Plants, Cells, Algae, and Cyanobacteria In Vitro and Cryobank Collections at the Institute of Plant Physiology, Russian Academy of Sciences—A Platform for Research and Production Center
Abstract
:Simple Summary
Abstract
1. Introduction
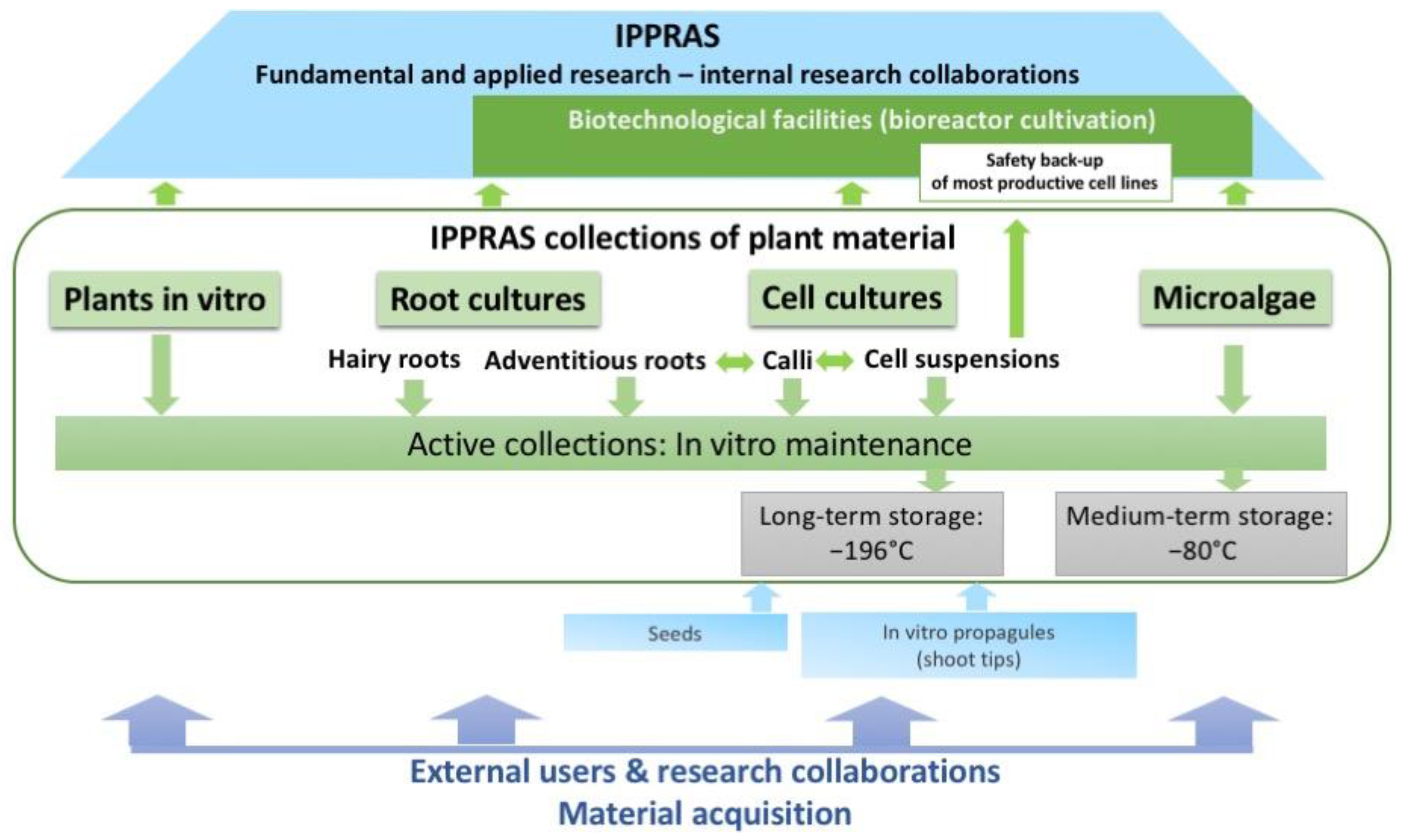
- Culture collection of microalgae and cyanobacteria IPPAS.
- In vitro collection of transgenic potato plants.
- All-Russian collection of plant cell cultures.
- Hairy root culture collection.
- Adventitious root culture collection.
- IPPRAS cryobank.
- Vitis spp. plant collection.
- Collection of succulent plants.
2. All-Russian Collection of Plant Cell Cultures
2.1. Plant Cell Culture Collections around the World
2.2. All-Russian Collection of Plant Cell Cultures—Historical Perspective and Current Composition
2.3. Using Plant Cell Culture Strains in the Research
2.4. Biotechnological Application of Plant Cell Strains from the Collection
| Cell Strain | Year Strain Induced/Received by Collection | Characteristics |
|---|---|---|
| Dioscorea deltoidea, strains DM-05 and DM-05-03 | 1972/1985 | Small-aggregated, rapidly growing cell strains developed through mutagenesis (single and double treatment with N-nitroso-N-methylurea) [77]; super-producer of steroidal glycosides protodioscin and deltoside and their 25(S)-isomers [60,78,79], adapted for large-scale bioreactor cultivation [80,81]. The total content of steroidal glycosides 4.6–5.7% DW [79] can be increased up to 13.9% DW in bioreactor production with a high aeration level [60,81]. Extensively used to study the regulation of steroidal glycoside biosynthesis in cell cultures [60]. Bioreactor-produced cell biomass was assessed for elemental composition [73], toxicology [82], and demonstrated positive effects in rats with induced type 2 diabetes mellitus and obesity [82,83]. |
| Polyscias filicifolia, strains BFT-01-95 and Pf-SH | 1991/1995; 2018/2023 | Cell strains adapted for large-scale bioreactor cultivation [70] with a total content of polysciosides and their derivatives up to 3% DW. Bioreactor-produced cell biomass of BFT-01-95 has adaptogenic and anti-teratogenic activities, and is currently used in commercial food supplements [76,84]. |
| Panax ginseng, strain G1 | 1959/1985 | One of the oldest cell strains with stable growth and chromosome number, a producer of ginsenosides. The strain is currently used in the commercial production of several cosmetic products. |
| Panax japonicus, strain 62 | 1995–97/1998 | Cell strain adapted for large-scale bioreactor cultivation [71] with a total content of ginsenosides (Rg1, malonyl-Rg1, Rb1, malonyl-Rb1, Rb2/Rb3, malonyl-Rb2/Rb3, Rd, malonyl-Rd, Rf, R0, chikusetsusaponin IVa) of 3.46% DW [85]. Bioreactor-produced cell biomass exhibited hypoglycemic and hypocholesterolemic activity in rats with diet-induced obesity [82]. |
| Tribulus terrestris, strain 8 | 2014/2014 | Cell strain adapted for laboratory bioreactor cultivation with a total content of furostanol glycosides 0.1% DW [75]. Bioreactor-produced cell biomass positively affected rats with induced type 2 diabetes mellitus and obesity [82,83]. |
3. Adventitious and Hairy Root Culture Collections
4. In Vitro Collection of Transgenic Potato Plants at IPPRAS
4.1. In Vitro Potato Collections in the World
4.2. Collection of Transgenic Potato at IPPRAS
- -
- Thirty-six transgenic lines of cv. Skoroplodny. Three lines demonstrated high blackleg resistance.
- -
- Six transgenic lines of cv. Jucovsky Ranny. The original (non-transgenic) cultivar is susceptible to early blight. Out of the six transgenic lines, one line demonstrated high resistance to early blight during five years of annual infection tests.
- -
- Forty-two transgenic lines of cv. Sarpo mira. Nine lines demonstrated high resistance to early blight and preserved this trait for five years, as confirmed by annual infection tests [112]. These perspective lines are currently used in the research programs at the Russian Potato Research Centre.
5. IPPRAS Cryobank of Plant Genetic Resources
6. Culture Collection of Microalgae and Cyanobacteria IPPAS at the Institute of Plant Physiology of Russian Academy of Sciences
6.1. Culture Collection of Microalgae and Cyanobacteria IPPAS—Historical Perspective and Composition
6.2. Use of the Collection Strains for Fundamental Studies on the Physiology of Photosynthetic Microorganisms
6.3. Biotechnological Application of Collection Strains and Links to Biotechnological Production Facilities
6.4. Taxonomic Identification
7. Quality Management System (QMS) at IPPRAS Collections
7.1. Documentation and Information Management
7.2. Contamination and Purity Control
7.3. Collection Duplication and Safety Back-Up at −70 °C and −196 °C
8. User Services
- -
- live material distribution;
- -
- deposition and secure storage of strains developed by users;
- -
- induction/isolation of new strains for specific applications;
- -
- culture evaluation, optimization, and passport development.
