The Utility of ADC First-Order Histogram Features for the Prediction of Metachronous Metastases in Rectal Cancer: A Preliminary Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Image Acquisition
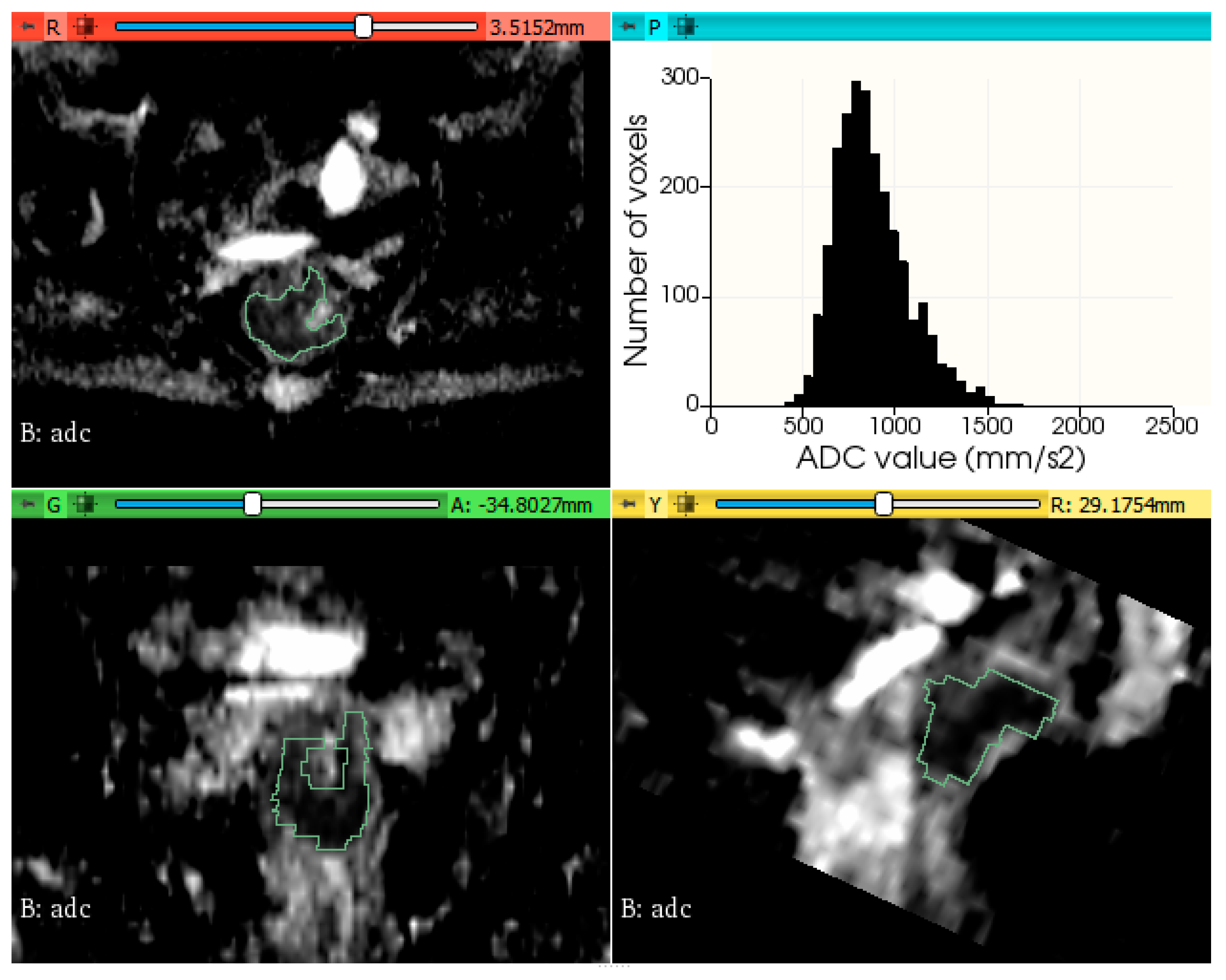
2.3. Tumor Segmentation and Feature Extraction
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal Cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Valentini, V.; van Stiphout, R.G.P.M.; Lammering, G.; Gambacorta, M.A.; Barba, M.C.; Bebenek, M.; Bonnetain, F.; Bosset, J.F.; Bujko, K.; Cionini, L.; et al. Selection of Appropriate End-Points (PCR vs. 2yDFS) for Tailoring Treatments with Prediction Models in Locally Advanced Rectal Cancer. Radiother. Oncol. 2015, 114, 302–309. [Google Scholar] [CrossRef]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.R.; Villanueva, M.T.; Witzigmann, H.; et al. Preoperative versus Postoperative Chemoradiotherapy for Locally Advanced Rectal Cancer: Results of the German CAO/ARO/AIO-94 Randomized Phase III Trial after a Median Follow-up of 11 Years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef]
- Peeters, K.C.M.J.; Marijnen, C.A.M.; Nagtegaal, I.D.; Kranenbarg, E.K.; Putter, H.; Wiggers, T.; Rutten, H.; Pahlman, L.; Glimelius, B.; Leer, J.W.; et al. The TME Trial after a Median Follow-up of 6 Years: Increased Local Control but No Survival Benefit in Irradiated Patients with Resectable Rectal Carcinoma. Ann. Surg. 2007, 246, 693–701. [Google Scholar] [CrossRef]
- Manfredi, S.; Lepage, C.; Hatem, C.; Coatmeur, O.; Faivre, J.; Bouvier, A.M. Epidemiology and Management of Liver Metastases from Colorectal Cancer. Ann. Surg. 2006, 244, 254–259. [Google Scholar] [CrossRef]
- Van Gijn, W.; Marijnen, C.A.M.; Nagtegaal, I.D.; Kranenbarg, E.M.K.; Putter, H.; Wiggers, T.; Rutten, H.J.T.; Påhlman, L.; Glimelius, B.; van de Velde, C.J.H. Preoperative Radiotherapy Combined with Total Mesorectal Excision for Resectable Rectal Cancer: 12-Year Follow-up of the Multicentre, Randomised Controlled TME Trial. Lancet Oncol. 2011, 12, 575–582. [Google Scholar] [CrossRef]
- Taylor, F.G.M.; Quirke, P.; Heald, R.J.; Moran, B.J.; Blomqvist, L.; Swift, I.R.; Sebag-Montefiore, D.; Tekkis, P.; Brown, G. Preoperative Magnetic Resonance Imaging Assessment of Circumferential Resection Margin Predicts Disease-Free Survival and Local Recurrence: 5-Year Follow-up Results of the MERCURY Study. J. Clin. Oncol. 2014, 32, 34–43. [Google Scholar] [CrossRef]
- Maas, M.; Nelemans, P.J.; Valentini, V.; Crane, C.H.; Capirci, C.; Rödel, C.; Nash, G.M.; Kuo, L.J.; Glynne-Jones, R.; García-Aguilar, J.; et al. Adjuvant Chemotherapy in Rectal Cancer: Defining Subgroups Who May Benefit after Neoadjuvant Chemoradiation and Resection: A Pooled Analysis of 3313 Patients. Int. J. Cancer 2015, 137, 212–220. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Y.; Sun, G.; Zheng, K.; Lou, Z.; Gao, X.-H.; Hao, L.-Q.; Liu, L.-J.; Meng, R.-G.; Zhang, W. Rectal Cancer Patients with Downstaging after Neoadjuvant Chemoradiotherapy and Radical Resection Do Not Benefit from Adjuvant Chemotherapy. Ann. Transl. Med. 2020, 8, 743. [Google Scholar] [CrossRef]
