef]
- Yañez-Yazlle, M.F.; Romano-Armada, N.; Acreche, M.M.; Rajal, V.B.; Irazusta, V.P. Halotolerant bacteria isolated from extreme environments induce seed germination and growth of chia (Salvia hispanica L.) and quinoa (Chenopodium quinoa Willd.) under saline stress. Ecotoxicol. Environ. Saf. 2021, 218, 112273. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, C.; Sayyed, R.Z.; El Enshasy, H.A.; Vaidya, H.; Sharma, D.; Patel, N.; Malek, R.A.; Syed, A.; Elgorban, A.M.; Ahmad, K.; et al. Halotolerant microbial consortia for sustainable mitigation of salinity stress, growth promotion, and mineral uptake in tomato plants and soil nutrient enrichment. Sustainability 2021, 13, 8369. [Google Scholar] [CrossRef]
- Kerbab, S.; Silini, A.; Chenari Bouket, A.; Cherif-Silini, H.; Eshelli, M.; El Houda Rabhi, N.; Belbahri, L. Mitigation of NaCl Stress in wheat by rhizosphere engineering using salt habitat adapted PGPR halotolerant bacteria. Appl. Sci. 2021, 11, 1034. [Google Scholar] [CrossRef]
- Jabborova, D.; Kannepalli, A.; Davranov, K.; Narimanov, A.; Enakiev, Y.; Syed, A.; Elgorban, A.M.; Bahkali, A.H.; Wirth, S.; Sayyed, R.Z.; et al. Co-inoculation of rhizobacteria promotes growth, yield, and nutrient contents in soybean and improves soil enzymes and nutrients under drought conditions. Sci. Rep. 2021, 11, 22081. [Google Scholar] [CrossRef] [PubMed]
- Mansour, E.; Mahgoub, H.A.M.; Mahgoub, S.A.; El-Sobky, E.-S.E.A.; Abdul-Hamid, M.I.; Kamara, M.M.; AbuQamar, S.F.; El-Rarabily, K.A.; Desoki, E.S.M. Enhancement of drought tolerance in diverse Vicia faba cultivars by inoculation with plant growth-promoting rhizobacteria under newly reclaimed soil conditions. Sci. Rep. 2021, 11, 24142. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Nawaz, F.; Hussain, M.B.; Ikram, R.M. Comparative effects of individual and consortia Plant Growth Promoting Bacteria on physiological and enzymatic mechanisms to confer drought tolerance in maize (Zea mays L.). J. Soil Sci. Plant Nutr. 2021, 21, 3461–3476. [Google Scholar] [CrossRef]
- Cortés-Patiño, S.; Vargas, C.; Álvarez-Flórez, F.; Bonilla, R.; Estrada-Bonilla, G. Potential of Herbaspirillum and Azospirillum consortium to promote growth of perennial ryegrass under water deficit. Microorganisms 2021, 1, 91. [Google Scholar] [CrossRef] [PubMed]
- Igiehon, O.N.; Babalola, O.O. Rhizobium and mycorrhizal fungal species improved soybean yield under drought stress conditions. Curr. Microbiol. 2021, 78, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Bashan, Y.; Prabhu, S.R.; de-Bashan, L.E.; Kloepper, J.W. Disclosure of exact protocols of fermentation, identity of microorganisms within consortia, formation of advanced consortia with microbe-based products. Biol. Fertil. Soils 2020, 56, 443–445. [Google Scholar] [CrossRef]
- Thomloudi, E.-E.; Tsalgatidou, P.C.; Douka, D.; Spantidos, T.-N.; Dimou, M.; Venieraki, A.; Katinakis, P. Multistrain versus single-strain plant growth promoting microbial inoculants-The compatibility issue. Hell. Plant Prot. J. 2019, 12, 61–77. [Google Scholar] [CrossRef]
- Alori, E.T.; Emmanuel, O.C.; Glick, B.R.; Babalola, O.O. Plant-archaea relationships: A potential means to improve crop production in arid and semiarid regions. World J. Microbiol. Biotechnol. 2020, 36, 133. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Glick, B.R. Halotolerant-plant growth-promoting bacteria: Prospects for mitigating salinity stress in plants. Environ. Exp. Bot. 2020, 178, 104124. [Google Scholar] [CrossRef]
