Simple Summary
The landmark annotation of hindwing venation is one of the most important methods in the hindwing morphological, functional, and evolutionary analysis of beetles, and the number of the landmark samples greatly affects the effectiveness of these analysis. However, large-scale manual landmark annotation is a time-consuming task that hinders the progress of wing morphology research. Some machine learning techniques have been applied to beetle image recognition, but the lack of data for beetle hindwing landmarks limits the use of machine learning for beetle hindwing landmark detection. In this study, we propose a new approach to solve the problem of insufficient training samples for beetle hindwing landmark detection, by fine-tuning a new deep high-resolution convolutional neural network pretrained on a natural image database to transfer it to the domain of beetle hindwings. The results of experiments shows the effectiveness of this new approach as it demonstrated small error in the detection of leaf beetle hindwing landmarks and required a very low number of samples.
Abstract
Hindwing venation is one of the most important morphological features for the functional and evolutionary analysis of beetles, as it is one of the key features used for the analysis of beetle flight performance and the design of beetle-like flapping wing micro aerial vehicles. However, manual landmark annotation for hindwing morphological analysis is a time-consuming process hindering the development of wing morphology research. In this paper, we present a novel approach for the detection of landmarks on the hindwings of leaf beetles (Coleoptera, Chrysomelidae) using a limited number of samples. The proposed method entails the transfer of a pre-existing model, trained on a large natural image dataset, to the specific domain of leaf beetle hindwings. This is achieved by using a deep high-resolution network as the backbone. The low-stage network parameters are frozen, while the high-stage parameters are re-trained to construct a leaf beetle hindwing landmark detection model. A leaf beetle hindwing landmark dataset was constructed, and the network was trained on varying numbers of randomly selected hindwing samples. The results demonstrate that the average detection normalized mean error for specific landmarks of leaf beetle hindwings (100 samples) remains below 0.02 and only reached 0.045 when using a mere three samples for training. Comparative analyses reveal that the proposed approach out-performs a prevalently used method (i.e., a deep residual network). This study showcases the practicability of employing natural images—specifically, those in ImageNet—for the purpose of pre-training leaf beetle hindwing landmark detection models in particular, providing a promising approach for insect wing venation digitization.
1. Introduction
As one of the most vital functional organs of insects, with implications for insect flight, wings are considered a key factor in the evolutionary success of insects [1,2]. The wing morphology, such as the wing length, wing shape, tip shape, and radial sector shape, reflects the functional adaptation [3,4,5] and evolutionary history of insects [6,7]. Beetles are the most diverse group of insects, and both their forewing specialization and hindwing folding [8] are regarded as meaningful key morphological indicators that are indispensable in the analysis of wing morphology evolutionary patterns [9].
Wing landmark annotation based morphological methods have been widely used in studies on beetles, in terms of aspects such as phylogeny [10], evolution [11,12], ecology [13], biogeography [14], and flight deformation [15]. The amount of landmark data on the wing largely determines the effectiveness of the subsequent morphological analysis [16]. However, with the use of software for the manual annotation of landmarks (e.g., tpsDig [17]), obtaining digitized morphological landmark datasets from a large volume of insect wing pictures is highly time-consuming. Hence, it is imperative to incorporate machine learning technology in order to address the problem of detecting landmarks in a large amount of beetle hindwing images.
Machine learning has already been used in insect morphology research, mainly for species identification [18,19]. The deep residual network (ResNet), as a convolutional neural network (CNN) and also one of the most prevalent machine learning methods at present, can be used to identify species of beetles [20]. To date, few machine learning methods have been used for the landmark detection of insect wings [21,22], especially beetle hindwings. In addition, conventional network training methods currently cannot obtain effective models on small training datasets, given the lack of publicly available large-scale beetle hindwing landmark datasets.
To solve the problems outlined above, and to improve the efficiency of obtaining landmarks and reliability of the subsequent morphological analysis, we propose the combination of the deep high-resolution network (HRNet) and transfer learning to detect leaf beetle hindwing venation landmarks. HRNet, as a derived type of CNN with high resolution, performs well in terms of landmark detection [23], but is dependent on a training set with a large number of leaf beetle hindwing samples. Transfer learning, which has previously been applied for beetle classification [20,24], can compensate for the inadequacy of hindwing data pertaining to leaf beetles through the migration of pre-trained models based on a readily available dataset [25,26]. ImageNet, which is presently the largest database for natural image recognition worldwide, comprises over 14,000,000 images and 20,000 categories (accessed on 11 April 2023 via http://www.image-net.org) [27], and is commonly used for pre-training in transfer learning [28]. Through the process of fine-tuning, the HRNet model parameters pre-trained with ImageNet on leaf beetle hindwing landmark datasets, we tested the detection performance for 36 landmarks on the leaf beetle hindwing venation with an extremely restricted number of training samples. The model exhibited promising usability for hindwing landmark detection. The main innovations of this study are as follows: (1) We created a hindwing landmark dataset for the family Chrysomelidae (HWLFC); (2) We combine HRNet with transfer learning to detect leaf beetle hindwing landmarks automatically. This approach provides a novel perspective for better understanding of the evolution of beetle hindwings.
2. Materials and Methods
2.1. Hardware and Software Environment
For the present investigation, we utilized Python 3.8.10 (developed by the Python Software Foundation; Beaverton, OR, USA) and PyTorch 1.12 (developed by Facebook, Inc.; Menlo Park, CA, USA) as foundational programming languages for both training and testing procedures. We employed the Adam algorithm [29] to optimize the network parameters, along with the learning schedule described in Ref. [30]. The base learning rate is set to 0.0001 [31], and is dropped to 0.00001 and 1 × 10 at the 40th and 55th epochs, respectively. The batch size was set to 8 and the training process was terminated within 80 epochs. We ran it on a graphics workstation with the Ubuntu 20.04.4 LTS OS, an AMD Ryzen Threadripper 2990WX 32-Core Processor, 128 GB RAM, and an NVIDIA GeForce RTX 2080Ti GPU. The versions of CUDA and cuDNN (both developed by the NVIDIA Corporation; Santa Clara, CA, USA) were 11.6 and 8.3.2, respectively.
2.2. Image and Dataset Preparation
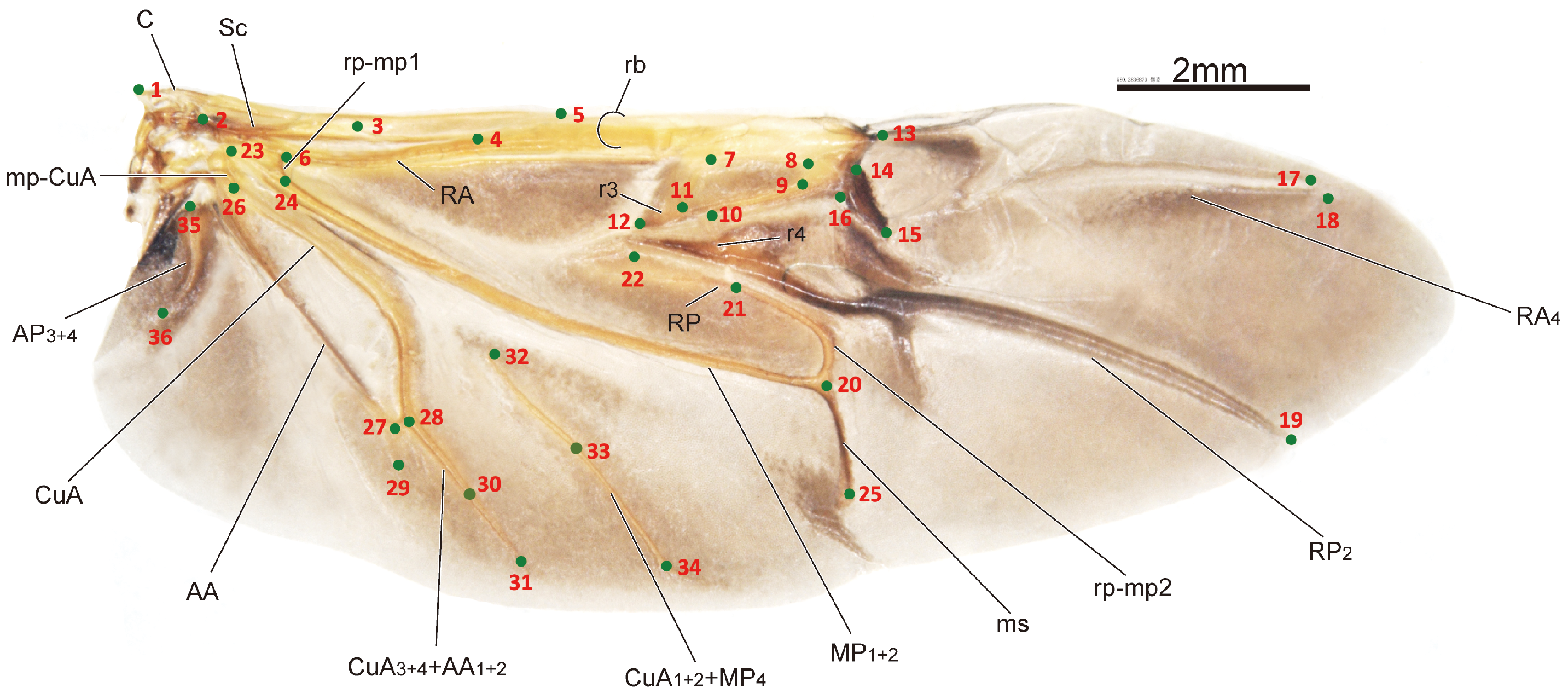
The sample processing and imaging of the leaf beetle hindwings used in this study have been described in detail in one of our previous papers [32]. Briefly, the hindwings of the specimens were obtained through examination and dissection using a LEICA MZ 12.5 dissecting microscope. Subsequently, images of the hindwings were captured using a Nikon D500s camera connected to a Zeiss Stereo Discovery V12 stereoscope. The HWLFC dataset contains images of 256 leaf beetle hindwings, representing 16 subfamilies and 231 genera. The principle of landmark selection is based on key points in the hindwing vein distribution, such as intersections, bases, and ends or beginnings of hindwing veins. The HWLFC dataset includes the same 36 landmarks (Figure 1, Table 1), and the annotation data are formatted according to the COCO dataset [33].
