Mass Start or Time Trial? Structure of the Nervous System and Neuroregeneration in Pygospio elegans (Spionidae, Annelida)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Collection and Keeping
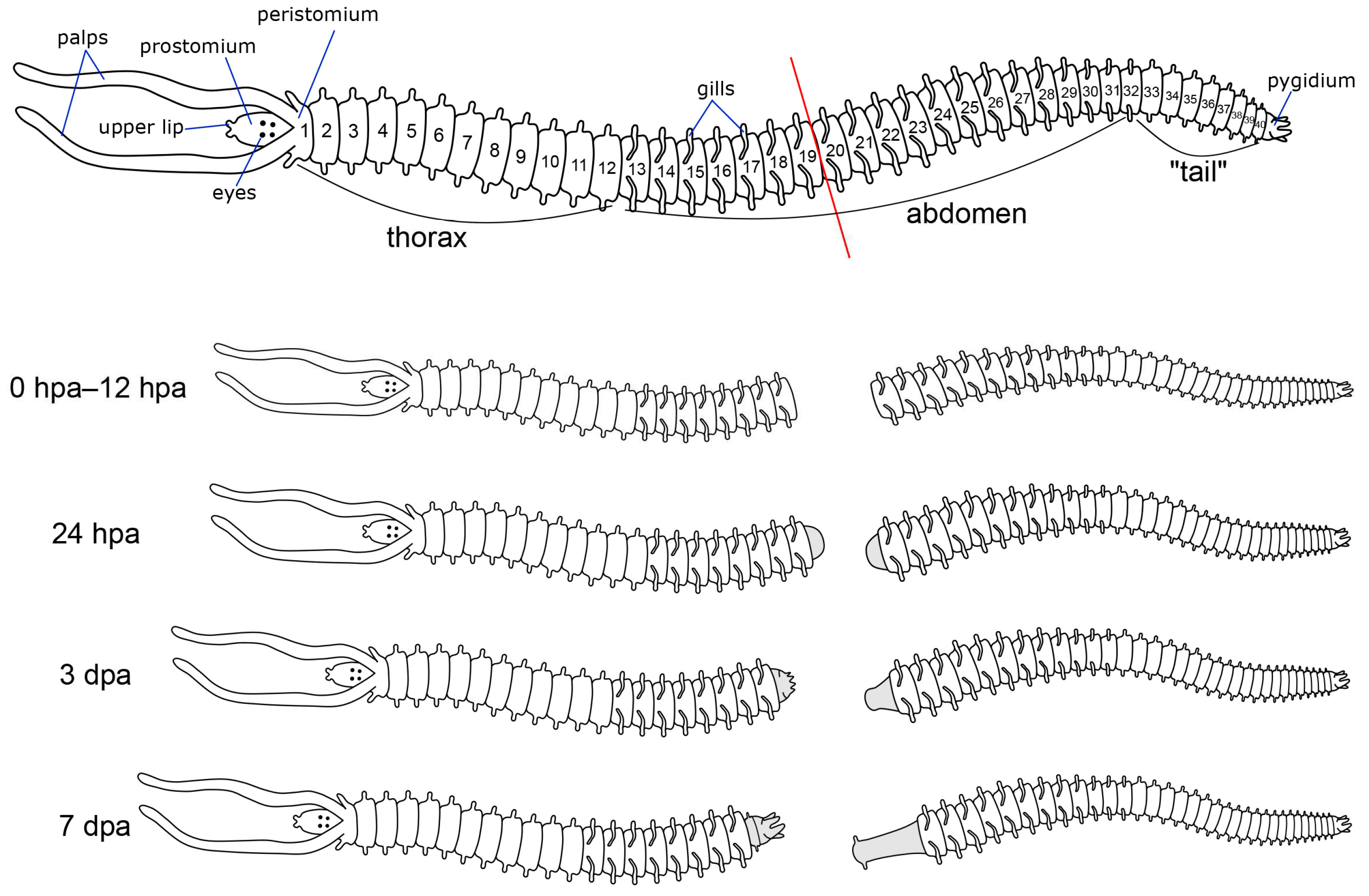
2.2. Sampling and Regeneration Experiments, Fixation
2.3. Immunochemistry
2.4. Microscopy and Image Processing
3. Results
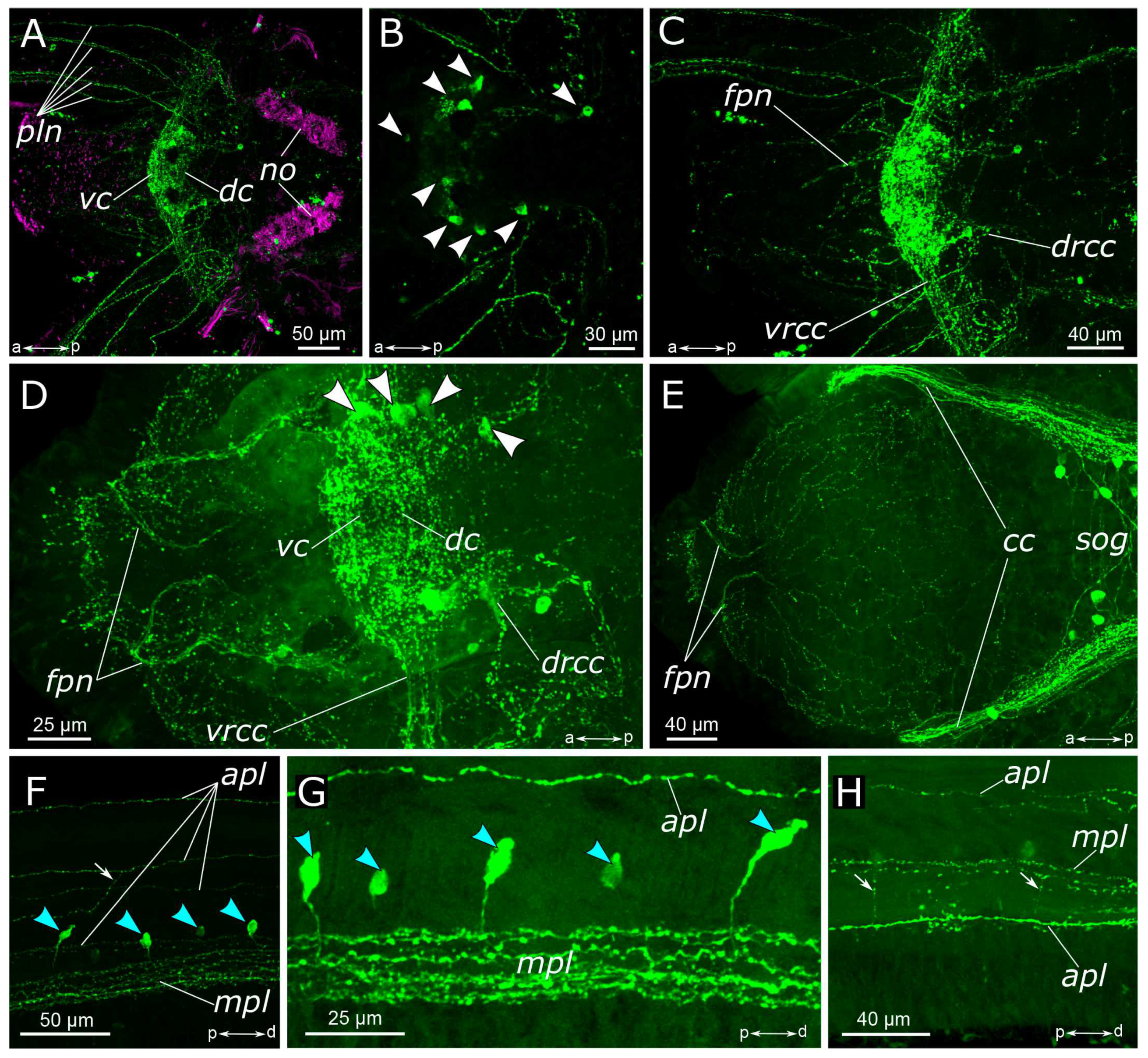
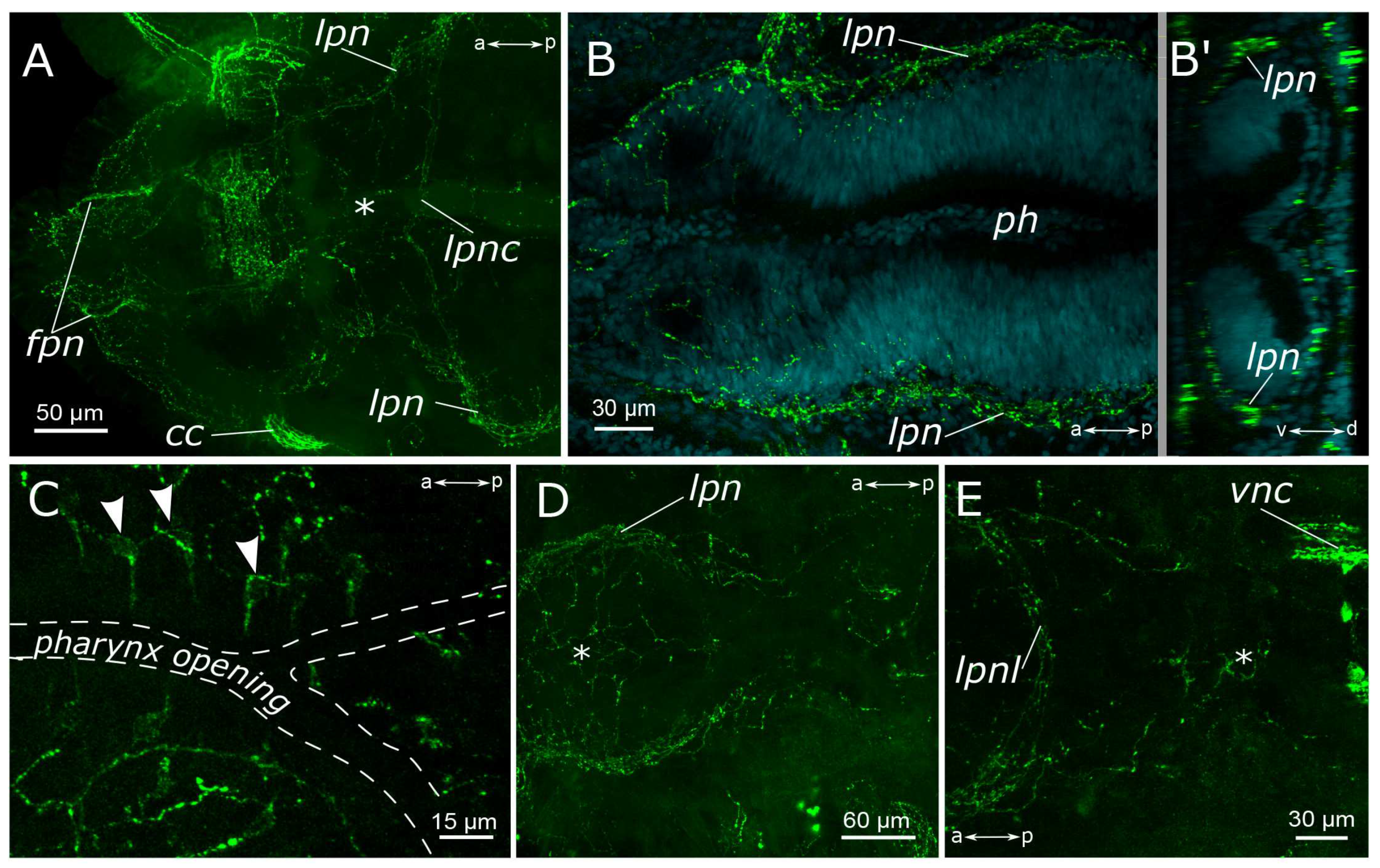
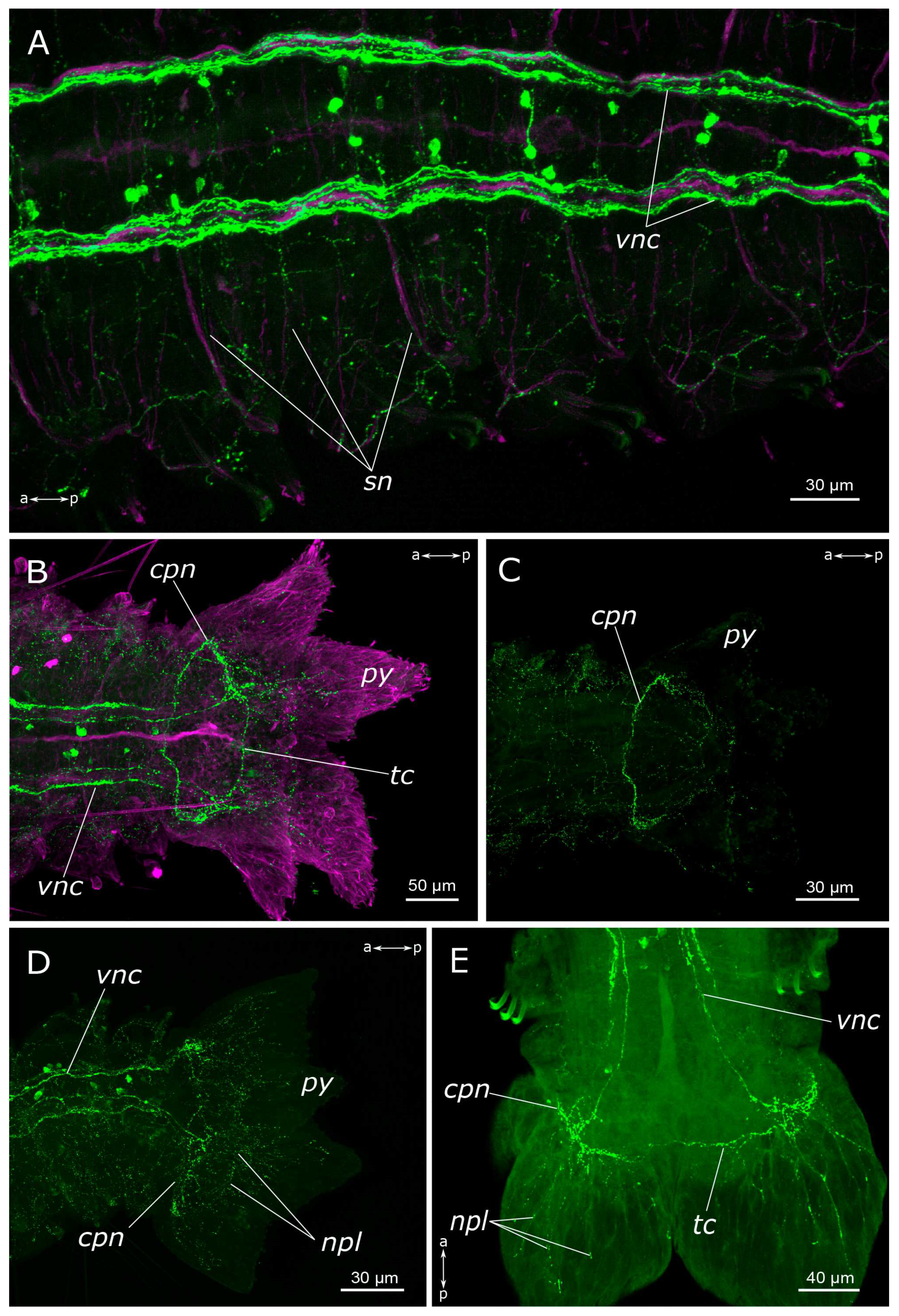
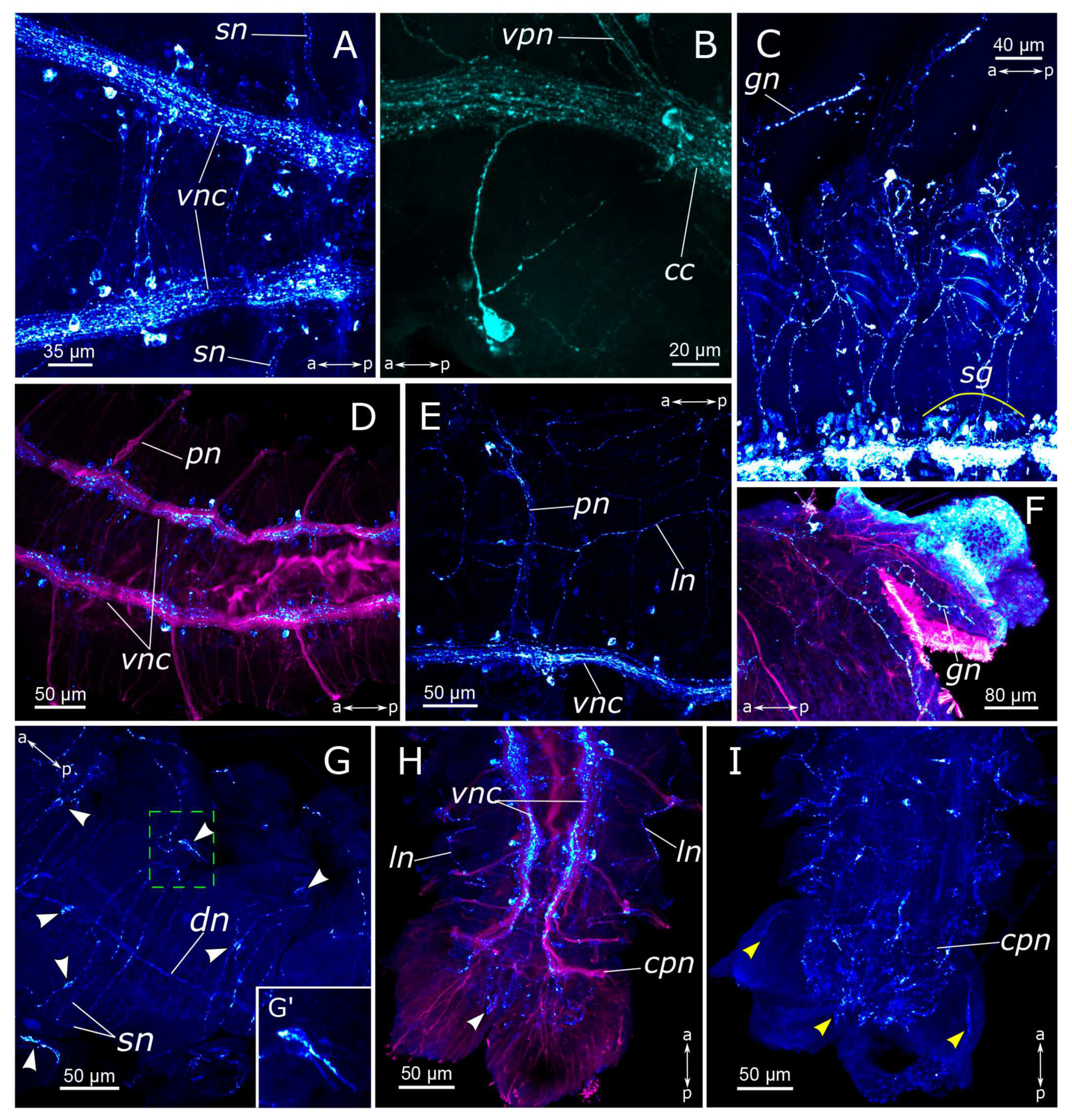
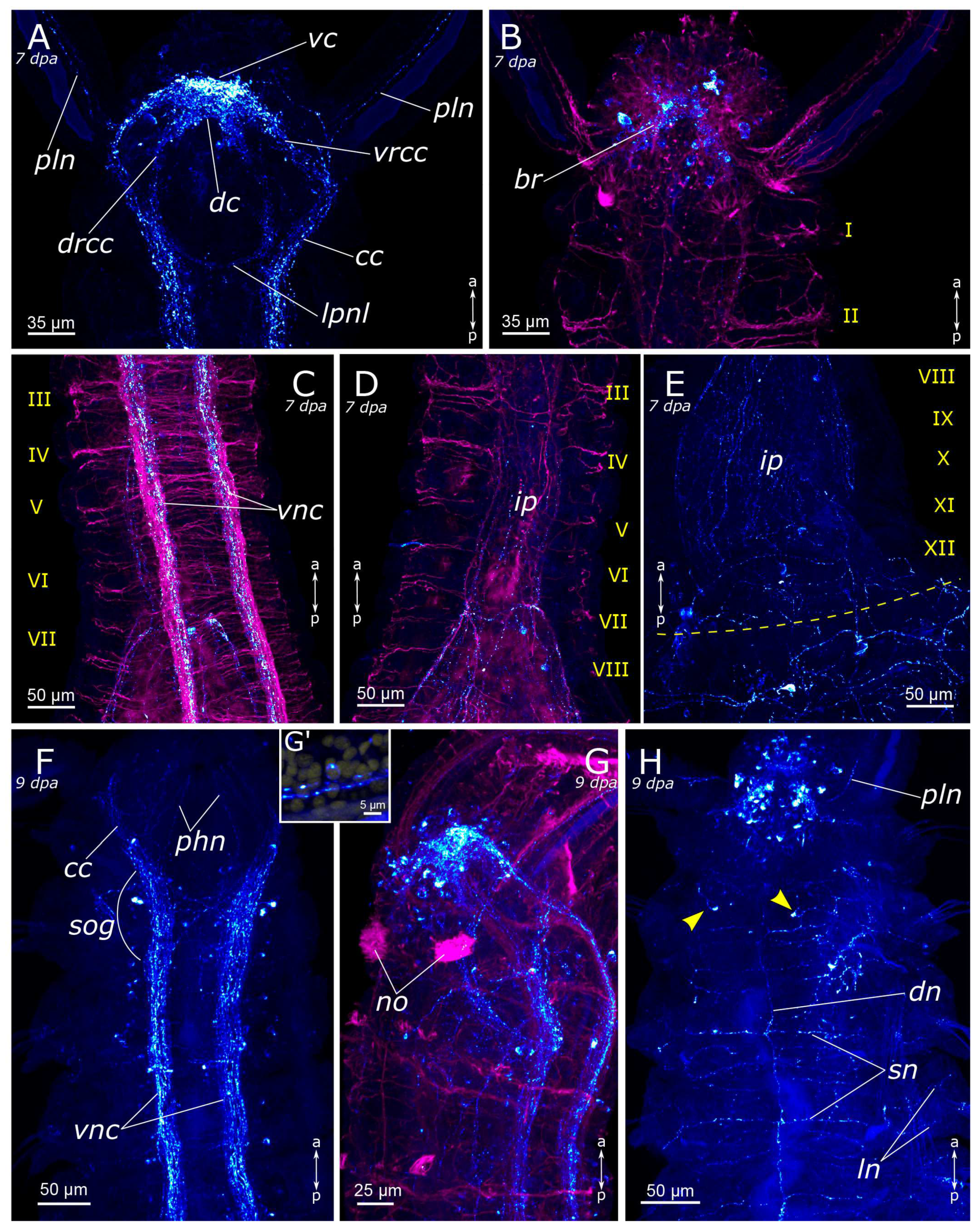
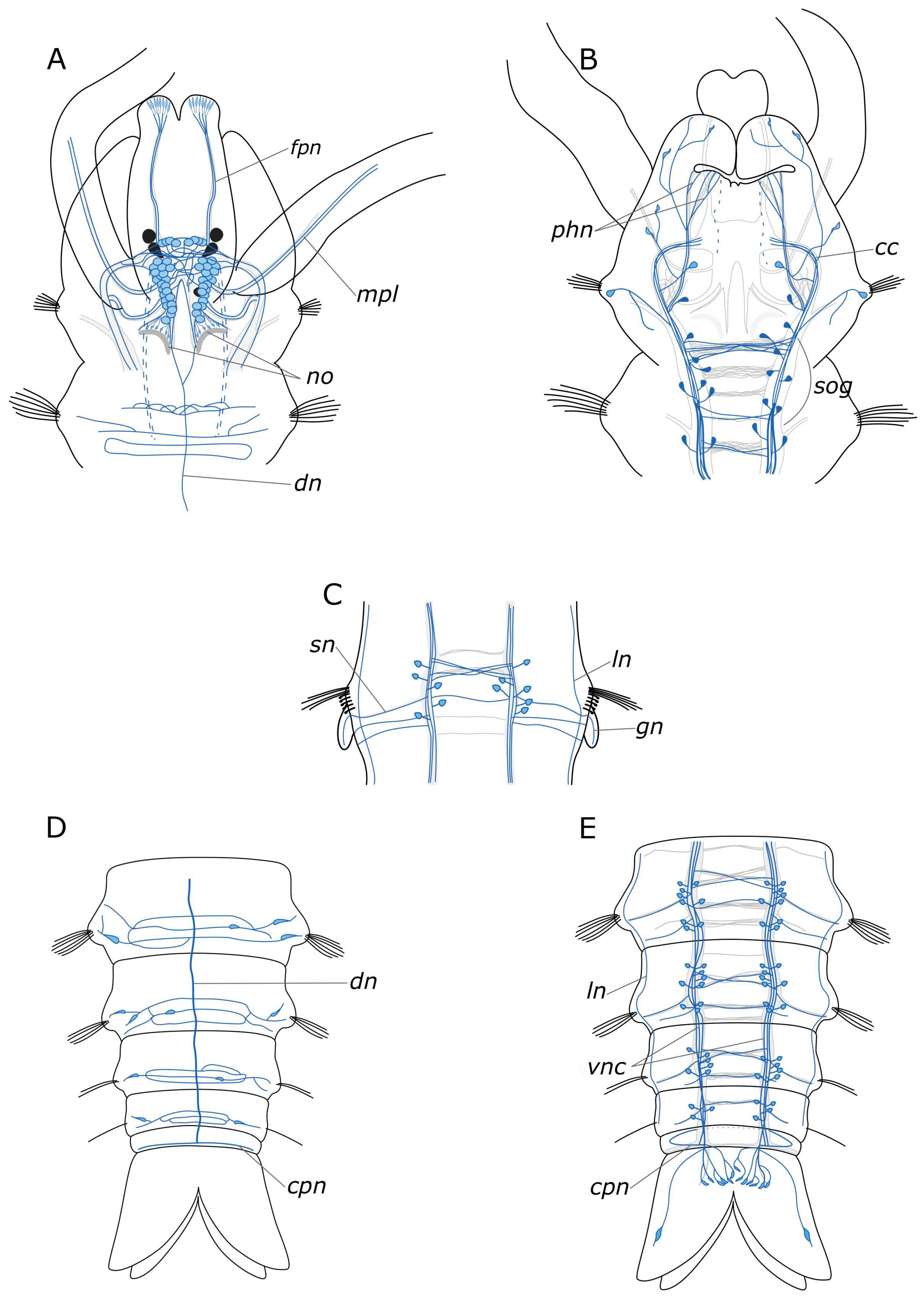
3.1. The General Structure of the Nervous System
3.1.1. 5-HT-Positive Immunoreactivity
3.1.2. FMRFamide-Positive Immunoreactivity
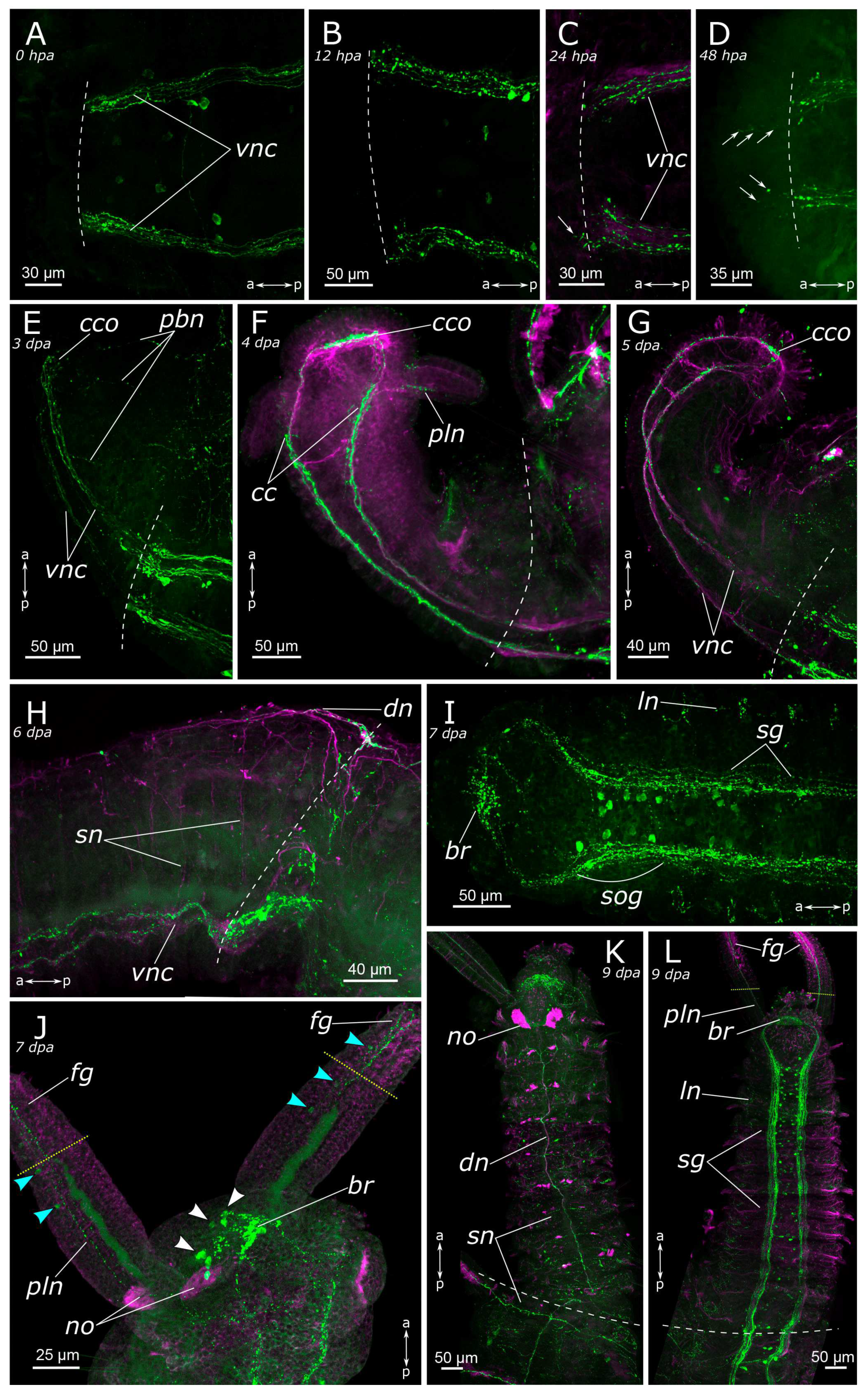
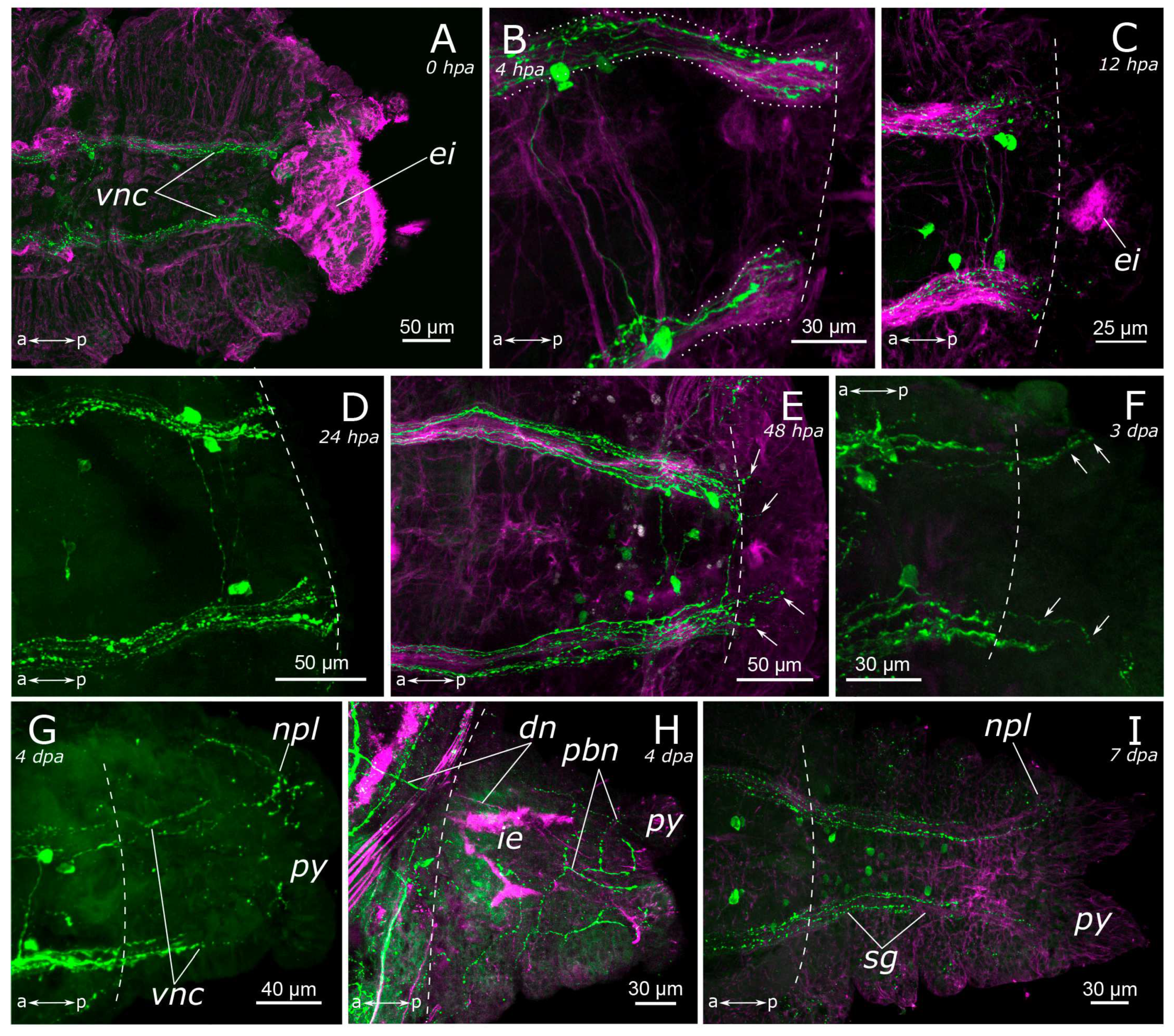
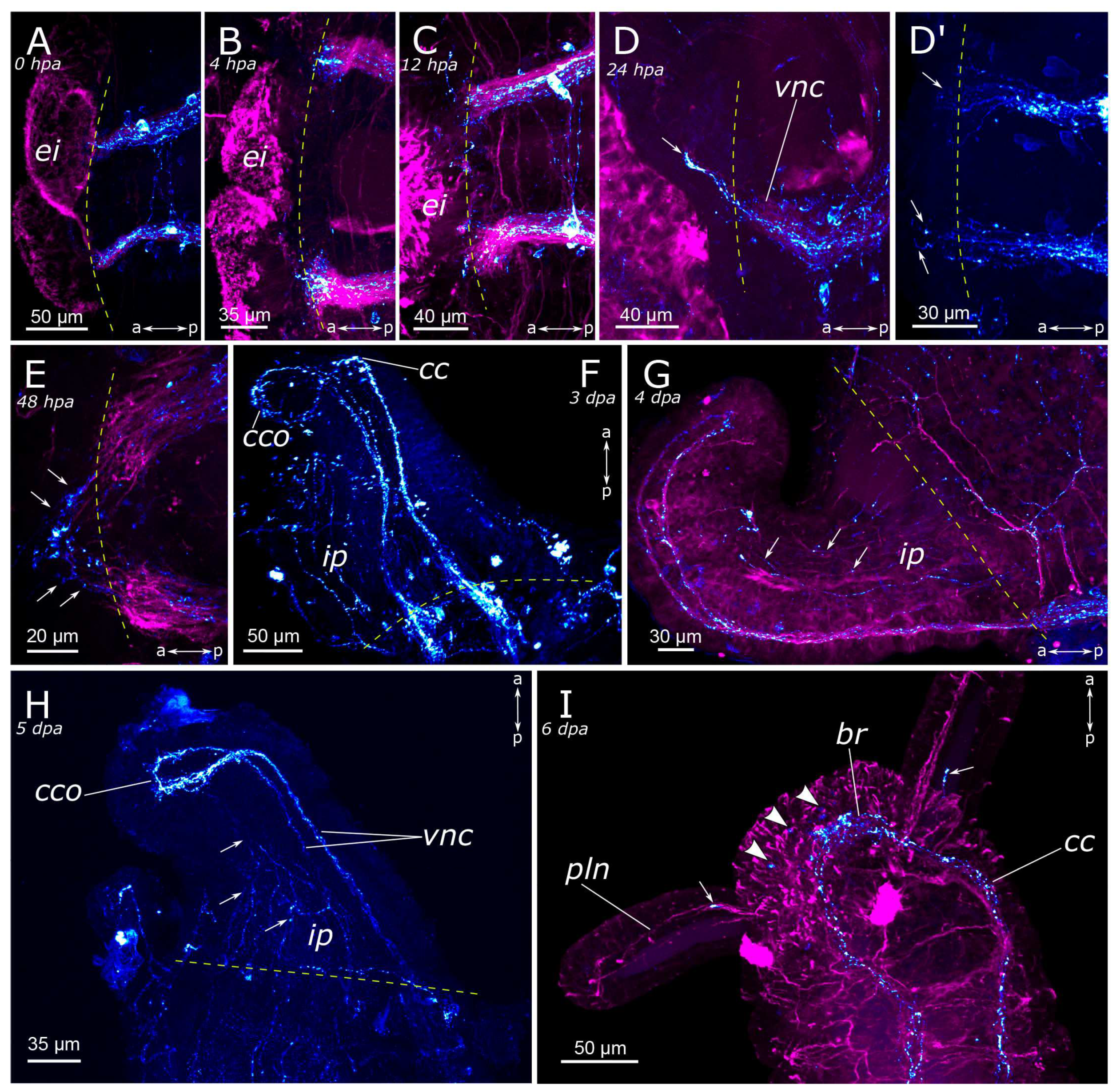
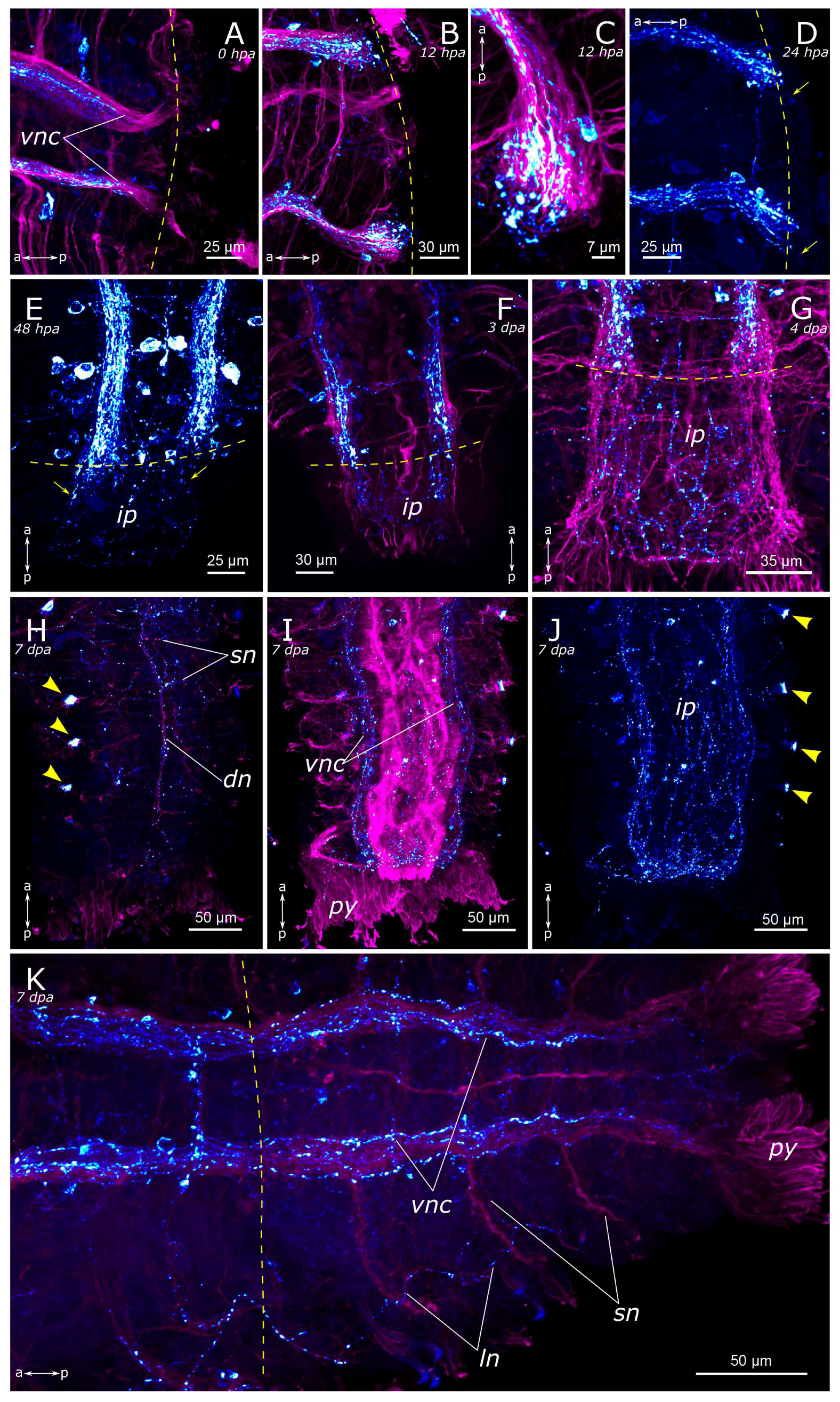
3.2. Regeneration of the Nervous System
3.2.1. 5-HT-Positive Nervous System Regeneration
3.2.2. FMRFamide-Positive Nervous System Regeneration
4. Discussion
4.1. The General Structure of the Nervous System
4.1.1. Central Nervous System (CNS)
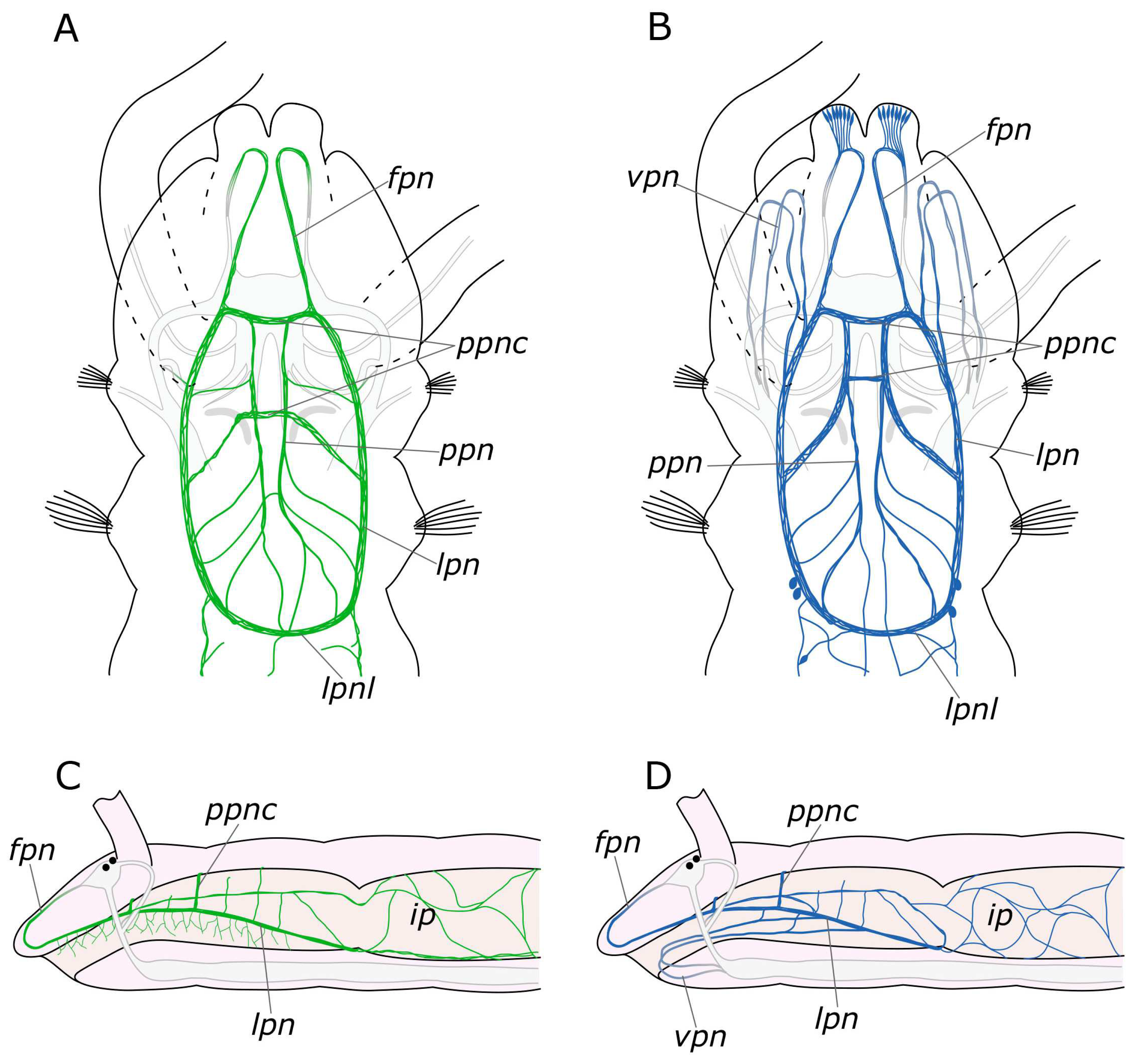
4.1.2. The Palps and Nuchal Organs
4.1.3. Innervation of the Pharynx
4.2. Regeneration
4.2.1. Anterior Regeneration and the Central Nervous System (CNS)
4.2.2. Posterior Regeneration
4.2.3. Regeneration of the Peripheral Nervous System (PNS)
4.2.4. Palp and Food Groove Regeneration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blake, J.A.; Arnofsky, P.L. Reproduction and Larval Development of the Spioniform Polychaeta with Application to Systematics and Phylogeny. Hydrobiologia 1999, 402, 57–106. [Google Scholar] [CrossRef]
- Gibson, G.D.; Harvey, J.M. Morphogenesis during Asexual Reproduction in Pygospio Elegans Claparede (Annelida, Polychaeta). Biol. Bull. 2000, 199, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.D.; Paterson, I.G. Morphogenesis during Sexual and Asexual Reproduction in Amphipolydora vestalis (Polychaeta:Spionidae). N. Z. J. Mar. Freshw. Res. 2003, 37, 741–752. [Google Scholar] [CrossRef]
- David, A.A.; Williams, J.D. Asexual Reproduction and Anterior Regeneration under High and Low Temperatures in the Sponge Associate Polydora colonia (Polychaeta:Spionidae). Invertebr. Reprod. Dev. 2012, 56, 315–324. [Google Scholar] [CrossRef]