- Larimer, A.L.; Clay, K.; Bever, J.D. Synergism and context dependency of interactions between arbuscular mycorrhizal fungi and rhizobia with a prairie legume. Ecology 2014, 95, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Raklami, A.; Bechtaoui, N.; Tahiri, A.I.; Anli, M.; Meddich, A.; Oufdou, K. Use of rhizobacteria and mycorrhizae consortium in the open field as a strategy for improving crop nutrition, productivity and soil fertility. Front. Microbiol. 2019, 10, 1106. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Paredes, A.; Valdés, G.; Nuti, M. Ecosystem functions of microbial consortia in sustainable sgriculture. Agronomy 2020, 10, 1902. [Google Scholar] [CrossRef]
- Veselaj, E.; Sallaku, G.; Balliu, A. Tripartite relationships in legume crops are plant-microorganism-specific and strongly influenced by salinity. Agriculture 2018, 8, 117. [Google Scholar] [CrossRef]
- Guzmán, P.G.; Troncoso, M.D.P.; Monfil, V.O.; Estrella, A.H. Trichoderma species: Versatile plant symbionts. Phytopathology 2019, 109, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Gill, R.; Trivedi, D.K.; Anjum, N.A.; Sharma, K.K.; Ansari, M.W.; Ansari, A.A.; Johri, A.K.; Prasad, R.; Pereira, E.; et al. Piriformospora indica: Potential and significance in plant stress tolerance. Front. Microbiol. 2016, 22, 332. [Google Scholar] [CrossRef] [PubMed]
- Miceli, A.; Moncada, A.; Vetrano, F. Use of microbial biostimulants to Increase the salinity tolerance of vegetable transplants. Agronomy 2021, 11, 143. [Google Scholar] [CrossRef]
- Liu, B.; Jing, D.; Liu, F.; Ma, H.; Liu, X.; Peng, L. Serendipita indica alleviates drought stress responses in walnut (Juglans regia L.) seedlings by stimulating osmotic adjustment and antioxidant defense system. Appl. Microbiol. Biotechnol. 2021, 105, 8951–8968. [Google Scholar] [CrossRef] [PubMed]
- Jangir, P.; Shekhawat, P.K.; Bishnoi, A.; Ram, H.; Soni, P. Role of Serendipita indica in enhancing drought tolerance in crops, Physiol. Mol. Plant Pathol. 2021, 116, 101691. [Google Scholar] [CrossRef]
- Li, L.; Li, L.; Wang, X.; Zhu, P.; Wu, H.; Qi, S. Plant growth-promoting endophyte Piriformospora indica alleviates salinity stress in Medicago truncatula. Plant Physiol. Biochem. 2017, 119, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Yun, P.; Xu, L.; Wang, S.S.; Shabala, L.; Shabala, S.; Zhang, W.Y. Piriformospora indica improves salinity stress tolerance in Zea mays L. plants by regulating Na+ and K+ loading in root and allocating K+ in shoots. Plant Growth Regul. 2018, 86, 323–331. [Google Scholar] [CrossRef]
- Ghorbani, A.; Omran, V.; Razavi, S.M.; Pirdashti, H.; Ranjbar, M. Piriformospora indica confers salinity tolerance on tomato (Lycopersicon esculentum Mill.) through amelioration of nutrient accumulation, K+/Na+ homeostasis and water status. Plant Cell Rep. 2019, 38, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Del Barrio-Duque, A.; Ley, J.; Samad, A.; Antonielli, L.; Sessitsch, A.; Compant, S. Beneficial endophytic bacteria-Serendipita indica interaction for crop enhancement and resistance to phytopathogens. Front. Microbiol. 2019, 19, 2888. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamalero, E.; Glick, B.R. Recent Advances in Bacterial Amelioration of Plant Drought and Salt Stress. Biology 2022, 11, 437. https://doi.org/10.3390/biology11030437
Gamalero E, Glick BR. Recent Advances in Bacterial Amelioration of Plant Drought and Salt Stress. Biology. 2022; 11(3):437. https://doi.org/10.3390/biology11030437
Chicago/Turabian StyleGamalero, Elisa, and Bernard R. Glick. 2022. "Recent Advances in Bacterial Amelioration of Plant Drought and Salt Stress" Biology 11, no. 3: 437. https://doi.org/10.3390/biology11030437
APA StyleGamalero, E., & Glick, B. R. (2022). Recent Advances in Bacterial Amelioration of Plant Drought and Salt Stress. Biology, 11(3), 437. https://doi.org/10.3390/biology11030437