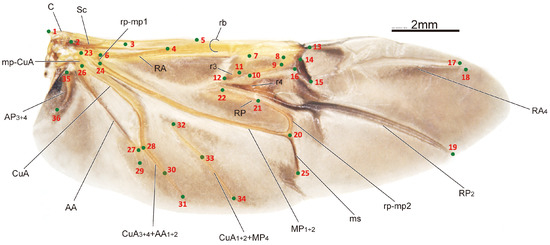
Figure 1.
The distribution of the hindwing landmarks and the names of the hindwing veins for the leaf beetle (Potaninia assamensis).

Table 1.
Basic information about the 36 landmarks of leaf beetle hindwings.
The annotated dataset consists of two parts: Image information and annotation information of landmarks. The image information includes the index (denoted as “id”), file path (denoted as “file_name”), width (denoted as “width”), and height (denoted as “height”) of each image. The annotation information of landmarks contains several fields. The landmark coordinate array (denoted as “Keypoints”) has a length of , where k is the number of landmarks on a leaf beetle hindwing. Each group of three components corresponds to a single landmark, with the first two components representing the x and y coordinates, respectively, and the third component representing a visibility flag bit (v) which is not used in HWLFC as all landmarks are visible. The landmark number (denoted as “num_keypoints”) indicates the total number of landmarks for a leaf beetle hindwing. The bounding box of the hindwing (denoted as “Bbox”) denotes the location of the hindwing in the image, with the first two components representing the upper-left coordinates of the bounding box and the last two components representing the width and height of the bounding box [33].
The images are in TIFF format and have a size of 4288 × 2848 pixels. The coordinates of the landmarks in the measurements take the lower left corner of the image as the origin, with the image height subtracted from the y coordinate of the feature point to obtain the coordinates of the annotation data, taking the top left corner as the origin. The value “num_keypoints” is equal to 36, and “Keypoints” is an array of length 108. “Bbox” represents the coordinates of the top left corner and the width and height of the bounding box in the image, with the distance from landmark 1 to landmark 18 used as the normalized length.
2.3. Network Architecture
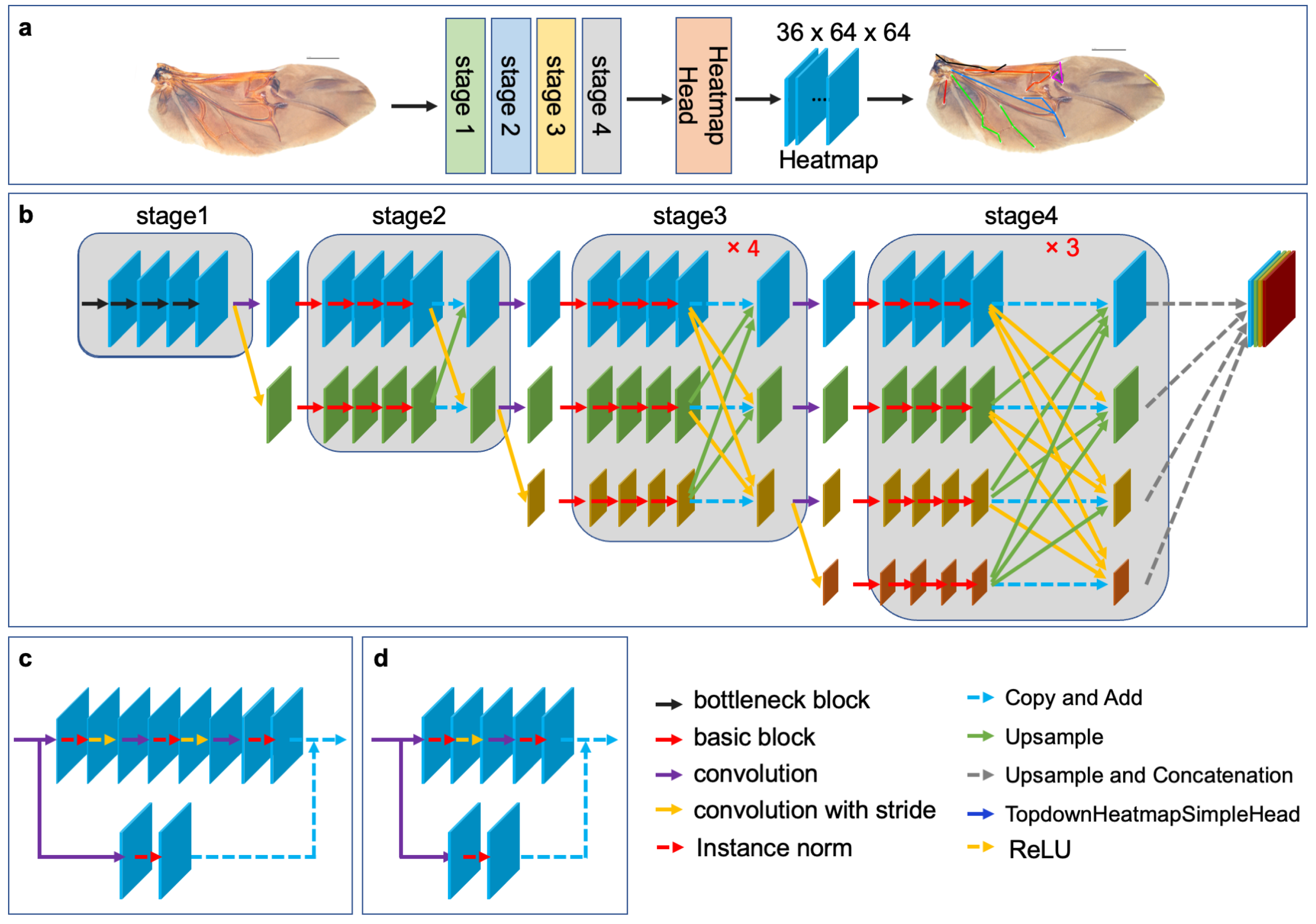
In this study, HRNet is used to learn a rich representation of wing morphology and improve the accuracy of hindwing landmark detection. The architecture of HRNet, as shown in Figure 2b, comprises four stages. Stage 1 consists of a high-resolution convolution branch, while subsequent stages add low-resolution convolution branches at the corresponding levels. Additionally, the different resolution convolution branches are parallel to each other, in order to achieve multi-resolution convolution. Stage 1 is composed of four consecutive bottleneck modules (Figure 2d), while Stages 2, 3, and 4 are composed of consecutive basic modules (Figure 2c). A fusion module is included after every four basic modules, in order to achieve multiresolution feature fusion. Both the bottleneck and basic modules are residual units, with residual connections consisting of a convolution with a kernel size of 1 × 1. The forward connection of the bottleneck module contains three convolutional layers with configurations (1 × 1, 1, 0), (3 × 3, 1, 1), and (1 × 1, 1, 0). The number of channels is first reduced and then enlarged. The forward connection of the basic module contains two convolution layers with configuration (3 × 3, 1, 1), and the number of channels remains constant. To facilitate information exchange between multi-resolution representations, Stages 2, 3, and 4 re-use the fusion module to obtain one, four, and three feature fusions, respectively. HRNet is followed by a representation head that concatenates the three low-resolution features into a high-resolution representation through up-sampling. The output of the representation head is then transferred to the top-down heatmap head to generate the predicted heatmap of the landmarks. The dimensions of HeatmapHead are (B, C, H, W), where B is the number of samples in the batch, C is the number of channels (which corresponds to the number of landmarks), and H and W are the height and width of the heatmap, respectively. For the hindwing venation of leaf beetles, C is equal to 36, while H and W are both equal to 64.
