Structural and Functional Aspects of the Spleen in Molly Fish Poecilia sphenops (Valenciennes, 1846): Synergistic Interactions of Stem Cells, Neurons, and Immune Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Histological and Histochemical Analysis
2.3. Semithin Sections and Transmission Electron Microscopy (TEM) Analysis
2.4. Immunohistochemical Analysis
2.5. Digitally Colored TEM Images
3. Results
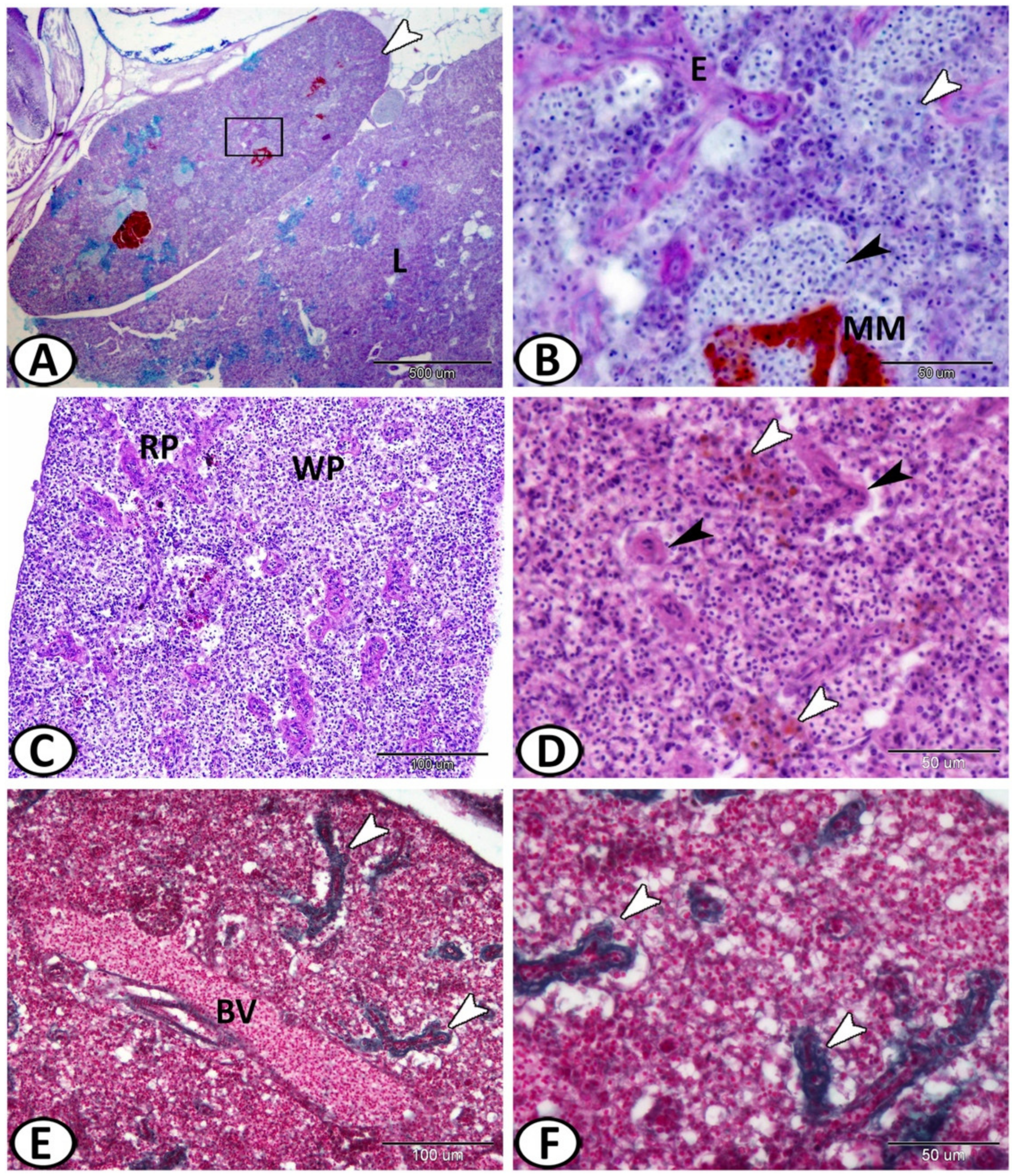
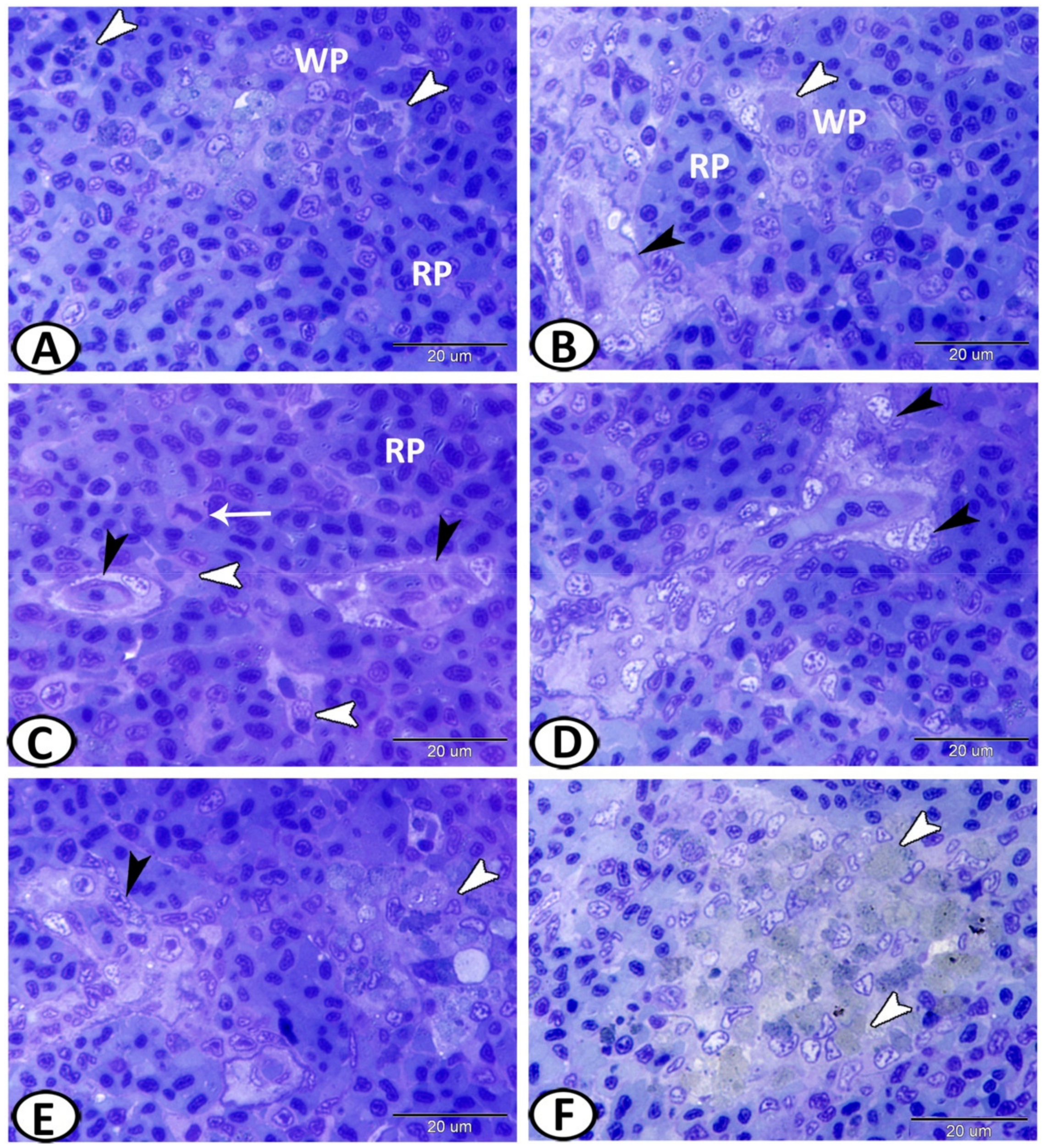
3.1. Histological and Histochemical Analysis
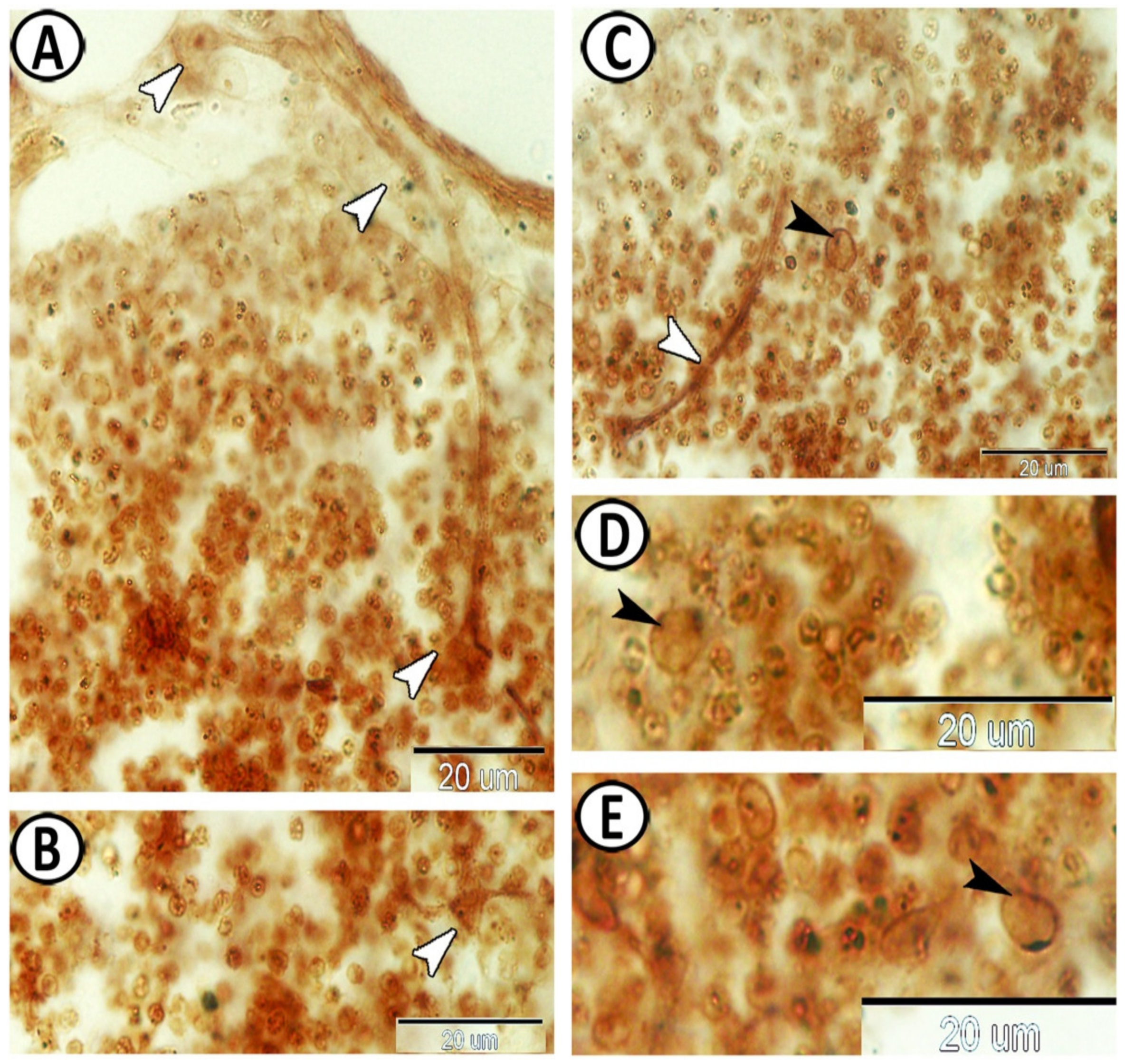
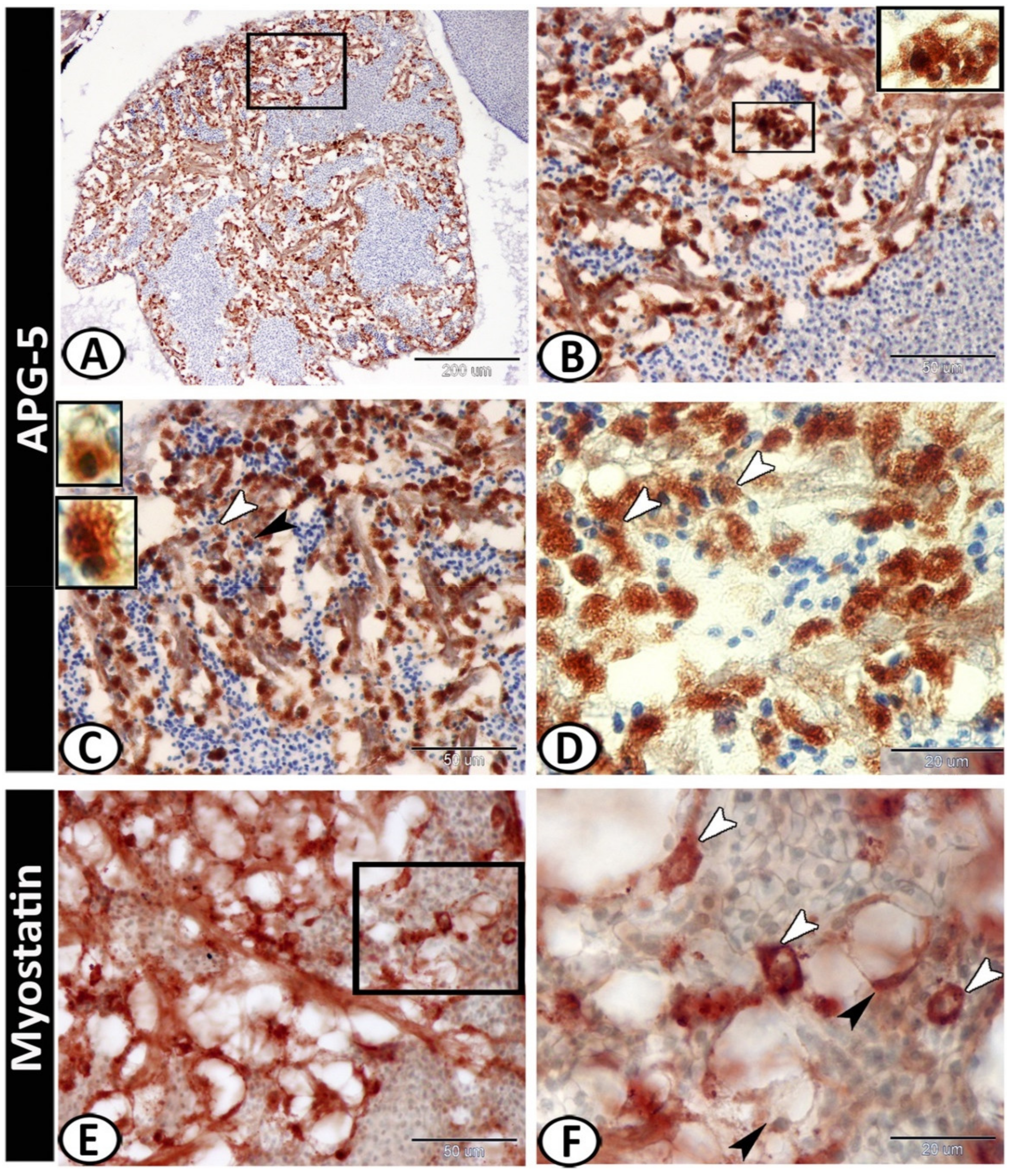
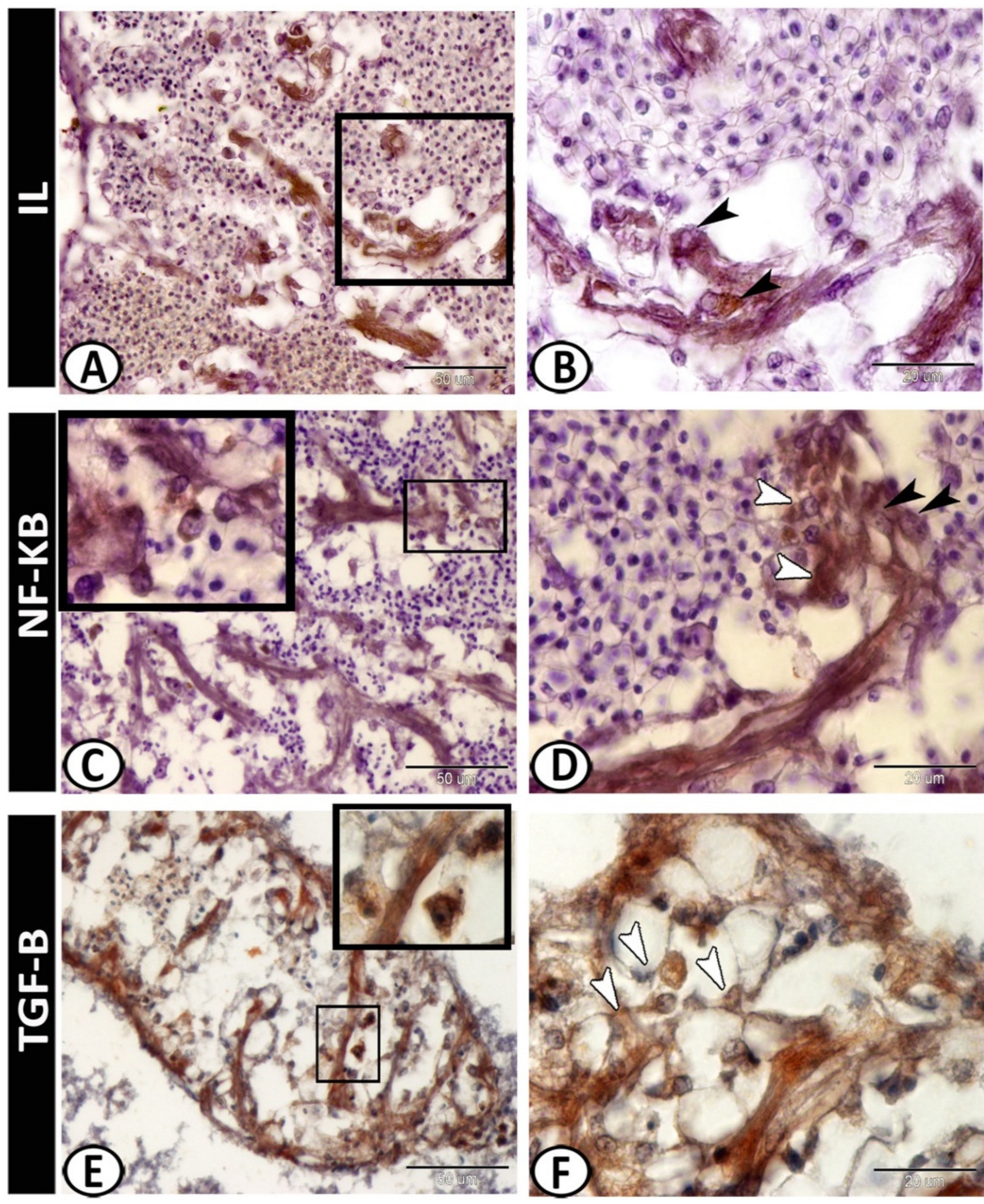
3.2. Immunohistochemistry
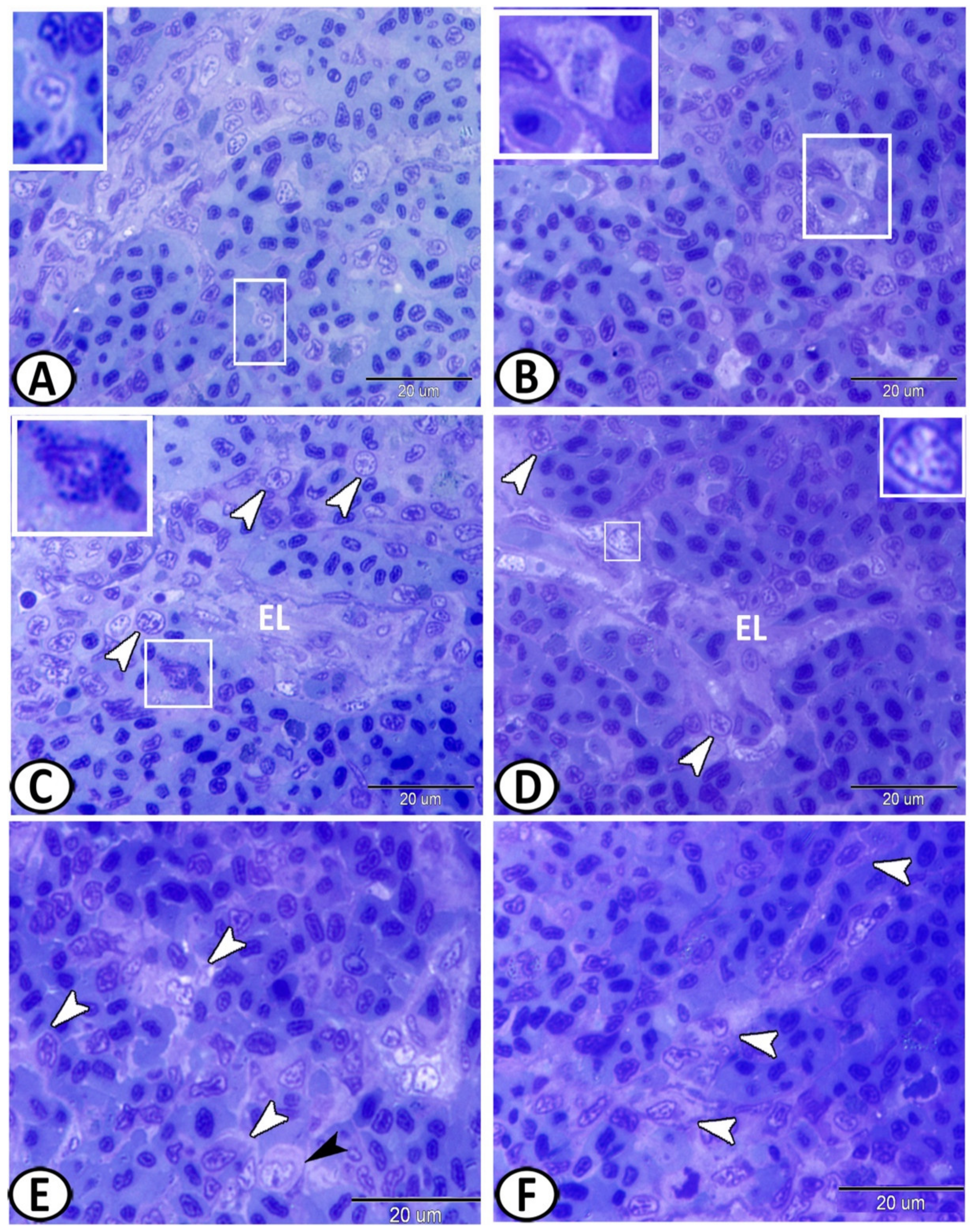
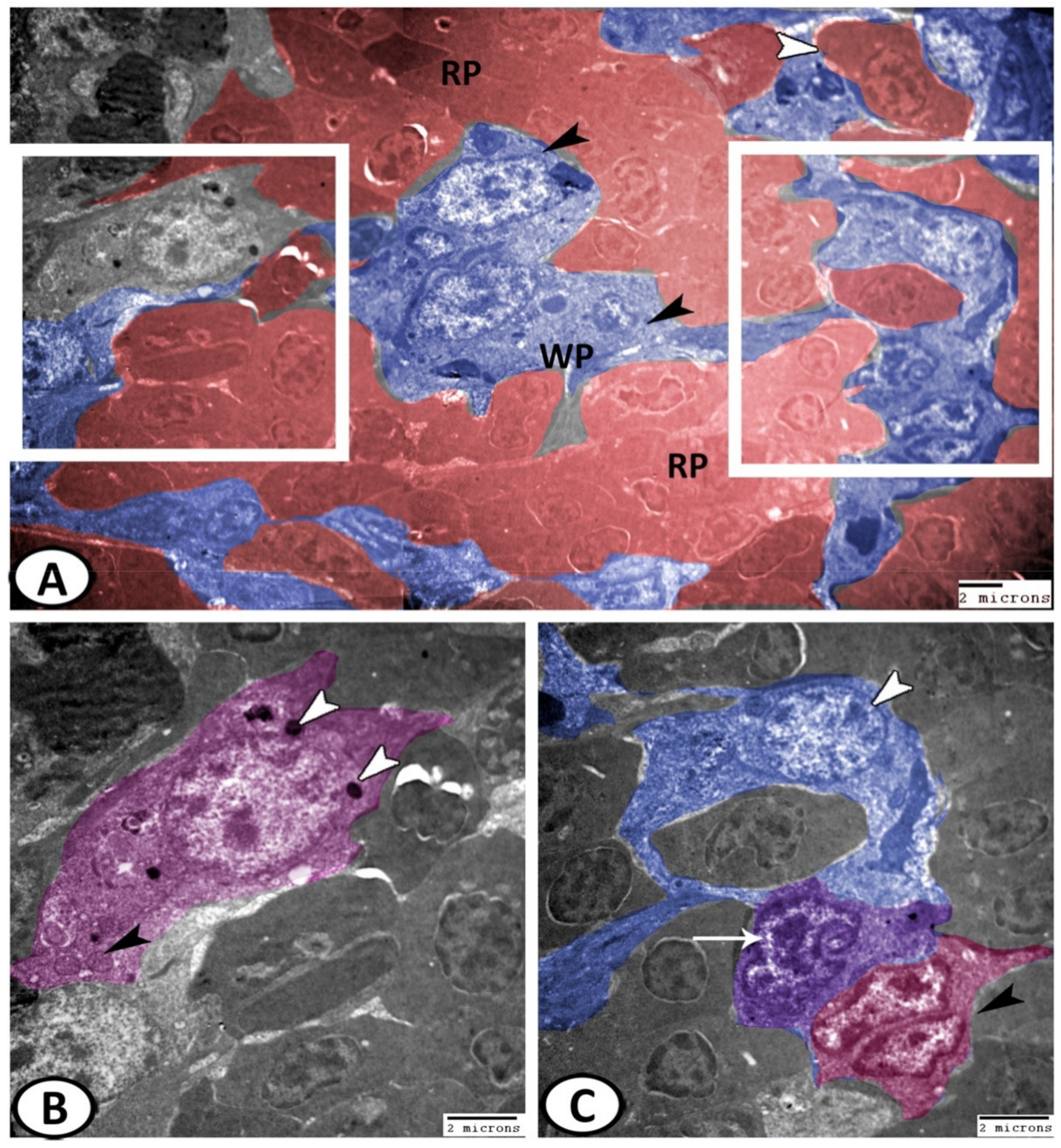
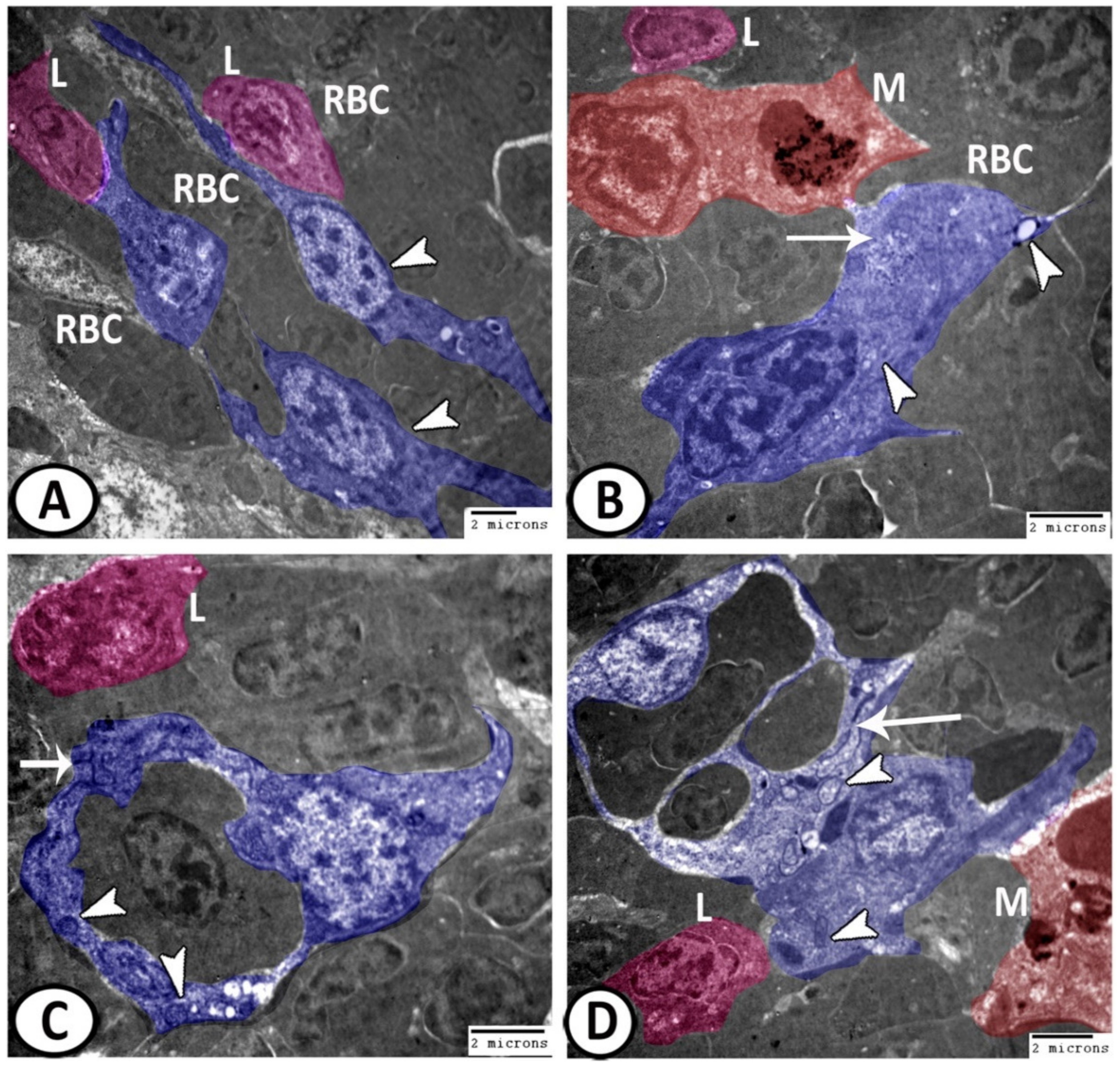
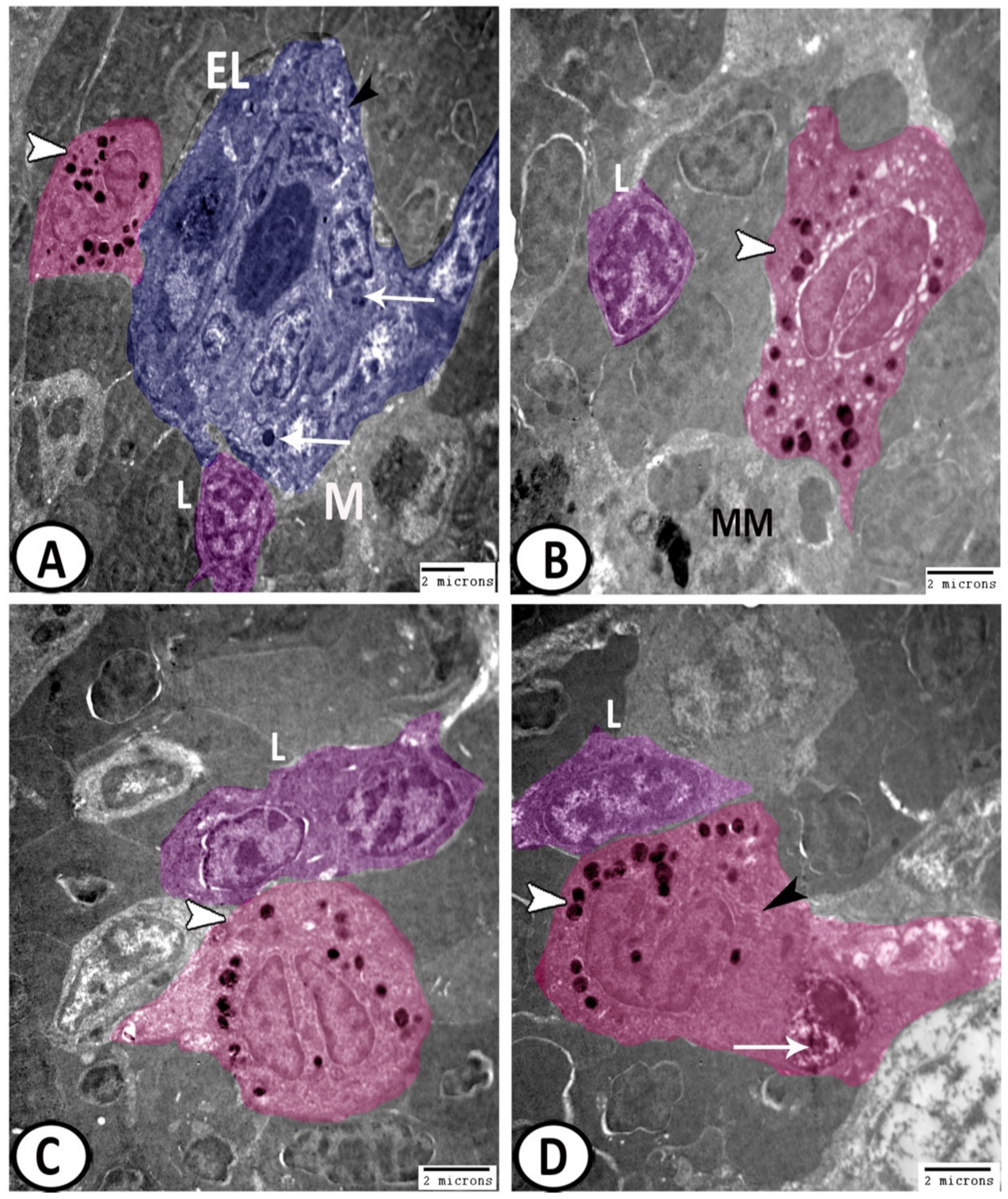
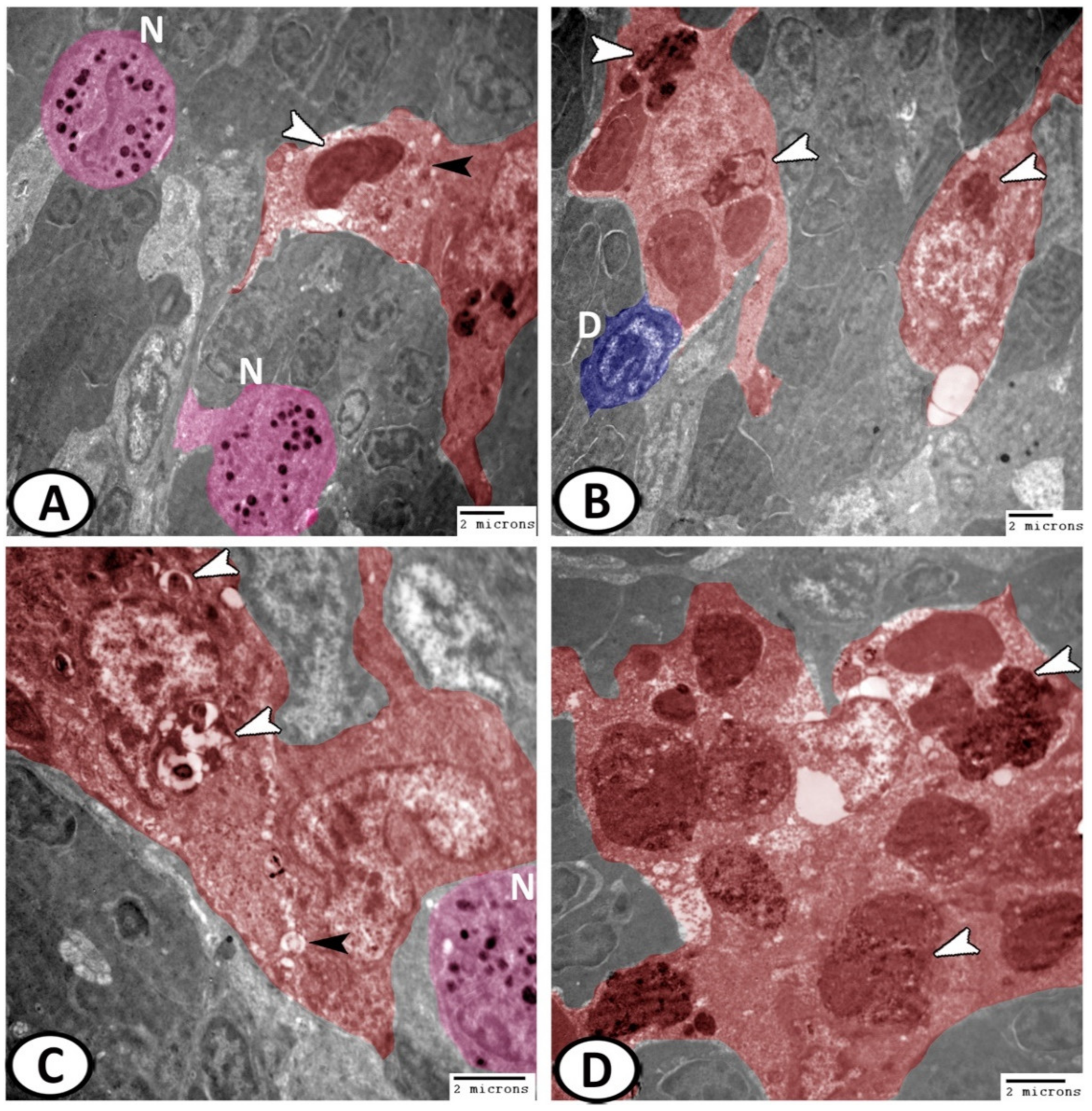
3.3. Electron Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rombout, J.H.W.M.; Huttenhuis, H.B.T.; Picchietti, S.; Scapigliati, G. Phylogeny and ontogeny of fish leucocytes. Fish Shellfish Immunol. 2005, 19, 441–455. [Google Scholar] [CrossRef] [PubMed]
- D’iglio, C.; Albano, M.; Tiralongo, F.; Famulari, S.; Rinelli, P.; Savoca, S.; Spanò, N.; Capillo, G. Biological and ecological aspects of the blackmouth catshark (Galeus melastomus rafinesque, 1810) in the southern tyrrhenian sea. J. Mar. Sci. Eng. 2021, 9, 967. [Google Scholar] [CrossRef]
- Nielsen, M.E.; Esteve-Gassent, M.D. The eel immune system: Present knowledge and the need for research. J. Fish Dis. 2006, 29, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Maina, J.N.; Icardo, J.M.; Zaccone, G.; Aragona, M.; Lauriano, E.R.; Alesci, A.; Albano, M.; Guerrera, M.C.; Germana, A.; Fernandes, J.M.O.; et al. Immunohistochemical and Ultrastructural Study of the Immune Cell System and Epithelial Surfaces of the Respiratory Organs in the Bimodally-Breathing African Sharptooth Catfish (Clarias gariepinus Burchell, 1822). Acta Zool. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Ellis, A.E. Innate host defense mechanisms of fish against viruses and bacteria. Dev. Comp. Immunol. 2001, 25, 827–839. [Google Scholar] [CrossRef]
- Karlsbakk, E.; Kristmundsson, Á.; Albano, M.; Brown, P.; Freeman, M.A. Redescription and phylogenetic position of Myxobolus ‘eglefini’ and Myxobolus platessae n. comb. (Myxosporea), parasites in the cartilage of some North Atlantic marine fishes, with notes on the phylogeny and classification of the Platysporina. Parasitol. Int. 2017, 66, 952–959. [Google Scholar] [CrossRef] [Green Version]