- Whitford, T.A.; Williams, J.D. Anterior Regeneration in the Polychaete Marenzelleria viridis (Annelida:Spionidae). Invertebr. Biol. 2016, 135, 357–369. [Google Scholar] [CrossRef]
- Bely, A.E. Distribution of Segment Regeneration Ability in the Annelida. Integr. Comp. Biol. 2006, 46, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Kube, J.; Powilleit, M. Factors Controlling the Distribution of Marenzelleria Cf. Viridis, Pygospio Elegans and Streblospio Shrubsoli (Polychaeta:Spionidae) in the Southern Baltic Sea, with Special Attention for the Response to an Event of Hypoxia. Aquat. Ecol. 1997, 31, 187–198. [Google Scholar] [CrossRef]
- Morgan, T.S.; Rogers, A.D.; Paterson, G.L.J.; Hawkins, L.E.; Sheader, M. Evidence for Poecilogony in Pygospio elegans (Polychaeta:Spionidae). Mar. Ecol. Prog. Ser. 1999, 178, 121–132. [Google Scholar] [CrossRef]
- Thonig, A.; Banta, G.T.; Gibon, S.; Kesäniemi, J.; Hansen, W.B.; Knott, K.E. Acute and Chronic Response to a Change in Salinity of the Euryhaline Polychaete Pygospio elegans (Claparède). J. Exp. Mar. Bio. Ecol. 2019, 516, 79–88. [Google Scholar] [CrossRef]
- Starunov, V.; Barmasova, G.A.; Nesterenko, M.A.; Kulakova, M.A.; Novikova, E.L. Pygospio elegans (Annelida:Spionidae)—An Annelid Model for Regeneration Studies. Invertebr. Zool. 2020, 17, 247–266. [Google Scholar] [CrossRef]
- Armitage, D.L. The Ecology and Reproductive Cycle of Pygospio Elegans Claparède (Polychaeta:Spionidae) from Tomales Bay; California University of the Pacific: Stockton, CA, USA, 1979. [Google Scholar]
- Planques, A.; Malem, J.; Parapar, J.; Vervoort, M.; Gazave, E. Morphological, Cellular and Molecular Characterization of Posterior Regeneration in the Marine Annelid Platynereis dumerilii. Dev. Biol. 2019, 445, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Dorresteijn, A. The Polychaete Platynereis dumerilii (Annelida): A Laboratory Animal with Spiralian Cleavage, Lifelong Segment Proliferation and a Mixed Benthic/Pelagic Life Cycle. Bioessays 2004, 26, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.A.; Jékely, G. Neuronal Cell Types in the Annelid Platynereis dumerilii. Curr. Opin. Neurobiol. 2019, 56, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, E.; Stockinger, A.W.; Girard, J.; Raible, F.; Duygu Özpolat, B. A Scalable Culturing System for the Marine Annelid Platynereis dumerilii. PLoS ONE 2019, 14, e0226156. [Google Scholar] [CrossRef] [PubMed]