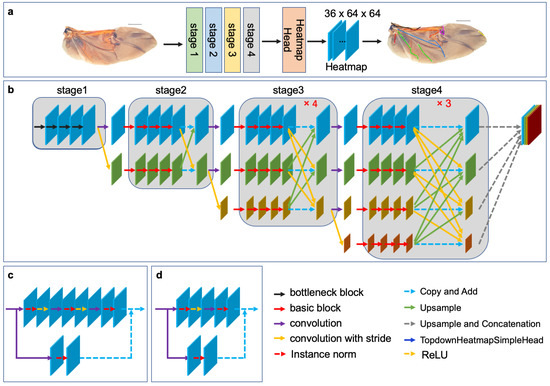
Figure 2.
The architecture of HRNet: (a) Leaf beetle hindwing landmark detection model based on transfer learning and HRNet; (b) the overall structure of HRNet, which consists of four stages. Each stage adds a low-resolution feature channel, the input resolution channels are processed in parallel, the resolution features are combined at the end of each stage, and four feature maps with different resolutions are the output; (c) the basic block; and (d) the bottleneck block.
For this study, we applied the HRNet-w18 model pre-trained on ImageNet [27,34] and developed a model for detecting landmarks on leaf beetle hindwings through parameter transfer. Specifically, we preserved the network structure and parameters of certain stages, while re-training the network structure of the remaining stages to optimize the parameters using a limited leaf beetle hindwing landmark dataset [35,36]. We implemented three distinct strategies to fine-tune the parameters of the HRNet model, as illustrated in Table 2.

Table 2.
Three strategies to fine-tune the parameters of the HRNet model.
In the training phase, the pretrained weights obtained from the natural image dataset were loaded into the model. The model was then trained on the leaf beetle hindwing landmark dataset. In particular, the pre-training parameters of HRNet obtained from ImageNet were loaded into the backbone network, while the parameters of the heatmap head network were initialized with random values. The hindwing images were loaded into HRNet, generating four feature maps with high to low resolution. The representation head fused these four features of different resolutions into a high-resolution representation through up-sampling and convolution, and the fused representation was input into the heatmap head to obtain the predicted heatmap of landmarks. Mean Squared Error (MSE) loss was then computed using the predicted heatmap and the ground truth heatmap.
3. Results
The test set was created by randomly selecting 80 samples from HWLFC, each of which included an image of a leaf beetle hindwing and its corresponding landmark annotation. To ensure the repeatability of the experiment and evaluate the performance of the model on training sets of varying size, the remaining samples were used to construct 10 groups of training sets, each containing a specific number (i.e., 1, 3, 5, 10, 50, 100) of randomly selected samples. To evaluate the performance of the model, we applied the Normalized Mean Error (NME) metric [37], which is a commonly used evaluation metric in landmark detection tasks [38]. The NME is defined as follows:
where P and represent the predicted and true coordinates of the landmarks, respectively; is the number of landmarks, which is 36 in the hindwing of a leaf beetle; and d is the reference distance used to normalize the absolute error. The NME metric measures the average Euclidean distance between the predicted and true landmarks, normalized by the reference distance. We used the distance between the proximal anterior point of the humeral plate (Landmark 1) and the distal point of Radius Anterior1 (Landmark 18) as the reference distance for HWLFC. This metric provides a measure of the accuracy of the model’s predictions and allows for quantitative comparison of the performance of different models.
