Enhanced Anxiety and Olfactory Microglial Activation in Early-Stage Familial Alzheimer’s Disease Mouse Model
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Open Field Test (OFT)
2.3. Total RNA Isolation and Real-Time Quantitative PCR (RT-qPCR)
2.4. Immunohistochemistry (IHC)
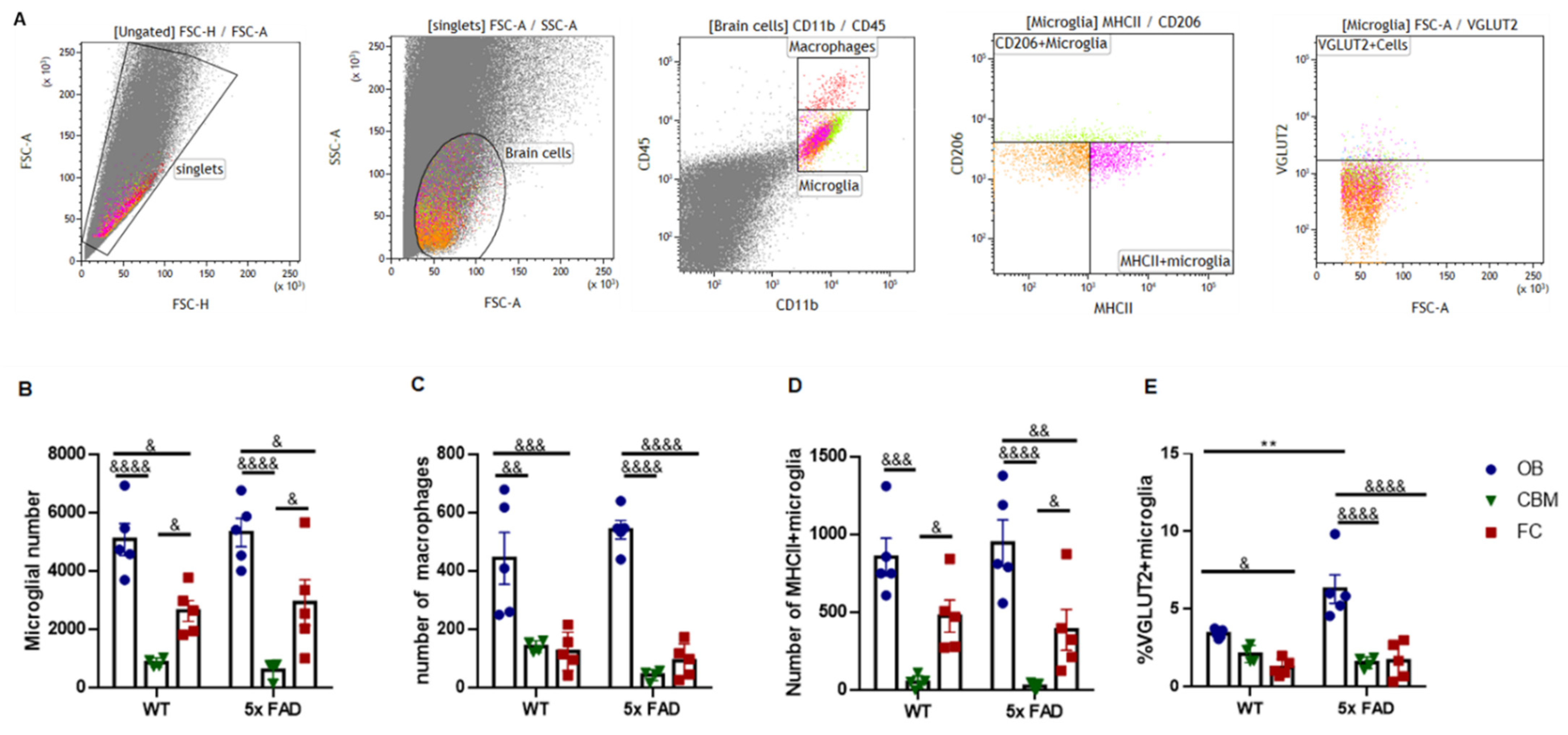
2.5. Flow Cytometry
2.6. Statistical Analysis
3. Results
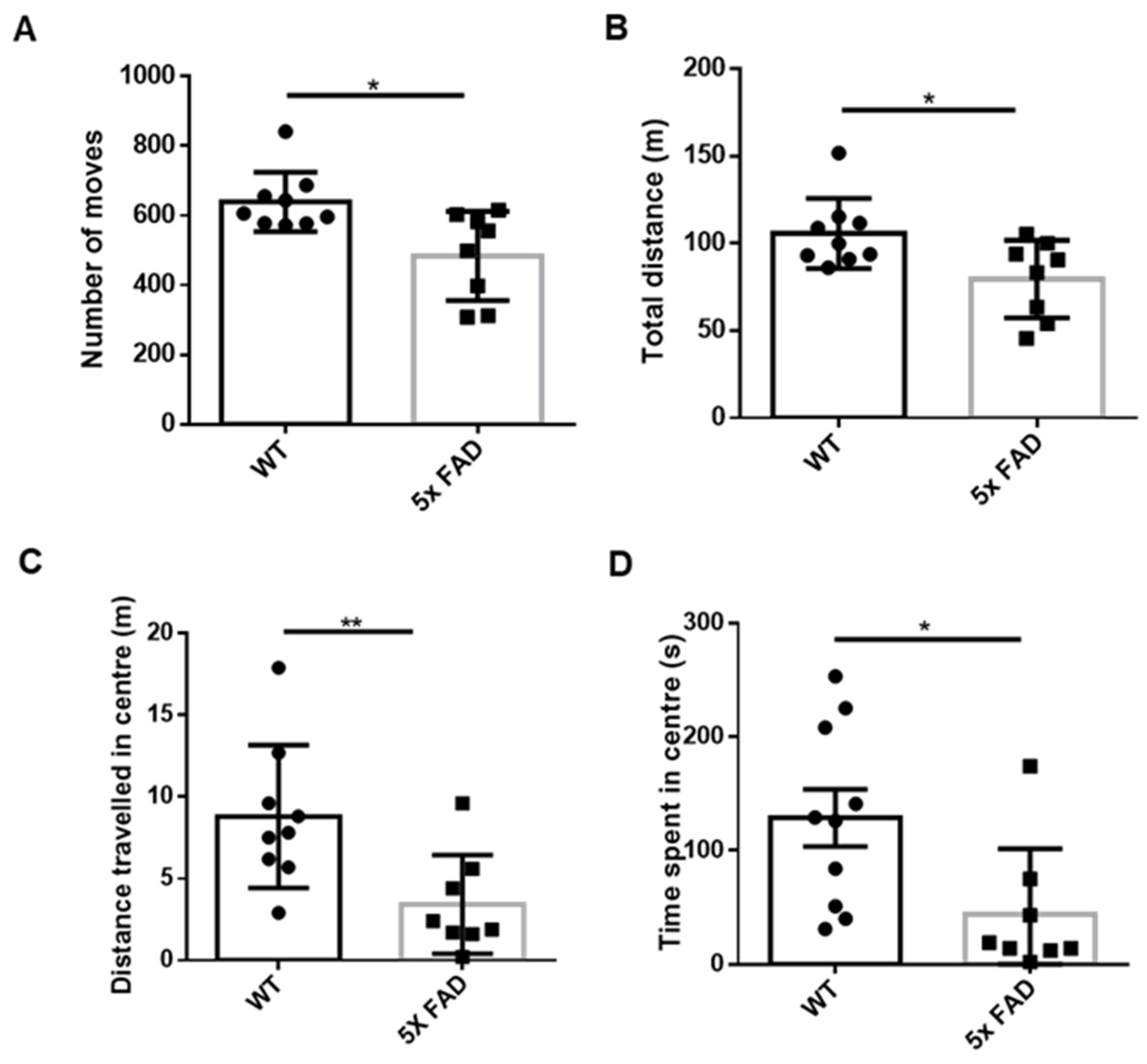
3.1. Young Adult FAD Mice Showed Enhanced Anxiety
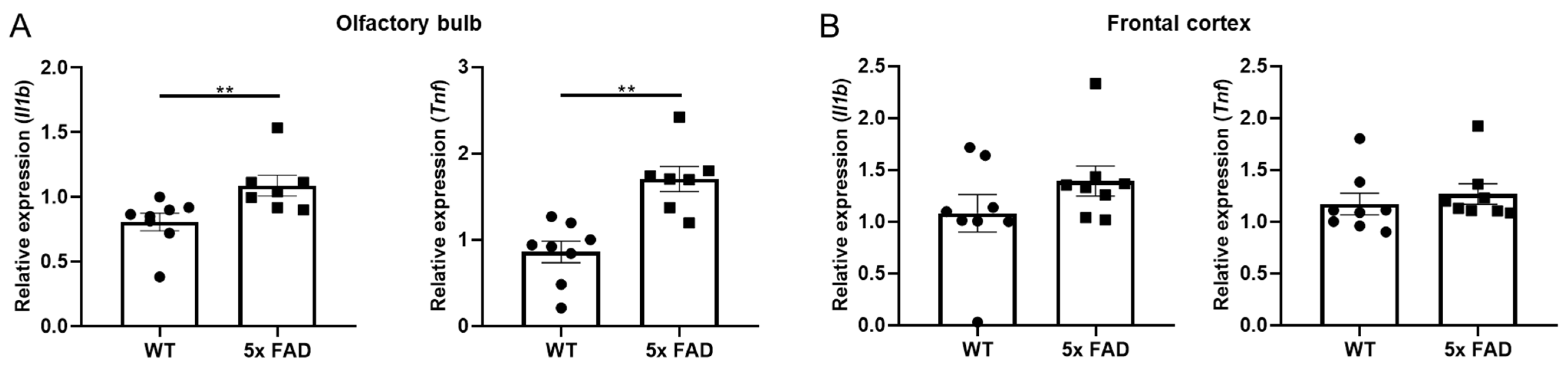
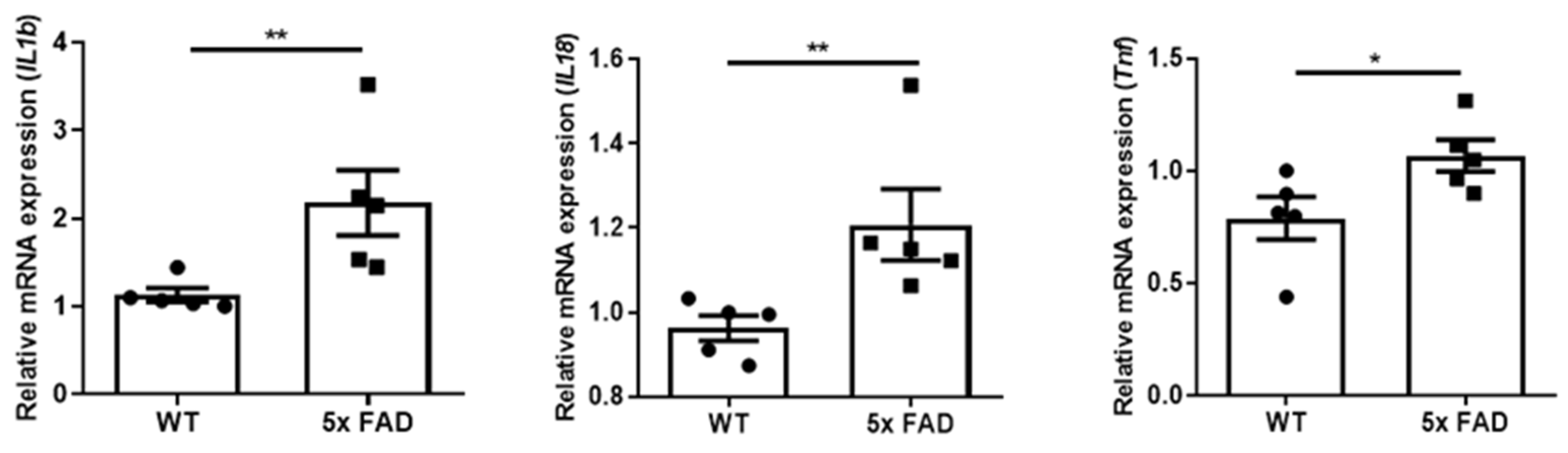
3.2. Pro-Inflammatory Cytokines Were Upregulated in the OB of Young 5 × FAD Male Mice
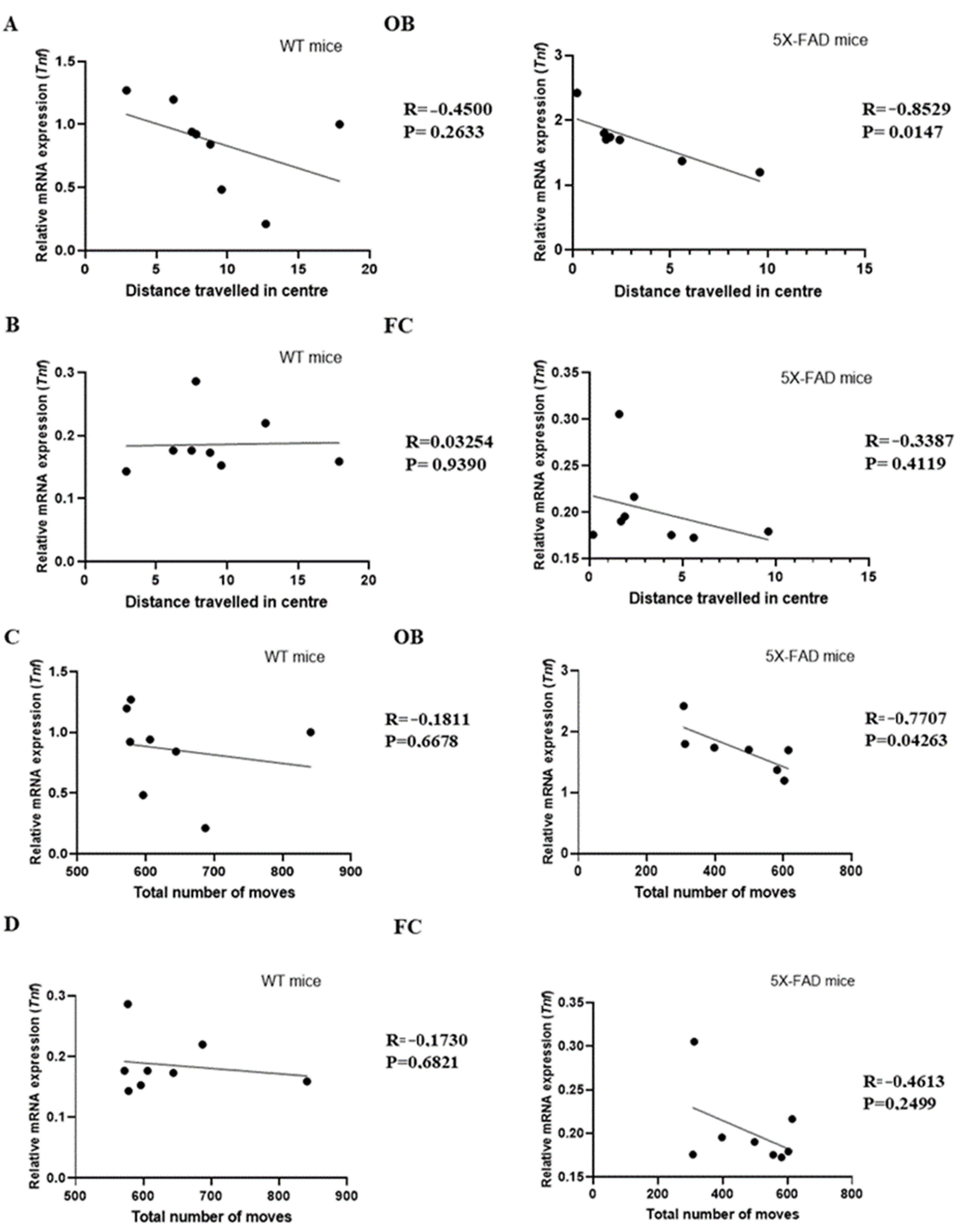
3.3. Tnf Was Associated with Anxiety in Young 5 × FAD Male Mice
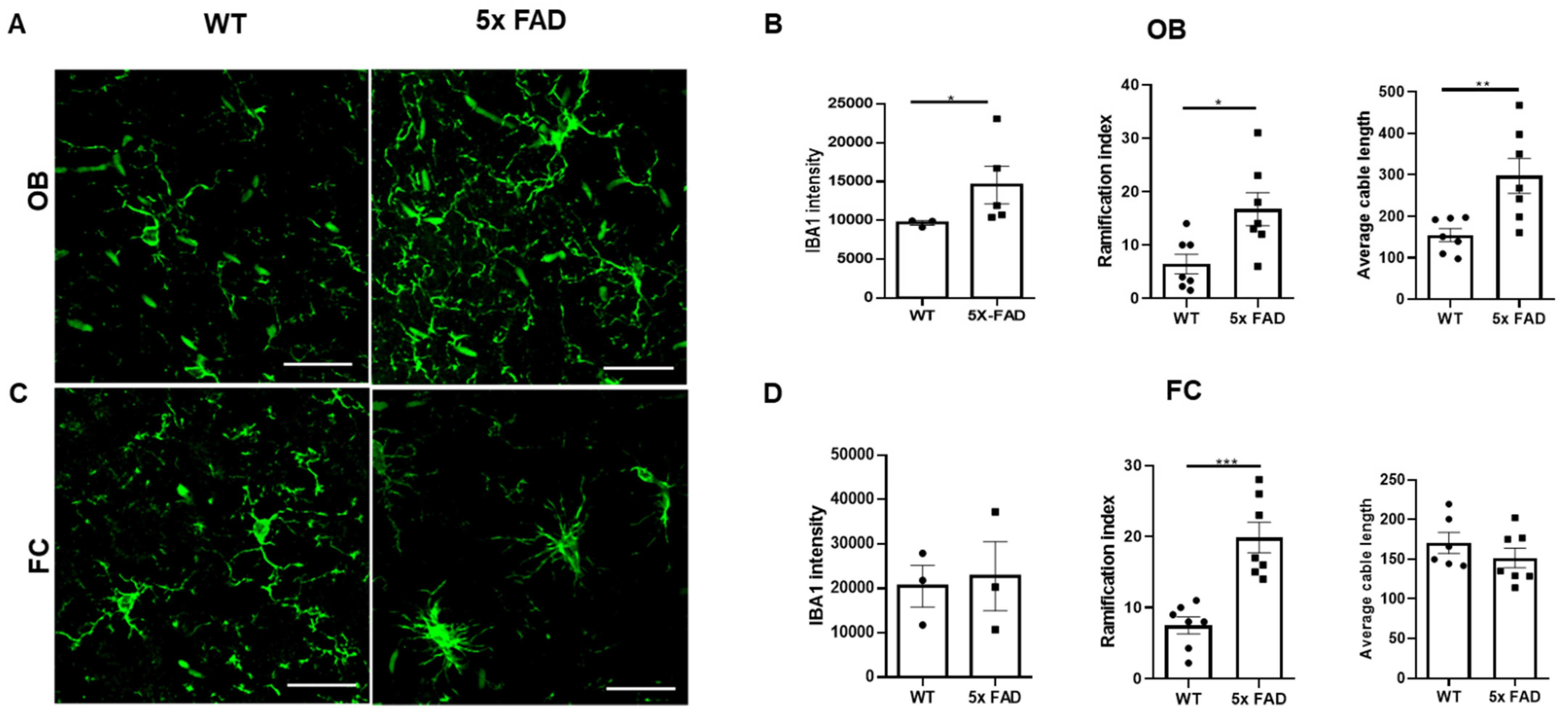
3.4. Microglial Activation and Morphological Changes in the OB and FC of Young 5 × FAD Male Mice
3.5. Elevated Pro-Inflammatory Cytokines in the FC of Older 5 × FAD Male Mice
3.6. Microglial Presynaptic Pruning Was Enhanced in the OB of Older 5 × FAD Male Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mendez, M.F. The relationship between anxiety and Alzheimer’s disease. J. Alzheimer’s Dis. Rep. 2021, 5, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.; Rios, C.L.O.; Lahmann, C.; Ruecker, G.; Bauer, J.; Boeker, M. Anxiety as a risk factor of Alzheimer’s disease and vascular dementia. Br. J. Psychiatry 2018, 213, 654–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, D.; Coen, R.; Kilroy, D.; Belinski, K.; Bruce, I.; Coakley, D.; Walsh, B.; Cunningham, C.; Lawlor, B.A. Anxiety and behavioural disturbance as markers of prodromal Alzheimer’s disease in patients with mild cognitive impairment. Int. J. Geriatr. Psychiatry 2011, 26, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Velayudhan, L. Neuropsychiatric symptoms in mild cognitive impairment: A literature review. Dement. Geriatr. Cogn. Disord. 2020, 49, 146–155. [Google Scholar] [CrossRef]
- Roberto, N.; Portella, M.J.; Marquié, M.; Alegret, M.; Hernández, I.; Mauleón, A.; Rosende-Roca, M.; Abdelnour, C.; Esteban de Antonio, E.; Tartari, J.P. Neuropsychiatric profile as a predictor of cognitive decline in mild cognitive impairment. Front. Aging Neurosci. 2021, 814. [Google Scholar] [CrossRef]
- Mah, L.; Binns, M.A.; Steffens, D.C. Alzheimer’s Disease Neuroimaging InitiativeAnxiety symptoms in amnestic mild cognitive impairment are associated with medial temporal atrophy and predict conversion to Alzheimer disease. Am. J. Geriatr. Psychiatry 2015, 23, 466–476. [Google Scholar] [CrossRef] [Green Version]