9. Conclusions
10. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Collection | IPPAS Collection of Microalgae and Cyanobacteria | In Vitro Collection of Potato Plants | All-Russian Collection of Plant Cell Cultures | Adventitious Root Collection * | Cryobank |
|---|---|---|---|---|---|
| Type of material conserved | Microalgae and cyanobacteria (wild types and mutants) | In vitro plants | Undifferentiated cell cultures (calli, cell suspensions) | Adventitious roots | Shoot apices of in vitro plants, cell cultures, in vitro cultured protocorms, seeds |
| Year collection established | 1958 | 2006 | 1965 | 2020 | 1977 |
| Age of the oldest samples | Over 80 years | 17 years | 64 years | 2 years | 46 years |
| Total number of taxa/lines in the collection | 91 genera/106 species/430 strains | Solanum tuberosum/202 lines | 15 families/51 species/117 cell strains | 7 families/12 species/12 strains | 74 families/457 species |
| Conservation methods | 1. In vitro with periodic subcultures (active collection) 2. Cold storage at −80 °C | In vitro with periodic subcultures (active collection) | 1. In vitro with periodic subcultures (active collection) 2. Cryopreservation at −196 °C (old and the most productive cell strains) | In vitro with periodic subcultures (active collection) | Storage in LN at −196 °C (long-term conservation) |
| Material acquisition sources | 1. External (research collections in and outside Russia) 2. Expeditions and strain isolation | 1. External (research institutes in Russia and Belarus) 2. In-house developed transgenic lines | 1. IPPRAS 2. Cell lines received from external users for maintenance (patent) purposes | IPPRAS | 1. In-house developed clones and cell lines 2. Agricultural research institutes 3. Botanic gardens |
| Fundamental research | Mechanisms of intracellular regulation and stress responses in photosynthetic cells | Molecular mechanisms of disease resistance in potato | Plant cell physiology and biochemistry, growth regulation, and secondary metabolism | Secondary metabolism and its regulation in adventitious roots | Mechanisms of plant cold- and cryo-tolerance development, cryopreservation-induced injuries |
| Applied research | Production of biofuels, pigments, feed, and food additives | 1. Development and optimization of potato transformation protocols 2. Development of disease-resistant potato lines | Production of vegetative biomass and bioactive compounds for food, cosmetics, and pharmacology | Biotechnological production of plant bioactive compounds | Viability of plant material during long-term storage, evaluation of material integrity and performance after cryopreservation |
| Services to external users | 1. Strain deposit (maintenance) for the patent purpose 2. Taxonomic identification 3. Evaluation of the biotechnological potential of the strains 4. Strain distribution with request to research and commercial organizations | Development of transgenic potato lines carrying specific genes | 1. Cell culture induction, optimization, and evaluation 2. Passport development for new cell lines 3. Cell culture deposit (maintenance) for the patent purpose | Currently not available | Long-term conservation of botanical, agricultural, and biotechnological collections |
| Parameter | Formula |
|---|---|
| Growth index, I | I = (Xi − X0)/X0 |
| where X0 and Xi are initial dry weight and dry weight at the end of the exponential growth phase, respectively (g·L−1). | |
| Specific growth rate, μ (d−1) | µ = (1nXi − lnX0)/∆t |
| where X0 and Xi are initial dry weight and dry weight at the end of the exponential growth phase, respectively (g·L−1); ∆t is the duration of the exponential growth phase specific for each culture. | |
| Economic coefficient, Y | Y = (Xmax − X0)/S0 |
| where X0 and Xmax are initial dry weight and maximum dry weight (g·L−1), and S0 is the initial sucrose concentration in culture medium (g·L−1). | |
| Productivity, P (g/(L d)) | P = (Xmax − X0)/(ti − t0) |
| where X0 and Xi are initial dry weight and maximum dry weight (g·L−1), and t0 and ti are the time points at inoculation (0 days) and at maximum dry weight accumulation, respectively. | |
| Taxon | Culture Medium Type | ||
|---|---|---|---|
| Family | Species | Agar-Solidified | Liquid |
| Araliaceae | Polyscias filicifolia L.H. Bailey | + | + |
| Polyscias scutellaria (Burm.f.) Fosberg | + | + | |
| Polyscias balfouriana L. H. Bailey | + | + | |
| Fabaceae (Leguminosae) | Sutherlandia frutescens (L.) W. T. Aiton | + | − |
| Maackia amurensis Rupr. | + | + | |
| Lamiaceae | Ziziphora pamiroalaica Juz. ex Nevski | + | − |
| Ziziphora interrupta Juz. | + | − | |
| Plantaginaceae | Digitalis lanata Ehrh. | + | + |
| Digitalis ciliata Trautv. | + | − | |
| Amaryllidaceae | Pancratium maritimum L. | + | + |
| Crassulaceae | Kalanchoe laciniata (L.) DC. | + | + |
| Betulaceae | Corylus avellane L. | + | + |
| Country of Origin | Number of Strains |
|---|---|
| Russia | 113 |
| USA | 20 |
| Vietnam | 7 |
| Mongolia | 7 |
| Kazakhstan | 7 |
| Switzerland | 6 |
| Czech Republic | 6 |
| Germany | 4 |
| Uzbekistan | 3 |
| Indonesia | 2 |
| United Kingdom | 2 |
| Japan | 2 |
| Moldova | 2 |
| Italy | 2 |
| Azerbaijan | 2 |
| Kenya | 2 |
| Republic of Chad | 2 |
| Ethiopia | 1 |
| Netherlands | 1 |
| Slovakia | 1 |
| Western Sahara | 1 |
| Australia | 1 |
| Belgium | 1 |
| Tajikistan | 1 |
| Peru | 1 |
| Tanzania | 1 |
| Republic of Palau | 1 |
| Turkmenistan | 1 |
| Georgia | 1 |
| India | 1 |
| Tajikistan | 1 |
| Ukraine | 1 |
| France | 1 |
| n/a | 225 |
| Collection/Institution, Country, Town | City, Country | Number of Strains (% in the Collection) | Year of the Deposition |
|---|---|---|---|
| Working collection of Dmitry Los and his group, Institute of plant physiology of Russian Academy of Sciences, http://cellreg.org/http-en-cellreg-org/, accessed on 20 April 2023 | Moscow, Russia | 91 (21%) | 2005–2022 |
| Collection staff expeditions and strain isolation, Institute of plant physiology of Russian Academy of Sciences | Moscow, Russia | 56 (13%) | 1968, 1983–1991, 2014–2021 |
| Collection of Chlamydomonas reinhardtii photosynthetic mutants of Vladimir Ladygin, Institute of Basic Biological Problems, Russian Academy of Sciences | Pushchino, Russia | 54 (13%) | 2009 |
| CALU, Collection of Algae St. Petersburg (Leningrad) State University, Centre for Culture Collection of Microorganisms, St. Petersburg State University, https://researchpark.spbu.ru/en/collection-ccem-eng/1930-ccem-kollekciya-calu-eng, accessed on 20 April 2023 | St. Petersburg, Russia | 16 (4%) | 1969, 1987–1993, 2011 |
| Pringsheim’s (Prat’s) algal collection, Charles University (currently does not exist) | Prague, Czech Republic | 16 (4%) | 1957–1964 |
| Working collections of Dr. Lyudmila Gerasimenko and Dr. Olga Samylina, Winogradsky Institute of Microbiology, Federal Research Center “Biotechnology”, Russian Academy of Sciences | Moscow, Russia | 14 (3%) | 1979, 1996, 1998, 2019 |
| Working collection of Olga Sentsova, Biology Faculty, Moscow State University | Moscow, Russia | 12 (3%) | 1990 |
| LABIK, Collection of Algal Cultures in Laboratory of Algology, Komarov Botanical Institute of Russian Academy of Sciences | St. Petersburg, Russia | 11 (3%) | 1989, 1993 |
| CCALA, Culture collection of Autotrophic Organisms (CCALA) of the Institute of Botany of the Czech Academy of Sciences, https://ccala.butbn.cas.cz/en, accessed on 20 April 2023 | Třeboň, Czech Republic | 10 (2%) | 1964, 1988–1989 |
| NAMSU, collection of biotechnologically relevant microalgae of Faculty of Biology, Moscow State University | Moscow, Russia | 10 (2%) | 2013–2014, 2017–2022 |
| Working collection of Dr. Hiroshi Nakamura, The Institute of Algological Research (currently does not exist) | Japan | 8 (2%) | 1960 |
| ACSSI, Algal Collection of Soil Science Institute, Institute of physicochemical and biological problems in soil science of the Russian Academy of Sciences | Pushchino, Russia | 8 (2%) | 2019–2021 |
| Working collection of Ziyadin Ramazanov, K. A. Timiryazev Institute of plant physiology Russian Academy of Sciences | Moscow, Russia | 7 (2%) | 1980, 1984, 1996 |
| Working collections from Institute of plant physiology (now Institute of plant physiology and genetics) of Bulgarian Academy of Sciences | Sofia, Bulgaria | 6 (1%) | 1970, 1978–1979, 1986 |
| Working collection of Prof. Bolatkhan Zayadan group, Faculty of Biology and Biotechnology of the Al-Farabi Kazakh National University | Almaty, Kazakhstan | 6 (1%) | 2013 |
| IAM, Algal Collection of Institute of Applied Microbiology, the University of Tokyo, Japan (does not exist know—moved to NIES, Microbial Culture Collection at the National Institute for Environmental Studies, https://mcc.nies.go.jp/07imformation_IAM_e.html, accessed on 20 April 2023) | Tsukuba, Japan | 5 (1%) | 1981, 1984, 1993 |
| SAG, Culture Collection of Algae at Göttingen University, https://uni-goettingen.de/en/45175.html, accessed on 20 April 2023 | Göttingen, Germany | 5 (1%) | 1983, 1990 |
| Working collections of the researchers of the Vavilov Institute of General Genetics, Russian Academy of Sciences | Moscow, Russia | 5 (1%) | 1961, 1967 |
References
- Gepts, P. Plant Genetic resources conservation and utilization: The accomplishments and future of a societal insurance policy. Crop Sci. 2006, 46, 2278–2292. [Google Scholar] [CrossRef]
- Dzyubenko, N.I. Vavilov’s collection of worldwide crop genetic resources in the 21st century. Biopreserv. Biobank. 2018, 16, 377–383. [Google Scholar] [CrossRef] [Green Version]
- Noriega, I.L.; Halewood, M.; Abberton, M.; Amri, A.; Angarawai, I.I.; Anglin, N.; Blümmel, M.; Bouman, B.; Campos, H.; Costich, D.; et al. CGIAR operations under the plant treaty framework. Crop Sci. 2019, 59, 819–832. [Google Scholar] [CrossRef] [Green Version]
- Ebert, A.W. The role of vegetable genetic resources in nutrition security and vegetable breeding. Plants 2020, 9, 736. [Google Scholar] [CrossRef]
- FAO. The Second Report on the State of the World’s Plant Genetic Resources for Food and Agriculture; FAO: Rome, Italy, 2010. [Google Scholar]