3.1. Performance of Transfer Strategies
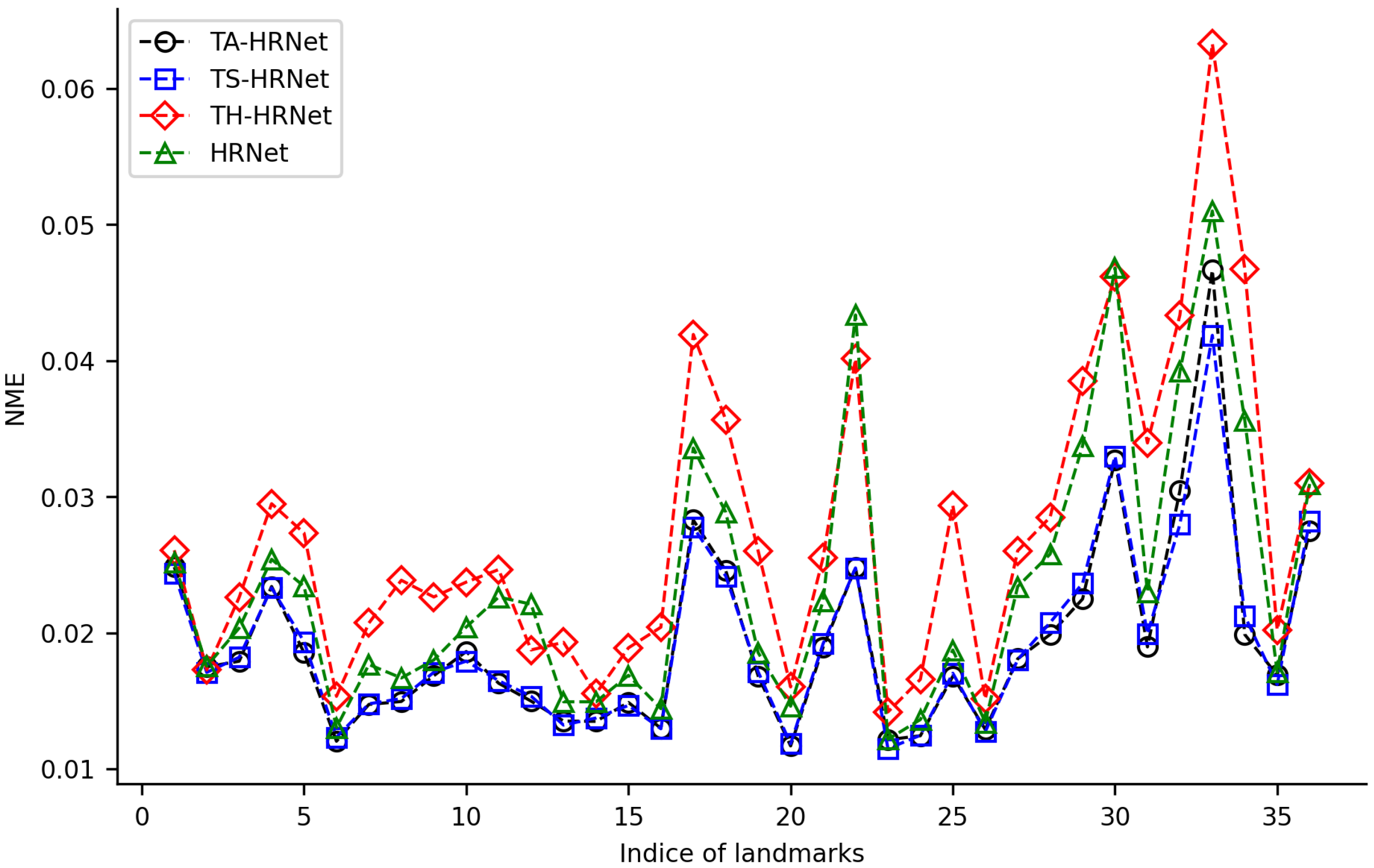
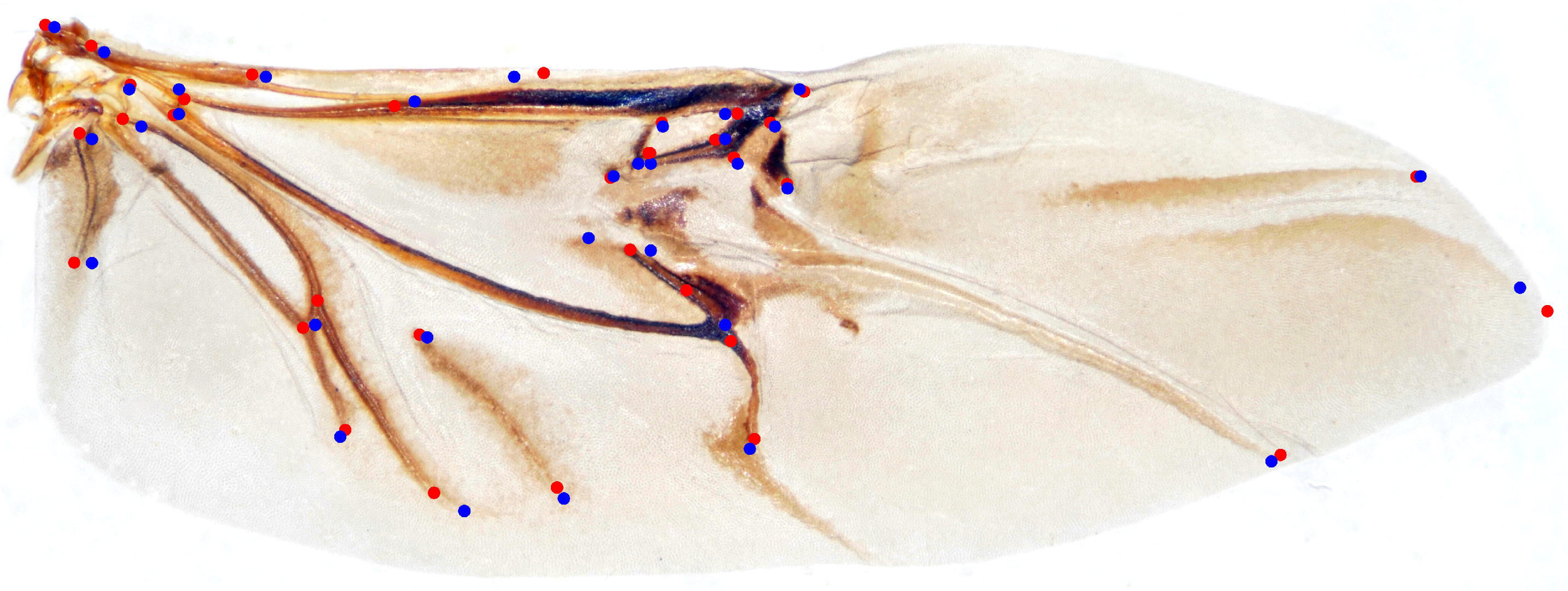
We randomly selected 100 samples from HWLFC as the training set, then compared the performance differences between the initial HRNet model and three HRNet models with different transfer strategies (TH-HRNet, TS-HRNet, and TA-HRNet in Table 2) using the unique test set (Figure 3 and Table S1 in the Supplementary Materials). The manually annotated (red) and TS-HRNet-predicted (blue) landmarks on a leaf beetle hindwing (Phratora bicolor) are shown in Figure 4. Only the results with the optimal strategy of TS-HRNet is detailed in Table S1, in which Stage 1 is retained with parameters as a general feature layer, while Stage 2, Stage 3, and Stage 4 are re-trained with parameters as specific feature layers. The test results for the other strategies are shown in Table S2.
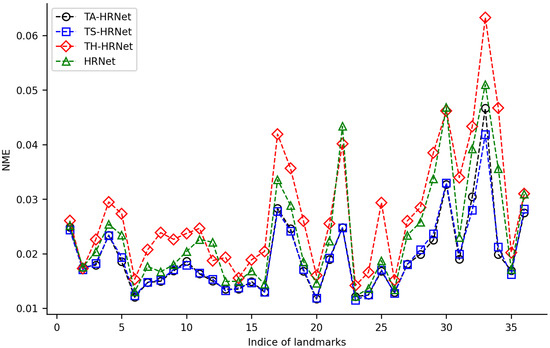
Figure 3.
NME changes for 36 landmarks on leaf beetle hindwings using 4 training methods.
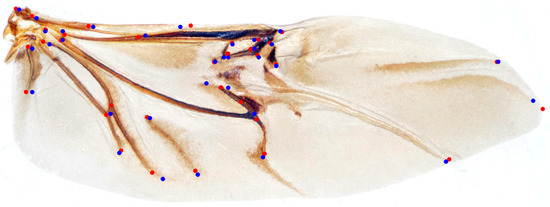
Figure 4.
The manually annotated (red) and predicted (blue) landmarks on a leaf beetle hindwing (Phratora bicolor).
Our experiments show that applying transfer strategies can effectively improve the performance of CNNs, and the average NME values for TS-HRNet and TA-HRNet were better than those of HRNet (Table S1). Among the three transfer strategies, TS-HRNet performed the best, in terms of the NME for each hindwing landmark (0.0115–0.0419; average 0.0193), while TA-HRNet presented evident fluctuations depending on the hindwing landmark, and TH-HRNet had relatively high NME (see Figure 3). In summary, TS-HRNet obtained the best NME and stability in the landmark detection tasks covering different landmarks on the hindwings of leaf beetle. Therefore, in subsequent experiments, we utilized TS-HRNet to test the parameter fine-tuning strategy.
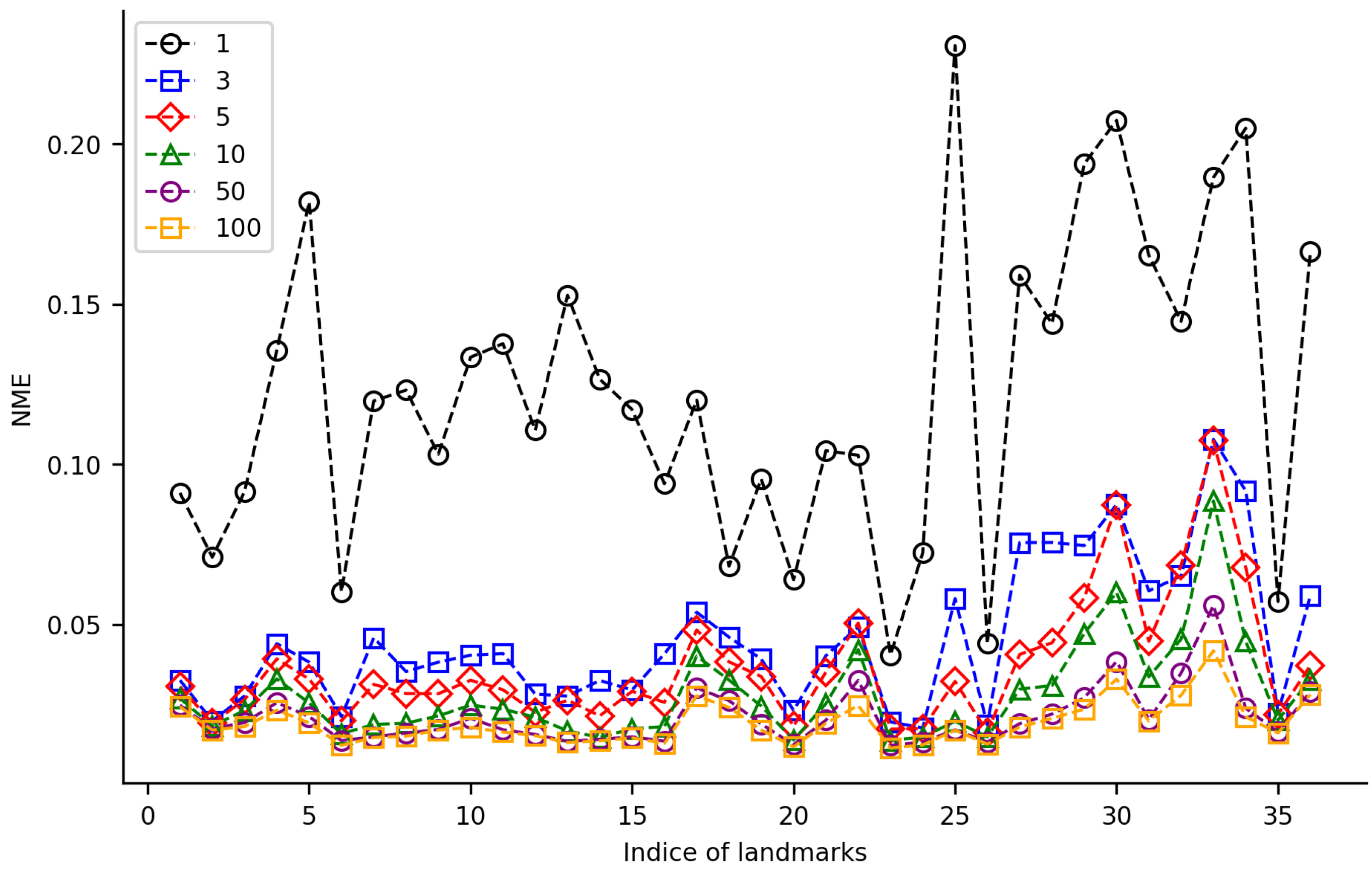
3.2. TS-HRNet Performance on Training Datasets of Varied Sizes
For the TS-HRNet model, we constructed different training datasets with 1, 3, 5, 10, 50, and 100 samples from HWLFC, then tested the impact of the training dataset size on the landmark detection performance (Figure 5, Table S3). When the training dataset contained 50 or more samples, the average NME of TS-HRNet was less than 0.022. Almost all landmarks were detected accurately in the test images, with only a slight fluctuation in NME (0.0213 ± 0.0091). When the size of the training dataset was reduced to three samples, the NME of most landmarks remained at a low level (<0.046). Nine specific landmarks—namely, 25, 27, 28, 29, 30, 31, 32, 33, and 36—led to an evident reduction in detection efficacy, although the normalized mean error (NME) remained below 0.110. When there was only one sample in the training dataset, the NME of all landmarks increased significantly (average NME was 0.123). As the size of the training dataset increased, there was a notable decrease in the NME of certain landmarks, such as the posterior of medial spur (Landmark 25), whose NME decreased from 0.23 to 0.02 as the size of the training dataset increased from 1 to 10.