- Toyota, Y.; Ikeda, M.; Shinagawa, S.; Matsumoto, T.; Matsumoto, N.; Hokoishi, K.; Fukuhara, R.; Ishikawa, T.; Mori, T.; Adachi, H. Comparison of behavioral and psychological symptoms in early-onset and late-onset Alzheimer’s disease. Int. J. Geriatr. Psychiatry A J. Psychiatry Late Life Allied Sci. 2007, 22, 896–901. [Google Scholar] [CrossRef]
- Murphy, C. Olfactory and other sensory impairments in Alzheimer disease. Nat. Rev. Neurol. 2019, 15, 11–24. [Google Scholar] [CrossRef]
- Chen, X.; Guo, W.; Yu, L.; Luo, D.; Xie, L.; Xu, J. Association Between Anxious Symptom Severity and Olfactory Impairment in Young Adults with Generalized Anxiety Disorder: A Case–Control Study. Neuropsychiatr. Dis. Treat. 2021, 17, 2877. [Google Scholar] [CrossRef]
- Glinka, M.E.; Samuels, B.A.; Diodato, A.; Teillon, J.; Mei, D.F.; Shykind, B.M.; Hen, R.; Fleischmann, A. Olfactory deficits cause anxiety-like behaviors in mice. J. Neurosci. 2012, 32, 6718–6725. [Google Scholar] [CrossRef] [Green Version]