- Smith, P. Building a global system for the conservation of all plant diversity. Sibbaldia Int. J. Bot. Gard. Hortic. 2016, 14, 5–13. [Google Scholar] [CrossRef]
- Howes, M.R.; Quave, C.L.; Collemare, J.; Tatsis, E.C.; Twilley, D.; Lulekal, E.; Farlow, A.; Li, L.; Cazar, M.; Leaman, D.J.; et al. Molecules from nature: Reconciling biodiversity conservation and global healthcare imperatives for sustainable use of medicinal plants and fungi. Plants People Planet 2020, 2, 463–481. [Google Scholar] [CrossRef]
- Hamilton, A.C. Medicinal plants, conservation and livelihoods. Biodivers. Conserv. 2004, 13, 1477–1517. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Available online: http://www.iucnredlist.org (accessed on 19 March 2023).
- Chen, S.-L.; Yu, H.; Luo, H.-M.; Wu, Q.; Li, C.-F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef] [Green Version]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef]
- Moraes, R.M.; Cerdeira, A.L.; Lourenço, M.V. Using micropropagation to develop medicinal plants into crops. Molecules 2021, 26, 1752. [Google Scholar] [CrossRef]
- Hasnain, A.; Naqvi, S.A.H.; Ayesha, S.I.; Khalid, F.; Ellahi, M.; Iqbal, S.; Hassan, M.Z.; Abbas, A.; Adamski, R.; Markowska, D.; et al. Plants in vitro propagation with its applications in food, pharmaceuticals and cosmetic industries; current scenario and future approaches. Front. Plant Sci. 2022, 13, 1009395. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, N. On the way to commercializing plant cell culture platform for biopharmaceuticals: Present Status and Prospect. Pharm. Bioprocess. 2014, 2, 499–518. [Google Scholar] [CrossRef] [Green Version]
- Máthé, C.; Demeter, Z.; Resetár, A.; Gonda, S.; Balázs, A.; Szôke, É.; Kiss, Z.; Simon, Á.; Székely, V.; Riba, M.; et al. The Plant Tissue Culture Collection at the Department of Botany, University of Debrecen. Acta Biol. Szeged. 2012, 56, 179–182. [Google Scholar]
- Kreis, W. Exploiting plant cell culture for natural product formation. J. Appl. Bot. Food Qual. 2019, 92, 216–225. [Google Scholar] [CrossRef]
- Stepanova, A.Y.; Malunova, M.V.; Gladkov, E.A.; Evsyukov, S.V.; Tereshonok, D.V.; Solov’eva, A.I. Collection of hairy roots as a basis for fundamental and applied research. Molecules 2022, 27, 8040. [Google Scholar] [CrossRef]
- de Oliveira Lourenço, S. Microalgae culture collections, strain maintenance, and propagation. In Handbook of Microalgae-Based Processes and Products; Jacob-Lopez, E., Queroz, M.J., Maroneze, M.M., Zepka, L.Q., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 49–84. [Google Scholar]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Chisti, Y. Raceways-based production of algal crude oil. Green 2013, 3, 195–216. [Google Scholar] [CrossRef]
- Jenderek, M.M.; Reed, B.M. Cryopreserved storage of clonal germplasm in the USDA national plant germplasm system. Vitr. Cell. Dev. Biol. Plant 2017, 53, 299–308. [Google Scholar] [CrossRef]
- Vollmer, R.; Villagaray, R.; Egusquiza, V.; Espirilla, J.; García, M.; Torres, A.; Rojas, E.; Panta, A.; Barkley, N.A.; Ellis, D. The potato cryobank at The International Potato Center (CIP): A model for long term conservation of clonal plant genetic resources collections of the future. Cryo Lett. 2016, 37, 318–329. [Google Scholar]
- Panis, B.; Nagel, M.; Van den Houwe, I. Challenges and prospects for the conservation of crop genetic resources in field genebanks, in in vitro collections and/or in liquid nitrogen. Plants 2020, 9, 1634. [Google Scholar] [CrossRef]
- Acker, J.P.; Adkins, S.; Alves, A.; Horna, D.; Toll, J. Feasibility study for a safety back-up cryopreservation facility. In Independent Expert Report: July 2017; Bioversity International: Rome, Italy, 2017. [Google Scholar]
- Panis, B. Sixty years of plant cryopreservation: From freezing hardy mulberry twigs to establishing reference crop collections for future generations. Acta Hortic 2019, 1234, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Normah, M.N.; Sulong, N.; Reed, B.M. Cryopreservation of shoot tips of recalcitrant and tropical species: Advances and strategies. Cryobiology 2019, 8, 1–14. [Google Scholar] [CrossRef]
- Benelli, C. Plant cryopreservation: A look at the present and the future. Plants 2021, 10, 2744. [Google Scholar] [CrossRef] [PubMed]
- Reinhoud, P.J.; Uragami, A.; Sakai, A.; Van Iren, F. Vitrification of plant cell suspensions. In Cryopreservation and Freeze-Drying Protocols; Day, J.G., Pennington, M.W., Eds.; Humana Press: Totowa, NJ, USA, 1995; pp. 113–120. [Google Scholar]
- Schumacher, H.M.; Westphal, M.; Heine-Dobbernack, E. Cryopreservation of plant cell lines. In Cryopreservation and Freeze-drying Protocols. Methods in Molecular Biology; Wolkers, W., Oldenhof, H., Eds.; Springer: New York, NY, USA, 2015; pp. 423–429. [Google Scholar]
- Popova, E.V.; Nosov, A.V.; Titova, M.V.; Kochkin, D.V.; Fomenkov, A.A.; Kulichenko, I.E.; Nosov, A.M. Advanced biotechnologies: Collections of plant cell cultures as a basis for development and production of medicinal preparations. Russ. J. Plant Physiol. 2021, 68, 385–400. [Google Scholar] [CrossRef]
- Nausch, H.; Buyel, J.F. Cryopreservation of plant cell cultures—diverse practices and protocols. N. Biotechnol. 2021, 62, 86–95. [Google Scholar] [CrossRef]
- Davoodi, A.; Khoshvishkaie, E.; Azadbakht, M. Plant cells technology as an effective biotechnological approach for high scale production of pharmaceutical natural compounds: A meta-analysis study. Pharm. Biomed. Res. 2019, 5, 1–9. [Google Scholar] [CrossRef]
- Nosov, A.M. Application of cell technologies for production of plant-derived bioactive substances of plant origin. Appl. Biochem. Microbiol. 2012, 48, 609–624. [Google Scholar] [CrossRef]
- Yue, W.; Ming, Q.; Lin, B.; Rahman, K.; Zheng, C.-J.; Han, T.; Qin, L. Medicinal plant cell suspension cultures: Pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef]
- Smart, N.J.; Fowler, M.W. An airlift column bioreactor suitable for large-scale cultivation of plant cell suspensions. J. Exp. Bot. 1984, 35, 531–537. [Google Scholar] [CrossRef]
- Sato, F.; Yamada, Y. High berberine-producing cultures of Coptis japonica cells. Phytochemistry 1984, 23, 281–285. [Google Scholar] [CrossRef]
- Tabata, M.; Fujita, Y. Production of shikonin by plant cell cultures. In Biotechnology in Plant Science; Zaitlin, M., Day, P., Hollaender, A., Eds.; Elsevier: Orlando, FL, USA, 1985; pp. 207–218. [Google Scholar]
- Sasson, A. Production of useful biochemicals by higher-plant cell cultures: Biotechnological and economic aspects. In Biotechnology: Economic and Social Aspects; Da Silva, E., Ratledge, C., Sasson, A., Eds.; Cambridge University Press: Cambridge, UK, 1992; pp. 81–109. [Google Scholar]
- Rittershaus, E.; Ulrich, J.; Weiss, A.; Westphal, K. Large scale industrial fermentation of plant cells: Experiences in cultivation of plant cells in a fermentation cascade up to a volume of 75,000 L. BioEngineering 1989, 5, 28–34. [Google Scholar]
- Kobayashi, Y.; Akita, M.; Sakamoto, K.; Liu, H.; Shigeoka, T.; Koyano, T.; Kawamura, M.; Furuya, T. Large-scale production of anthocyanin by Aralia cordata cell suspension cultures. Appl. Microbiol. Biotechnol. 1993, 40, 215–218. [Google Scholar] [CrossRef]
- Ulbrich, B.; Wiesner, W.; Arens, H. Large-scale production of rosmarinic acid from plant cell cultures of Coleus blumei Benth. In Primary and Secondary Metabolism of Plant Cell Cultures; Proceedings in Life Sciences; Neumann, K.H., Barz, W., Reinhard, E., Eds.; Springer: Berlin, Germany, 1985; pp. 293–303. [Google Scholar] [CrossRef]
- Yazaki, K. Lithospermum erythrorhizon cell cultures: Present and future aspects. Plant Biotechnol. 2017, 34, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Krasteva, G.; Georgiev, V.; Pavlov, A. Recent applications of plant cell culture technology in cosmetics and foods. Eng. Life Sci. 2021, 21, 68–76. [Google Scholar] [CrossRef]
- Barbulova, A.; Apone, F.; Colucci, G. Plant cell cultures as source of cosmetic active ingredients. Cosmetics 2014, 1, 94–104. [Google Scholar] [CrossRef]
- Trehan, S.; Michniak-Kohn, B.; Beri, K. Plant stem cells in cosmetics: Current trends and future directions. Futur. Sci. OA 2017, 3, FSO226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eibl, R.; Meier, P.; Stutz, I.; Schildberger, D.; Hühn, T.; Eibl, D. Plant cell culture technology in the cosmetics and food industries: Current state and future trends. Appl. Microbiol. Biotechnol. 2018, 102, 8661–8675. [Google Scholar] [CrossRef] [Green Version]
- Nohynek, L.; Bailey, M.; Tähtiharju, J.; Seppänen-Laakso, T.; Rischer, H.; Oksman-Caldentey, K.; Puupponen-Pimiä, R.; Seppänen-Laakso, T.; Rischer, H.; Oksman-Caldentey, K.; et al. Cloudberry (Rubus chamaemorus) cell culture with bioactive substances: Establishment and mass propagation for industrial use. Eng. Life Sci. 2014, 14, 667–675. [Google Scholar] [CrossRef]
- Nordlund, E.; Lille, M.; Silventoinen, P.; Nygren, H.; Seppänen-Laakso, T.; Mikkelson, A.; Aura, A.-M.; Heiniö, R.-L.; Nohynek, L.; Puupponen-Pimiä, R.; et al. Plant cells as food—a concept taking shape. Food Res. Int. 2018, 107, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Suvanto, J.; Nohynek, L.; Seppänen-Laakso, T.; Rischer, H.; Salminen, J.P.; Puupponen-Pimiä, R. Variability in the production of tannins and other polyphenols in cell cultures of 12 nordic plant species. Planta 2017, 246, 227–241. [Google Scholar] [CrossRef] [Green Version]
- Korkina, L.G.; Mikhal’chik, E.; Suprun, M.V.; Pastore, S.; Dal Toso, R. Molecular mechanisms underlying wound healing and anti-inflammatory properties of naturally occurring biotechnologically produced phenylpropanoid glycosides. Cell. Mol. Biol. (Noisy-Le-Grand) 2007, 53, 84–91. [Google Scholar]
- Di Paola, R.; Esposito, E.; Mazzon, E.; Riccardi, L.; Caminiti, R.; Dal Toso, R.; Pressi, G.; Cuzzocrea, S. Teupolioside, a phenylpropanoid glycosides of Ajuga reptans, biotechnologically produced by IRBN22 plant cell line, exerts beneficial effects on a rodent model of colitis. Biochem. Pharmacol. 2009, 77, 845–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Active botanicals research stem cells. Extract of Ajuga reptans Titrated in TEUPOLIOSIDE. Available online: https://abres.it/en/teupolioside/ (accessed on 14 April 2023).