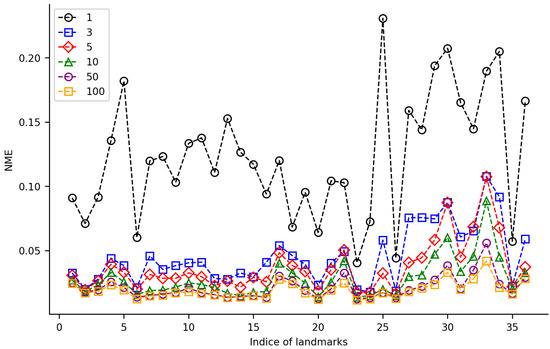
Figure 5.
NME values for 36 landmarks on leaf beetle hindwings using TS-HRNet on 6 datasets of varying size.
3.3. Performance Comparison of TS-HRNet with TS-ResNet
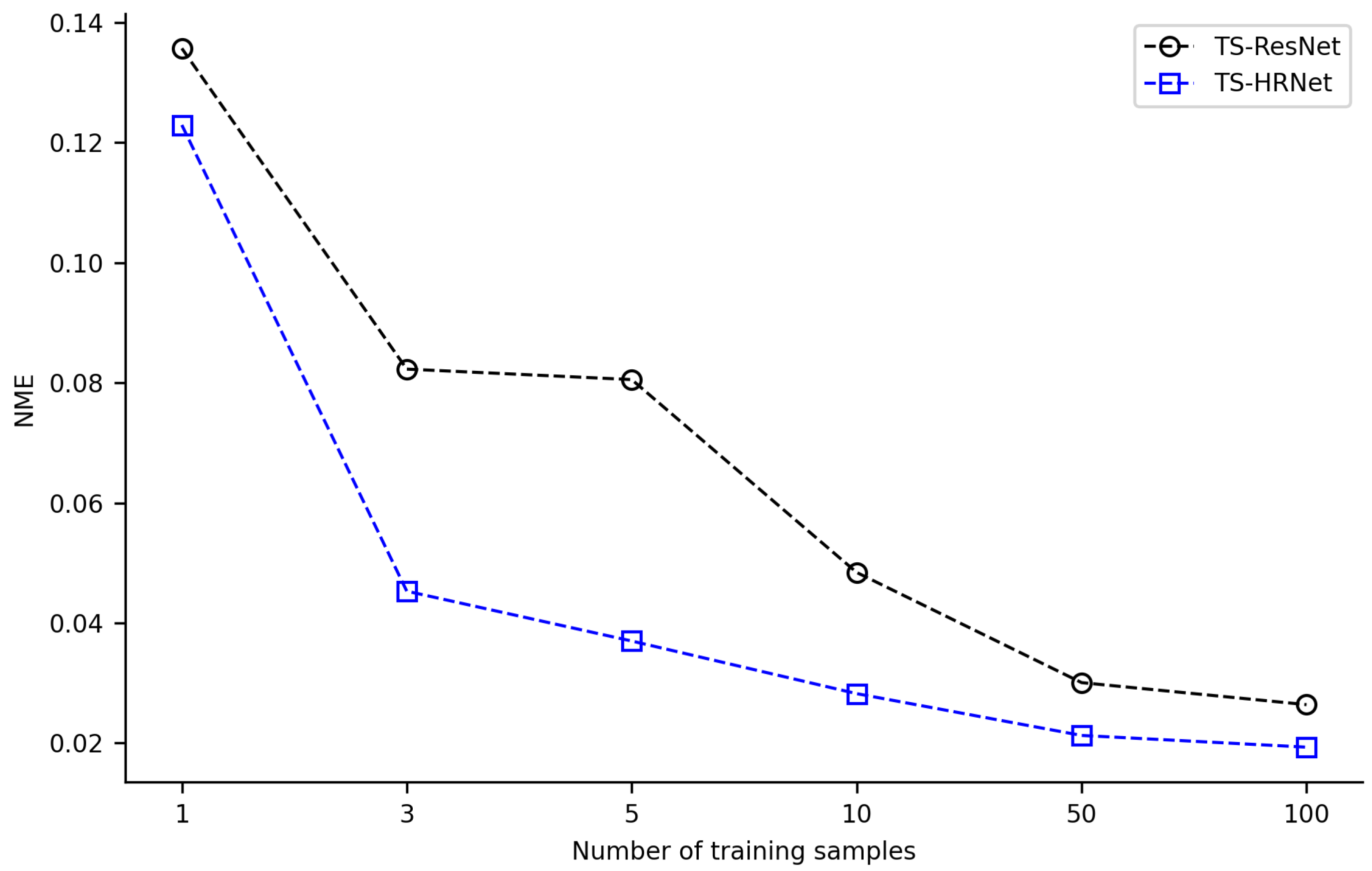
In order to verify the performance advantage of TS-HRNet in detecting multiple landmarks on leaf beetle hindwings under small-sample conditions, we conducted an ablation experiment on TS-HRNet with the baseline deep learning method as ResNet. ResNet has been widely used in deep learning research and is well-suited for facilitating transfer learning [31]. Utilizing the ResNet model pre-trained on ImageNet, we obtained the corresponding TS-ResNet by using the identical transfer strategy, parameter configuration, and training and testing datasets as with TS-HRNet, and then recorded the testing results.
The combination of transfer learning with TS-ResNet and TS-HRNet was effective in scenarios with few training samples (1 or 3). Acceptable detection NME (<0.03) could be achieved with only a few training samples, where TS-ResNet required about 50 samples, while TS-HRNet required only 10 samples (Figure 6, Supplementary Tables S3 and S4). By expanding the training dataset to over 100 images, the models detected landmarks with very low NME (TS-ResNet: 0.0264, TS-HRNet: 0.0193). Across all scenarios with varying numbers of training samples, TS-HRNet outperformed TS-ResNet in terms of NME.
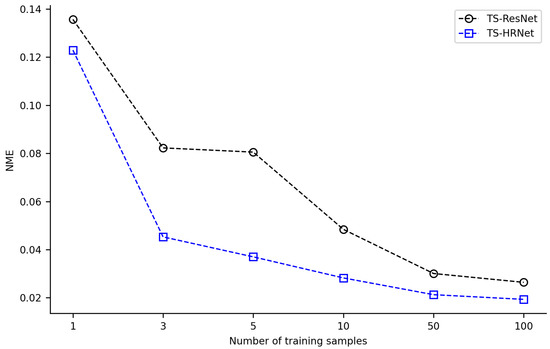
Figure 6.