- Hendriksen, H.; Korte, S.M.; Olivier, B.; Oosting, R.S. The olfactory bulbectomy model in mice and rat: One story or two tails? Eur. J. Pharmacol. 2015, 753, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Gu, M.Q.; Yang, Z.Y.; Xia, J.; Li, P.; Vasar, E.; Tian, L.; Song, C. Endogenous n-3 PUFAs attenuated olfactory bulbectomy-induced behavioral and metabolomic abnormalities in Fat-1 mice. Brain Behav. Immun. 2021, 96, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.-A.; Zhang, J.; Zhou, M.; Wei, Z.; Wu, X.-L.; Dai, X.-M.; Zhu, Y.-G.; Chen, X.-C. Reduction of glucose metabolism in olfactory bulb is an earlier Alzheimer’s disease-related biomarker in 5 × FAD mice. Chin. Med. J. 2015, 128, 2220–2227. [Google Scholar] [CrossRef] [PubMed]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef]
- Cai, Y.; Xue, Z.Q.; Zhang, X.M.; Li, M.B.; Wang, H.; Luo, X.G.; Cai, H.; Yan, X.X. An age-related axon terminal pathology around the first olfactory relay that involves amyloidogenic protein overexpression without plaque formation. Neuroscience 2012, 26, 160–173. [Google Scholar] [CrossRef]
- Girard, S.D.; Jacquet, M.; Baranger, K.; Migliorati, M.; Escoffier, G.; Bernard, A.; Khrestchatisky, M.; Féron, F.; Rivera, S.; Roman, F.S.; et al. Onset of hippocampus-dependent memory impairments in 5 × FAD transgenic mouse model of Alzheimer’s disease. Hippocampus 2014, 24, 762–772. [Google Scholar] [CrossRef]