- Choi, H.-K.; Son, J.-S.; Na, G.-H.; Hong, S.-S.; Park, Y.-S.; Song, J.-Y. Mass production of paclitaxel by plant cell culture. J. Plant Biotechnol. 2002, 29, 59–62. [Google Scholar] [CrossRef] [Green Version]
- VTT Technical Research Centre of Finland Ltd. VTT Culture Collection. Available online: http://culturecollection.vtt.fi (accessed on 14 April 2023).
- Experimental Plant Division. RIKEN BioResource Research Center. Plant Cultured Cell Resources. Available online: https://epd.brc.riken.jp/en/pcellc (accessed on 14 April 2023).
- Laboratory of Plant Biotechnologies. Available online: http://lpb.ueb.cas.cz/About.html (accessed on 14 April 2023).
- Pinaev, G.P.; Poljanskaya, G.G. Creation and development of the Russian collection of human, animal and plant cell cultures. In Cell Cultures. Information Bulletin. V. 26; Bogdanova, M.S., Ed.; Polytechnical University: Saint-Petersburg, Russia, 2010; Volume 61, pp. 3–61. [Google Scholar]
- Butenko, R.G. The tissue culture of medical plants and its possible use in pharmacy. Vopr. Farmakogn. 1967, 21, 184. [Google Scholar]
- Slepyan, L.I.; Grushevitskii, I.V.; Butenko, R.G. Ginseng as an object for introduction into tissue culture in vitro. Vopr. Farmakogn. 1967, 21, 198. [Google Scholar]
- Nosov, A.M.; Popova, E.V.; Kochkin, D.V. Isoprenoid production via plant cell cultures: Biosynthesis, accumulation and scaling-up to bioreactors. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Paek, K.Y., Murthy, H.N., Zhong, J.J., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 563–623. [Google Scholar]
- Novikova, G.V.; Stepanchenko, N.S.; Zorina, A.A.; Nosov, A.V.; Rakitin, V.Y.; Moshkov, I.E.; Los, D.A. Coupling of cell division and differentiation in Arabidopsis thaliana cultured cells with interaction of ethylene and ABA signaling pathways. Life 2020, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Orlova, Y.V.; Majorova, O.V.; Khalilova, L.A.; Voronkov, A.S.; Fomenkov, A.A.; Nosov, A.V.; Popova, L.G.; Balnokin, Y.V. Participation of endocytosis in sodium ion uptake by the cells of Arabidopsis thaliana (L.) Heynh in the suspension culture. Biochem. (Moscow), Suppl. Ser. A Membr. Cell Biol. 2018, 12, 382–389. [Google Scholar] [CrossRef]
- Orlova, Y.V.; Sergienko, O.V.; Khalilova, L.A.; Voronkov, A.S.; Fomenkov, A.A.; Nosov, A.V.; Popova, L.G.; Shuvalov, A.V.; Ryabova, A.V.; Balnokin, Y.V. Sodium transport by endocytic vesicles in cultured Arabidopsis thaliana (L.) Heynh. cells. Vitr. Cell. Dev. Biol. Plant 2019, 55, 359–370. [Google Scholar] [CrossRef]
- Novikova, G.V.; Mur, L.A.J.; Nosov, A.V.; Fomenkov, A.A.; Mironov, K.S.; Mamaeva, A.S.; Shilov, E.S.; Rakitin, V.Y.; Hall, M.A. Nitric oxide has a concentration-dependent effect on the cell cycle acting via EIN2 in Arabidopsis thaliana cultured cells. Front. Physiol. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zlobin, I.E.; Pashkovskiy, P.P.; Kartashov, A.V.; Nosov, A.V.; Fomenkov, A.A.; Kuznetsov, V.V. The relationship between cellular Zn status and regulation of Zn homeostasis genes in plant cells. Environ. Exp. Bot. 2020, 176, 104104. [Google Scholar] [CrossRef]
- Kochkin, D.V.; Demidova, E.V.; Globa, E.B.; Nosov, A.M. Profiling of taxoid compounds in plant cell cultures of different species of yew (Taxus Spp.). Molecules 2023, 28, 2178. [Google Scholar] [CrossRef] [PubMed]
- Nosov, A.V.; Titova, M.V.; Fomenkov, A.A.; Kochkin, D.V.; Galishev, B.A.; Sidorov, R.A.; Medentsova, A.A.; Kotenkova, E.A.; Popova, E.V.; Nosov, A.M. Callus and suspension cell cultures of Sutherlandia frutescens and preliminary screening of their phytochemical composition and antimicrobial activity. Acta Physiol. Plant. 2023, 45, 42. [Google Scholar] [CrossRef]
- Titova, M.V.; Kochkin, D.V.; Sobolkova, G.I.; Fomenkov, A.A.; Sidorov, R.A.; Nosov, A.M. Obtainment and characterization of Alhagi persarum Boiss. et Buhse callus cell cultures that produce isoflavonoids. Appl. Biochem. Microbiol. 2021, 57, 20–30. [Google Scholar] [CrossRef]
- Glagoleva, E.S.; Konstantinova, S.V.; Kochkin, D.V.; Ossipov, V.; Titova, M.V.; Popova, E.V.; Nosov, A.M.; Paek, K.-Y. Predominance of oleanane-type ginsenoside R0 and malonyl esters of protopanaxadiol-type ginsenosides in the 20-year-old suspension cell culture of Panax japonicus C.A. Meyer. Ind. Crops Prod. 2022, 177, 114417. [Google Scholar] [CrossRef]
- Titova, M.V.; Popova, E.V.; Shumilo, N.A.; Kulichenko, I.E.; Chernyak, N.D.; Ivanov, I.M.; Klushin, A.G.; Nosov, A.M. Stability of cryopreserved Polyscias filicifolia suspension cell culture during cultivation in laboratory and industrial bioreactors. Plant Cell Tissue Organ Cult. 2021, 145, 591–600. [Google Scholar] [CrossRef]
- Demidova, E.V.; Reshetnyak, O.V.; Oreshnikov, A.V.; Nosov, A.M. Growth and biosynthetic characteristics of ginseng (Panax japonicus Var. Repens) deep-tank cell culture in bioreactors. Russ. J. Plant Physiol. 2006, 53, 134–140. [Google Scholar] [CrossRef]
- Titova, M.V.; Shumilo, N.A.; Kulichenko, I.E.; Ivanov, I.M.; Sukhanova, E.S.; Nosov, A.M. Features of respiration and formation of steroidal glycosides in Dioscorea deltoidea cell suspension culture grown in flasks and bioreactors. Russ. J. Plant Physiol. 2015, 62, 557–563. [Google Scholar] [CrossRef]
- Titova, M.V.; Popova, E.V.; Konstantinova, S.V.; Kochkin, D.V.; Ivanov, I.M.; Klyushin, A.G.; Titova, E.G.; Nebera, E.A.; Vasilevskaya, E.R.; Tolmacheva, G.S.; et al. Suspension cell culture of Dioscorea deltoidei—a renewable source of biomass and furostanol glycosides for food and pharmaceutical industry. Agronomy 2021, 11, 394. [Google Scholar] [CrossRef]
- Titova, M.V.; Reshetnyak, O.V.; Osipova, E.A.; Osip’yants, A.I.; Shumilo, N.A.; Oreshnikov, A.V.; Nosov, A.M. Submerged cultivation of Stephania glabra (Roxb.) Miers cells in different systems: Specific features of growth and accumulation of alkaloid stepharine. Appl. Biochem. Microbiol. 2012, 48, 645–649. [Google Scholar] [CrossRef]
- Khandy, M.T.; Kochkin, D.V.; Tomilova, S.V.; Galishev, B.A.; Sukhanova, E.S.; Klyushin, A.G.; Ivanov, I.M.; Nosov, A.M. Obtaining and study of callus and suspension plant cell cultures of Tribulus terrestris L., a producer of steroidal glycosides. Appl. Biochem. Microbiol. 2017, 53, 800–806. [Google Scholar] [CrossRef]
- Kotin, A.M.; Bichevaya, N.K. Anti-Teratogenic Agent. Patent No. WO1996002266A1, 1 February 1996. [Google Scholar]
- Karanova, S.L.; Shamina, Z.B.; Rapoport, I.A. Effect of N-NMU on the variability of cell population of Dioscorea deltoidea in vitro. Genetica 1975, 11, 35–40. [Google Scholar]
- Khandy, M.T.; Titova, M.V.; Konstantinova, S.V.; Kochkin, D.V.; Ivanov, I.M.; Nosov, A.M. Formation of protodioscin and deltoside isomers in suspension cultures of nepal yam (Dioscorea deltoidea Wall.) cells. Appl. Biochem. Microbiol. 2016, 52, 657–662. [Google Scholar] [CrossRef]
- Kochkin, D.V.; Khandy, M.T.; Zaitsev, G.P.; Tolkacheva, N.V.; Shashkov, A.S.; Titova, M.V.; Chirva, V.Y.; Nosov, A.M. Protodioscin in Dioscorea deltoidea suspension cell culture. Chem. Nat. Compd. 2016, 52, 664–668. [Google Scholar] [CrossRef]
- Lipsky, A.K.; Nosov, A.M.; Paukov, V.N.; Karanova, S.L. Growth and metabolism of the Dioscorea deltoidea cell culture on submerged cultivation. In Plant Cell Culture; Butenko, R.G., Ed.; MIR Publishers: Moscow, Russia, 1985; pp. 76–107. [Google Scholar]
- Titova, M.V.; Khandy, M.T.; Konstantinova, S.V.; Kulichenko, I.E.; Sukhanova, E.S.; Kochkin, D.V.; Nosov, A.M. Effect of inhibitors of two isoprenoid biosynthetic pathways on physiological and biosynthetic characteristics of Dioscorea deltoidea cell suspension culture. Russ. J. Plant Physiol. 2016, 63, 894–900. [Google Scholar] [CrossRef]
- Povydysh, M.N.; Titova, M.V.; Ivkin, D.Y.; Krasnova, M.V.; Vasilevskaya, E.R.; Fedulova, L.V.; Ivanov, I.M.; Klushin, A.G.; Popova, E.V.; Nosov, A.M. The hypoglycemic and hypocholesterolemic activity of Dioscorea deltoidea, Tribulus terrestris and Panax japonicus cell culture biomass in rats with high-fat diet-induced obesity. Nutrients 2023, 15, 656. [Google Scholar] [CrossRef] [PubMed]
- Povydysh, M.N.; Titova, M.V.; Ivanov, I.M.; Klushin, A.G.; Kochkin, D.V.; Galishev, B.A.; Popova, E.V.; Ivkin, D.Y.; Luzhanin, V.G.; Krasnova, M.V.; et al. Effect of phytopreparations based on bioreactor-grown cell biomass of Dioscorea deltoidea, Tribulus terrestris and Panax japonicus on carbohydrate and lipid metabolism in Type 2 diabetes mellitus. Nutrients 2021, 13, 3811. [Google Scholar] [CrossRef]
- Kotin, O.A.; Kotin, A.M.; Emel’yanov, M.O. Use of a Preparation of the Plant Polyscias filicifolia for Treating Mineral Deficiencies. Patent WO2022010387A1, 13 January 2022. [Google Scholar]
- Kochkin, D.V.; Kachala, V.V.; Shashkov, A.S.; Chizhov, A.O.; Chirva, V.Y.; Nosov, A.M. Malonyl-ginsenoside content of a cell-suspension culture of Panax japonicus Var. Repens. Phytochemistry 2013, 93, 18–26. [Google Scholar] [CrossRef]
- Titova, M.V.; Kochkin, D.V.; Fomenkov, A.A.; Ivanov, I.M.; Kotenkova, E.A.; Kocharyan, G.L.; Dzhivishev, E.G.; Mekhtieva, N.P.; Popova, E.V.; Nosov, A.M. Obtaining and characterization of suspension cell culture of Alhagi persarum Boiss. et Buhse: A producer of isoflavonoids. Russ. J. Plant Physiol. 2021, 68, 652–660. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Agostini, E.; Ludwig-Müller, J.; Xu, J. Genetically transformed roots: From plant disease to biotechnological resource. Trends Biotechnol. 2012, 30, 528–537. [Google Scholar] [CrossRef]
- Baque, M.A.; Moh, S.-H.; Lee, E.-J.; Zhong, J.-J.; Paek, K.-Y. Production of biomass and useful compounds from adventitious roots of high-value added medicinal plants using bioreactor. Biotechnol. Adv. 2012, 30, 1255–1267. [Google Scholar] [CrossRef]
- Baque, M.A.; Hahn, E.-J.; Paek, K.-Y. Induction mechanism of adventitious root from leaf explants of Morinda citrifolia as affected by auxin and light quality. Vitr. Cell. Dev. Biol. Plant 2010, 46, 71–80. [Google Scholar] [CrossRef]