- Whyte, S.K. The innate immune response of finfish—A review of current knowledge. Fish Shellfish Immunol. 2007, 23, 1127–1151. [Google Scholar] [CrossRef]
- Ángeles Esteban, M. An Overview of the Immunological Defenses in Fish Skin. ISRN Immunol. 2012, 2012, 853470. [Google Scholar] [CrossRef] [Green Version]
- Cooper, E.L. Comparative immunology. Integr. Comp. Biol. 2003, 43, 278–280. [Google Scholar] [CrossRef] [Green Version]
- Fänge, R.; Nilsson, S. The fish spleen: Structure and function. Experientia 1985, 41, 152–158. [Google Scholar] [CrossRef]
- Lauriano, E.R.; Pergolizzi, S.; Aragona, M.; Montalbano, G.; Guerrera, M.C.; Crupi, R.; Faggio, C.; Capillo, G. Intestinal immunity of dogfish Scyliorhinus canicula spiral valve: A histochemical, immunohistochemical and confocal study. Fish Shellfish Immunol. 2019, 87, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Capillo, G.; Zaccone, G.; Cupello, C.; Fernandes, J.M.O.; Viswanath, K.; Kuciel, M.; Zuwala, K.; Guerrera, M.C.; Aragona, M.; Icardo, J.M.; et al. Expression of acetylcholine, its contribution to regulation of immune function and O2 sensing and phylogenetic interpretations of the African butterfly fish Pantodon buchholzi (Osteoglossiformes, Pantodontidae). Fish Shellfish Immunol. 2021, 111, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Uribe, C.; Folch, H.; Enriquez, R.; Moran, G. Innate and adaptive immunity in teleost fish: A review. Vet. Med. 2011, 56, 486–503. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-García, A.; Ramírez-Herrejón, J.P.; Medina-Nava, M.; Hernández-Morales, R.; Domínguez-Domínguez, O. Reproductive biology of the invasive species Pseudoxiphophorus bimaculatus and Poecilia sphenops in the Teuchitlán River, México. J. Appl. Ichthyol. 2018, 34, 81–90. [Google Scholar] [CrossRef]
- Alda, F.; Reina, R.G.; Doadrio, I.; Bermingham, E. Phylogeny and biogeography of the Poecilia sphenops species complex (Actinopterygii, Poeciliidae) in Central America. Mol. Phylogenet. Evol. 2013, 66, 1011–1026. [Google Scholar] [CrossRef] [PubMed]
- Tembo, R.N. The sublethal effects of low-pH exposure on the chemoreception of poecilia sphenops. Arch. Environ. Contam. Toxicol. 2009, 57, 157–163. [Google Scholar] [CrossRef]
- Drury, R. Theory and Practice of Histological Techniques. J. Clin. Pathol. 1983, 36, 609. [Google Scholar] [CrossRef] [Green Version]
