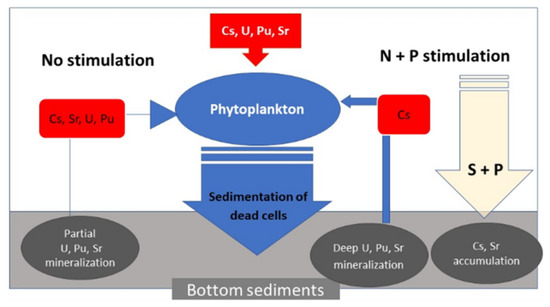
Biogeochemical Factors of Cs, Sr, U, Pu Immobilization in Bottom Sediments of the Upa River, Located in the Zone of Chernobyl Accident
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
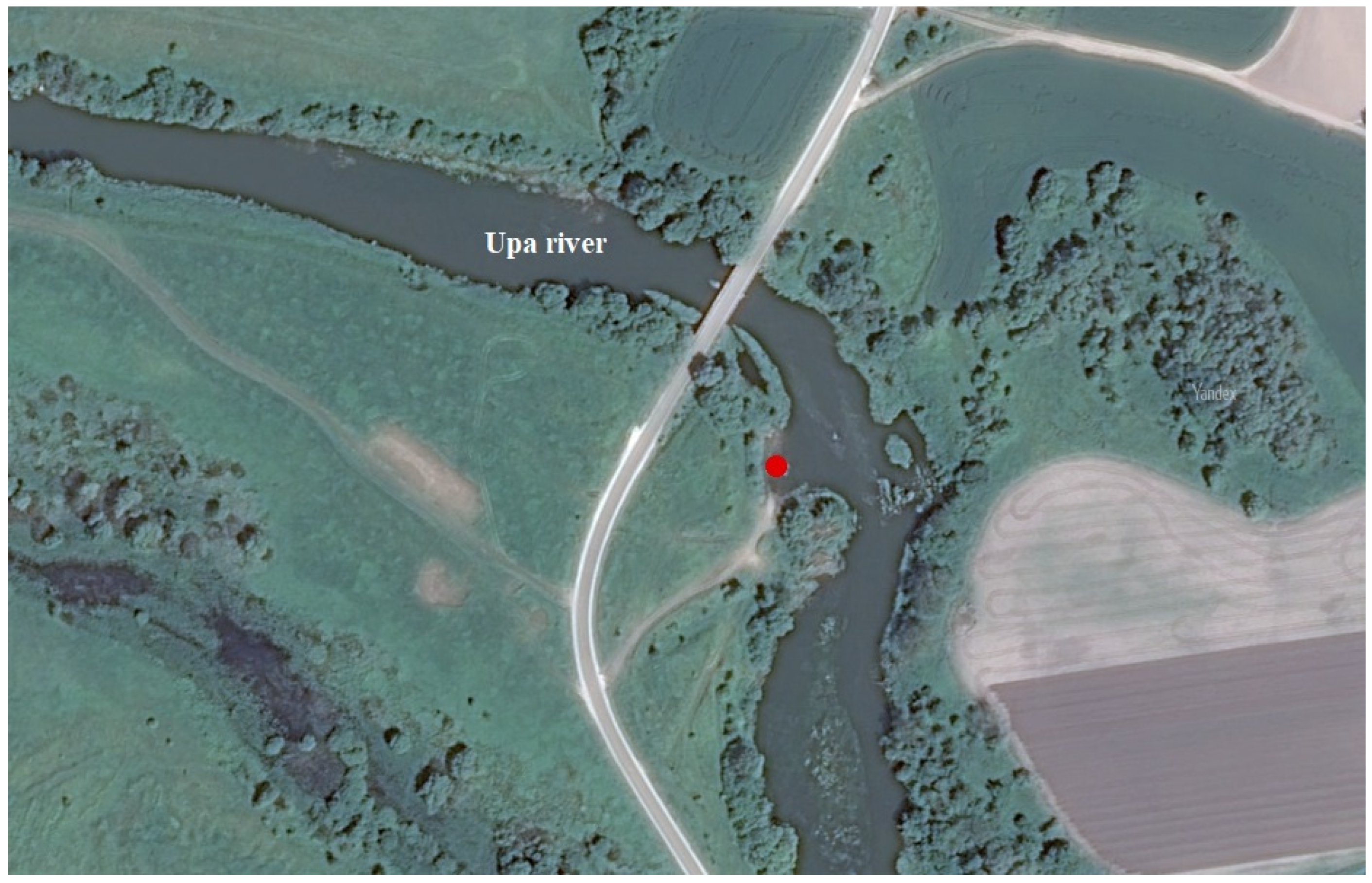
2.1. Description of the Site
2.2. Experiment
2.2.1. Cultivation of a Phototrophic Enrichment Culture
2.2.2. Determining the Effect of Mineral Additives on Accelerating Biomass Growth
2.2.3. Assessment of Radionuclide Immobilization Efficiency in Phytoplankton Development
2.2.4. Modeling Radionuclide Behavior in Sludge
2.3. Analytical Methods
2.4. Geochemical Modeling
3. Results and Discussion
3.1. Sample Characteristics
3.1.1. Chemical and Radiochemical Composition of Liquid Samples
3.1.2. Chemical, Radiochemical and Mineral Composition of Sludge Samples
3.2. Phytoplankton and Microbial Diversity
3.2.1. Phyto- and Bacterioplankton Diversity in the Water Samples
3.2.2. Microbial Diversity of the Bottom Sediment
3.3. Phytoplankton Activation by Additives
Biomass Accumulation and Number of Morphotypes
3.4. Laboratory Modeling of Radionuclide Behavior in the Water-Bottom Sediment System
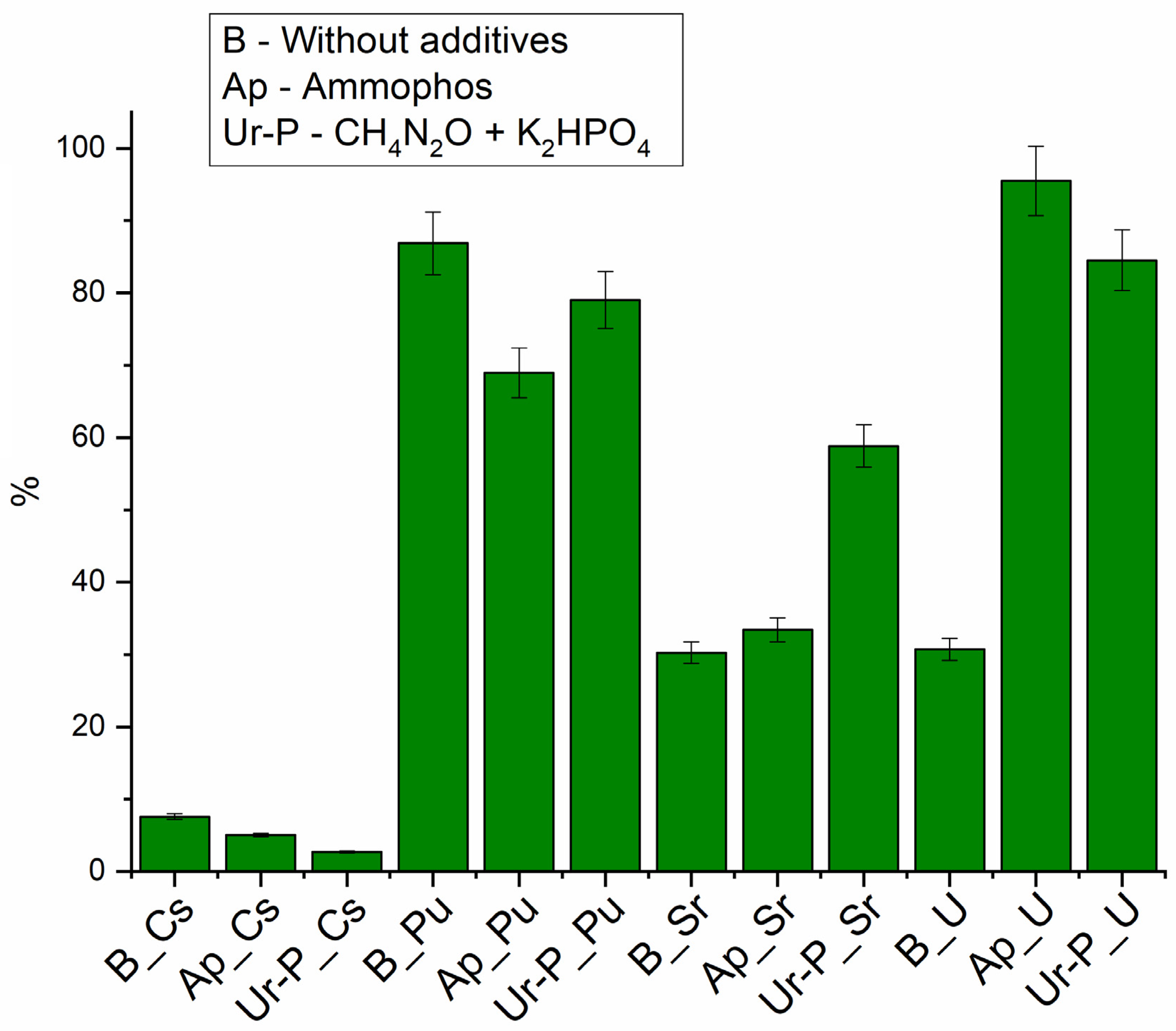
3.4.1. Evaluation of Radionuclide Removal by Phytoplankton Activation with Stimulant Additives
3.4.2. Assessment of Binding Force of Radionuclides to Bottom Sludge
3.5. Thermodynamic Modeling of Radionuclide Behavior in Sludge
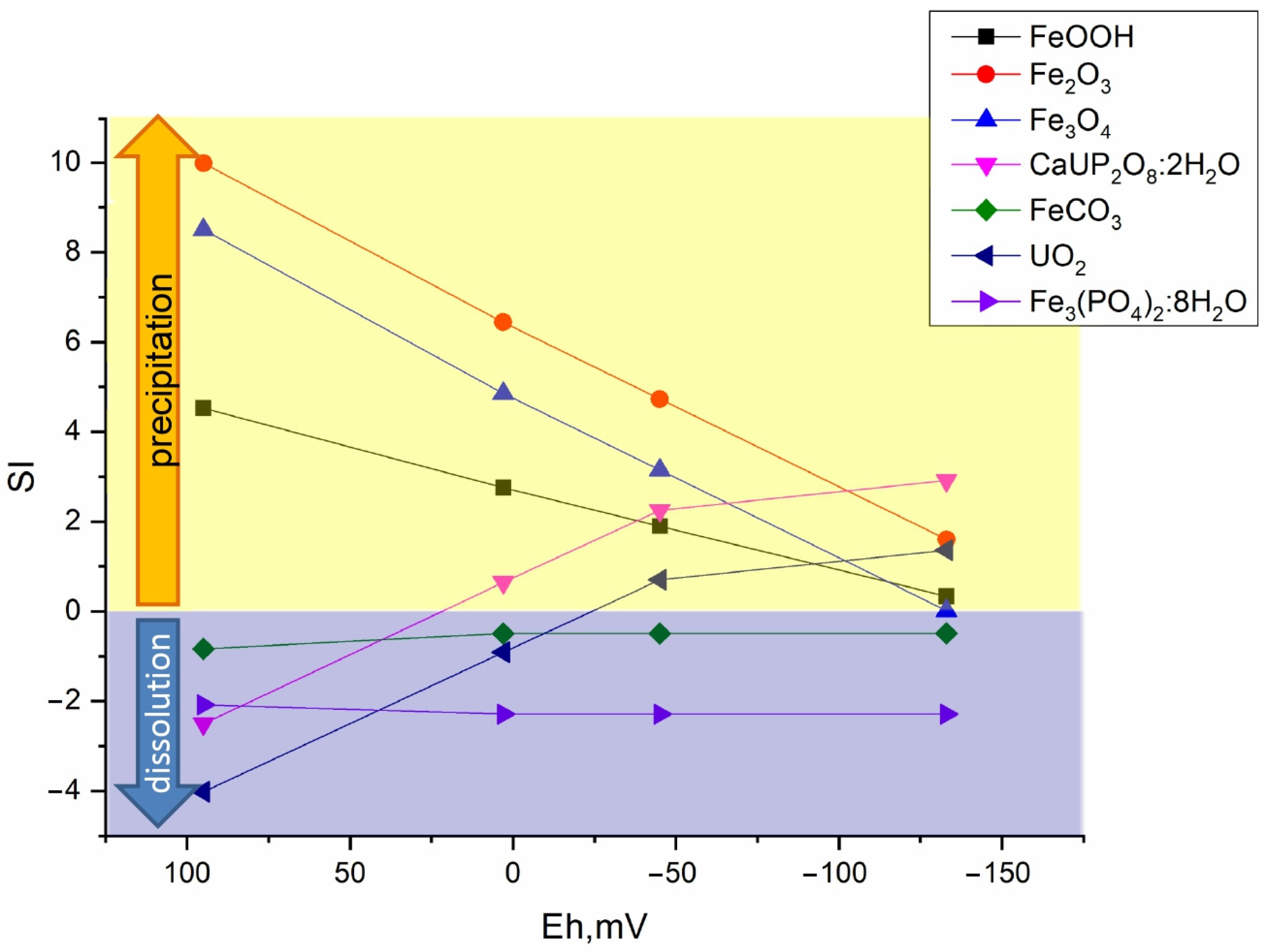
3.5.1. Changing Physical and Chemical Conditions in Bottom Sediments