- Donini, M.; Marusic, C. Hairy roots as bioreactors for the production of biopharmaceuticals. In Hairy Roots (an Effective Tool of Plant Biotechnology); Srivastava, V., Mehrotra, S., Mishra, S., Eds.; Springer: Singapore, 2018; pp. 213–225. ISBN 9789811325625. [Google Scholar]
- Murthy, H.N.; Hahn, E.J.; Paek, K.-Y. Adventitious roots and secondary metabolism. Chin. J. Biotechnol. 2008, 24, 711–716. [Google Scholar] [CrossRef]
- Wawrosch, C.; Zotchev, S.B. Production of bioactive plant secondary metabolites through in vitro technologies—status and outlook. Appl. Microbiol. Biotechnol. 2021, 105, 6649–6668. [Google Scholar] [CrossRef]
- Skarjinskaia, M.; Ruby, K.; Araujo, A.; Taylor, K.; Gopalasamy-Raju, V.; Musiychuk, K.; Chichester, J.A.; Palmer, G.A.; de la Rosa, P.; Mett, V.; et al. Hairy roots as a vaccine production and delivery system. Adv. Biochem. Eng. Biotechnol. 2013, 134, 115–134. [Google Scholar] [CrossRef]
- Kuzovkina, I.N.; Gohar, A.; Alterman, I.E. Production of β-carboline alkaloids in transformed root cultures of Peganum harmala L. Z. Für Naturforsch. C 1990, 45, 727–728. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, J.; Facchini, P.J. Alkaloid biosynthesis: Metabolism and trafficking. Annu. Rev. Plant Biol. 2008, 59, 735–769. [Google Scholar] [CrossRef] [Green Version]
- Schepers, H.; Hausladen, H.; Hansen, J.G.; Bain, R.A.; Ritchie, F.; Gaucher, D.; Ivanović, Ž.; Blagojević, J.; Kildea, S.; Filippov, A.; et al. Epidemics and Control of Early & Late Blight, 2015 & 2016 in Europe; 16th EuroBlight Workshop: Aarhus, Denmark, 2017; pp. 11–32. [Google Scholar]
- Yellareddygari, S.K.R.; Taylor, R.J.; Pasche, J.S.; Zhang, A.; Gudmestad, N.C. Predicting potato tuber yield loss due to early blight severity in the Midwestern United States. Eur. J. Plant Pathol. 2018, 152, 71–79. [Google Scholar] [CrossRef]
- Steward, F.C.; Caplin, S.M. A tissue culture from potato tuber: The synergistic action of 2,4-D and of coconut milk. Science. 1951, 113, 518–520. [Google Scholar] [CrossRef]
- Chapman, H.W. Potato tissue cultures. Am. Potato J. 1955, 32, 207–210. [Google Scholar] [CrossRef]
- Goodwin, P.B.; Kim, Y.C.; Adisarwanto, T. Propagation of potato by shoot-tip culture. 1. Shoot multiplication. Potato Res. 1980, 23, 9–18. [Google Scholar] [CrossRef]
- Roca, W.M.; Espinoza, N.O.; Roca, M.R.; Bryan, J.E. A tissue culture method for the rapid propagation of potatoes. Am. Potato J. 1978, 55, 691–701. [Google Scholar] [CrossRef]
- Kämäräinen-Karppinen, T.; Virtanen, E.; Rokka, V.-M.; Pirttilä, A.M. Novel bioreactor technology for mass propagation of potato microtubers. Plant Cell, Tissue Organ Cult. 2010, 101, 245–249. [Google Scholar] [CrossRef]
- Bamberg, J.B.; Martin, M.W.; Abad, J.; Jenderek, M.M.; Tanner, J.; Donnelly, D.J.; Nassar, A.M.K.; Veilleux, R.E.; Novy, R.G. In vitro technology at the US Potato Genebank. Vitr. Cell. Dev. Biol. Plant 2016, 52, 213–225. [Google Scholar] [CrossRef]
- Vollmer, R.; Villagaray, R.; Cárdenas, J.; Castro, M.; Chávez, O.; Anglin, N.L.; Ellis, D. A large-scale viability assessment of the potato cryobank at the International Potato Center (CIP). In Vitro Cell. Dev. Biol. Plant 2017, 53, 309–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonova, O.Y.; Apalikova, O.V.; Ukhatova, Y.V.; Krylova, E.A.; Shuvalov, O.Y.; Shuvalova, A.R.; Gavrilenko, T.A. Eradication of viruses in microplants of three cultivated potato species (Solanum tuberosum L., S. phureja Juz. & Buk., S. stenotomum Juz. & Buk.) using combined thermo-chemotherapy method. Sel’skokhozyaistvennaya Biol. 2017, 52, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Bettoni, J.C.; Mathew, L.; Pathirana, R.; Wiedow, C.; Hunter, D.A.; McLachlan, A.; Khan, S.; Tang, J.; Nadarajan, J. Eradication of potato virus S, potato virus A, and potato virus M from infected in vitro-grown potato shoots using in vitro therapies. Front. Plant Sci. 2022, 13, 878733. [Google Scholar] [CrossRef] [PubMed]
- Oves, E.V.; Gaitova, N.A.; Shishkina, O.A. Maintenance of potato varieties in in vitro and field collections of the Russian Potato Research Centre. Plant Biotechnol. Breed. 2022, 5, 28–41. [Google Scholar] [CrossRef]
- Zhevora, S.V. (Ed.) Potato; Russian Potato Resarch Centre: Moscow, Russia, 2022. [Google Scholar]
- Yuorieva, N.O.; Voronkov, A.S.; Tereshonok, D.V.; Osipova, E.S.; Platonova, E.V.; Belyaev, D.V. An assay for express screening of potato transformants by GFP fluorescence. Moscow Univ. Biol. Sci. Bull. 2018, 73, 69–75. [Google Scholar] [CrossRef]
- Beliaev, D.V.; Yuorieva, N.O.; Tereshonok, D.V.; Tashlieva, I.I.; Derevyagina, M.K.; Meleshin, A.A.; Rogozhin, E.A.; Kozlov, S.A. High resistance of potato to early blight is achieved by expression of the Pro-SmAMP1 gene for hevein-like antimicrobial peptides from common chickweed (Stellaria media). Plants 2021, 10, 1395. [Google Scholar] [CrossRef]
- Rogozhin, E.A.; Oshchepkova, Y.I.; Odintsova, T.I.; Khadeeva, N.V.; Veshkurova, O.N.; Egorov, T.A.; Grishin, E.V.; Salikhov, S.I. Novel antifungal defensins from Nigella sativa L. seeds. Plant Physiol. Biochem. 2011, 49, 131–137. [Google Scholar] [CrossRef]
- Beliaev, D.; Rogozhin, E.A.; Meleshin, A.A.; Tereshonok, D.V.; Derevyagina, M.K.; Yuorieva, N.O.; Tashlieva, I.I.; Djalilov, F.S.; Voronkova, E.V. NsD3, a defensin from Nigella Sativa, confers high resistance of several commercial potato varieties to fungi and bacteria NsD3. Vitr. Cell. Dev. Biol. Anim. 2020, 56, 42–65. [Google Scholar] [CrossRef]
- Jur’eva, N.O.; Egorov, T.A.; Beljaev, D.V.; Sobol’kova, G.I.; Derevjagina, M.K.; Rogozhin, E.A.; Tereshonok, D.V.; Meleshin, A.A.; Shelukhin, P.G. Method of Production In Vitro of Potato Forms Resistant to Phytophthora and Alternaria Spot Causative Agents. RU 2 524 424 C1, 27 July 2014. [Google Scholar]
- Fukalova, T.; García Martínez, M.D.; Raigón, M.D. Five undervalued edible species inherent to autumn-winter season: Nutritional composition, bioactive constituents and volatiles profile. PeerJ 2021, 9, e12488. [Google Scholar] [CrossRef]
- Benson, D.E.; Benson, E. (Eds.) Plant Conservation Biotechnology; CRC Press: London, UK, 1999; ISBN 9781482273038. [Google Scholar] [CrossRef]
- Reed, B.M.; Sarasan, V.; Kane, M.; Bunn, E.; Pence, V.C. Biodiversity conservation and conservation biotechnology tools. Vitr. Cell. Dev. Biol. Plant 2011, 47, 1–4. [Google Scholar] [CrossRef]
- Kulus, D.; Zalewska, M. Cryopreservation as a tool used in long-term storage of ornamental species—A review. Sci. Hortic. (Amsterdam) 2014, 168, 88–107. [Google Scholar] [CrossRef]
- Pence, V.C.; Ballesteros, D.; Walters, C.; Reed, B.M.; Philpott, M.; Dixon, K.W.; Pritchard, H.W.; Culley, T.M.; Vanhove, A.-C. Cryobiotechnologies: Tools for expanding long-term ex situ conservation to all plant species. Biol. Conserv. 2020, 250, 108736. [Google Scholar] [CrossRef]
- Walters, C.; Pence, V.C. The unique role of seed banking and cryobiotechnologies in plant conservation. Plants People Planet 2021, 3, 83–91. [Google Scholar] [CrossRef]
- Kulak, V.; Longboat, S.; Brunet, N.D.; Shukla, M.; Saxena, P. In vitro technology in plant conservation: Relevance to biocultural diversity. Plants 2022, 11, 503. [Google Scholar] [CrossRef] [PubMed]
- Whelehan, L.M.; Dalziell, E.L.; Bunn, E.; Mancera, R.L.; Funnekotter, B. How does metabolic rate in plant shoot tips change after cryopreservation? Cryobiology 2022, 109, 1–9. [Google Scholar] [CrossRef]
- Popov, A.S.; Butenko, R.G.; Glukhova, I.N. Effect of the preparation and deep-freezing conditions on the regeneration of the suspension culture of wild carrot cells. Sov. Plant Physiol. 1978, 25, 972–979. [Google Scholar]
- Popov, A.S.; Popova, E.V.; Nikishina, T.V.; Vysotskaya, O.N. Cryobank of plant genetic resources in Russian Academy of Sciences. Int. J. Refrig. 2006, 29, 403–410. [Google Scholar] [CrossRef]
- IPPRAS Cryobank. Home Page. Available online: https://ippras.ru/institut/nauchnye_podrazdeleniya/gruppa-kriosokhraneniya-rasteniy/kriobank-ifr-ran/ (accessed on 26 October 2022).
- Maximov, N.A. Chemische schutzmittel der pflanzen gegen erfrieren. Ber. Dtsch. Bot. Ges. 1912, 30, 52–65, 293–305, 509–516. [Google Scholar]
- Maximov, N.A. Internal factors of frost and drought resistance in plants. Protoplasma 1929, 7, 259–291. [Google Scholar] [CrossRef]
- Tumanov, I.I.; Trunova, T.I. Hardening tissues of winter plants with sugar absorbed from the external solution. Sov. Plant Physiol. 1957, 4, 379–388. [Google Scholar]
- Tumanov, I.I.; Krasavtsev, O.A.; Khvalin, N.N. Increase of frost resistance of birch and black currant to −254 °C by hardening. Reports Acad. Sci. USSR 1959, 127, 1301–1303. [Google Scholar]
- Tumanov, I.I.; Butenko, R.G.; Ogolevets, I.V. Use of the isolated tissue method for studying hardening of plant cells. Sov. Plant Physiol. 1968, 15, 625–630. [Google Scholar]
- Tumanov, I.I.; Butenko, R.G.; Ogolevets, I.V.; Smetyuk, V.V. Increasing the frost resistance of spruce callus tissue culture by freezing out the less resistant cells. Sov. Plant Physiol. 1977, 24, 728–732. [Google Scholar]
- Samygin, G.A.; Varlamov, V.N.; Matveeva, N.M. The ability of seeds to endure ultra low temperatures. Sov. Plant Physiol. 1960, 7, 76–78. [Google Scholar]
- Butenko, R.G.; Grushevitskii, I.V.; Slepyan, L.I. Organogenesis and somatic embryogenesis in tissue culture of ginseng and other members of genus Panax L. Bot. Zh. 1968, 53, 906. [Google Scholar]
- Butenko, R.G.; Popov, A.S.; Volkova, L.A.; Chernyak, N.D.; Nosov, A.M. Recovery of cell cultures and their biosynthetic capacity after storage of Dioscorea deltoidea and Panax ginseng cells in liquid nitrogen. Plant Sci. Lett. 1984, 33, 285–292. [Google Scholar] [CrossRef]
- Volkova, L.A.; Urmantseva, V.V.; Popova, E.V.; Nosov, A.M. Physiological, cytological and biochemical stability of Medicago sativa L. cell culture after 27 years of cryogenic storage. Cryo Lett. 2015, 36, 252–263. [Google Scholar]
- Vysotskaya, O.N.; Sementsova, M.V.; Osipova, E.S. Cryobank of Timiryazev Institute of Plant Physiology, Russian Academy of Sciences and plant material recovery after long-term preservation in liquid nitrogen. In Proceedings of the All-Russian Scien. Conf. with Intern. Participation and a School for Young Scientists: “Experimental Plant Biology and Biotechnology: History and a Look into the Future”. Moscow, Russia, 27 September–1 October 2021; Los, D.A., Ed.; Timiryazev Institute of Plant Physiology, Russian Academy of Sciences: Moscow, Russia, 2021; p. 359. [Google Scholar]