- Nezlin, L.P. The Golden Age of Comparative Morphology: Laser Scanning Microscopy and Neurogenesis in Trochophore Animals. Russ. J. Dev. Biol. 2010, 41, 381–390. [Google Scholar] [CrossRef]
- Horridge, G.A. Analysis of the Rapid Responses of Nereis and Harmothoe (Annelida). Proc. R. Soc. Lond. Ser. B-Biol. Sci. 1959, 150, 245–262. [Google Scholar] [CrossRef]
- Hanson, J. The Blood-System in the Serpulimorpha (Annelida, Polychaeta). Q. J. Microsc. Sci. 1951, 3, 255–274. [Google Scholar]
- Müller, M.C.M. Polychaete Nervous Systems: Ground Pattern and Variations—CLS Microscopy and the Importance of Novel Characteristics in Phylogenetic Analysis. Integr. Comp. Biol. 2006, 46, 125–133. [Google Scholar] [CrossRef]
- Weidhase, M.; Beckers, P.; Bleidorn, C.; Aguado, M.T. Nervous System Regeneration in Typosyllis Antoni (Annelida:Syllidae). Zool. Anz. 2017, 269, 57–67. [Google Scholar] [CrossRef]
- Kumar, S.; Tumu, S.C.; Helm, C.; Hausen, H. The Development of Early Pioneer Neurons in the Annelid Malacoceros fuliginosus. BMC Evol. Biol. 2020, 20, 117. [Google Scholar] [CrossRef]
- Heuer, C.M.; Müller, C.H.; Todt, C.; Loesel, R. Comparative Neuroanatomy Suggests Repeated Reduction of Neuroarchitectural Complexity in Annelida. Front. Zool. 2010, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- López-Vera, E.; Aguilar, M.B.; Heimer de la Cotera, E.P. FMRFamide and Related Peptides in the Phylum Mollusca. Peptides 2008, 29, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.J.; Papaioannou, S.; Holden-Dye, L. A Review of FMRFamide- and RFamide-like Peptides in Metazoa. Invert. Neurosci. 2009, 9, 111–153. [Google Scholar] [CrossRef] [PubMed]
- Rimskaya-Korsakova, N.N.; Kristof, A.; Malakhov, V.V.; Wanninger, A. Neural Architecture of Galathowenia oculata Zach, 1923 (Oweniidae, Annelida). Front. Zool. 2016, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Beckers, P.; Helm, C.; Bartolomaeus, T. The Anatomy and Development of the Nervous System in Magelonidae (Annelida)—Insights into the Evolution of the Annelid Brain. BMC Evol. Biol. 2019, 19, 173. [Google Scholar] [CrossRef] [PubMed]
- Helm, C.; Beckers, P.; Bartolomaeus, T.; Drukewitz, S.H.; Kourtesis, I.; Weigert, A.; Purschke, G.; Worsaae, K.; Struck, T.H.; Bleidorn, C. Convergent Evolution of the Ladder-like Ventral Nerve Cord in Annelida. Front. Zool. 2018, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Weidhase, M.; Helm, C.; Bleidorn, C. Morphological Investigations of Posttraumatic Regeneration in Timarete cf. punctata (Annelida:Cirratulidae). Zool. Lett. 2015, 1, 20. [Google Scholar] [CrossRef]