Average NME of TS-HRNet and TS-ResNet under different numbers of training samples.
4. Discussion
4.1. Entomological Significance of Wing Landmark Detection
The landmark detection of leaf beetle hindwings is important for better understanding the evolution of beetles. Beetle hindwing venation is one of the most important features, in terms of evolution and diversity [15,39], and wing landmarks reflect changes in wing structure and the morphological evolution of beetles and other pterygote insects [40]. For example, the evolution of the forewing shape and size is independent of hindwing shape and size in Papilio butterflies, which is reflected by wing landmark data [16]. The variation in wing venation reflects the diversity of beetles [40,41]; for instance, differences in the number and distribution of wing veins are closely related to flight, and feeding habits [42,43]. In addition, wing venation landmarks can be used to analyze the geographical distribution and evolutionary history of insects [7,9]. Differences in wing venation between populations or species reflect their adaptation to various environments. By comparing the wing venation landmarks of different populations or species, their evolutionary relationships and habitat preferences can be analyzed [12].
The distribution of beetle hindwing venation is a key feature for analyzing beetle flight performance, which is important for designing beetle-inspired micro aerial vehicles [44,45]. Hindwing landmarks can provide important information about the structural and functional features of beetle hindwings related to flight performance [12]; for example, the number and position of veins can affect the stiffness and elasticity of the hindwing, which consequently affects the lift and maneuverability of beetles [46,47]. Furthermore, the shape and size of hindwing veins can affect the aerodynamic performance parameters of the hindwings [48], such as drag and lift coefficients [49]. Therefore, by analyzing the hindwing landmarks of different beetle species, we can gain insights into the relationships between wing structure and flight performance, and may identify the key parameters for optimizing the flight performance of beetle-like flapping wing micro aerial vehicle [45,50].
The study of landmarks, especially the joints of wing veins, is the premise of folding mechanisms study and deployable wing design. By automatically detecting the landmarks of hindwing veins with the proposed approach, designing linkage mechanisms by simulating insect veins becomes a feasible solution. A rigid and flexible coupled bionic deployable wing may use linkage mechanisms as artificial veins according to the detected locations of joints to achieve flapping wing folding/unfolding and vein joints rotating [44]. Coordinates of landmarks can be obtained quickly with the proposed approach, and discriminant space of landmark-based wing venation geometric morphometrics can be used to distinguish morphologically similar species to explore intraspecific variation among populations and determine sexual dimorphism in various insects [3]. It can also be used to identify damaged specimens faster than traditional morphological methods and is less expensive than molecular methods [4].
4.2. Improving Landmark Detection Models with Transfer Learning
In the computer vision field, deep learning typically requires a large amount of data for training in order to extract useful features from images. When the training dataset is too small, the model may fail to learn landmark features sufficiently, resulting in poor generalization to new datasets and a decrease in performance [51]. Similarly, the performance of a landmark detection model is tightly linked to the size of the dataset; however, for certain tasks such as leaf beetle hindwing landmark detection, the dataset may be very limited, making it difficult to train an accurate model.
Transfer learning is a method that is commonly used to address this problem [20], which involves using a pre-trained model as a starting point and fine-tuning it on a small target task dataset [52]. By enabling the model to maintain its previous knowledge base while learning new feature space, transfer learning provides an effective solution to address dataset scarcity [53]. Ideally, leaf beetle landmark data would be the best pre-training dataset in the present context, however, there are currently no publicly available datasets for leaf beetle hindwing landmarks. ImageNet, which has been widely used in the computer vision field as a publicly available large-scale image data set, has been shown to be effective in pre-training deep learning models for different tasks [54]. The combination of the ImageNet dataset and transfer learning has already shown great potential for beetle species recognition [20,24] and can therefore be used as a pre-training dataset for beetle hindwing landmark detection.
In this study, three different transfer learning models were used to improve the landmark detection performance of leaf beetle hindwings. The results showed that two of the models achieved an average detection error less than 0.02 on the 36 leaf beetle hindwing landmarks (Figure 3), indicating a significant improvement in detection performance. This suggests that transfer learning is particularly useful with a limited dataset, as it allows the model to leverage pre-trained features from other tasks to improve its performance. Through transfer learning the low-level features trained on natural images are also effective for leaf beetle hindwing images. In contrast, models without transfer learning may not be able to adjust all model parameters on limited data and thus may not leverage pre-trained models from other tasks for low-level feature re-usage, leading to sub-optimal weights and poor performance. Therefore, the combination of transfer learning and the HRNet model for leaf beetle hindwing landmark detection presents significant advantages over more traditional approaches. In summary, our results demonstrate that transfer learning can greatly improve the detection performance of CNNs with a small amount of training data for hindwing landmarks, both across domains (i.e., from natural images to leaf beetle hindwing images) and tasks (i.e., from classification to landmark detection). This technology may even be applied to various other insect groups.
There are potential challenges related to domain adaptation and dataset bias in using transfer learning for landmark detection. The morphological features of insect wing veins may vary due to different species, ages, genders, etc., which requires addressing domain adaptation. Moreover, the pre-trained model used for transfer learning may have been trained on a dataset that is biased towards certain features or characteristics, which may not be relevant to the target dataset. This can lead to a decrease in performance and accuracy of the model. Therefore, while transfer learning is a powerful tool for addressing data scarcity in landmark detection, it is important to carefully consider the limitations and challenges associated with this approach and to choose the appropriate method based on the specific requirements of the task at hand. Future research in this area could focus on developing more robust and adaptive transfer learning methods that can effectively address these challenges and improve the performance of landmark detection models.
4.3. Performance Analysis of TS-HRNet
In classification, CNNs typically extract high-level features that capture the overall shape, texture, and color of objects or images, which are useful for discriminating between different image categories. Meanwhile, in landmark detection, low-level features that capture the edges, corners, and other location-sensitive features of images are extracted [55], which are meaningful for identifying specific landmarks in the image. Freezing all stages may limit the new-task learning ability of the HRNet model pre-trained on the ImageNet classification task [54], especially for landmark detection requiring significantly different features from the classification task. By fine-tuning some of the stages, the model has a greater capacity to learn new features relevant to the landmark detection task. Therefore, the performance of TH-HRNet was slightly worse than that of HRNet, as it is inconsistent with the classification results of cross-domain transfer learning [31], but TS-HRNet and TA-HRNet performed better than HRNet (Figure 3).
In our experiments, HRNet in combination with transfer learning (TS-HRNet) demonstrated competitive performance in the leaf beetle hindwing landmark detection, even when using a limited training dataset. The average NME remained below 0.045 when the number of training samples was reduced to only 3, and below 0.020 when the sample size was increased to 100 (Figure 5). For one sample, TS-HRNet presented poor detection performance and large fluctuations, although this is an extreme case. Under various training samples of different sizes, the average NME of TS-HRNet in the landmark detection task was significantly lower than that of TS-ResNet, which employed the same transfer learning strategy (Figure 6).
The performance of TS-HRNet is heavily dependent on the amount and quality of the training data. With a relatively small number of samples, the model may not have enough data to learn the complex features of hindwing landmarks, resulting in poor detection performance. This was particularly evident in the case of Landmarks 25, 27–34, and 36 located in the cubitus-anal region (Figure 5, Table 1 and Table S3), which may be too variable to learn with limited data as most of the morphological differences among different sub-families and genera occur in this region of the hindwing [32]. Furthermore, in the membranous zone the veins in the distal region of the leaf beetle hindwing degenerate into traces, rather than tubular structures. This membranization resulted in TS-HRNet achieving low detection accuracy on a few end point landmarks (i.e., Landmarks 17, 18, and 31) and marginal point landmarks (i.e., Landmarks 4 and 5) on the veins that were not obvious (Figure 5, Table 1 and Table S3).
On the other hand, TS-HRNet performed well in detecting landmarks on both radial (R) and medial (M) veins (i.e., Landmarks 2, 3, 6–16, 20, 23, 24, and 26; Figure 5, Table 1 and Table S3). As the hindwing morphology of leaf beetles has not undergone substantial changes throughout the evolution of leaf beetles, the R and M veins—which are crucial for flight as they stabilize the radial and apical wing fields—have remained essentially constant throughout their evolution [32].