- Diettrich, B.; Haack, U.; Popov, A.S.; Butenko, R.G.; Luckner, M. Long-term storage in liquid nitrogen of an embryogenic cell strain of Digitalis lanata. Biochem. Physiol. Pflanz. 1985, 180, 33–43. [Google Scholar] [CrossRef]
- Volkova, L.A.; Gorskaya, N.V.; Popov, A.S.; Paukov, V.N.; Urmantseva, V.V. Preservation of the main characteristics of Dioscorea mutant cell strains after storage at extremely low temperatures. Sov. Plant Physiol. 1986, 33, 598–605. [Google Scholar]
- Popov, A.S. Mechanisms of in vitro cryoinjury in plant cell cryopreservation. Russ. J. Plant Physiol. 1993, 40, 421–430. [Google Scholar]
- Chulafich, L.; Grubishich, D.; Vuiichich, R.; Volkova, L.A.; Popov, A.S. Somatic embryo production in vitro in Dioscorea caucasica Lipsky and Dioscorea balcanica Kosanin and cryopreservation of their organogenic callus tissues. Russ. J. Plant Physiol. 1994, 41, 821–826. [Google Scholar]
- Vysotskaya, O.N.; Popov, A.S.; Butenko, R.G. The advantage of glucose over sucrose in cryopreservation of strawberry meristems. Russ. J. Plant Physiol. 1999, 46, 255–257. [Google Scholar]
- Vysotskaya, O.N.; Mochammed, A.I.; Butenko, R.G. Cryopreservation of red raspberry meristems (Rubus idaeus L.) isolated from in vitro plantlets. Biol. Bull. 1999, 26, 19–22. [Google Scholar]
- Balekin, A.Y.; Vysotskaya, O.N. Rare cacti collection from liquid nitrogen. In Proceedings of the 85th International Conference Dedicated to Anniversary of the Central Botanical Garden of the National Academy of Sciences of Belarus: Role of Botanical Gardens and Arboretums in Conservation, Investigation and Sustainable Using Diversity of the Plant Word, Minsk, Belarus, 6–8 July 2017; Titov, V.V., Ed.; Medocont: Minsk, Belarus, 2017; pp. 31–34. [Google Scholar]
- Sementsova, M.V. Preservation of rare species from genus of Veratrum L. in IPPRAS Cryobank. In Proceedings of the XXVI International Scientific Conference of Students, Postgraduates and Young Scientists “Lomonosov”, Lomonosov, Moscow State University, Moscow, Russia, 8–12 April 2019; Aleshkovsky, I.A., Andriyanov, A.V., Antipov, E.A., Eds.; MAKS Press: Moscow, Russia, 2019. [Google Scholar]
- Kolomeitseva, G.L.; Nikishina, T.V.; Babosha, A.V.; Ryabchenko, A.S.; Vysotskaya, O.N. Morphophysiology and cryopreservation of seeds of Dendrobium nobile Lindl. (Orchidaceae) at different stages of development. Acta Physiol. Plant. 2022, 44, 36. [Google Scholar] [CrossRef]
- Koroleva, O.V.; Molkanova, O.I.; Vysotskaya, O.N. Cryoresistance of lilac meristems isolated from in vitro culture. In Proceedings of the III International Scientific and Practical Conference: “GenBio2022”, Yalta, Russia, 3–8 October 2022; Book of Abstracts «Genomics and Modern Biotechnology in Plant Propagation, Breeding and Conservation»; IT “ARIAL”: Simpheropol, Russia, 2022; pp. 99–100. [Google Scholar] [CrossRef]
- Nikishina, T.V.; Popov, A.S.; Kolomeitseva, G.L.; Golovkin, B.N. Effect of cryopreservation on seed germination of rare tropical orchids. Russ. J. Plant Physiol. 2001, 48, 810–815. [Google Scholar] [CrossRef]
- Nikishina, T.V.; Vakhrameeva, M.G.; Varlygina, T.I.; Pavlov, V.N.; Shirokov, A.I.; Burov, A.V.; Popovich, E.A.; Popov, A.S. Cryoconservation of seeds of rare russian orchids. Dokl. Biol. Sci. 2006, 408, 214–216. [Google Scholar] [CrossRef]
- Nikishina, T.V.; Kozlova, O.N.; Levitskaya, G.E.; Vysotskaya, O.N. Study of Dactylorhiza seeds (D. baltica and D. maculata) from the orchid collection of the cryobank at Timiryazev Institute of Plant Physiology, Russian Academy of Sciences. Biol. Bull. 2019, 46, 242–250. [Google Scholar] [CrossRef]
- Solov’eva, A.I.; Dolgikh, Y.I.; Vysotskaya, O.N.; Popov, A.S. Patterns of ISSR and REMAP DNA markers after cryogenic preservation of spring wheat calli by dehydration method. Russ. J. Plant Physiol. 2011, 58, 423–430. [Google Scholar] [CrossRef]
- Solov’eva, A.I.; Vysotskaya, O.N.; Dolgikh, Y.I. Effect of dehydration duration of apices on characteristics of in vitro plants of Fragaria vesca after cryopreservation. Russ. J. Plant Physiol. 2016, 63, 243–251. [Google Scholar] [CrossRef]
- Vysotskaya, O.N. Resistance of strawberry cultivars (Fragaria L.) and post cryogenic recovery plantlets in vitro. In Book of Abstracts VII International Strawberry Symposium ISHS; Zhang, L., Ed.; Agricultural Press: Beijing, China, 2012; p. 273. ISBN 9787109163898. [Google Scholar]
- Vysotskaya, O.N.; Sprinchanou, E.K.; Vysotskiy, V.A. The verification of long-term storage techniques for in vitro strawberry collections. Pomic. Small Fruits Cult. Russ. 2016, 45, 50–53. [Google Scholar]
- Vysotskaya, O.N. The Technique for Cryopreservation of Meristematic Shoot Tips Isolated from Plants In Vitro. Eurasian Patent no. 036602 (RU). Prior., 13 June 2019. [Google Scholar]
- Solov’eva, A.I.; Vysotskaya, O.N. Genetic diversity of a post-cryogenic Fragaria vesca L. clone after long-term cultivation in vitro and influenced by abiotic factors. Biol. Bull. 2020, 47, 253–258. [Google Scholar] [CrossRef]
- Vysotskiy, V.A.; Karpova, O.V.; Vysotskaya, O.N. Stability of major features in berry plants regenerated after cryopreservation. Hortic. Vitic. 2002, 3, 9–12. [Google Scholar]
- Bukhov, N.G.; Popova, E.V.; Popov, A.S. Photochemical activities of two photosystems in Bratonia orchid protocorms cryopreserved by vitrification method. Russ. J. Plant Physiol. 2006, 53, 793–799. [Google Scholar] [CrossRef]
- Baskakova, O.Y.; Voinkova, N.M.; Nikishina, T.V.; Osipova, E.A.; Popov, A.S. Resistance and cryopreservation of cell strains of Rhaponticum carthamoides and Thalictrum minus. Russ. J. Plant Physiol. 2003, 50, 666–671. [Google Scholar] [CrossRef]
- Osipova, E.S.; Kolomeitseva, G.N.; Vysotskaya, O.N. The algorithm for the formation of the orchid seed cryocollection. In Proceedings of the XII Inter. Conf.: “Conservation and Cultivation of Orchids”, Moscov, Russia, 7–10 June 2022; Varligina, T.I., Ed.; Moscow University Press: Moscow, Russia, 2022; pp. 185–193. [Google Scholar]
- Nikishina, T.V.; Kolomeitseva, G.N.; Antipina, V.A.; Bubnova, D.S.; O.N., V. Orchid seeds: 12-years cryopreservation experiment. In Proceedings of the X International Conference: “Conservation and Cultivation of Orchids”, Minsk, Belarus, 1–5 June 2015; Titok, V.V., Ed.; Varaksin, A.N.: Minsk, Belarus, 2015; pp. 177–181.
- Vysotskaya, O.N.; Popov, A.S. Cryopreservation technique for meristems, isolated from in vitro strawberry plantlets (Fragaria L.). RF Patent no. 2220563 (RU). Prior., 24 May 2002. [Google Scholar]
- Day, J.G.; Lukavský, J.; Friedl, T.; Brand, J.J.; Campbell, C.N.; Lorenz, M.; Elster, J. Pringsheim’s living legacy: CCALA, CCAP, SAG and UTEX culture collections of algae. Nov. Hedwigia 2004, 79, 27–37. [Google Scholar] [CrossRef]
- Culture Collection of Algae and Protozoa Home Page. Available online: https://www.ccap.ac.uk/ (accessed on 14 April 2023).
- Georg-August-Universität Göttingen. Sammlung von Algenkulturen (SAG) Home Page. Available online: https://uni-goettingen.de/en/www.uni-goettingen.de/de/184982.html (accessed on 14 April 2023).
- Biological resource center of Institut Pasteur (CRBIP). Pasteur Culture Collection of Cyanobacteria. Available online: https://webext.pasteur.fr/cyanobacteria/ (accessed on 14 April 2023).
- Institute of Botany of the AS CR. Culture Collection of Autotrophic Organisms (CCALA). Available online: https://ccala.butbn.cas.cz/en (accessed on 14 April 2023).
- The University of Texas at Austin. Culture Collection of Algae. Available online: https://utex.org/pages/about-utex#history (accessed on 14 April 2023).
- Pronina, N.A.; Tsoglin, L.N.; Los, D.A. Victor E. Semenenko (1932–1998). In Viktor E. Semenenko; Vladimirova, M.G., Los, D.A., Pronina, N.A., Tsoglin, L.N., Eds.; Admiral-Print: Moscow, Russia, 2012; pp. 45–60. [Google Scholar]
- Vladimirova, M.G.; Bartsevich, E.D.; Zholdakov, I.A.; Epifanova, O.O.; Markelova, A.G.; Maslova, I.P.; Kuptsova, E.S. IPPAS—collection of microalgae of Timiryazev institute of plant physiology, Acad. Sci. USSR. In Katalog Kul’tur Mikrovodoroslei v Kollektsiyakh SSSR (Catalogue of Microalgal Cultures in the Collections of USSR); Semenenko, V.E., Ed.; Russian Academy Science: Moscow, Russia, 1991; pp. 8–61. [Google Scholar]
- IPPAS on The European Culture Collections’ Organisation (ECCO) Website. Available online: https://www.eccosite.org/portfolio-item/ippas/ (accessed on 24 November 2022).
- IPPAS on Culture Collections Information Worldwide Website. Available online: https://ccinfo.wdcm.org/details?regnum=596 (accessed on 24 November 2022).
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: https://www.algaebase.org (accessed on 20 October 2022).
- Vladimirova, M.G.; Semenenko, V.E. Intensivnaya Kul’tura Odnokletochnykh Vodoroslei (Intense Culture of Unicellular Algae); Akad. Nauk SSSR: Moscow, Russia, 1962. [Google Scholar]
- Andersen, R.A. Algal Culturing Techniques; Elsevier Academic Press: London, UK, 2005. [Google Scholar]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef]
- Gabrielyan, D.A.; Sinetova, M.A.; Gabel, B.V.; Gabrielian, A.K.; Markelova, A.G.; Rodionova, M.V.; Bedbenov, V.S.; Shcherbakova, N.V.; Los, D.A. Cultivation of Chlorella sorokiniana IPPAS C-1 in flat-panel photobioreactors: From a laboratory to a pilot scale. Life 2022, 12, 1309. [Google Scholar] [CrossRef]
- Sarsekeyeva, F.K.; Usserbaeva, A.A.; Zayadan, B.K.; Mironov, K.S.; Sidorov, R.A.; Kozlova, A.Y.; Kupriyanova, E.V.; Sinetova, M.A.; Los, D.A. Isolation and characterization of a new cyanobacterial strain with a unique fatty acid composition. Adv. Microbiol. 2014, 04, 1033–1043. [Google Scholar] [CrossRef] [Green Version]
- Andersen, R.A.; Kawachi, M. Traditional microalgae isolation techniques. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elsevier Academic Press: London, UK, 2005; pp. 83–100. [Google Scholar]
- Online Catalog of the Collection IPPAS. Available online: http://cellreg.org/Catalog/ (accessed on 26 November 2022).