- O’Leary, T.P.; Stover, K.R.; Mantolino, H.M.; Darvesh, S.; Brown, R.E. Intact olfactory memory in the 5 × FAD mouse model of Alzheimer’s disease from 3 to 15 months of age. Behav. Brain Res. 2020, 393, 112731. [Google Scholar] [CrossRef]
- Boza-Serrano, A.; Yang, Y.; Paulus, A.; Deierborg, T. Innate immune alterations are elicited in microglial cells before plaque deposition in the Alzheimer’s disease mouse model 5 × FAD. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.-M.; Chiou, W.-F.; Su, T.-P.; Li, C.-T.; Chen, M.-H. Pro-inflammatory cytokine associated with somatic and pain symptoms in depression. J. Affect. Disord. 2014, 155, 28–34. [Google Scholar] [CrossRef]
- Alshammari, M.A.; Khan, M.R.; Mahmood, H.M.; Alshehri, A.O.; Alasmari, F.F.; Alqahtani, F.M.; Alasmari, A.F.; Alsharari, S.D.; Alhossan, A.; Ahmad, S.F.; et al. Systemic TNF-α blockade attenuates anxiety and depressive-like behaviors in db/db mice through downregulation of inflammatory signaling in peripheral immune cells. Saudi Pharm. J. 2020, 28, 621–629. [Google Scholar] [CrossRef]