- Marino, F.; Licata, L.; Albano, M.; Ieni, A.; di Caro, G.; Macrì, B. Angioleiomyoma in a conger (Conger conger). Dis. Aquat. Organ. 2016, 119, 85–89. [Google Scholar] [CrossRef]
- Karnovsky, M.J. A formaldehyde glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 1965, 27, 1A–149A. [Google Scholar]
- Reynolds, E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [Green Version]
- Biller-Takahashi, J.D.; Urbinati, E.C. Fish immunology. The modification and manipulation of the innate immune System:Brazilian studies. An. Acad. Bras. Cienc. 2014, 86, 1483–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benhamed, S.; Guardiola, F.A.; Mars, M.; Esteban, M.Á. Pathogen bacteria adhesion to skin mucus of fishes. Vet. Microbiol. 2014, 171, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zapata, A.; Diez, B.; Cejalvo, T.; Gutiérrez-De Frías, C.; Cortés, A. Ontogeny of the immune system of fish. Fish Shellfish Immunol. 2006, 20, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Salinas, I. The mucosal immune system of teleost fish. Biology 2015, 4, 525–539. [Google Scholar] [CrossRef] [Green Version]
- Rauta, P.R.; Nayak, B.; Das, S. Immune system and immune responses in fish and their role in comparative immunity study: A model for higher organisms. Immunol. Lett. 2012, 148, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Agius, C.; Roberts, R.J. Melano-macrophage centres and their role in fish pathology. J. Fish Dis. 2003, 26, 499–509. [Google Scholar] [CrossRef]
- Steinel, N.C.; Bolnick, D.I. Melanomacrophage centers as a histological indicator of immune function in fish and other poikilotherms. Front. Immunol. 2017, 8, 827. [Google Scholar] [CrossRef] [Green Version]
- King, R.; Beso, A.J.G.; Candelaria, V.Y.; Dela Cruz, J.G.; Margie, S.; Tameta, A.D.C.; Espinosa, A.A. Effects of Unleaded Petroleum on the Macrophage Aggregates ( MA ). bioRxiv 2016, 044537. [Google Scholar] [CrossRef] [Green Version]
- Manrique, W.G.; da Silva Claudiano, G.; Petrillo, T.R.; Pardi de Castro, M.; Pereira Figueiredo, M.A.; de Andrade Belo, M.A.; Engracia de Moraes, J.R.; de Moraes, F.R. Response of splenic melanomacrophage centers of Oreochromis niloticus (Linnaeus, 1758) to inflammatory stimuli by BCG and foreign bodies. J. Appl. Ichthyol. 2014, 30, 1001–1006. [Google Scholar] [CrossRef]
- Manrique, W.G.; Pereira Figueiredo, M.A.; Charlie-Silva, I.; Antonio de Andrade Belo, M.; Dib, C.C. Spleen melanomacrophage centers response of Nile tilapia during Aeromanas hydrophila and Mycobacterium marinum infections. Fish Shellfish Immunol. 2019, 95, 514–518. [Google Scholar] [CrossRef]