3.5.2. Thermodynamic Calculation of Radionuclide Forms in Bottom Sediments
3.6. Verification of Mineral Phase Formation Calculations
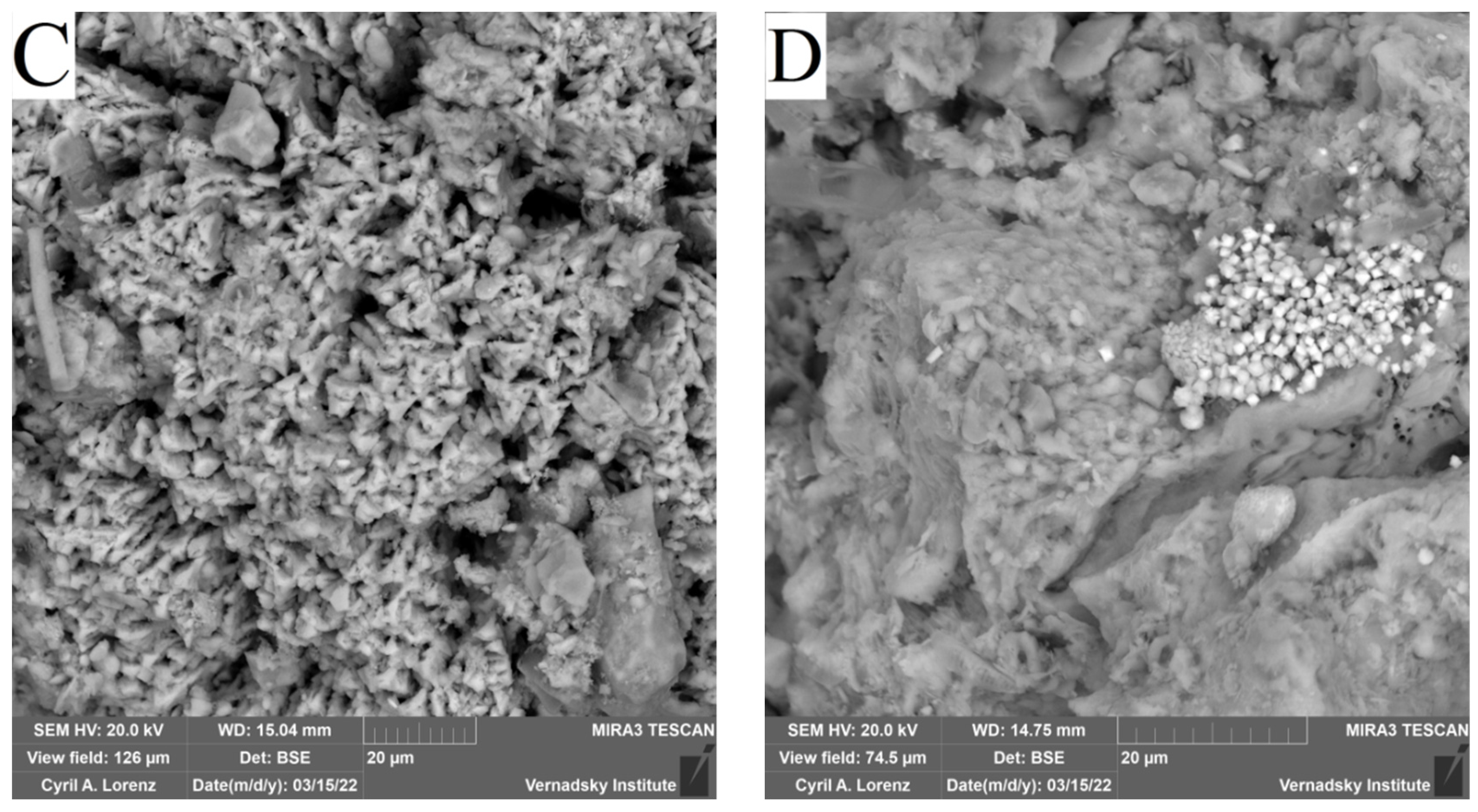
SEM EDX Sludge Analysis after Biostimulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Voelkle, H. A brief History of Nuclear Disasters: Prevention, Consequences and Re-coverage. Planet@Risk 2015, 3, 271–280. [Google Scholar]
- Schwantes, J.M.; Orton, C.R.; Clark, R.A. Analysis of a nuclear accident: Fission and activation product releases from the fukushima daiichi nuclear facility as remote indicators of source identification, extent of release, and state of damaged spent nuclear fuel. Env. Sci. Technol. Am. Chem. Soc. 2012, 46, 8621–8627. [Google Scholar] [CrossRef]
- Smirnov, M.O.; Merzlaya, G.E.; Afanas’ev, R.A. Methods of Reducing the Content of Radionuclides in Agricultural Plants after the Accident at the Chernobyl Nuclear Power Plant. In Environmental Technology and Engineering Techniques; Apple Academic Press: New York, NY, USA, 2020; pp. 271–282. [Google Scholar]
- Maslov, V.P. Aftermath of the Chernobyl Catastrophe from the Point of View of the Security Concept. Math. Notes 2019, 106, 757–770. [Google Scholar] [CrossRef]
- Eidemüller, D. Radioactive Incidents and Disasters. In Nuclear Power Explained; Springer: Cham, Switzerland, 2021; pp. 195–240. [Google Scholar]
- Rowan, D.J. Anthropogenic Radionuclides in Ottawa River Sediment near Chalk River Laboratories. Nucl. Rev. 2014, 1, 67–70. [Google Scholar] [CrossRef]