- Ribeiro, R.P.; Ponz-Segrelles, G.; Bleidorn, C.; Aguado, M.T. Comparative Transcriptomics in Syllidae (Annelida) Indicates That Posterior Regeneration and Regular Growth Are Comparable, While Anterior Regeneration Is a Distinct Process. BMC Genom. 2019, 20, 855. [Google Scholar] [CrossRef]
- Zattara, E.E.; Bely, A.E. Evolution of a Novel Developmental Trajectory: Fission Is Distinct from Regeneration in the Annelid Pristina leidyi. Evol. Dev. 2011, 13, 80–95. [Google Scholar] [CrossRef]
- Lindsay, S.M.; Jackson, J.L.; He, S.Q. Anterior Regeneration in the Spionid Polychaetes Dipolydora quadrilobata and Pygospio elegans. Mar. Biol. 2007, 150, 1161–1172. [Google Scholar] [CrossRef]
- Hentschel, B.T.; Harper, N.S. Effects of Simulated Sublethal Predation on the Growth and Regeneration Rates of a Spionid Polychaete in Laboratory Flumes. Mar. Biol. 2006, 149, 1175–1183. [Google Scholar] [CrossRef]
- Bely, A.E.; Nyberg, K.G. Evolution of Animal Regeneration: Re-Emergence of a Field. Trends Ecol. Evol. 2010, 25, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, S.M. Frequency of Injury and the Ecology of Regeneration in Marine Benthic Invertebrates. Integr. Comp. Biol. 2010, 50, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Özpolat, B.D.; Bely, A.E. Developmental and Molecular Biology of Annelid Regeneration: A Comparative Review of Recent Studies. Curr. Opin. Genet. Dev. 2016, 40, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.C.M. Nerve Development, Growth and Differentiation during Regeneration in Enchytraeus fragmentosus and Stylaria lacustris (Oligochaeta). Dev. Growth Differ. 2004, 46, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.C.M.; Henning, L. Ground Plan of the Polychaete Brain—I. Patterns of Nerve Development during Regeneration in Dorvillea bermudensis (Dorvilleidae). J. Comp. Neurol. 2004, 471, 49–58. [Google Scholar] [CrossRef]
- Lindsay, S.M.; Jackson, J.L.; Forest, D.L. Morphology of Anterior Regeneration in Two Spionid Polychaete Species: Implications for Feeding Efficiency. Invertebr. Biol. 2008, 127, 65–79. [Google Scholar] [CrossRef]