- Dossa, F.; Acuna, S.A.; Rickles, A.S.; Berho, M.; Wexner, S.D.; Quereshy, F.A.; Baxter, N.N.; Chadi, S.A. Association Between Adjuvant Chemotherapy and Overall Survival in Patients with Rectal Cancer and Pathological Complete Response After Neoadjuvant Chemotherapy and Resection. JAMA Oncol. 2018, 4, 930–937. [Google Scholar] [CrossRef] [Green Version]
- Polanco, P.M.; Mokdad, A.A.; Zhu, H.; Choti, M.A.; Huerta, S. Association of Adjuvant Chemotherapy with Overall Survival in Patients with Rectal Cancer and Pathologic Complete Response Following Neoadjuvant Chemotherapy and Resection. JAMA Oncol. 2018, 4, 938–943. [Google Scholar] [CrossRef]
- Breugom, A.J.; Swets, M.; Bosset, J.F.; Collette, L.; Sainato, A.; Cionini, L.; Glynne-Jones, R.; Counsell, N.; Bastiaannet, E.; van den Broek, C.B.M.; et al. Adjuvant Chemotherapy after Preoperative (Chemo)Radiotherapy and Surgery for Patients with Rectal Cancer: A Systematic Review and Meta-Analysis of Individual Patient Data. Lancet. Oncol. 2015, 16, 200–207. [Google Scholar] [CrossRef]
- Chung, M.J.; Lee, J.H.; Lee, J.H.; Kim, S.H.; Song, J.H.; Jeong, S.; Yu, M.; Nam, T.K.; Jeong, J.U.; Jang, H.S. Adjuvant Chemotherapy in Rectal Cancer Patients Treated with Preoperative Chemoradiation and Total Mesorectal Excision: A Multicenter and Retrospective Propensity-Score Matching Study. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 438–448. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Cederquist, L.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; et al. Rectal Cancer, Version 2.2018 Clinical Practice Guidelines in Oncology. JNCCN J. Natl. Compr. Cancer Netw. 2018, 16, 874–901. [Google Scholar] [CrossRef] [Green Version]
- Brown, G.; Radcliffe, A.G.; Newcombe, R.G.; Dallimore, N.S.; Bourne, M.W.; Williams, G.T. Preoperative Assessment of Prognostic Factors in Rectal Cancer Using High-Resolution Magnetic Resonance Imaging. Br. J. Surg. 2003, 90, 355–364. [Google Scholar] [CrossRef]
- Lee, E.S.; Kim, M.J.; Park, S.C.; Hur, B.Y.; Hyun, J.H.; Chang, H.J.; Baek, J.Y.; Kim, S.Y.; Kim, D.Y.; Oh, J.H. Magnetic Resonance Imaging-Detected Extramural Venous Invasion in Rectal Cancer before and after Preoperative Chemoradiotherapy: Diagnostic Performance and Prognostic Significance. Eur. Radiol. 2018, 28, 496–505. [Google Scholar] [CrossRef]
- Cienfuegos, J.A.; Rotellar, F.; Baixauli, J.; Beorlegui, C.; Sola, J.J.; Arbea, L.; Pastor, C.; Arredondo, J.; Hernández-Lizoáin, J.L. Impact of Perineural and Lymphovascular Invasion on Oncological Outcomes in Rectal Cancer Treated with Neoadjuvant Chemoradiotherapy and Surgery. Ann. Surg. Oncol. 2015, 22, 916–923. [Google Scholar] [CrossRef]