- Kimura, R.; Ohno, M. Impairments in remote memory stabilization precede hippocampal synaptic and cognitive failures in 5 × FAD Alzheimer mouse model. Neurobiol. Dis. 2009, 33, 229–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, F.; Baldauf, K.; Wetzel, W.; Reymann, K. Behavioral and EEG changes in male 5 × FAD mice. Physiol. Behav. 2014, 135, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Sochocka, M.; Zwolinska, K.; Leszek, J. The infectious etiology of Alzheimer’s disease. Curr. Neuropharmacol. 2017, 15, 996–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bu, X.L.; Yao, X.Q.; Jiao, S.S.; Zeng, F.; Liu, Y.H.; Xiang, Y.; Liang, C.R.; Wang, Q.H.; Wang, X.; Cao, H.Y. A study on the association between infectious burden and A lzheimer’s disease. Eur. J. Neurol. 2015, 22, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Prinz, M.; Masuda, T.; Wheeler, M.A.; Quintana, F.J. Microglia and central nervous system–associated macrophages—From origin to disease modulation. Annu. Rev. Immunol. 2021, 39, 251–277. [Google Scholar] [CrossRef]
- Salvador, A.F.; de Lima, K.A.; Kipnis, J. Neuromodulation by the immune system: A focus on cytokines. Nat. Rev. Immunol. 2021, 21, 526–541. [Google Scholar] [CrossRef]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases. Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef] [Green Version]