- Bánvölgyi, T.; Barna, J.; Csoknya, M.; Hámori, J.; Elekes, K. Reorganization of Peptidergic Systems during Brain Regeneration in Eisenia fetida (Oligochaeta, Annelida). Acta Biol. Hung. 2000, 51, 409–416. [Google Scholar] [CrossRef]
- Yoshida-Noro, C.; Myohara, M.; Kobari, F.; Tochinai, S. Nervous System Dynamics during Fragmentation and Regeneration in Enchytraeus japonensis (Oligochaeta, Annelida). Dev. Genes Evol. 2000, 210, 311–319. [Google Scholar] [CrossRef]
- Koritsanszky, S.; Hartwig, H.G. The Regeneration of the Monoaminergic System in the Cerebral Ganglion of the Earthworm Allolobophora caliginosa: A Morphological and Microspectrofluorometrical Analysis. Cell Tissue Res. 1974, 151, 171–186. [Google Scholar] [CrossRef]
- Csoknya, M.; Barna, J.; Elekes, K. Reorganization of the GABAergic System Following Brain Extirpation in the Earthworm (Eisenia Fetida, Annelida, Oligochaeta). Acta Biol. Hung. 2002, 53, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Starunova, Z.I.; Shunkina, K.V.; Novikova, E.L.; Starunov, V.V. Histamine and Gamma-Aminobutyric Acid in the Nervous System of Pygospio elegans (Annelida:Spionidae): Structure and Recovery during Reparative Regeneration. BMC Zool. 2022, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Barmasova, G.A.; Starunova, Z.I.; Novikova, E.L.; Starunov, V.V. Organization of Catecholaminergic System of Pygospio elegans and Platynereis dumerilii (Annelida). Invertebr. Zool. 2022, 19, 335–350. [Google Scholar] [CrossRef]
- Orrhage, L.; Müller, M.C.M. Morphology of the Nervous System of Polychaeta (Annelida). Hydrobiologia 2005, 535, 79–111. [Google Scholar] [CrossRef]
- Orrhage, L. On the Microanatomy of the Cephalic Nervous System of Nereidae (Polychaeta), with a Preliminary Discussion of Some Earlier Theories on the Segmentation of the Polychaete Brain. Acta Zool. 1993, 74, 145–172. [Google Scholar] [CrossRef]
- Orrhage, L. Anatomische Und Morphologische Studien Uber Die Polychaetenfamilien Spionidae, Disomidae Und Poecilochaetidae. Zool. Bidr. fran Uppsala 1964, 36, 335–405. [Google Scholar]
- Anctil, M.; Waele, J.-P.; Miron, M.-J.; Pani, A.K. Monoamines in the Nervous System of the Tube-WormChaetopterus variopedatus (Polychaeta): Biochemical Detection and Serotonin Immunoreactivity. Cell Tissue Res. 1990, 259, 81–92. [Google Scholar] [CrossRef]