- Bhanot, K. Preliminary fact finding mission following the accident at the nuclear fuel processing facility in Tokaimura, Japan (Vienna: IAEA). J. Radiol. Prot. 2000, 20, 73. [Google Scholar] [CrossRef]
- Novikov, A.P.; Kalmykov, S.N.; Utsunomiya, S.; Ewing, R.C.; Horreard, F.; Merkulov, A.; Clark, S.B.; Tkachev, V.V.; Myasoedov, B.F. Colloid transport of plutonium in the far-field of the Mayak Production Association, Russia. Science 2006, 314, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Pryakhin, E.A.; Mokrov, Y.G.; Tryapitsina, G.A.; Ivanov, I.A.; Osipov, D.I.; Atamanyuk, N.I.; Deryabina, L.V.; Shaposhnikova, I.A.; Shishkina, E.A.; Obvintseva, N.A.; et al. Characterization of biocenoses in the storage reservoirs of liquid radioactive wastes of Mayak PA. Initial descriptive report. J. Env. Radioact. 2016, 151, 449–460. [Google Scholar] [CrossRef]
- Bugai, D.; Skalskyy, A.; Dzhepo, S.; Kubko, Y.; Kashparov, V.; van Meir, N.; Stammose, D.; Simonucci, C.; Martin-Garin, A. Radionuclide migration at experimental polygon at Red Forest waste site in Chernobyl zone. Part 2: Hydrogeological characterization and groundwater transport modeling. Appl. Geochem. 2012, 27, 1359–1374. [Google Scholar] [CrossRef]
- Novikov, A.P.; Vlasova, I.E.; Safonov, A.V.; Ermolaev, V.M.; Zakharova, E.V.; Kalmykov, S.N. Speciation of actinides in groundwater samples collected near deep nuclear waste repositories. J. Environ. Radioact. 2018, 192, 334–341. [Google Scholar] [CrossRef]
- Kobayashi, T.; Otosaka, S.; Togawa, O.; Hayashi, K. Development of a Non-conservative Radionuclides Dispersion Model in the Ocean and its Application to Surface Cesium-137 Dispersion in the Irish Sea. J. Nucl. Sci. Technol. 2007, 44, 238–247. [Google Scholar] [CrossRef]