- Denizet, M.; Cotter, L.; Lledo, P.-M.; Lazarini, F. Sensory deprivation increases phagocytosis of adult-born neurons by activated microglia in the olfactory bulb. Brain Behav. Immun. 2017, 60, 38–43. [Google Scholar] [CrossRef]
- Herbert, R.P.; Harris, J.; Chong, K.P.; Chapman, J.; West, A.K.; Chuah, M.I. Cytokines and olfactory bulb microglia in response to bacterial challenge in the compromised primary olfactory pathway. J. Neuroinflammat. 2012, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wallace, J.; Lord, J.; Dissing-Olesen, L.; Stevens, B.; Murthy, V.N. Microglial depletion disrupts normal functional development of adult-born neurons in the olfactory bulb. Elife 2020, 9, e50531. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.W.; Wohleb, E.S. How Stress Shapes Neuroimmune Function: Implications for the Neurobiology of Psychiatric Disorders. Biol. Psychiatry 2021, 90, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Roy, E.R.; Wang, B.; Wan, Y.-W.; Chiu, G.; Cole, A.; Yin, Z.; Propson, N.E.; Xu, Y.; Jankowsky, J.L.; Liu, Z. Type I interferon response drives neuroinflammation and synapse loss in Alzheimer disease. J. Clin. Investig. 2020, 130, 1912–1930. [Google Scholar] [CrossRef] [PubMed]