- Forest, D.L.; Lindsay, S.M. Observations of Serotonin and FMRFamide-like Immunoreactivity in Palp Sensory Structures and the Anterior Nervous System of Spionid Polychaetes. J. Morphol. 2008, 269, 544–551. [Google Scholar] [CrossRef]
- Worsaae, K. The Systematic Significance of Palp Morphology in the Polydora Complex (Polychaeta:Spionidae). Zool. Anz. 2001, 240, 47–59. [Google Scholar] [CrossRef]
- Lindsay, S.M.; Riordan, T.J.; Forest, D. Identification and Activity-Dependent Labeling of Peripheral Sensory Structures on a Spionid Polychaete. Biol. Bull. 2004, 206, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Schlötzer-Schrehardt, U. Ultrastructural Investigation of the Nuchal Organs of Pygospio elegans (Polychaeta). II. Adult Nuchal and Dorsal Organs. Zoomorphology 1987, 107, 169–179. [Google Scholar] [CrossRef]
- Purschke, G. Anatomy and Ultrastructure of Ventral Pharyngeal Organs and Their Phylogenetic Importance in Polychaeta (Annelida). IV. The Pharynx and Jaws of the Dorvilleidae. Acta Zool. 1987, 68, 83–105. [Google Scholar] [CrossRef]
- Winchell, C.J.; Valencia, J.E.; Jacobs, D.K. Confocal Analysis of Nervous System Architecture in Direct-Developing Juveniles of Neanthes arenaceodentata (Annelida, Nereididae). Front. Zool. 2010, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Schlawny, A.; Hamann, T.; Müller, M.A.; Pfannenstiel, H.-D. The Catecholaminergic System of an Annelid (Ophryotrocha Puerilis, Polychaeta). Cell Tissue Res. 1991, 265, 175–184. [Google Scholar] [CrossRef]
- Zanol, J. Homology of Prostomial and Pharyngeal Structures in Eunicida (Annelida) Based on Innervation and Morphological Similarities. J. Morphol. 2010, 271, 1023–1043. [Google Scholar] [CrossRef] [PubMed]
- Purschke, G.; Tzetlin, A.B. Dorsolateral Ciliary Folds in the Polychaete Foregut: Structure, Prevalence and Phylogenetic Significance. Acta Zool. 1996, 77, 33–49. [Google Scholar] [CrossRef]
- Tzetlin, A.; Purschke, G. Pharynx and Intestine. Hydrobiologia 2005, 535, 199–225. [Google Scholar] [CrossRef]
- Weidhase, M.; Bleidorn, C.; Helm, C. Structure and Anterior Regeneration of Musculature and Nervous System in Cirratulus cf. cirratus (Cirratulidae, Annelida). J. Morphol. 2014, 275, 1418–1430. [Google Scholar] [CrossRef]
- Csoknya, M.; Barna, J.; Hiripi, L.; Hámori, J.; Elekes, K. Reorganization of Monoaminergic Systems in the Earthworm, Eisenia Fetida, Following Brain Extirpation. J. Exp. Zool. A. Comp. Exp. Biol. 2003, 296, 18–29. [Google Scholar] [CrossRef]