- Bugai, D.A.; Olegov, D.; Kubko, Y. Monitoring Observations and Analyses of 90 Sr Migration Regime in the Aquifer (2000 Year Studies); Institute of Geological Sciences: Kiev, Ukraine, 2001. [Google Scholar] [CrossRef]
- Lavrik, V.I. Principles of Mathematical Modeling of Processes of Physicochemical and Biological Self-purification in Aquatic Ecosystems. Hydrobiol. J. 2001, 37, 87–111. [Google Scholar] [CrossRef]
- Kanivets, V.; Laptev, G.; Konoplev, A.; Lisovyi, H.; Derkach, G.; Voitsekhovych, O. Distribution and Dynamics of Radionuclides in the Chernobyl Cooling Pond. In Behavior of Radionuclides in the Environment II; Springer: Singapore, 2020; pp. 349–405. [Google Scholar]
- Sasaki, T.; Kauri, T.; Kudo, A. Effect of pH and temperature on the sorption of Np and Pa to mixed anaerobic bacteria. Appl. Radiat. Isot. 2001, 55, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, X.Y.; Chen, T.; Tian, W.Y.; Zheng, Z.; Wang, L.H.; Liu, C.L. The influence of pH on the sorption and diffusion of 99TcO 4- in Beishan granite. Radiochim. Acta 2012, 100, 449–455. [Google Scholar] [CrossRef]
- Buckau, G. Humic Substances in Performance Assessment of Nuclear Waste Disposal: Actinide and Iodine Migration in the Far-Field Second Technical Progress Report; Institut für Nukleare Entsorgung: Eggenstein-Leopoldshafen, Germany, 2004; Available online: https://publikationen.bibliothek.kit.edu/270060838 (accessed on 12 November 2022).