- Tjahjaningsih, W.; Pursetyo, K.T.; Sulmartiwi, L. Melanomacrophage centers in kidney, spleen and liver: A toxic response in carp fish (Cyprinus carpio) exposed to mercury chloride. AIP Conf. Proc. 2017, 1813, 020012. [Google Scholar] [CrossRef]
- Bols, N.C.; Brubacher, J.L.; Ganassin, R.C.; Lee, L.E.J. Ecotoxicology and innate immunity in fish. Dev. Comp. Immunol. 2001, 25, 853–873. [Google Scholar] [CrossRef]
- Jordanova, M.; Miteva, N.; Rocha, E. A qualitative and quantitative study of the hepatic pigmented macrophage aggregates during the breeding cycle of ohrid trout, Salmo letnica Kar. (Teloestei, Salmonidae). Microsc. Res. Tech. 2008, 71, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Wolke, R.E. Piscine macrophage aggregates: A review. Annu. Rev. Fish Dis. 1992, 2, 91–108. [Google Scholar] [CrossRef]
- Micale, V.; Perdichizzi, F. A quantitative and histochemical study on melano-macrophage centres in the spleen of the teleost fish Diplodus annularis L. J. Fish Biol. 1990, 37, 191–197. [Google Scholar] [CrossRef]
- Fishelson, L. Ontogenesis and functional metamorphosis of the head-kidney in bottomspawner and mouthbrooder cichlid fishes (Cichlidae, teleostei). J. Morphol. 1996, 229, 1–21. [Google Scholar] [CrossRef]
- Agius, C. The Melano-Macrophage Centres of Fish: A Review. Fish Immunol. 1985, 85–105. [Google Scholar] [CrossRef]
- Meseguer, J.; López-Ruiz, A.; Esteban, M.A. Melano-macrophages of the seawater teleosts, sea bass (Dicentrarchus labrax) and gilthead seabream (Sparus aurata): Morphology, formation and possible function. Cell Tissue Res. 1994, 277, 1–10. [Google Scholar] [CrossRef]
- Thorsen, J.; Høyheim, B.; Koppang, E.O. Isolation of the Atlantic salmon tyrosinase gene family reveals heterogenous transcripts in a leukocyte cell line. Pigment. Cell Res. 2006, 19, 327–336. [Google Scholar] [CrossRef]
- Udroiu, I.; Sgura, A. The phylogony of the spleen. Q. Rev. Biol. 2017, 92, 411–443. [Google Scholar] [CrossRef]
- Espenes, A.; Press, C.M.L.; Dannevig, B.H.; Landsverk, T. Investigation of the structural and functional features of splenic ellipsoids in rainbow trout (Oncorhynchus mykiss). Cell Tissue Res. 1995, 279, 469–474. [Google Scholar] [CrossRef]