- Ramón y Cajal, S.; Sesé, M.; Capdevila, C.; Aasen, T.; de Mattos-Arruda, L.; Diaz-Cano, S.J.; Hernández-Losa, J.; Castellví, J. Clinical Implications of Intratumor Heterogeneity: Challenges and Opportunities. J. Mol. Med. 2020, 98, 161–177. [Google Scholar] [CrossRef] [Green Version]
- Jamal-Hanjani, M.; Quezada, S.A.; Larkin, J.; Swanton, C. Translational Implications of Tumor Heterogeneity. Clin. Cancer Res. 2015, 21, 1258–1266. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, J.P.B.; Rose, C.J.; Waterton, J.C.; Carano, R.A.D.; Parker, G.J.M.; Jackson, A. Imaging Intratumor Heterogeneity: Role in Therapy Response, Resistance, and Clinical Outcome. Clin. Cancer Res. 2015, 21, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Surov, A.; Meyer, H.J.; Wienke, A. Associations between Apparent Diffusion Coefficient (ADC) and KI 67 in Different Tumors: A Meta-Analysis. Part 1: ADC Mean. Oncotarget 2017, 8, 75434–75444. [Google Scholar] [CrossRef] [Green Version]
- Surov, A.; Meyer, H.J.; Wienke, A. Correlation between Apparent Diffusion Coefficient (ADC) and Cellularity Is Different in Several Tumors: A Meta-Analysis. Oncotarget 2017, 8, 59492–59499. [Google Scholar] [CrossRef] [Green Version]
- Surov, A.; Pech, M.; Powerski, M.; Woidacki, K.; Wienke, A. Pretreatment Apparent Diffusion Coefficient Cannot Predict Histopathological Features and Response to Neoadjuvant Radiochemotherapy in Rectal Cancer: A Meta-Analysis. Dig. Dis. 2021, 40, 33–49. [Google Scholar] [CrossRef]
- Just, N. Improving Tumour Heterogeneity MRI Assessment with Histograms. Br. J. Cancer 2014, 111, 2205–2213. [Google Scholar] [CrossRef] [Green Version]
- Donati, O.F.; Mazaheri, Y.; Afaq, A.; Vargas, H.A.; Zheng, J.; Moskowitz, C.S.; Hricak, H.; Akin, O. Prostate Cancer Aggressiveness: Assessment with Whole-Lesion Histogram Analysis of the Apparent Diffusion Coefficient. Radiology 2014, 271, 143–152. [Google Scholar] [CrossRef]
- Park, G.E.; Kim, S.H.; Kim, E.J.; Kang, B.J.; Park, M.S. Histogram Analysis of Volume-Based Apparent Diffusion Coefficient in Breast Cancer. Acta Radiol. 2017, 58, 1294–1302. [Google Scholar] [CrossRef]
- Xue, H.; Ren, C.; Yang, J.; Sun, Z.; Li, S.; Jin, Z.; Shen, K.; Zhou, W. Histogram Analysis of Apparent Diffusion Coefficient for the Assessment of Local Aggressiveness of Cervical Cancer. Arch. Gynecol. Obstet. 2014, 290, 341–348. [Google Scholar] [CrossRef]
- Zhang, K.; Zheng, Y.; Huang, H.; Lei, J. Preliminary Study on Predicting Pathological Staging and Immunohistochemical Markers of Rectal Cancer Based on ADC Histogram Analysis. Acad. Radiol. 2021, 28 (Suppl. 1), S184–S191. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Tang, H.; Hu, X.; Shen, Y.; Kamel, I.; Li, Z.; Hu, D. Rectal Cancer Invasiveness: Whole-Lesion Diffusion-Weighted Imaging (DWI) Histogram Analysis by Comparison of Reduced Field-of-View and Conventional DWI Techniques. Sci. Rep. 2019, 9, 18760. [Google Scholar] [CrossRef] [PubMed]