- Adam, C.; Garnier-Laplace, J. Bioaccumulation of silver-110m, cobalt-60, cesium-137, and manganese-54 by the freshwater algae Scenedesmus obliquus and Cyclotella meneghiana and by suspended matter collected during a summer bloom event. Limnol. Oceanogr. 2003, 48, 2303–2313. [Google Scholar] [CrossRef]
- German, K.E.; Firsova, E.V.; Peretrukhin, V.F.; Khizhnyak, T.V.; Simonoff, M. Bioaccumulation of Tc, Pu, and Np on Bottom Sediments in Two Types of Freshwater Lakes of the Moscow Oblast. Radiochemistry 2003, 45, 250–256. [Google Scholar] [CrossRef]
- Suresh Kumar, K.; Dahms, H.W.; Won, E.J.; Lee, J.S.; Shin, K.H. Microalgae—A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef] [PubMed]
- German, K.E.; Safonov, A.V.; Zelenina, D.A.; Sitanskaya, A.V.; Boldyrev, K.A.; Belova, E.V. Hypolimnion behavior of technetium in freshwater at various stages of eutrophication. J. Environ. Radioact. 2021, 237, 106716. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Garrido, I. Microalgae immobilization: Current techniques and uses. Bioresour. Technol. 2008, 99, 3949–3964. [Google Scholar] [CrossRef] [PubMed]
- Vogel, M.; Günther, A.; Rossberg, A.; Li, B.; Bernhard, G.; Raff, J. Biosorption of U(VI) by the green algae Chlorella vulgaris in dependence of pH value and cell activity. Sci. Total Environ. 2010, 409, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Zinicovscaia, I.; Safonov, A.; Zelenina, D.; Ershova, Y.; Boldyrev, K. Evaluation of biosorption and bioaccumulation capacity of cyanobacteria Arthrospira (spirulina) platensis for radionuclides. Algal Res. 2020, 51, 102075. [Google Scholar] [CrossRef]
- Safonov, A.V.; Ognistaya, A.V.; Boldyrev, K.A.; Zelenina, D.A.; Bondareva, L.G.; Tananaev, I.G. The Role of Phytoplankton in Self-Purification of Water Bodies with Radionuclide Pollutants. Radiochem. Pleiades J. 2022, 64, 120–132. [Google Scholar] [CrossRef]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Jessat, J.; Sachs, S.; Moll, H.; John, W.; Steudtner, R.; Hübner, R.; Bok, F.; Stumpf, T. Bioassociation of U(VI) and Eu(III) by Plant (Brassica napus) Suspension Cell Cultures—A Spectroscopic Investigation. Environ. Sci. Technol. 2021, 55, 6718–6728. [Google Scholar] [CrossRef]
- Gill, G.; Geesey, C.R.A.L.N. Pyrrhotite: An Iron Sulfide Mineral Formed During Growth of Sulfate-Reducing Bacteria at a Hematite Surface. In AGU Fall Meeting Abstracts; American Geophysical Union: Washington, DC, USA, 2008. [Google Scholar]
- Berg, J.S.; Jézéquel, D.; Duverger, A.; Lamy, D.; Laberty-Robert, C.; Miot, J. Microbial diversity involved in iron and cryptic sulfur cycling in the ferruginous, low-sulfate waters of Lake Pavin. PLoS ONE 2019, 14, e0212787. [Google Scholar] [CrossRef] [PubMed]
- Popa, R.; Kinkle, B.K. Isolation of Thiomonas thermosulfatus Strain 51, a Species Capable of Coupling Biogenic Pyritization with Chemiosmotic Energy Transduction. Geomicrobiol. J. 2004, 21, 297–309. [Google Scholar] [CrossRef]
- Schippers, A.; Jorgensen, B.B. Biogeochemistry of pyrite and iron sulfide oxidation in marine sediments. Geochim. Cosmochim. Acta 2002, 66, 85–92. [Google Scholar] [CrossRef]
- Stolyar, S.V.; Kolenchukova, O.A.; Boldyreva, A.V.; Kudryasheva, N.S.; Gerasimova, Y.V.; Krasikov, A.A.; Yaroslavtsev, R.N.; Bayukov, O.A.; Ladygina, V.P.; Birukova, E.A. Biogenic Ferrihydrite Nanoparticles: Synthesis, Properties In Vitro and In Vivo Testing and the Concentration Effect. Biomedicines 2021, 9, 323. [Google Scholar] [CrossRef]
- Safonov, A.V.; Boguslavskii, A.E.; Boldyrev, K.A.; Zayceva, L.V. Biogenic Factors of Formation of Geochemical Uranium Anomalies near the Sludge Storage of the Novosibirsk Chemical Concentrate Plant. Geochem. Int. 2019, 57, 709–715. [Google Scholar] [CrossRef]
- Borch, T.; Masue, Y.; Kukkadapu, R.K.; Fendorf, S. Phosphate Imposed Limitations on Biological Reduction and Alteration of Ferrihydrite. Environ. Sci. Technol. 2007, 41, 166–172. [Google Scholar] [CrossRef]
- Townsend, L.T.; Shaw, S.; Ofili, N.E.R.; Kaltsoyannis, N.; Walton, A.S.; Mosselmans, J.F.W.; Neill, T.S.; Lloyd, J.R.; Heath, S.; Hibberd, R.; et al. Formation of a U(VI)—Persulfide Complex during Environmentally Relevant Sulfidation of Iron (Oxyhydr)oxides. Environ. Sci. Technol. 2019, 54, 129–136. [Google Scholar] [CrossRef]
- Lee, S.Y.; Baik, M.H.; Cho, H.R.; Jung, E.C.; Jeong, J.T.; Choi, J.W.; Lee, Y.B.; Lee, Y.J. Abiotic reduction of uranium by mackinawite (FeS) biogenerated under sulfate-reducing condition. J. Radioanal. Nucl. Chem. 2013, 296, 1311–1319. [Google Scholar] [CrossRef]
- Philp, J.C.; Atlas, R.M. Bioremediation of Contaminated Soils and Aquifers. In Bioremediation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 139–236. [Google Scholar]