- Sinetova, M.A.; Kupriyanova, E.V.; Markelova, A.G.; Allakhverdiev, S.I.; Pronina, N.A. Identification and functional role of the carbonic anhydrase Cah3 in thylakoid membranes of pyrenoid of Chlamydomonas reinhardtii. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 1248–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupriyanova, E.V.; Sinetova, M.A.; Cho, S.M.; Park, Y.-I.; Markelova, A.G.; Los, D.A.; Pronina, N.A. Specific features of the system of carbonic anhydrases of alkaliphilic cyanobacteria. Russ. J. Plant Physiol. 2013, 60, 465–471. [Google Scholar] [CrossRef]
- Kupriyanova, E.V.; Sinetova, M.A.; Mironov, K.S.; Novikova, G.V.; Dykman, L.A.; Rodionova, M.V.; Gabrielyan, D.A.; Los, D.A. Highly active extracellular α-class carbonic anhydrase of Cyanothece sp. ATCC 51142. Biochimie 2019, 160, 200–209. [Google Scholar] [CrossRef]
- Zorina, A.A.; Mironov, K.S.; Stepanchenko, N.S.; Sinetova, M.A.; Koroban, N.V.; Zinchenko, V.V.; Kupriyanova, E.V.; Allakhverdiev, S.I.; Los, D.A. Regulation systems for stress responses in cyanobacteria. Russ. J. Plant Physiol. 2011, 58, 749–767. [Google Scholar] [CrossRef]
- Zorina, A.A.; Novikova, G.V.; Los, D.A. Participation of Ser-Thr protein kinases in regulation of heat stress responses in Synechocystis. In Stress and Environmental Regulation of Gene Expression and Adaptation in Bacteria; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 766–780. [Google Scholar]
- Sinetova, M.A.; Los, D.A. New insights in cyanobacterial cold stress responses: Genes, sensors, and molecular triggers. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 2391–2403. [Google Scholar] [CrossRef]
- Fedurayev, P.V.; Mironov, K.S.; Gabrielyan, D.A.; Bedbenov, V.S.; Zorina, A.A.; Shumskaya, M.; Los, D.A. Hydrogen peroxide participates in perception and transduction of cold stress signal in Synechocystis. Plant Cell Physiol. 2018, 59, 1255–1264. [Google Scholar] [CrossRef] [Green Version]
- Mironov, K.; Sinetova, M.; Shumskaya, M.; Los, D. Universal molecular triggers of stress responses in cyanobacterium Synechocystis. Life 2019, 9, 67. [Google Scholar] [CrossRef] [Green Version]
- Gabrielyan, D.A.; Sinetova, M.A.; Gabrielian, A.K.; Bobrovnikova, L.A.; Bedbenov, V.S.; Starikov, A.Y.; Zorina, A.A.; Gabel, B.V.; Los, D.A. Laboratory system for intensive cultivation of microalgae and cyanobacteria. Russ. J. Plant Physiol. 2023, 70, 1–11. [Google Scholar] [CrossRef]
- Gorelova, O.; Baulina, O.; Ismagulova, T.; Kokabi, K.; Lobakova, E.; Selyakh, I.; Semenova, L.; Chivkunova, O.; Karpova, O.; Scherbakov, P.; et al. Stress-induced changes in the ultrastructure of the photosynthetic apparatus of green microalgae. Protoplasma 2019, 256, 261–277. [Google Scholar] [CrossRef]
- Solovchenko, A.E.; Ismagulova, T.T.; Lukyanov, A.A.; Vasilieva, S.G.; Konyukhov, I.V.; Pogosyan, S.I.; Lobakova, E.S.; Gorelova, O.A. Luxury phosphorus uptake in microalgae. J. Appl. Phycol. 2019, 31, 2755–2770. [Google Scholar] [CrossRef]
- Semenenko, V.E.; Vladimirova, M.G.; Orleanskaya, O.B. The Physiological Characteristics of Chlorella Sp. K at High Temperature Extremes. I. Uncoupling Effect of Extreme Temperatures on Cellular Functions of Chlorella. Sov. Plant Physiol. 1967, 14, 612–625. [Google Scholar]
- Semenenko, V.E.; Vladimirova, M.G.; Orleanskaya, O.B.; Raikov, N.J.; Kovanova, E.S. Physiological Characteristics of Chlorella sp. K at high extremal temperatures. II. Alteration in biosynthesis, ultrastructure, and activity of photosynthetic apparatus in Chlorella upon dissociation of cell functions by extreme temperature. Sov. Plant Physiol. 1969, 16, 172–181. [Google Scholar]
- Zhukova, T.S.; Klyachko-Gurvich, G.L.; Vladimirova, M.G.; Kurnosova, T.A. A comparative characterization of growth and biosynthesis direction in different Chlorella strains under nitrogen starvation: 2. Carbohydrate and lipid production. Sov. Plant Physiol. 1969, 16, 96–101. [Google Scholar]
- Vladimirova, M.G.; Klyachko-Gurvich, G.L.; Kurnosova, T.A.; Zhukova, T.S. A Comparative Characterization of growth and biosynthesis direction in different Chlorella strains under nitrogen starvation: 1. Investigation of growth and productivity. Sov. Plant Physiol. 1968, 15, 993–1001. [Google Scholar]
- Shebanova, A.; Ismagulova, T.; Solovchenko, A.; Baulina, O.; Lobakova, E.; Ivanova, A.; Moiseenko, A.; Shaitan, K.; Polshakov, V.; Nedbal, L.; et al. Versatility of the green microalga cell vacuole function as revealed by analytical transmission electron microscopy. Protoplasma 2017, 254, 1323–1340. [Google Scholar] [CrossRef]
- Ismagulova, T.; Shebanova, A.; Gorelova, O.; Baulina, O.; Solovchenko, A. A new simple method for quantification and locating P and N reserves in microalgal cells based on energy-filtered transmission electron microscopy (EFTEM) elemental maps. PLoS ONE 2018, 13, e0208830. [Google Scholar] [CrossRef] [Green Version]
- Schreiber, C.; Behrendt, D.; Huber, G.; Pfaff, C.; Widzgowski, J.; Ackermann, B.; Müller, A.; Zachleder, V.; Moudříková, Š.; Mojzeš, P.; et al. Growth of algal biomass in laboratory and in large-scale algal photobioreactors in the temperate climate of western germany. Bioresour. Technol. 2017, 234, 140–149. [Google Scholar] [CrossRef]
- Schreiber, C.; Schiedung, H.; Harrison, L.; Briese, C.; Ackermann, B.; Kant, J.; Schrey, S.D.; Hofmann, D.; Singh, D.; Ebenhöh, O.; et al. Evaluating potential of green alga Chlorella vulgaris to accumulate phosphorus and to fertilize nutrient-poor soil substrates for crop plants. J. Appl. Phycol. 2018, 30, 2827–2836. [Google Scholar] [CrossRef]
- Gabrielyan, D.A.; Gabel, B.V.; Sinetova, M.A.; Gabrielian, A.K.; Markelova, A.G.; Shcherbakova, N.V.; Los, D.A. Optimization of CO2 supply for the intensive cultivation of Chlorella sorokiniana IPPAS C-1 in the laboratory and pilot-scale flat-panel photobioreactors. Life 2022, 12, 1469. [Google Scholar] [CrossRef]
- Piligaev, A.V.; Sorokina, K.N.; Shashkov, M.V.; Parmon, V.N. Screening and comparative metabolic profiling of high lipid content microalgae strains for application in wastewater treatment. Bioresour. Technol. 2018, 250, 538–547. [Google Scholar] [CrossRef]
- Sukačová, K.; Búzová, D.; Trávníček, P.; Červený, J.; Vítězová, M.; Vítěz, T. Optimization of microalgal growth and cultivation parameters for increasing bioenergy potential: Case study using the oleaginous microalga Chlorella pyrenoidosa Chick (IPPAS C2). Algal Res. 2019, 40, 101519. [Google Scholar] [CrossRef]
- Semenenko, V.; Vladimirova, M. Effect of cosmic flight conditions in the sputnik-ship on the viability of Chlorella. Fiziol. Rastenii 1961, 8, 743–749. [Google Scholar]
- Sinetova, M.A.; Sidorov, R.A.; Starikov, A.Y.; Voronkov, A.S.; Medvedeva, A.S.; Krivova, Z.V.; Pakholkova, M.S.; Bachin, D.V.; Bedbenov, V.S.; Gabrielyan, D.A.; et al. Assessment of the biotechnological potential of cyanobacterial and microalgal strains from IPPAS culture collection. Appl. Biochem. Microbiol. 2020, 56, 794–808. [Google Scholar] [CrossRef]
- Krivina, E.S.; Bobrovnikova, L.A.; Temraleeva, A.D.; Markelova, A.G.; Gabrielyan, D.A.; Sinetova, M.A. Description of Neochlorella semenenkoi Gen. et. sp. nov. (Chlorophyta, Trebouxiophyceae), a novel Chlorella-like alga with high biotechnological potential. Diversity 2023, 15, 513. [Google Scholar] [CrossRef]
- Červený, J.; Sinetova, M.A.; Valledor, L.; Sherman, L.A.; Nedbal, L. Ultradian metabolic rhythm in the diazotrophic cyanobacterium Cyanothece sp. ATCC 51142. Proc. Natl. Acad. Sci. USA 2013, 110, 13210–13215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starikov, A.Y.; Usserbaeva, A.A.; Sinetova, M.A.; Sarsekeyeva, F.K.; Zayadan, B.K.; Ustinova, V.V.; Kupriyanova, E.V.; Los, D.A.; Mironov, K.S. Draft genome sequence of Cyanobacterium sp. strain IPPAS B-1200 with a unique fatty acid composition. Genome Announc. 2016, 4, e01306-16. [Google Scholar] [CrossRef] [Green Version]
- Starikov, A.Y.; Usserbaeva, A.A.; Mironov, K.S.; Sidorov, R.A.; Zayadan, B.K.; Bedbenov, V.S.; Sinetova, M.A.; Los, D.A. Substrate specificity of acyl-lipid Δ9-desaturase from Cyanobacterium sp. IPPAS B-1200, a cyanobacterium with unique fatty acid composition. Russ. J. Plant Physiol. 2018, 65, 490–497. [Google Scholar] [CrossRef]
- Sinetova, M.A.; Bolatkhan, K.; Sidorov, R.A.; Mironov, K.S.; Skrypnik, A.N.; Kupriyanova, E.V.; Zayadan, B.K.; Shumskaya, M.; Los, D.A. Polyphasic characterization of the thermotolerant cyanobacterium Desertifilum sp. strain IPPAS B-1220. FEMS Microbiol. Lett. 2017, 364, fnx027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mironov, K.S.; Sinetova, M.A.; Bolatkhan, K.; Zayadan, B.K.; Ustinova, V.V.; Kupriyanova, E.V.; Skrypnik, A.N.; Gogoleva, N.E.; Gogolev, Y.V.; Los, D.A. Draft genome sequence of the thermotolerant cyanobacterium Desertifilum sp. IPPAS B-1220. Genome Announc. 2016, 4, e01304-16. [Google Scholar] [CrossRef] [Green Version]
- Samylina, O.S.; Sinetova, M.A.; Kupriyanova, E.V.; Starikov, A.Y.; Sukhacheva, M.V.; Dziuba, M.V.; Tourova, T.P. Ecology and biogeography of the ‘marine Geitlerinema’ cluster and a description of Sodalinema orleanskyi sp. nov., Sodalinema gerasimenkoae sp. nov., Sodalinema stali sp. nov. and Baaleninema simplex gen. et sp. nov. (Oscillatoriales, Cyanobacteria). FEMS Microbiol. Ecol. 2021, 97, fiab104. [Google Scholar] [CrossRef]
- Zavřel, T.; Sinetova, M.A.; Búzová, D.; Literáková, P.; Červený, J. Characterization of a model cyanobacterium Synechocystis sp. PCC 6803 autotrophic growth in a flat-panel photobioreactor. Eng. Life Sci. 2015, 15, 122–132. [Google Scholar] [CrossRef]
- Zavřel, T.; Faizi, M.; Loureiro, C.; Poschmann, G.; Stühler, K.; Sinetova, M.; Zorina, A.; Steuer, R.; Červený, J. Quantitative insights into the cyanobacterial cell economy. Elife 2019, 8, e42508. [Google Scholar] [CrossRef]
- Sinetova, M.A.; Sidorov, R.A.; Medvedeva, A.A.; Starikov, A.Y.; Markelova, A.G.; Allakhverdiev, S.I.; Los, D.A. Effect of salt stress on physiological parameters of microalgae Vischeria punctata strain IPPAS H-242, a superproducer of eicosapentaenoic acid. J. Biotechnol. 2021, 331, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Mironov, K.S.; Sinetova, M.A.; Kupriyanova, E.V.; Ustinova, V.V.; Kozlova, A.Y.; Messineva, E.M.; Gabrielyan, D.A.; Bedbenov, V.S.; Zayadan, B.K.; Los, D.A. Draft genome sequences of two thermotolerant cyanobacterial strains isolated from hot springs. Genome Announc. 2018, 6, e01548-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mironov, K.S.; Leusenko, P.A.; Ustinova, V.V.; Bolatkhan, K.; Zayadan, B.K.; Kupriyanova, E.V.; Shumskaya, M.; Sinetova, M.A.; Los, D.A. Draft genome sequences of a putative prokaryotic consortium (IPPAS B-1204) consisting of a cyanobacterium (Leptolyngbya Sp.) and an alphaproteobacterium (Porphyrobacter sp.). Microbiol. Resour. Announc. 2019, 8, e01637-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Los, D.; Mironov, K. Modes of fatty acid desaturation in cyanobacteria: An update. Life 2015, 5, 554–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starikov, A.Y.; Sidorov, R.A.; Mironov, K.S.; Goriainov, S.V.; Los, D.A. Delta or omega? Δ12 (ω6) fatty acid desaturases count 3C after the pre-existing double bond. Biochimie 2020, 179, 46–53. [Google Scholar] [CrossRef]
- Amiri, R.M.; Yur’eva, N.O.; Shimshilashvili, K.R.; Goldenkova-Pavlova, I.V.; Pchelkin, V.P.; Kuznitsova, E.I.; Tsydendambaev, V.D.; Trunova, T.I.; Los, D.A.; Jouzani, G.S.; et al. Expression of acyl-lipid Δ12-desaturase gene in prokaryotic and eukaryotic cells and its effect on cold stress tolerance of potato. J. Integr. Plant Biol. 2010, 52, 289–297. [Google Scholar] [CrossRef]
- González-Resendiz, L.; Johansen, J.R.; León-Tejera, H.; Sánchez, L.; Segal-Kischinevzky, C.; Escobar-Sánchez, V.; Morales, M. A bridge too far in naming species: A total evidence approach does not support recognition of four species in Desertifilum (Cyanobacteria). J. Phycol. 2019, 55, 898–911. [Google Scholar] [CrossRef]