- Staal, F.C.R.; van der Reijd, D.J.; Taghavi, M.; Lambregts, D.M.J.; Beets-Tan, R.G.H.; Maas, M. Radiomics for the Prediction of Treatment Outcome and Survival in Patients with Colorectal Cancer: A Systematic Review. Clin. Colorectal Cancer 2021, 20, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Di Re, A.M.; Sun, Y.; Sundaresan, P.; Hau, E.; Toh, J.W.T.; Gee, H.; Or, M.; Haworth, A. MRI Radiomics in the Prediction of Therapeutic Response to Neoadjuvant Therapy for Locoregionally Advanced Rectal Cancer: A Systematic Review. Expert Rev. Anticancer Ther. 2021, 21, 425–449. [Google Scholar] [CrossRef]
- Nardone, V.; Boldrini, L.; Grassi, R.; Franceschini, D.; Morelli, I.; Becherini, C.; Loi, M.; Greto, D.; Desideri, I. Radiomics in the Setting of Neoadjuvant Radiotherapy: A New Approach for Tailored Treatment. Cancers 2021, 13, 3590. [Google Scholar] [CrossRef]
- Chiloiro, G.; Rodriguez-Carnero, P.; Lenkowicz, J.; Casà, C.; Masciocchi, C.; Boldrini, L.; Cusumano, D.; Dinapoli, N.; Meldolesi, E.; Carano, D.; et al. Delta Radiomics Can Predict Distant Metastases in Locally Advanced Rectal Cancer: The Challenge to Personalize the Cure. Front. Oncol. 2020, 10, 595012. [Google Scholar] [CrossRef]
- Jeon, S.H.; Song, C.; Chie, E.K.; Kim, B.; Kim, Y.H.; Chang, W.; Lee, Y.J.; Chung, J.H.; Chung, J.B.; Lee, K.W.; et al. Delta-Radiomics Signature Predicts Treatment Outcomes after Preoperative Chemoradiotherapy and Surgery in Rectal Cancer. Radiat. Oncol. 2019, 14, 43. [Google Scholar] [CrossRef] [Green Version]
- Liang, M.; Cai, Z.; Zhang, H.; Huang, C.; Meng, Y.; Zhao, L.; Li, D.; Ma, X.; Zhao, X. Machine Learning-Based Analysis of Rectal Cancer MRI Radiomics for Prediction of Metachronous Liver Metastases. Acad. Radiol. 2019, 26, 1495–1504. [Google Scholar] [CrossRef]
- Traverso, A.; Kazmierski, M.; Shi, Z.; Kalendralis, P.; Welch, M.; Nissen, H.D.; Jaffray, D.; Dekker, A.; Wee, L. Stability of Radiomic Features of Apparent Diffusion Coefficient (ADC) Maps for Locally Advanced Rectal Cancer in Response to Image Pre-Processing. Phys. Med. 2019, 61, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Gourtsoyianni, S.; Doumou, G.; Prezzi, D.; Taylor, B.; Stirling, J.J.; Taylor, N.J.; Siddique, M.; Cook, G.J.R.; Glynne-Jones, R.; Goh, V. Primary Rectal Cancer: Repeatability of Global and Local-Regional MR Imaging Texture Features. Radiology 2017, 284, 552–561. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Yang, X.; Du, X.; Zhuo, Z.; Xin, L.; Cheng, X. Whole-Tumour Diffusion Kurtosis MR Imaging Histogram Analysis of Rectal Adenocarcinoma: Correlation with Clinical Pathologic Prognostic Factors. Eur. Radiol. 2018, 28, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.; Xu, L.; Li, Z.; Lv, H.; Dong, N.; Li, W.; Yang, Z.; Wang, Z.; Jin, E. Application of Texture Analysis Based on Apparent Diffusion Coefficient Maps in Discriminating Different Stages of Rectal Cancer. J. Magn. Reson. Imaging 2017, 45, 1798–1808. [Google Scholar] [CrossRef]