- Alessi, D.S.; Lezama-Pacheco, J.S.; Janot, N.; Suvorova, E.I.; Cerrato, J.M.; Giammar, D.E.; Davis, J.A.; Fox, P.M.; Williams, K.H.; Long, P.E.; et al. Speciation and reactivity of uranium products formed during in situ bioremediation in a shallow alluvial aquifer. Environ. Sci. Technol. 2014, 48, 12842–12850. [Google Scholar] [CrossRef] [PubMed]
- Wufuer, R.; Duo, J.; Li, W.; Fan, J.; Pan, X. Bioremediation of uranium-and nitrate-contaminated groundwater after the in situ leach mining of uranium. Water 2021, 13, 3188. [Google Scholar] [CrossRef]
- Safonov, A.V.; Babich, T.L.; Sokolova, D.S.; Grouzdev, D.S.; Tourova, T.P.; Poltaraus, A.B.; Zakharova, E.V.; Merkel, A.Y.; Novikov, A.P.; Nazina, T.N. Microbial community and in situ bioremediation of groundwater by nitrate removal in the zone of a radioactive waste surface repository. Front. Microbiol. 2018, 9, 1985. [Google Scholar] [CrossRef]
- Wu, W.M.; Carley, J.; Gentry, T.; Ginder-Vogel, M.A.; Fienen, M.; Mehlhorn, T.; Yan, H.; Caroll, S.; Pace, M.N.; Nyman, J.; et al. Pilot-scale in situ bioremedation of uranium in a highly contaminated aquifer. 2. Reduction of U(VI) and geochemical control of U(VI) bioavailability. Environ. Sci. Technol. 2006, 40, 3986–3995. [Google Scholar] [CrossRef]
- Konoplev, A.; Kanivets, V.; Laptev, G.; Voitsekhovich, O.; Zhukova, O.; Germenchuk, M. Long-Term Dynamics of the Chernobyl-Derived Radionuclides in Rivers and Lakes. In Behavior of Radionuclides in the Environment II; Springer: Singapore, 2020; pp. 323–348. [Google Scholar]
- Belyaev, V.R.; Golosov, V.N.; Markelov, M.V.; Evrard, O.; Ivanova, N.N.; Paramonova, T.A.; Shamshurina, E.N. Using Chernobyl-derived 137Cs to document recent sediment deposition rates on the River Plava floodplain (Central European Russia). Hydrol. Process 2013, 27, 807–821. [Google Scholar] [CrossRef]
- Konoplev, A.V.; Ivanov, M.M.; Golosov, V.N.; Konstantinov, E.A. Reconstruction of long-term dynamics of Chernobyl-derived 137Cs in the Upa River using bottom sediments in the Scheckino reservoir and semi-empirical modelling. Proc. Int. Assoc. Hydrol. Sci. 2019, 381, 95–99. [Google Scholar]
- Golosov, V.N.; Ivanov, M.M.; Tsyplenkov, A.S.; Ivanov, M.A.; Wakiyama, Y.; Konoplev, A.V.; Konstantinov, E.A.; Ivanova, N.N. Erosion as a Factor of Transformation of Soil Radioactive Contamination in the Basin of the Shchekino Reservoir (Tula Region). Eurasian Soil Sci. 2021, 54, 291–303. [Google Scholar] [CrossRef]
- Hughes, E.O.; Gorham, P.R.; Zehnder, A. Toxicity of a Unialgal Culture of Microcystis Aeruginosa. Can. J. Microbiol. 2011, 4, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.M. Simple conditions for growth of unicellular blue-green algae on plates1, 2. J. Phycol. 1968, 4, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Deruelles, J.; Waterbury, J.B. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. User’s guide to phreeqc (Version 2)—A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. USGS Rep. 1999, 99, 312. [Google Scholar] [CrossRef]
- Fernández-Gómez, B.; Richter, M.; Schüler, M.; Pinhassi, J.; Acinas, S.G.; González, J.M.; Pedrós-Alió, C. Ecology of marine Bacteroidetes: A comparative genomics approach. ISME J. 2013, 7, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Alvarez, V.; Schrantz, K.A.; Pressman, J.G.; Speitel, G.E.; Wahman, D.G. Pyrosequencing analysis of bench-scale nitrifying biofilters removing trihalomethanes. Environ. Eng. Sci. 2013, 30, 582–588. [Google Scholar] [CrossRef]
- Gong, X.; Chen, Z.; Deng, Y.; Zhao, D.; Gao, P.; Zhang, L.; Tu, Q.; Qu, L.; Zheng, L.; Zhang, Y.; et al. Contrasting archaeal and bacterial community assembly processes and the importance of rare taxa along a depth gradient in shallow coastal sediments. Sci. Total Environ. 2022, 852, 158411. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Safonov, A.V.; Khijniak, T.V. Biosorption of Hexavalent Chromium and Uranium by Bacteria, Microalga, and Fungi. In Heavy Metals and Other Pollutants in the Environment; CRC Press: Boca Raton, FL, USA, 2016; pp. 333–360. [Google Scholar]
- Vieto, S.; Rojas-Gätjens, D.; Jiménez, J.I.; Chavarría, M. The potential of Pseudomonas for bioremediation of oxyanions. Environ. Microbiol. Rep. 2021, 13, 773–789. [Google Scholar] [CrossRef]
- Otsuka, O.; Yamashita, M. Selenium recovery from wastewater using the selenate-reducing bacterium Pseudomonas stutzeri NT-I. Hydrometallurgy 2020, 197, 105470. [Google Scholar] [CrossRef]
- Geckeis, H.; Salbu, B.; Schafer, T.; Zavarin, M. Environmental chemistry of plutonium. In Plutonium Futures-the Science; American Nuclear Society: La Grange Park, IL, USA, 2018; p. 113. [Google Scholar]
- Foster, R.I.; Kim, K.W.; Oh, M.K.; Lee, K.Y. Effective removal of uranium via phosphate addition for the treatment of uranium laden process effluents. Water Res. 2019, 158, 82–93. [Google Scholar] [CrossRef]