- Voronezhskaya, E.; Glebov, K.; Khabarova, M.; Ponimaskin, E.; Nezlin, L. Adult-to-Embryo Chemical Signaling in the Regulation of Larval Development in Trochophore Animals: Cellular and Molecular Mechanisms. Acta Biol. Hung. 2008, 59 (Suppl. S2), 117–122. [Google Scholar] [CrossRef] [PubMed]
- Croll, R.P.; Voronezhskaya, E.E. Early Elements in Gastropod Neurogenesis. Dev. Biol. 1996, 173, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Voronezhskaya, E.E.; Tsitrin, E.B.; Nezlin, L.P. Neuronal Development in Larval Polychaete Phyllodoce Maculata (Phyllodocidae). J. Comp. Neurol. 2003, 455, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Voronezhskaya, E.E.; Nezlin, L.P.; Odintsova, N.A.; Plummer, J.T.; Croll, R.P. Neuronal Development in Larval Mussel Mytilus trossulus (Mollusca:Bivalvia). Zoomorphology 2008, 127, 97–110. [Google Scholar] [CrossRef]
- Koza, A.; Wilhelm, M.; Hiripi, L.; Elekes, K.; Csoknya, M. Embryogenesis of the Serotonergic System in the Earthworm Eisenia fetida (Annelida, Oligochaeta): Immunohistochemical and Biochemical Studies. J. Comp. Neurol. 2006, 497, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, N.; Wanninger, A. Larval Neurogenesis in Sabellaria Alveolata Reveals Plasticity in Polychaete Neural Patterning. Evol. Dev. 2008, 10, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Baguñà, J.; Romero, R.; Saló, E.; Collet, J.; Auladell, C.; Ribas, M.; Riutort, M.; García-Fernàndez, J.; Burgaya, F.; Bueno, D. Growth, Degrowth and Regeneration as Developmental Phenomena in Adult Freshwater Planarians. In Experimental Embryology in Aquatic Plants and Animals; Springer: Berlin/Heidelberg, Germany, 1990; pp. 129–162. [Google Scholar]
- Seshan, K.R.; Bittner, G.D. Developmental and Other Factors Affecting Regeneration of Crayfish CNS Axons. J. Comp. Neurol. 1987, 262, 535–545. [Google Scholar] [CrossRef]
- Müller, M.C.M.; Berenzen, A. Experiments on Anterior Regeneration in Eurythoe complanata (“Polychaeta”, Amphinomidae): Reconfiguration of the Nervous System and Its Function for Regeneration. Zoomorphology 2003, 122, 95–103. [Google Scholar] [CrossRef]
- Matthews, J.A.; Hentschel, B.T. In Situ Rates of Body Growth and Palp Regeneration of a Spionid Polychaete Following Simulated Sublethal Predation. Mar. Biol. 2012, 159, 1039–1048. [Google Scholar] [CrossRef]




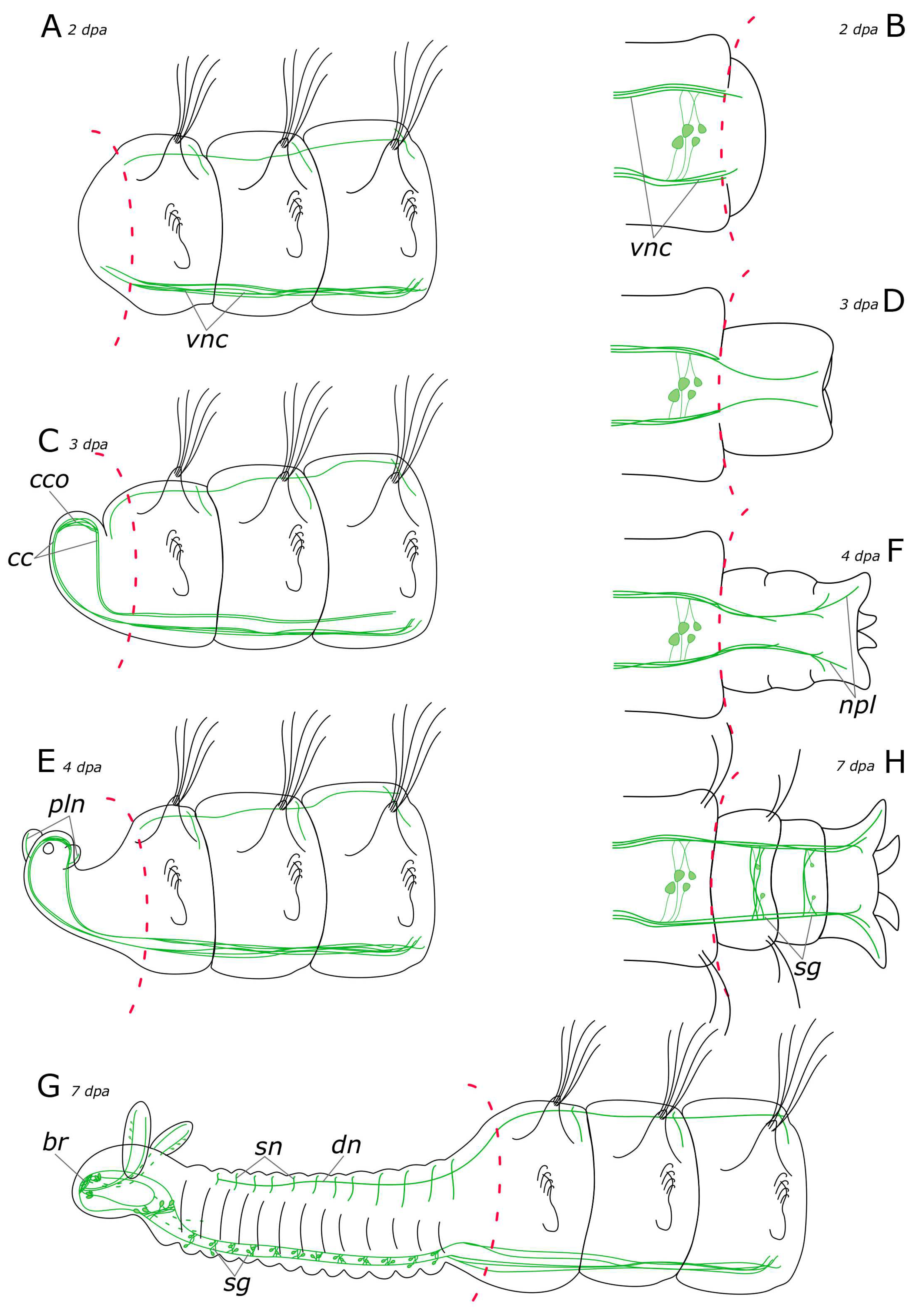