- Ruddle, N.H.; Akirav, E.M. Secondary Lymphoid Organs: Responding to Genetic and Environmental Cues in Ontogeny and the Immune Response. J. Immunol. 2009, 183, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.; Utke, K.; Somamoto, T.; Köllner, B.; Ototake, M.; Nakanishi, T. Cytotoxic activities of fish leucocytes. Fish Shellfish Immunol. 2006, 20, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Kondera, E. Cell composition of the head kidney of European chub (Squalius cephalus L. ). Arch. Polish Fish. 2014, 22, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Mokhtar, D.M. Fish Histology: From Cells to Organs; Apple Academic Press: New York, NY, USA, 2017; pp. 1–246. [Google Scholar] [CrossRef]
- Mokhtar, D.M.; Abdelhafez, E.A. An overview of the structural and functional aspects of immune cells in teleosts. Histol. Histopathol. 2021, 36, 399–414. [Google Scholar] [CrossRef]
- Mokhtar, D.M. Characterization of the fish ovarian stroma during the spawning season: Cytochemical, immunohistochemical and ultrastructural studies. Fish Shellfish Immunol. 2019, 94, 566–579. [Google Scholar] [CrossRef]
- Dezfuli, B.S.; Capuano, S.; Manera, M. A description of rodlet cells from the alimentary canal of Anguilla anguilla and their relationship with parasitic helminths. J. Fish Biol. 1998, 53, 1084–1095. [Google Scholar] [CrossRef]
- Bassity, E.; Clark, T.G. Functional identification of dendritic cells in the teleost model, rainbow trout (Oncorhynchus mykiss). PLoS ONE 2012, 7, e33196. [Google Scholar] [CrossRef] [Green Version]
- Mokhtar, D.M.; Abd-Elhafez, E.A.; Hassan, A.H.S. Microanalysis of the Intestinal Bulb of Grass Carp (Ctenopharyngodon Idella): Histological, Histochemical, Immunohistochemical, and Scanning Electron Microscopical Studies. Microsc. Microanal. 2021, 27, 1564–1572. [Google Scholar] [CrossRef]
- Meseguer, J.; López-Ruiz, A.; Garcí-Ayala, A. Reticulo-endothelial stroma of the head-kidney from the seawater teleost gilthead seabream (Sparus aurata L.): An ultrastructural and cytochemical study. Anat. Rec. 1995, 241, 303–309. [Google Scholar] [CrossRef]
- Yao, Z.; Delorme-Axford, E.; Backues, S.K.; Klionsky, D.J. Atg41/Icy2 regulates autophagosome formation. Autophagy 2015, 11, 2288–2299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierdominici, M.; Vomero, M.; Barbati, C.; Colasanti, T.; Maselli, A.; Vacirca, D.; Giovannetti, A.; Malorni, W.; Ortona, E. Role of autophagy in immunity and autoimmunity, with a special focus on systemic lupus erythematosus. FASEB J. 2012, 26, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Radaelli, G.; Rowlerson, A.; Mascarello, F.; Patruno, M.; Funkenstein, B. Myostatin precursor is present in several tissues in teleost fish: A comparative immunolocalization study. Cell Tissue Res. 2003, 311, 239–250. [Google Scholar] [CrossRef] [PubMed]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Elliott, B.; Renshaw, D.; Getting, S.; Mackenzie, R. The central role of myostatin in skeletal muscle and whole body homeostasis. Acta Physiol. 2012, 205, 324–340. [Google Scholar] [CrossRef]