- Daopuye, S.I.; Grace, A.C.; Charles, C.O.; Kelechi, G.A. Sedimentation Profile of Strontium Phosphate Precipitate in Aqueous Medium. NIPES J. Sci. Technol. Res. 2020, 2, 113–118. [Google Scholar] [CrossRef]
- Gupta, D.K.; Schulz, W.; Steinhauser, G.; Walther, C. Radiostrontium transport in plants and phytoremediation. Environ. Sci. Pollut. Res. 2018, 25, 29996–30008. [Google Scholar] [CrossRef]
- Kalin, M.; Wheeler, W.N.; Meinrath, G. The removal of uranium from mining waste water using algal/microbial biomass. J. Environ. Radioact. 2005, 78, 151–177. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Ahmed, B.; Beyenal, H. Immobilization of uranium in groundwater using biofilms. Emerg. Environ. Technol. 2010, 2, 1–37. [Google Scholar]
- Neu, M.P.; Icopini, G.A.; Boukhalfa, H. Plutonium speciation affected by environmental bacteria. Radiochim. Acta 2005, 93, 705–714. [Google Scholar] [CrossRef]
- Saito, K.; Kuroda, K.; Suzuki, R.; Kino, Y.; Sekine, T.; Shinoda, H.; Yamashiro, H.; Fukuda, T.; Kobayashi, J.; Abe, Y.; et al. Intestinal Bacteria as Powerful Trapping Lifeforms for the Elimination of Radioactive Cesium. Front. Vet Sci. 2019, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Balboni, E.; Smith, K.F.; Moreau, L.M.; Li, T.T.; Maloubier, M.; Booth, C.H.; Kersting, A.B.; Zavarin, M. Transformation of Ferrihydrite to Goethite and the Fate of Plutonium. ACS Earth Space Chem. 2020, 4, 1993–2006. [Google Scholar] [CrossRef]
- Kirsch, R.; Fellhauer, D.; Altmaier, M.; Neck, V.; Rossberg, A.; Fanghänel, T.; Charlet, L.; Scheinost, A.C. Oxidation State and Local Structure of Plutonium Reacted with Magnetite, Mackinawite, and Chukanovite. Environ. Sci. Technol. 2011, 45, 7267–7274. [Google Scholar] [CrossRef]
- Safonov, A.V.; Boguslavsky, A.E.; Gaskova, O.L.; Boldyrev, K.A.; Shvartseva, O.S.; Khvashchevskaya, A.A.; Popova, N.M. Biogeochemical Modelling of Uranium Immobilization and Aquifer Remediation Strategies Near NCCP Sludge Storage Facilities. Appl. Sci. 2021, 11, 2875. [Google Scholar] [CrossRef]
- Safonov, A.; Tregubova, V.; Ilin, V.; Boldyrev, K.; Zinicovscaia, I.; Frontasyeva, M.; Khijniak, T. Comparative Study of Lanthanum, Vanadium, and Uranium Bioremoval Using Different Types of Microorganisms. Water Air Soil Pollut. 2018, 229, 82. [Google Scholar] [CrossRef]
- Safonov, A.; Lavrinovich, E.; Emel’yanov, A.; Boldyrev, K.; Kuryakov, V.; Rodygina, N.; Zakharova, E.; Novikov, A. Risk of colloidal and pseudo-colloidal transport of actinides in nitrate contaminated groundwater near a radioactive waste repository after bioremediation. Sci. Rep. 2022, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Safonov, A.; Popova, N.; Boldyrev, K.; Lavrinovich, E.; Boeva, N.; Artemiev, G.; Kuzovkina, E.; Emelyanov, A.; Myasnikov, I.; Zakharova, E.; et al. The microbial impact on U, Pu, Np, and Am immobilization on aquifer sandy rocks, collected at the deep LRW injection site. J. Geochem. Explor. 2022, 240, 107052. [Google Scholar] [CrossRef]
- Kong, L.; Ruan, Y.; Zheng, Q.; Su, M.; Diao, Z.; Chen, D.; Hou, L.; Chang, X.; Shih, K. Uranium extraction using hydroxyapatite recovered from phosphorus containing wastewater. J. Hazard Mater. 2020, 382, 120784. [Google Scholar] [CrossRef] [PubMed]
- Romanchuk, A.Y.; Vlasova, I.E.; Kalmykov, S.N. Speciation of Uranium and Plutonium from Nuclear Legacy Sites to the Environment: A Mini Review. Front. Chem. 2020, 8, 630. [Google Scholar] [CrossRef] [PubMed]
- Macaskie, L.E.; Jeong, B.C.; Tolley, M.R. Enzymically accelerated biomineralization of heavy metals: Application to the removal of americium and plutonium from aqueous flows. FEMS Microbiol. Rev. 1994, 14, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, R.; Robinson, K.G.; Reed, G.D.; Sayler, G.S. Reduction of hexavalent uranium from organic complexes by sulfate- and iron-reducing bacteria. Appl. Environ. Microbiol. 1997, 63, 4385–4391. [Google Scholar] [CrossRef]
- Wall, J.D.; Krumholz, L.R. Uranium Reduction. Annu. Rev. Microbiol. 2006, 60, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Shelobolina, E.S.; Vrionis, H.A.; Findlay, R.H.; Lovley, D.R. Geobacter uraniireducens sp. nov., isolated from subsurface sediment undergoing uranium bioremediation. Int. J. Syst. Evol. Microbiol. 2008, 58, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Yuan, Y.; Feng, L.; Sun, W.; Lin, K.; Zhang, J.; Zhang, Y.; Wang, H.; Wang, N.; Peng, Q. Highly efficient immobilization of environmental uranium contamination with Pseudomonas stutzeri by biosorption, biomineralization, and bioreduction. J. Hazard Mater. 2022, 424, 127758. [Google Scholar] [CrossRef]
- Ithurbide, A.; Peulon, S.; Miserque, F.; Beaucaire, C.; Chaussé, A. Interaction between uranium(VI) and siderite (FeCO3) surfaces in carbonate solutions. Radiochim. Acta 2009, 97, 177–180. [Google Scholar] [CrossRef]