Although TS-HRNet showed a reduction in the detection efficacy on a few landmarks in specific regions when the size of the training dataset was reduced to three samples or less, most landmarks still remained at a low NME level. When the training dataset contained 50 or more samples, the average NME of TS-HRNet was consistently low, with almost all landmarks being accurately detected in the test images. Therefore, the accuracy was not significantly affected by the differences in detection performance across different regions as the training dataset increases, and the proposed method is robust and effective for detecting landmarks on leaf beetle hindwings.
4.4. Implications for Future Studies
In this study, we detailed the construction of a dataset for the hindwing landmarks of leaf beetles and the combination of HRNet and transfer learning for the detection of these landmarks. Our method accurately predicted the hindwing landmarks (Figure 3), providing valuable data for morphological analysis. The method showed promising results on a sample of limited size consisting of leaf beetle hindwing landmark data (Figure 5). Together with the construction of the hindwing landmark dataset, the proposed approach allows a large amount of beetle hindwing landmark data to be quickly obtained using only a small number of manually annotated samples. The construction of the hindwing landmark dataset and the landmark detection method can be extended to the whole order of Coleoptera and even other insects, providing a more efficient way to study their morphological evolution and diversity.
However, the detection errors for the landmarks in the cubitus–anal area, as well as in the membranous distal area of the leaf beetle hindwings, were relatively large (Figure 3). Further research is required to analyze and understand the structural features that are important for automatic detection of these landmarks, in order to further improve the detection performance on these landmarks. Methods that can be used for this purpose involve the visualization of CNNs, such as random input sampling explanation (RISE) [56], which can visualize the key areas of the method used in this study for wing landmark detection, thus allowing for a better understanding of the important structural features required to detect these landmarks.
Based on the coordinates of landmarks on the leaf beetle and even the beetle hindwings predicted by HRNet in combination with transfer learning, further analyses and studies can be carried out. Procrustes alignment provides the means to minimize the non-shape differences between different samples and obtain Procrustes coordinates for morphological analysis. Furthermore, principal component analysis (PCA) can be applied to analyze the main trends of beetle hindwing evolution [57,58], and the ancestral morphology of hindwings can be reconstructed based on phylogenetic analysis [9].
Although the method presented in this study can be applied to the detection of hindwing landmarks in other beetle groups, it should be noted that the existing dataset is not comprehensive and cannot fully represent the diversity of beetle hindwing morphology [32]. Further data collection and landmark annotation is still required to obtain a more comprehensive beetle hindwing training dataset for the proposed method, which would provide richer landmark data for the study of beetle wing morphology, function, evolution, and diversity.
In summary, this study has important implications for the field of coleopterology and other fields. We provided a new method for obtaining quantitative data on beetle hindwing morphology, and which can be further applied to the study of other insect wings. Further data collection and research are still required to improve the wing landmark detection accuracy within specific areas, extend the species application range of the proposed method, and understand the diversity and evolution of beetle wing morphology. The combination of HRNet and transfer learning provides a promising foundation for such future research.
5. Conclusions
In this study, the HRNet architecture fine-tuned on a specific domain through transfer learning demonstrated stronger leaf beetle hindwing landmark detection ability, with a lower dependence on the size of the training dataset compared to the current popular method. The proposed approach greatly improved the detection performance of convolutional neural networks in tasks with limited training data. When the model was trained on a small dataset of 100 hindwing images, the average detection error on 36 hindwing landmarks was less than 0.02, while the error only reached 0.045 when the training set was reduced to 3 hindwing samples. The proposed approach can significantly reduce the time spent by entomologists on landmark labeling, helping to accelerate research aimed at better understanding beetle evolution and gaining insights into key parameters for the optimization of biomimetic beetle-inspired micro aerial vehicles. This study provides a new method for obtaining quantitative data on beetle hindwing morphology, which can be further applied to the study of a wider range of insect wings. Further data collection and research should be carried out to improve the detection accuracy for specific landmarks on beetle wings, expand its application range to more insects, and better understand the diversity and evolution of insect wing morphology.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biology12071006/s1.
Author Contributions
Y.Y. conceived the study, performed the algorithm design and coding, participated in preparing the dataset, data analysis, and manuscript writing. X.L. and C.L. participated in manuscript revision. W.L., G.M., G.Y. and J.R. participated in preparing the data. S.G. conceived the study and participated in manuscript revision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Science Foundation of China [Grant No. 32270460 to S.Q.G.]. The funders had no role in the design of the study, in the collection, analysis, and interpretation of the data, or in the writing of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data and code applied in this research is accessible through the following link: https://gitlab.com/mgcyung/hrnet-wing.git (accessed on 13 July 2023).
Acknowledgments
We thank Zhong Du (Fujian Normal University) and Zhengzhong Huang (Institute of Zoology, Chinese Academy of Sciences) for a helpful discussion. We would like to thank Wu Pengxiang for English language editing.
Conflicts of Interest
The authors declare that they have no conflict of interest related to this work.
References
- Kukalová-Peck, J. Origin of the Insect Wing and Wing Articulation from the Arthropodan Leg. Can. J. Zool. 1983, 61, 1618–1669. [Google Scholar] [CrossRef]
- Hörnschemeyer, T.; Willkommen, J. The Contribution of Flight System Characters to the Reconstruction of the Phylogeny of the Pterygota. Arthropod Syst. Phylogeny 2007, 65, 15–23. [Google Scholar]
- Changbunjong, T.; Prakaikowit, N.; Maneephan, P.; Kaewwiset, T.; Weluwanarak, T.; Chaiphongpachara, T.; Dujardin, J.P. Landmark Data to Distinguish and Identify Morphologically Close Tabanus Spp. (Diptera: Tabanidae). Insects 2021, 12, 974. [Google Scholar] [CrossRef] [PubMed]
- Simões, R.F.; Wilke, A.B.B.; Chagas, C.R.F.; de Menezes, R.M.T.; Suesdek, L.; Multini, L.C.; Silva, F.S.; Grech, M.G.; Marrelli, M.T.; Kirchgatter, K. Wing Geometric Morphometrics as a Tool for the Identification of Culex Subgenus Mosquitoes of Culex (Diptera: Culicidae). Insects 2020, 11, 567. [Google Scholar] [CrossRef]
- Hamilton, C.A.; Winiger, N.; Rubin, J.J.; Breinholt, J.; Rougerie, R.; Kitching, I.J.; Barber, J.R.; Kawahara, A.Y. Hidden Phylogenomic Signal Helps Elucidate Arsenurine Silkmoth Phylogeny and the Evolution of Body Size and Wing Shape Trade-Offs. Syst. Biol. 2022, 71, 859–874. [Google Scholar] [CrossRef]
- Bai, Y.; Dong, J.; Guan, D.; Xie, J.; Xu, S. Geographic Variation in Wing Size and Shape of the Grasshopper Trilophidia Annulata (Orthoptera: Oedipodidae): Morphological Trait Variations Follow an Ecogeographical Rule. Sci. Rep. 2016, 6, 32680. [Google Scholar] [CrossRef]