- Helterline, D.L.I.; Garikipati, D.; Stenkamp, D.L.; Rodgers, B.D. Embryonic and tissue-specific regulation of myostatin-1 and -2 gene expression in zebrafish. Gen. Comp. Endocrinol. 2007, 151, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.; Secombes, C.J. The function of fish cytokines. Biology 2016, 5, 23. [Google Scholar] [CrossRef]
- Zhu, L.; Nie, L.; Zhu, G.; Xiang, L.; Shao, J. Advances in research of fish immune-relevant genes: A comparative overview of innate and adaptive immunity in teleosts. Dev. Comp. Immunol. 2013, 39, 39–62. [Google Scholar] [CrossRef]
- Dinarello, C.A. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef]
- Netea, M.G.; Simon, A.; van de Veerdonk, F.; Kullberg, B.J.; van der Meer, J.W.M.; Joosten, L.A.B. IL-1β processing in host defense: Beyond the inflammasomes. PLoS Pathog. 2010, 6, e1000661. [Google Scholar] [CrossRef] [Green Version]
- Sims, J.E.; Smith, D.E. The IL-1 family: Regulators of immunity. Nat. Rev. Immunol. 2010, 10, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.A.M.; Zou, J.; Houlihan, D.F.; Secombes, C.J. Directional responses following recombinant cytokine stimulation of rainbow trout (Oncorhynchus mykiss) RTS-11 macrophage cells as revealed by transcriptome profiling. BMC Genom. 2007, 8, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.J.; Chen, J.; He, Y.Q.; Shi, Y.H. Molecular characterization of an IL-1β gene from ayu, Plecoglossus altivelis. Fish Shellfish Immunol. 2013, 34, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, D.; Li, J.; Liu, Z. Molecular characterization, recombinant expression and bioactivity analysis of the interleukin-1β from the yellowfin sea bream, Acanthopagrus latus (Houttuyn). Fish Shellfish Immunol. 2008, 24, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Vojtech, L.N.; Scharping, N.; Woodson, J.C.; Hansen, J.D. Roles of inflammatory caspases during processing of zebrafish interleukin-1β in Francisella noatunensis infection. Infect. Immun. 2012, 80, 2878–2885. [Google Scholar] [CrossRef] [Green Version]
- Watzke, J.; Schirmer, K.; Scholz, S. Bacterial lipopolysaccharides induce genes involved in the innate immune response in embryos of the zebrafish (Danio rerio). Fish Shellfish Immunol. 2007, 23, 901–905. [Google Scholar] [CrossRef]