- Hammond, T.R.; Marsh, S.E.; Stevens, B. Immune signaling in neurodegeneration. Immunity 2019, 50, 955–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chithanathan, K.; Xuan, F.-L.; Hickey, M.A.; Tian, L. Enhanced Anxiety and Olfactory Microglial Activation in Early-Stage Familial Alzheimer’s Disease Mouse Model. Biology 2022, 11, 938. https://doi.org/10.3390/biology11060938
Chithanathan K, Xuan F-L, Hickey MA, Tian L. Enhanced Anxiety and Olfactory Microglial Activation in Early-Stage Familial Alzheimer’s Disease Mouse Model. Biology. 2022; 11(6):938. https://doi.org/10.3390/biology11060938
Chicago/Turabian StyleChithanathan, Keerthana, Fang-Ling Xuan, Miriam Ann Hickey, and Li Tian. 2022. "Enhanced Anxiety and Olfactory Microglial Activation in Early-Stage Familial Alzheimer’s Disease Mouse Model" Biology 11, no. 6: 938. https://doi.org/10.3390/biology11060938
APA StyleChithanathan, K., Xuan, F.-L., Hickey, M. A., & Tian, L. (2022). Enhanced Anxiety and Olfactory Microglial Activation in Early-Stage Familial Alzheimer’s Disease Mouse Model. Biology, 11(6), 938. https://doi.org/10.3390/biology11060938