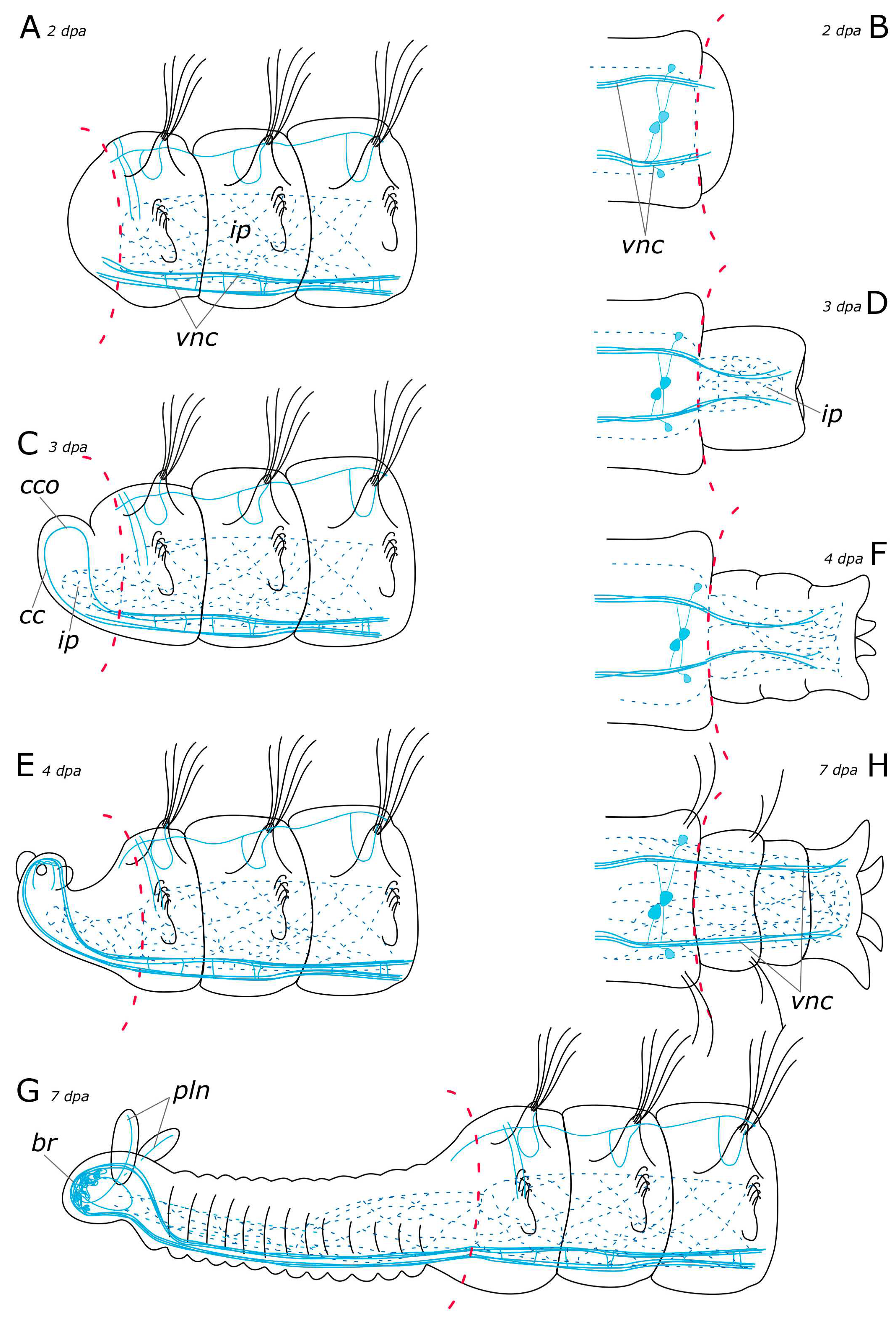
| 5-HT-pos. NS | FMRFamide-pos. NS | GABA-pos. NS (after [43]) | HA-pos. NS (after [43]) | CA-pos. NS (after [10]) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AR | PR | AR | PR | AR | PR | AR | PR | AR | PR | |
| 1 dpa, wound healing | Single fibers of VNC | – | Single fibers of VNC | – | – | – | – | – | – | – |
| 2 dpa, blastema | Fibers of VNC | Fibers of VNC | Fibers of VNC | Fibers of VNC | – | – | – | – | Single fibers of VNC | – |
| 3 dpa, primordia | Neuropil, cc, VNC, pbn | VNC | Neuropil, cc, VNC, intestinal plexus | VNC, intestinal plexus | Single fibers of VNC | Dorsal longitudinal nerve | Single fibers of VNC | Single fibers of VNC | Sensory cells and fibers, fibers of VNC | Fibers of VNC |
| 4–6 dpa, segmentation | (4–6 dpa) Neuropil, cc, VNC, pln, dn, sn | (4–6 dpa) VNC, npl, pbn | (4–6 dpa) Neuropil, cc, VNC, brain neurons, intestinal plexus, pln | (4–6 dpa) VNC, intestinal plexus | (4 dpa) VNC, brain neurons | (4 dpa) VNC | (4 dpa) VNC, intestinal nerve | (4 dpa) VNC | (4 dpa) Sensory cells, dorsal nerve | (4 dpa) VNC, nerve elements of the pygidium |
| 7–9 dpa, differentiation | (7–9 dpa) Cerebral ganglion, SG, pln, prc, dn, sn, ln | (7–9 dpa) SG, npl | (7–9 dpa) Cerebral ganglion, VNC, pln, prc, dn, sn, ln | (7–9 dpa) SG, ln, sn | (7 dpa) Cerebral ganglion, SG, pln, prc | (7 dpa) SG | (7 dpa) Cerebral ganglion, VNC, pln | (7 dpa) VNC | (7 dpa) Cerebral ganglion, VNC, pln | (7 dpa) VNC, nerve elements of the pygidium, sn |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shunkina, K.V.; Starunova, Z.I.; Novikova, E.L.; Starunov, V.V. Mass Start or Time Trial? Structure of the Nervous System and Neuroregeneration in Pygospio elegans (Spionidae, Annelida). Biology 2023, 12, 1412. https://doi.org/10.3390/biology12111412
Shunkina KV, Starunova ZI, Novikova EL, Starunov VV. Mass Start or Time Trial? Structure of the Nervous System and Neuroregeneration in Pygospio elegans (Spionidae, Annelida). Biology. 2023; 12(11):1412. https://doi.org/10.3390/biology12111412
Chicago/Turabian StyleShunkina, Ksenia V., Zinaida I. Starunova, Elena L. Novikova, and Viktor V. Starunov. 2023. "Mass Start or Time Trial? Structure of the Nervous System and Neuroregeneration in Pygospio elegans (Spionidae, Annelida)" Biology 12, no. 11: 1412. https://doi.org/10.3390/biology12111412