- Peng, Y.; Tang, H.; Meng, X.; Shen, Y.; Hu, D.; Kamel, I.; Li, Z. Histological Grades of Rectal Cancer: Whole-Volume Histogram Analysis of Apparent Diffusion Coefficient Based on Reduced Field-of-View Diffusion-Weighted Imaging. Quant. Imaging Med. Surg. 2020, 10, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiang, Z.; Guan, Y.; Chen, Y.; Huang, X.; Liu, S.; He, J.; Zhou, Z.; Ge, Y. Whole-Lesion Apparent Diffusion Coefficient First- and Second-Order Texture Features for the Characterization of Rectal Cancer Pathological Factors. J. Comput. Assist. Tomogr. 2018, 42, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, Y.; Wang, X.; Yu, Y.; Zhou, X.; Luan, K. Histogram Analysis of Diffusion-Weighted Magnetic Resonance Imaging as a Biomarker to Predict Lymph Node Metastases in T3 Stage Rectal Carcinoma. Cancer Manag. Res. 2021, 13, 2983–2993. [Google Scholar] [CrossRef]
- Chidambaram, V.; Brierley, J.D.; Cummings, B.; Bhayana, R.; Menezes, R.J.; Kennedy, E.D.; Kirsch, R.; Jhaveri, K.S. Investigation of Volumetric Apparent Diffusion Coefficient Histogram Analysis for Assessing Complete Response and Clinical Outcomes Following Pre-Operative Chemoradiation Treatment for Rectal Carcinoma. Abdom. Radiol. 2017, 42, 1310–1318. [Google Scholar] [CrossRef]
- Van Heeswijk, M.M.; Lambregts, D.M.J.; Maas, M.; Lahaye, M.J.; Ayas, Z.; Slenter, J.M.G.M.; Beets, G.L.; Bakers, F.C.H.; Beets-Tan, R.G.H. Measuring the Apparent Diffusion Coefficient in Primary Rectal Tumors: Is There a Benefit in Performing Histogram Analyses? Abdom. Radiol. 2017, 42, 1627–1636. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.H.; Oh, S.N.; Rha, S.E.; Choi, J.I.; Lee, S.H.; Jang, H.S.; Kim, J.G.; Grimm, R.; Son, Y. Diffusion-Weighted Imaging: Apparent Diffusion Coefficient Histogram Analysis for Detecting Pathologic Complete Response to Chemoradiotherapy in Locally Advanced Rectal Cancer. J. Magn. Reson. Imaging 2016, 44, 212–220. [Google Scholar] [CrossRef]
- Xie, H.; Wu, G. Application of Diffusion Kurtosis Imaging and Histogram Analysis for Assessing Preoperative Stages of Rectal Cancer. Gastroenterol. Res. Pract. 2018, 2018, 9786932. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Lin, M.B.; Xu, J.L.; Zhang, L.H.; Zuo, X.M.; Zhang, Z.S.; Liu, M.X.; Xu, J.M. Are ADC Values of Readout-Segmented Echo-Planar Diffusion-Weighted Imaging (RESOLVE) Correlated with Pathological Prognostic Factors in Rectal Adenocarcinoma? World J. Surg. Oncol. 2018, 16, 138. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.Y.; Chen, M.D.; Zhao, X.X.; Yan, C.G.; Mei, Y.J.; Xu, Y.K. Multiple Mathematical Models of Diffusion-Weighted Magnetic Resonance Imaging Combined with Prognostic Factors for Assessing the Response to Neoadjuvant Chemotherapy and Radiation Therapy in Locally Advanced Rectal Cancer. Eur. J. Radiol. 2019, 110, 249–255. [Google Scholar] [CrossRef]