- Krivina, E.; Sinetova, M.; Savchenko, T.; Degtyaryov, E.; Tebina, E.; Temraleeva, A. Micractinium lacustre and M. thermotolerans spp. nov. (Trebouxiophyceae, Chlorophyta): Taxonomy, temperature-dependent growth, photosynthetic characteristics and fatty acid composition. Algal Res. 2023, 71, 103042. [Google Scholar] [CrossRef]
- Lorenz, M.; Friedl, T.; Day, J.G. Perpetual maintenance of actively metabolizing microalgal cultures. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elsevier Academic Press: London, UK, 2005; pp. 145–156. [Google Scholar]
- Kan, Y.; Pan, J. A one-shot solution to bacterial and fungal contamination in the green alga Chlamydomonas reinhardtii culture by using an antibiotic cocktail. J. Phycol. 2010, 46, 1356–1358. [Google Scholar] [CrossRef]
- Day, J.G.; Brand, J.J. Cryopreservation methods for maintaining cultures. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elsevier Academic Press: London, UK, 2005; pp. 165–187. [Google Scholar]
- Day, J.G.; Benson, E.E.; Harding, K.; Knowles, B.; Idowu, M.; Bremner, D.; Santos, L.; Santos, F.; Friedl, T.; Lorenz, M.; et al. Cryopreservation and Conservation of Microalgae: The Development of a Pan-European Scientific and Biotechnological Resource (the COBRA Project). Cryo Lett. 2005, 26, 231–238. [Google Scholar]
- Müller, J.; Friedl, T.; Hepperle, D.; Lorenz, M.; Day, J.G. Distinction between mutliple isolates of Chlorella vulgaris (Chlorophyta, Trebouxiophyceae) and testing for conspecificity using amplified fragment length polymorphism and ITS RDNA sequences. J. Phycol. 2005, 41, 1236–1247. [Google Scholar] [CrossRef]
- Muller, J.; Day, J.G.; Harding, K.; Hepperle, D.; Lorenz, M.; Friedl, T. Assessing genetic stability of a range of terrestrial microalgae after cryopreservation using amplified fragment length polymorphism (AFLP). Am. J. Bot. 2007, 94, 799–808. [Google Scholar] [CrossRef]
- Krivokharchenko, A.S.; Chernyak, N.D.; Nosov, A.M. Cryopreservation of suspension cultures of plant cells by the freezing technique elaborated for mammalian embryos. Russ. J. Plant Physiol. 1999, 46, 831–834. [Google Scholar]
- Temraleeva, A. Validation of Spongiosarcinopsis terrestris gen. et sp. nov. (Protosiphonaceae, Chlorophyta). Not. Algarum 2019, 98, 1–2. [Google Scholar]
- Krivina, E.; Temraleeva, A.; Sinetova, M. New species Micractinium kostikovii (Chlorellaceae, Trebouxiophyceae) from Russia. Phycol. Res. 2022, 70, 22–34. [Google Scholar] [CrossRef]
- Temraleeva, A.; Krivina, E.; Boldina, O. Edaphochloris, gen. nov.: A new genus of soil green algae (Trebouxiophyceae, Chlorophyta) with simple morphology. Plant Syst. Evol. 2022, 308, 4. [Google Scholar] [CrossRef]
- Krivina, E.S.; Boldina, O.N.; Bukin, Y.S.; Bykova, S.V.; Temraleeva, A.D. Species Delimitation Polyphasic Approach Reveals Meyerella similis sp. nov.: A new species of “small green balls” within the Chlorella-clade (Trebouxiophyceae, Chlorophyta). Org. Divers. Evol. 2023, 23, 25–40. [Google Scholar] [CrossRef]
- Iwamoto, K.; Fukuyo, S.; Okuda, M.; Kobayashi, M.; Shiraiwa, Y. Cryopreservation of the chlorophyll D-containing cyanobacterium Acaryochloris marina. Procedia Environ. Sci. 2012, 15, 118–125. [Google Scholar] [CrossRef] [Green Version]














| Subset | Cultivars | Provider of the Original Material | No. of Lines | Foreigner Genes Expressed | Use | Reference |
|---|---|---|---|---|---|---|
| Non-transformed | ||||||
| 1 | Cultivated Russian varieties, haploid inducer line, dihaploids and foreign commercial cultivars | Russian Potato Research Centre (Russia), Institute of Genetics and Cytology NASB (Belarus) | 46 | No | Initial material for transgenic plant production | [108] |
| Transformed | ||||||
| 2.1. | cv. Skoroplodny, cv. Jucovsky Ranny | Russian Potato Research Centre | 9 | hGFP reporter gene | Express-test for transgenic plant identification | [109] |
| 2.2. | cv. Udacha, cv. Jucovsky Ranny | Russian Potato Research Institute | 12 | Pro-SmAMP1 gene | Breeding programs: early blight resistance | [110] |
| 2.3. | cv. Udacha, cv. Niculinsky | Russian Potato Research Centre | 9 | NPT gene | Controls in experimental studies | no |
| 2.4. | cv. Skoroplodny | Russian Potato Research Centre | 36 | NsD3 (from Nigella sativa L.), a defensin-encoding gene | Blackleg resistance studies | [111] |
| cv. Jucovsky Ranny (early blight susceptible) | Russian Potato Research Centre | 6 | NsD3 (from Nigella sativa), a defensin-encoding gene | Early blight resistance studies | [112] | |
| cv. Sarpo mira | Russian Potato Research Centre | 42 | NsD3 (from Nigella sativa), a defensin-encoding gene | Early blight resistance studies | [112] | |
| 2.5. | T1 hybrids | Institute of Genetics and Cytology NASB | 42 | NsD3 (from Nigella sativa), a defensin-encoding gene | Breeding programs: early blight resistance | no |
| Taxon | Material Type | Method of Cryopreservation | Period of Storage | Viability/Regrowth after Cryopreservation | Traits Evaluated | Reference |
|---|---|---|---|---|---|---|
| Medicago sativa | Cell culture | PF | 27 years | 20% | The growth index, mitotic index, and peroxidase activity were fully restored | [134] |
| Polyscias filicifolia | Cell culture | PF | 5 years | 40% | Growth parameters in 20-L, 75-L, and 630-L bioreactors were fully restored | [70] |
| Rhaponticum carthamoides and Thalictrum minus, two strains | Cell culture | PF | Several months | 60–80% | The growth index and protoberberine content were fully restored | [157] |
| Dioscorea deltoidea | Cell culture | PF | Several months | 30% | The growth index and production of diosgenin, sitosterol, and stigmasterol were fully recovered | [133] |
| Orchidaceae, 130 species | Seeds | Fast-freezing | Several days to several years | 0–100% | In vitro germination | [135,144,146,147,148,158,159] |
| Asparagaceae, Campanulaceae, Ericaceae, Iridaceae, Fabaceae, Liliaceae, Melanthiaceae, Poaceae | Seeds | Fast-freezing and PF | Several days to 30 years | 0–100% | In vitro and in soil germination | [135,143] |
| Cactaceae | Seeds | Fast-freezing | 22 days | 23–81% | In soil germination | [142] |
| Fragaria, 28 cultivars | In vitro shoot meristems | PF | 1 h to 36 months | 0–100% | In vitro recovery | [140] |
| Fragaria, 50 cultivars | In vitro shoot meristems | Fast-freezing | 5 to 10 years | 75–100% | In vitro recovery; stability confirmed by RAPD, REMAP, and ISSR analyses | [135,150,151,152,153,154] |
| Rubus, two cultivars | In vitro shoot meristems | Fast-freezing and PF | >1 h | 10–86% | In vitro recovery | [141] |
| Sorbus, two cultivars | In vitro shoot meristems | Fast-freezing | >1 h | 79–83% | In vitro recovery | [153] |
| Rubus, four mericlones | In vitro shoot meristems | Fast-freezing | >1 h | 63–85% | In vitro recovery | [153] |
| Syringa, two cultivars | In vitro shoot meristems | Fast-freezing | Several weeks | 83–84% | In vitro recovery | [145] |
| Total Number of Strains | 430 |
|---|---|
| Identified to family level | 27 |
| Identified to genus level | 403 |
| Identified to species level | 236 |
| Total number of genera | 91 |
| Total number of species | 106 |
| Strain | Year Strain Isolated/Received by Collection | Characteristics |
|---|---|---|
| Chlorella sorokiniana IPPAS C-1 | 1961/1961 | Unicellular coccoid green microalgae with robust growth characteristics; thermotolerant [187]; used in studies on endogenous regulation of photosynthesis and metabolism [188,189,190,191,192,193,194,195]; under stressed conditions, it accumulates mainly starch [191,192]; acts as a reference strain for biotechnological applications [175,188,189,196,197,198]. |
| Chlorella vulgaris IPPAS C-2 | n.a./1957 | Unicellular coccoid green microalgae with robust growth characteristics; under stressed conditions, it accumulates mainly lipids [192]; acts as a reference strain for biotechnological applications [172,192,194,199,200]. The world’s first culture that was exposed to space flight conditions on the second spacecraft satellite [201]. |
| Neochlorella semenenkoi Krivina, Temraleeva, Bobrovnikova and Sinetova IPPAS C-1210 | 2014/2014 | Unicellular coccoid green microalgae with robust growth characteristics; thermotolerant, halotolerant, and alkaliphilic; accumulates lipids enriched with α-linolenic FA [202,203]. |
| Crocosphaera subtropica IPPAS B-1603 Synonym: ‘Cyanothece sp.’ ATCC 51142 | 1992/2012 | Unicellular marine nitrogen-fixing cyanobacterium; a model organism for studying circadian and ultradian rhythms [204]; possesses highly active extracellular α-class carbonic anhydrase [181]. |
| Cyanobacterium sp. Rippka and Cohen-Bazire IPPAS B-1200 | 2013/2013 | A unicellular alkaliphilic cyanobacterium with a wide temperature optimum (24–34 °C); contains many short saturated and monounsaturated C14 and C16 FAs, which can be used for biofuels [176]; draft genome sequenced [205]; possesses a unique desaturase, DesC, that nonspecifically introduces double bonds in C14, C16, and C18 FAs [206]. |
| Desertifilum tharense IPPAS B-1220 | 2013/2013 | A filamentous thermophilic cyanobacterium with a wide temperature optimum (28–40 °C); contains many 16:2Δ7,10 FAs rarely found in cyanobacteria [207]; draft genome sequence available [208]. |
| Sodalinema gerasimenkoae IPPAS B-353 | 1996/1996 | A filamentous haloalkaliphilic cyanobacterium; reference strain for the species [209]; a model organism for studying CO2 -concentrating mechanisms [180]. |
| Synechocystis sp. IPPAS B-1400 | 1968/2005 | Strain PCC 6803 GT-L, a model cyanobacterium for studying mechanisms of intracellular regulation and stress responses of photosynthetic cells [182,183,184,185,186]; wild type for many regulatory mutants; was used for the development of a new method for the growth characterization and optimization [210] and for studying quantitative growth properties and resource allocation [211]. |
| Vischeria punctata IPPAS H-242 | 1941/1958 | A unicellular coccoid eustigmatophycean microalgae; rich in eicosapentaenoic FAs [202,212]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuorieva, N.; Sinetova, M.; Messineva, E.; Kulichenko, I.; Fomenkov, A.; Vysotskaya, O.; Osipova, E.; Baikalova, A.; Prudnikova, O.; Titova, M.; et al. Plants, Cells, Algae, and Cyanobacteria In Vitro and Cryobank Collections at the Institute of Plant Physiology, Russian Academy of Sciences—A Platform for Research and Production Center. Biology 2023, 12, 838. https://doi.org/10.3390/biology12060838
Yuorieva N, Sinetova M, Messineva E, Kulichenko I, Fomenkov A, Vysotskaya O, Osipova E, Baikalova A, Prudnikova O, Titova M, et al. Plants, Cells, Algae, and Cyanobacteria In Vitro and Cryobank Collections at the Institute of Plant Physiology, Russian Academy of Sciences—A Platform for Research and Production Center. Biology. 2023; 12(6):838. https://doi.org/10.3390/biology12060838
Chicago/Turabian StyleYuorieva, Natalya, Maria Sinetova, Ekaterina Messineva, Irina Kulichenko, Artem Fomenkov, Olga Vysotskaya, Ekaterina Osipova, Angela Baikalova, Olga Prudnikova, Maria Titova, and et al. 2023. "Plants, Cells, Algae, and Cyanobacteria In Vitro and Cryobank Collections at the Institute of Plant Physiology, Russian Academy of Sciences—A Platform for Research and Production Center" Biology 12, no. 6: 838. https://doi.org/10.3390/biology12060838
APA StyleYuorieva, N., Sinetova, M., Messineva, E., Kulichenko, I., Fomenkov, A., Vysotskaya, O., Osipova, E., Baikalova, A., Prudnikova, O., Titova, M., Nosov, A. V., & Popova, E. (2023). Plants, Cells, Algae, and Cyanobacteria In Vitro and Cryobank Collections at the Institute of Plant Physiology, Russian Academy of Sciences—A Platform for Research and Production Center. Biology, 12(6), 838. https://doi.org/10.3390/biology12060838