- Chen, X.; Romaniello, S.J.; Herrmann, A.D.; Wasylenki, L.E.; Anbar, A.D. Uranium isotope fractionation during coprecipitation with aragonite and calcite. Geochim. Cosmochim. Acta 2016, 188, 189–207. [Google Scholar] [CrossRef]
- Dong, W.; Ball, W.P.; Liu, C.X.; Wang, Z.M.; Stone, A.T.; Bai, J.; Zachara, J.M. Influence of calcite and dissolved calcium on uranium(VI) sorption to a Hanford subsurface sediment. Environ. Sci. Technol. 2005, 39, 7949–7955. [Google Scholar] [CrossRef]
- Reeder, R.J.; Nugent, M.; Tait, C.D.; Morris, D.E.; Heald, S.M.; Beck, K.M.; Hess, W.P.; Lanzirotti, A. Coprecipitation of uranium(VI) with calcite: XAFS, micro-XAS, and luminescence characterization. Geochim. Cosmochim. Acta 2001, 65, 3491–3503. [Google Scholar] [CrossRef]
- Boguslavsky, A.E.; Gaskova, O.L.; Naymushina, O.S.; Popova, N.M.; Safonov, A.V. Environmental monitoring of low-level radioactive waste disposal in electrochemical plant facilities in Zelenogorsk, Russia. Appl. Geochem. 2020, 119, 104598. [Google Scholar] [CrossRef]
- Safonov, A.V.; Andryushchenko, N.D.; Ivanov, P.V.; Boldyrev, K.A.; Babich, T.L.; German, K.E.; Zakharova, E.V. Biogenic Factors of Radionuclide Immobilization on Sandy Rocks of Upper Aquifers. Radiochemistry 2019, 61, 99–108. [Google Scholar] [CrossRef]
- Sood, A.; Renuka, N.; Prasanna, R.; Ahluwalia, A.S. Cyanobacteria as potential options for wastewater treatment. In Phytoremediation: Management of Environmental Contaminants; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; Volume 2, pp. 83–93. [Google Scholar]







| Ions | Values |
|---|---|
| HCO3− | 92.1 ± 2.3 |
| Cl− | 92.7 ± 2.32 |
| Mg2+ | 5.8 ± 0.15 |
| Na+ | 67.9 ± 1.70 |
| Ca2+ | 4.5 ± 0.10 |
| K+ | 11.8 ± 0.30 |
| NO3− | 1.7 ± 0.04 |
| SO42− | 2.9 ± 0.07 |
| Microcomponents | Values |
|---|---|
| Li | 350 ± 8.8 |
| B | 230 ± 5.8 |
| Al | 1400 ± 35 |
| Si | 1200 ± 30 |
| P | 380 ± 9.5 |
| Mn | 4200 ± 105 |
| Fe | 3200 ± 80 |
| Co | 10 ± 0.3 |
| Ni | 290 ± 7.3 |
| Cu | 17 ± 0.4 |
| Zn | 49 ± 1.2 |
| Rb | 3.2 ± 0.1 |
| Sr | 550 ± 13.8 |
| Y | 4600 ± 115 |
| Zr | 3100 ± 77.5 |
| Cs | 520 ± 13 |
| Ba | 360 ± 9 |
| Nd | 11 ± 0.3 |
| Pb | 2.3 ± 0.1 |
| U | 0.62 ± 0.02 |
| LOI * | Na2O | MgO | Al2O3 | SiO2 | K2O | CaO | TiO2 | MnO | Fe2O3 | P2O5 | S |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 71.5 | 1.20 | 0.96 | 2.60 | 17.56 | 1.20 | 0.46 | 0.570 | 0.06 | 2.84 | 0.08 | 0.03 |
| ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ||
| 0.35 | 0.006 | 0.004 | 0.01 | 0.087 | 0.06 | 0.023 | 0.028 | 0.003 | 0.014 | 0.004 | 0.0015 |
| No Additives | Ammophos | Urea + K2HPO4 | ||||
|---|---|---|---|---|---|---|
| Biomass, mg/L | Number of Morphotypes, pcs. | Biomass, mg/L | Number of Morphotypes, pcs. | Biomass, mg/L | Number of Morphotypes, pcs. | |
| K | 20 ± 2 | 9 | 75 ± 7.5 | 14 | 165 ± 16.5 | 15 |
| U | 9 ± 0.9 | 1 | 60 ± 6 | 5 | 75 ± 7.5 | 3 |
| Pu | 7 ± 0.7 | 1 | 50 ± 5 | 4 | 125 ± 12.5 | 6 |
| Cs | 8 ± 0.8 | 4 | 25 ± 2.5 | 3 | 50 ± 5 | 4 |
| Sr | 9 ± 0.9 | 3 | 35 ± 3.5 | 2 | 75 ± 7.5 | 2 |
| Photo | O | Mg | Al | Si | P | S | K | Ca | Fe |
|---|---|---|---|---|---|---|---|---|---|
| A (SO4) | 14.9 | - | 0.8 | 1.9 | - | 47.7 | 0.2 | 0.3 | 42.7 |
| B (Ap) | 32.6 | - | 1.3 | 4.1 | 0.8 | 31.4 | 0.14 | 1.5 | 35.7 |
| C (Ap + SO4) | 41.3 | 0.4 | 0.6 | 0.8 | - | - | - | 31.4 | 0.3 |
| D (Ap + SO4) | 22.9 | - | 0.4 | 1.2 | 1.2 | 41.8 | - | 1.7 | 30.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelenina, D.; Kuzmenkova, N.; Sobolev, D.; Boldyrev, K.; Namsaraev, Z.; Artemiev, G.; Samylina, O.; Popova, N.; Safonov, A. Biogeochemical Factors of Cs, Sr, U, Pu Immobilization in Bottom Sediments of the Upa River, Located in the Zone of Chernobyl Accident. Biology 2023, 12, 10. https://doi.org/10.3390/biology12010010
Zelenina D, Kuzmenkova N, Sobolev D, Boldyrev K, Namsaraev Z, Artemiev G, Samylina O, Popova N, Safonov A. Biogeochemical Factors of Cs, Sr, U, Pu Immobilization in Bottom Sediments of the Upa River, Located in the Zone of Chernobyl Accident. Biology. 2023; 12(1):10. https://doi.org/10.3390/biology12010010
Chicago/Turabian StyleZelenina, Darya, Natalia Kuzmenkova, Denis Sobolev, Kirill Boldyrev, Zorigto Namsaraev, Grigoriy Artemiev, Olga Samylina, Nadezhda Popova, and Alexey Safonov. 2023. "Biogeochemical Factors of Cs, Sr, U, Pu Immobilization in Bottom Sediments of the Upa River, Located in the Zone of Chernobyl Accident" Biology 12, no. 1: 10. https://doi.org/10.3390/biology12010010
APA StyleZelenina, D., Kuzmenkova, N., Sobolev, D., Boldyrev, K., Namsaraev, Z., Artemiev, G., Samylina, O., Popova, N., & Safonov, A. (2023). Biogeochemical Factors of Cs, Sr, U, Pu Immobilization in Bottom Sediments of the Upa River, Located in the Zone of Chernobyl Accident. Biology, 12(1), 10. https://doi.org/10.3390/biology12010010