- Palmisano, A.; Di Chiara, A.; Esposito, A.; Rancoita, P.M.V.; Fiorino, C.; Passoni, P.; Albarello, L.; Rosati, R.; del Maschio, A.; de Cobelli, F. MRI Prediction of Pathological Response in Locally Advanced Rectal Cancer: When Apparent Diffusion Coefficient Radiomics Meets Conventional Volumetry. Clin. Radiol. 2020, 75. [Google Scholar] [CrossRef] [PubMed]
- Nougaret, S.; Vargas, H.A.; Lakhman, Y.; Sudre, R.; Do, R.K.G.; Bibeau, F.; Azria, D.; Assenat, E.; Molinari, N.; Pierredon, M.A.; et al. Intravoxel Incoherent Motion-Derived Histogram Metrics for Assessment of Response after Combined Chemotherapy and Radiation Therapy in Rectal Cancer: Initial Experience and Comparison between Single-Section and Volumetric Analyses. Radiology 2016, 280, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Choi, S.H.; Kim, Y.J.; Kim, K.G.; Sohn, C.H.; Kim, J.H.; Yun, T.J.; Chang, K.H. Gliomas: Histogram Analysis of Apparent Diffusion Coefficient Maps with Standard- or High-b-Value Diffusion-Weighted MR Imaging-Correlation with Tumor Grade. Radiology 2011, 261, 882–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, M.; Kozawa, E.; Tanisaka, M.; Hasegawa, K.; Yasuda, M.; Sakai, F. Utility of Histogram Analysis of Apparent Diffusion Coefficient Maps Obtained Using 3.0T MRI for Distinguishing Uterine Carcinosarcoma from Endometrial Carcinoma. J. Magn. Reson. Imaging 2016, 43, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Radiomic Features—Pyradiomics v3.0.1.post11+g03d23f7 Documentation. Available online: https://pyradiomics.readthedocs.io/en/latest/features.html#module-radiomics.firstorder (accessed on 31 December 2021).
- Lu, Z.; Wang, L.; Xia, K.; Jiang, H.; Weng, X.; Jiang, J.; Wu, M. Prediction of Clinical Pathologic Prognostic Factors for Rectal Adenocarcinoma: Volumetric Texture Analysis Based on Apparent Diffusion Coefficient Maps. J. Med. Syst. 2019, 43, 331. [Google Scholar] [CrossRef]
- Lu, Z.H.; Jiang, H.; Xia, K.J.; Jiang, J.L.; Wu, M. Textural Differences Based on Apparent Diffusion Coefficient Maps for Discriminating PT3 Subclasses of Rectal Adenocarcinoma. World J. Clin. Cases 2021, 9, 6987. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhang, C.; Zou, S.; Zhao, X.; Xu, K.; Zhang, H.; Zhou, C. MRI Texture Analysis in Predicting Treatment Response to Neoadjuvant Chemoradiotherapy in Rectal Cancer. Oncotarget 2018, 9, 11999–12008. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Huang, D.Y.; Li, Y.; Dai, X.; Shi, H.-B. Correlation of Standard Diffusion-Weighted Imaging and Diffusion Kurtosis Imaging with Distant Metastases of Rectal Carcinoma. J. Magn. Reson. Imaging 2016, 44, 221–229. [Google Scholar] [CrossRef]


| MRI Parameter | TSE T2-Weighted Image | DWI | ||
|---|---|---|---|---|
| Sagittal | HR Coronal Oblique | HR Axial Oblique | ||
| TR (ms) | 3500 | 3500 | 4000 | 5800 |
| TE (ms) | 91 | 91 | 80 | 96 |
| Slice no | 28 | 25 | 25 | 30 |
| Bandwidth (Hz/pixel) | 391 | 391 | 391 | 1132 |