- Secombes, C.J.; Wang, T.; Bird, S. Vertebrate Cytokines and Their Evolution. Evol. Immune Syst. Conserv. Diversif. 2016, 87–150. [Google Scholar] [CrossRef]
- Viatour, P.; Merville, M.P.; Bours, V.; Chariot, A. Phosphorylation of NF-κB and IκB proteins: Implications in cancer and inflammation. Trends Biochem. Sci. 2005, 30, 43–52. [Google Scholar] [CrossRef]
- Pasparakis, M. Role of NF-κB in epithelial biology. Immunol. Rev. 2012, 246, 346–358. [Google Scholar] [CrossRef]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-κB and rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef]
- Correa, R.G.; Tergaonkar, V.; Ng, J.K.; Dubova, I.; Izpisua-Belmonte, J.C.; Verma, I.M. Characterization of NF-κΒ/IκΒ Proteins in Zebra Fish and Their Involvement in Notochord Development. Mol. Cell. Biol. 2004, 24, 5257–5268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Sabatino, A.; Pickard, K.M.; Rampton, D.; Kruidenier, L.; Rovedatti, L.; Leakey, N.A.B.; Corazza, G.R.; Monteleone, G.; MacDonald, T.T. Blockade of transforming growth factor β upregulates T-box transcription factor T-bet, and increases T helper cell type 1 cytokine and matrix metalloproteinase-3 production in the human gut mucosa. Gut 2008, 57, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Takehara, K. Growth regulation of skin fibroblasts. J. Dermatol. Sci. 2000, 24, S70–S77. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Cua, D.J. T Cells Doing It for Themselves: TGF-β Regulation of Th1 and Th17 Cells. Immunity 2007, 26, 547–549. [Google Scholar] [CrossRef] [Green Version]
- Ashcroft, G.S. Bidirectional regulation of macrophage function by TGF-β. Microbes Infect. 1999, 1, 1275–1282. [Google Scholar] [CrossRef] [Green Version]
- Qi, P.; Xie, C.; Guo, B.; Wu, C. Dissecting the role of transforming growth factor-β1 in topmouth culter immunobiological activity: A fundamental functional analysis. Sci. Rep. 2016, 6, 27179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q. Role of Nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zhu, Z. Nrf2 is involved in osmoregulation, antioxidation and immunopotentiation in Coilia nasus under salinity stress. Biotechnol. Biotechnol. Equip. 2019, 33, 1453–1463. [Google Scholar] [CrossRef] [Green Version]
- Wen, Z.; Liu, W.; Li, X.; Chen, W.; Liu, Z.; Wen, J.; Liu, Z. A protective role of the Nrf2-Keap1 pathway in maintaining intestinal barrier function. Oxid. Med. Cell. Longev. 2019, 2019, 1759149. [Google Scholar] [CrossRef] [Green Version]