| FOV (mm) | 220 | 220 | 200 | 250 |
| Slice thickness (mm) | 3 | 4 | 3 | 4 |
| Matrix | 256 × 256 | 256 × 256 | 256 × 256 | 136 × 160 |
| Acquisition time (min) | 4 | 5.5 | 6 | 4.5 |
| ADC First-Order Histogram Feature | Description |
|---|---|
| Minimum | The minimum ADC value within the VOI. |
| Maximum | The maximum ADC value within the VOI. |
| Mean | The average ADC value within the VOI. |
| Median | The ADC value below 50% of all ADC voxel values lie. |
| 10th percentile | The ADC value below 10% of all ADC voxel values lie. |
| 90th percentile | The ADC value below 90% of all ADC voxel values lie. |
| Skewness | Measures the asymmetry of the distribution of ADC values around the mean value. |
| Kurtosis | Measures the ‘peakedness’ of the distribution of ADC values within the VOI. |
| Interquartile range | Measures the spread of the distribution of ADC values, defined as the difference between 75th and 25th percentile. |
| Entropy | Measures the inherent randomness in the ADC values within the VOI. |
| Energy | Measures the squared magnitude of ADC values within the VOI. |
| Uniformity | Measures the homogeneity in the ADC values within the VOI. |
| Variance | Measures squared distances of each ADC value of a histogram from the mean. |
| Mean absolute deviation | Mean distance of all ADC values from the mean value of the image array. |
| Robust mean absolute deviation | Mean distance of all ADC values from the mean value calculated on the subset of image array with ADC in between, or equal to the 10th and 90th percentile. |
| Range | Measures difference between the highest and lowest ADC values. |
| RootMeanSquared | Square root of the mean of all the squared ADC values of the histogram. This feature is another measure of the magnitude of a histogram. |
| Variable | Non Metastases (MM-) Group (n = 37) | Metachronous Metastases (MM+) Group (n = 15) | p Value |
|---|---|---|---|
| Age (years) * | 59.27 ± 11.11 | 61.87 ± 9.85 | 0.43 |
| Gender | 0.29 | ||
| Male | 27 | 8 | |
| Female | 10 | 7 | |
| Tumor length (mm) * | 58.22 ± 19.74 | 53.93 ± 18.57 | 0.47 |
| Tumor differentiation grade | 0.41 | ||
| G1–G2 | 36 | 13 | |
| G3 | 1 | 2 | |
| Clinical tumor stage (cT) | |||
| T2 | 9 | 3 | 0.98 |
| T3–T4 | 28 | 12 | |
| Clinical nodal stage (cN) | 0.93 | ||
| N1 | 17 | 6 | |
| N2 | 20 | 9 | |
| Mesorectal fascia (MRF) involvement | 0.80 | ||
| Positive | 29 | 12 | |
| Negative | 8 | 3 | |
| Extramural vascular invasion (EMVI) | 0.95 | ||
| Positive | 4 | 1 | |
| Negative | 33 | 14 | |
| Pathological tumor stage (pT) | 0.15 | ||
| pT0-pT2 | 17 | 3 | |
| pT3 | 20 | 12 | |
| Pathological nodal stage (pN) | 0.30 | ||
| pN0 | 27 | 8 | |
| pN1-N2 | 10 | 7 |
| ADC First-Order Feature | MM- | MM+ | p Value |
|---|---|---|---|
| Minimum ^ | 310.84 ± 193.73 | 243.87 ± 210.26 | 0.28 |
| Maximum ^ | 1972.22 ± 284.54 | 2047.13 ± 294.47 | 0.40 |
| Mean ^ | 927.20 ± 100.45 | 974.48 ± 93.91 | 0.12 |
| Median ^ | 901.96 ± 98.87 | 949.07 ± 104.04 | 0.13 |
| 10th percentile ^ | 679.14 ± 101.11 | 694.65 ± 91.58 | 0.61 |
| 90th percentile ^ | 1210.20 ± 112.90 | 1293.60 ± 103.65 | 0.02 * |
| Skewness | 0.72 ± 0.41 | 0.60 ± 0.29 | 0.32 |
| Kurtosis | 4.52 ± 1.11 | 3.90 ± 0.74 | 0.05 |
| Interquartile Range | 269.62 ± 33.63 | 308.42 ± 51.64 | 0.002 * |
| Entropy | 5.04 ± 0.15 | 5.20 ± 0.22 | 0.005 * |
| Energy | 1,465,303,600.05 ± 1,764,343,232.40 | 1,814,657,493.13 ± 1,913,810,213.47 | 0.53 |
| Uniformity | 0.037 ± 0.004 | 0.032 ± 0.005 | 0.004 * |
| Variance | 48,432.81 ± 11,167.16 | 59,287.71 ± 18,590.48 | 0.01 * |
| Mean absolute deviation | 168.38 ± 19.22 | 188.70 ± 29.88 | 0.005 * |
| Robust mean Absolute deviation | 112.90 ± 13.60 | 129.40 ± 21.38 | 0.002 * |
| Range | 1661.38 ± 340.22 | 1803.27 ± 467.76 | 0.23 |
| RootMeanSquared | 953.14 ± 98.66 | 1004.59 ± 92.28 | 0.08 |
| ADC First-Order Feature | Cut-Off Value | AUC [95% CI] | Se [95% CI] | Sp [95% CI] | PPV [95% CI] | NPV [95% CI] |
|---|---|---|---|---|---|---|
| 90th percentile | >1236.2 * | 0.74 [0.60–0.85] | 80.0 [51.9–95.7] | 64.86 [47.5–79.8] | 48.0 [27.8–68.7] | 88.9 [70.8–97.6] |
| Interquartile range | >287.25 | 0.72 [0.58–0.83] | 73.33 [38.4–88.2] | 75.68 [58.8–88.2] | 52.6 [28.9–75.6] | 84.8 [68.1–94.9] |
| Entropy | >5.125 | 0.7 [0.56–0.82] | 60.00 [32.3–83.7] | 83.78 [68.0–93.8] | 60.00 [32.3–83.7] | 83.8 [68.0–93.8] |
| Uniformity | ≤0.0344 | 0.74 [0.60–0.85] | 73.33 [44.9–92.2] | 78.38 [61.8–90.2] | 57.9 [33.5–79.7] | 87.9 [71.8–96.6] |
| Variance | >57046 | 0.65 [0.51–0.78] | 53.33 [26.6–78.7] | 86.49 [71.2–95.5] | 61.5 [31.6–86.1] | 82.1 [66.5–92.5] |
| Mean absolute deviation | >175.89 | 0.70 [0.55–0.82] | 66.67 [38.4–88.2] | 78.38 [61.8–90.2] | 55.6 [30.8–78.5] | 85.3 [68.9–95.0] |
| Robust mean absolute deviation | >119.2689 | 0.73 [0.59–0.84] | 73.33 [44.9–92.2] | 78.38 [61.8–90.2] | 57.9 [33.5–79.7] | 87.9 [71.8–96.6] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boca, B.; Caraiani, C.; Popa, L.; Lebovici, A.; Feier, D.S.; Bodale, C.; Buruian, M.M. The Utility of ADC First-Order Histogram Features for the Prediction of Metachronous Metastases in Rectal Cancer: A Preliminary Study. Biology 2022, 11, 452. https://doi.org/10.3390/biology11030452
Boca B, Caraiani C, Popa L, Lebovici A, Feier DS, Bodale C, Buruian MM. The Utility of ADC First-Order Histogram Features for the Prediction of Metachronous Metastases in Rectal Cancer: A Preliminary Study. Biology. 2022; 11(3):452. https://doi.org/10.3390/biology11030452
Chicago/Turabian StyleBoca (Petresc), Bianca, Cosmin Caraiani, Loredana Popa, Andrei Lebovici, Diana Sorina Feier, Carmen Bodale, and Mircea Marian Buruian. 2022. "The Utility of ADC First-Order Histogram Features for the Prediction of Metachronous Metastases in Rectal Cancer: A Preliminary Study" Biology 11, no. 3: 452. https://doi.org/10.3390/biology11030452