- Spokony, R.F.; Aoki, Y.; Saint-Germain, N.; Magner-Fink, E.; Saint-Jeannet, J.P. The transcription factor Sox9 is required for cranial neural crest development in Xenopus. Development 2002, 129, 421–432. [Google Scholar] [CrossRef]
- Bastide, P.; Darido, C.; Pannequin, J.; Kist, R.; Robine, S.; Marty-Double, C.; Bibeau, F.; Scherer, G.; Joubert, D.; Hollande, F.; et al. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J. Cell Biol. 2007, 178, 635–648. [Google Scholar] [CrossRef]
- Pritchett, J.; Athwal, V.; Roberts, N.; Hanley, N.A.; Hanley, K.P. Understanding the role of SOX9 in acquired diseases: Lessons from development. Trends Mol. Med. 2011, 17, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Jo, A.; Denduluri, S.; Zhang, B.; Wang, Z.; Yin, L.; Yan, Z.; Kang, R.; Shi, L.L.; Mok, J.; Lee, M.J.; et al. The versatile functions of Sox9 in development, stem cells, and human diseases. Genes Dis. 2014, 1, 149–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, A.; Hochedlinger, K. The Sox family of transcription factors: Versatile regulators of stem and progenitor cell fate. Cell Stem Cell 2013, 12, 15–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayed, R.K.A.; Zaccone, G.; Capillo, G.; Albano, M.; Mokhtar, D.M. Structural and Functional Aspects of the Spleen in Molly Fish Poecilia sphenops (Valenciennes, 1846): Synergistic Interactions of Stem Cells, Neurons, and Immune Cells. Biology 2022, 11, 779. https://doi.org/10.3390/biology11050779
Sayed RKA, Zaccone G, Capillo G, Albano M, Mokhtar DM. Structural and Functional Aspects of the Spleen in Molly Fish Poecilia sphenops (Valenciennes, 1846): Synergistic Interactions of Stem Cells, Neurons, and Immune Cells. Biology. 2022; 11(5):779. https://doi.org/10.3390/biology11050779
Chicago/Turabian StyleSayed, Ramy K. A., Giacomo Zaccone, Gioele Capillo, Marco Albano, and Doaa M. Mokhtar. 2022. "Structural and Functional Aspects of the Spleen in Molly Fish Poecilia sphenops (Valenciennes, 1846): Synergistic Interactions of Stem Cells, Neurons, and Immune Cells" Biology 11, no. 5: 779. https://doi.org/10.3390/biology11050779
APA StyleSayed, R. K. A., Zaccone, G., Capillo, G., Albano, M., & Mokhtar, D. M. (2022). Structural and Functional Aspects of the Spleen in Molly Fish Poecilia sphenops (Valenciennes, 1846): Synergistic Interactions of Stem Cells, Neurons, and Immune Cells. Biology, 11(5), 779. https://doi.org/10.3390/biology11050779