- Oliveira-Christe, R.; Wilke, A.B.B.; Marrelli, M.T. Microgeographic Wing-Shape Variation in Aedes Albopictus and Aedes Scapularis (Diptera: Culicidae) Populations. Insects 2020, 11, 862. [Google Scholar] [CrossRef]
- Saito, K.; Nomura, S.; Yamamoto, S.; Niiyama, R.; Okabe, Y. Investigation of Hindwing Folding in Ladybird Beetles by Artificial Elytron Transplantation and Microcomputed Tomography. Proc. Natl. Acad. Sci. USA 2017, 114, 5624–5628. [Google Scholar] [CrossRef]
- Bai, M.; Beutel, R.G.; Song, K.; Liu, W.; Malqin, H.; Li, S.; Hu, X.; Yang, X. Evolutionary Patterns of Hind Wing Morphology in Dung Beetles (Coleoptera: Scarabaeinae). Arthropod Struct. Dev. 2012, 41, 505–513. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, H.Y.; Ge, X.Y.; Yang, Y.X. Evaluating the Significance of Wing Shapes in Inferring Phylogenetic Proximity among the Generic Taxa: An Example of Cantharinae (Coleoptera, Cantharidae). Arthropod Syst. Phylogeny 2023, 81, 303–316. [Google Scholar] [CrossRef]
- Bai, M.; Beutel, R.G.; Shih, C.; Ren, D.; Yang, X. Septiventeridae, a New and Ancestral Fossil Family of Scarabaeoidea (Insecta: Coleoptera) from the Late Jurassic to Early Cretaceous Yixian Formation. J. Syst. Palaeontol. 2013, 11, 359–374. [Google Scholar] [CrossRef]
- Ospina-Garcés, S.M.; Escobar, F.; Baena, M.L.; Davis, A.L.V.; Scholtz, C.H. Do Dung Beetles Show Interrelated Evolutionary Trends in Wing Morphology, Flight Biomechanics and Habitat Preference? Evol. Ecol. 2018, 32, 663–682. [Google Scholar] [CrossRef]
- Tocco, C.; Dacke, M.; Byrne, M. Eye and Wing Structure Closely Reflects the Visual Ecology of Dung Beetles. J. Comp. Physiol. A 2019, 205, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Ariza-Marín, E.R.; Amat-García, G. Morphometric Changes in Wings of Bess Beetles (Coleoptera: Passalidae) Related to Elevation: A Case of Study in the Colombian Andes. Stud. Neotrop. Fauna Environ. 2021, 1–11. [Google Scholar] [CrossRef]
- Meresman, Y.; Husak, J.F.; Ben-Shlomo, R.; Ribak, G. Morphological Diversification Has Led to Inter-Specific Variation in Elastic Wing Deformation during Flight in Scarab Beetles. R. Soc. Open Sci. 2020, 7, 200277. [Google Scholar] [CrossRef]
- Owens, H.L.; Lewis, D.S.; Condamine, F.L.; Kawahara, A.Y.; Guralnick, R.P. Comparative Phylogenetics of Papilio Butterfly Wing Shape and Size Demonstrates Independent Hindwing and Forewing Evolution. Syst. Biol. 2020, 69, 813–819. [Google Scholar] [CrossRef]
- Rohlf, F.J. tpsDig, Digitize Landmarks and Outlines; Department of Ecology and Evolution, State University of New York: New York, NY, USA, 2006. [Google Scholar]
- Venegas, P.; Pérez, N.; Zapata, S.; Mosquera, J.D.; Augot, D.; Rojo-Álvarez, J.L.; Benítez, D. An Approach to Automatic Classification of Culicoides Species by Learning the Wing Morphology. PLoS One 2020, 15, e0241798. [Google Scholar] [CrossRef]
- Bellin, N.; Calzolari, M.; Callegari, E.; Bonilauri, P.; Grisendi, A.; Dottori, M.; Rossi, V. Geometric Morphometrics and Machine Learning as Tools for the Identification of Sibling Mosquito Species of the Maculipennis Complex (Anopheles). Infect. Genet. Evol. 2021, 95, 105034. [Google Scholar] [CrossRef]
- Valan, M.; Makonyi, K.; Maki, A.; Vondráček, D.; Ronquist, F. Automated Taxonomic Identification of Insects with Expert-Level Accuracy Using Effective Feature Transfer from Convolutional Networks. Syst. Biol. 2019, 68, 876–895. [Google Scholar] [CrossRef]
- Rodrigues, P.J.; Gomes, W.; Pinto, M.A. DeepWings©: Automatic Wing Geometric Morphometrics Classification of Honey Bee (Apis mellifera) Subspecies Using Deep Learning for Detecting Landmarks. Big Data Cogn. Comput. 2022, 6, 70. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Ho, B.H.; Lai, H.P.; Tran, H.T.; Bañuls, A.L.; Prudhomme, J.; Le, H.T. A Lightweight Keypoint Matching Framework for Insect Wing Morphometric Landmark Detection. Ecol. Inform. 2022, 70, 101694. [Google Scholar] [CrossRef]
- Sun, K.; Xiao, B.; Liu, D.; Wang, J. Deep High-Resolution Representation Learning for Human Pose Estimation. In Proceedings of the Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, Long Beach, CA, 15–20 June 2019; pp. 5693–5703. [CrossRef]
- Arroyo, J.C.T. Coleoptera Classification Using Convolutional Neural Network and Transfer Learning. IJETT 2021, 69, 1–5. [Google Scholar] [CrossRef]
- Pan, S.J.; Yang, Q. A Survey on Transfer Learning. IEEE Trans. Knowl. Data Eng. 2010, 10, 1345–1359. [Google Scholar] [CrossRef]
- Ribani, R.; Marengoni, M. A survey of transfer learning for convolutional neural networks. In Proceedings of the 2019 32nd SIBGRAPI Conference on Graphics, Patterns and Images Tutorials (SIBGRAPI-T), Rio de Janeiro, Brazil, 28–31 October 2019; pp. 47–57. [Google Scholar] [CrossRef]
- Deng, J.; Dong, W.; Socher, R.; Li, L.J.; Li, K.; Fei-Fei, L. ImageNet: A large-scale hierarchical image database. In Proceedings of the 2009 IEEE Conference on Computer Vision and Pattern Recognition, Miami, FL, USA, 20–25 June 2009; pp. 248–255. [Google Scholar] [CrossRef]
- He, K.; Girshick, R.; Dollar, P. Rethinking ImageNet pre-training. In Proceedings of the Proceedings of the IEEE/CVF International Conference on Computer Vision, Seoul, Republic of Korea, 27 October–2 November 2019; pp. 4918–4927.
- Kingma, D.P.; Ba, J.L. Adam: A Method for Stochastic Optimization. In Proceedings of the 3rd International Conference on Learning Representations, ICLR 2015—Conference Track Proceedings, San Diego, CA, USA, 7–9 May 2015. [Google Scholar]
- Xiao, B.; Wu, H.; Wei, Y. Simple baselines for human pose estimation and tracking. In Proceedings of the Computer Vision—ECCV, Munich, Germany, 8–14 September 2018; Springer: Cham, Switzerland, 2018; pp. 472–487. [Google Scholar] [CrossRef]
- Wang, B.; Sun, R.; Yang, X.; Niu, B.; Zhang, T.; Zhao, Y.; Zhang, Y.; Zhang, Y.; Han, J. Recognition of Rare Microfossils Using Transfer Learning and Deep Residual Networks. Biology 2023, 12, 16. [Google Scholar] [CrossRef]
- Ren, J.; Bai, M.; Yang, X.K.; Zhang, R.Z.; Ge, S.Q. Geometric Morphometrics Analysis of the Hind Wing of Leaf Beetles: Proximal and Distal Parts Are Separate Modules. Zookeys 2017, 685, 131–149. [Google Scholar] [CrossRef]
- Lin, T.Y.; Maire, M.; Belongie, S.; Hays, J.; Perona, P.; Ramanan, D.; Dollár, P.; Zitnick, C.L. Microsoft COCO: Common Objects in Context. In Proceedings of the European Conference on Computer Vision; Lecture Notes in Computer Science; Fleet, D., Pajdla, T., Schiele, B., Tuytelaars, T., Eds.; Springer: Zurich, Switzerland, 2014; pp. 740–755. [Google Scholar] [CrossRef]
- Russakovsky, O.; Deng, J.; Su, H.; Krause, J.; Satheesh, S.; Ma, S.; Huang, Z.; Karpathy, A.; Khosla, A.; Bernstein, M.; et al. ImageNet Large Scale Visual Recognition Challenge. Int. J. Comput. Vis. 2015, 115, 211–252. [Google Scholar] [CrossRef]
- Oquab, M.; Bottou, L.; Laptev, I.; Sivic, J. Learning and Transferring Mid-Level Image Representations Using Convolutional Neural Networks. In Proceedings of the Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Columbus, OH, USA, 23–28 June 2014; pp. 1717–1724.
- Tan, C.; Sun, F.; Kong, T.; Zhang, W.; Yang, C.; Liu, C. A survey on deep transfer learning. In Proceedings of the Artificial Neural Networks and Machine Learning—ICANN, Rhodes, Greece, 4–7 October 2018; Lecture Notes in Computer Science. Kůrková, V., Manolopoulos, Y., Hammer, B., Iliadis, L., Maglogiannis, I., Eds.; Springer: Cham, Switzerland, 2018; pp. 270–279. [Google Scholar] [CrossRef]
- Lan, X.; Hu, Q.; Cheng, J. ATF: An Alternating Training Framework for Weakly Supervised Face Alignment. IEEE Trans. Multimed. 2022, 25, 1798–1809. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, H.; Li, C.; Kim, J.; Wei, F. ADNet: Leveraging error-bias towards normal direction in face alignment. In Proceedings of the 2021 IEEE/CVF International Conference on Computer Vision (ICCV), Montreal, QC, Canada, 10–17 October 2021; pp. 3060–3070. [Google Scholar] [CrossRef]
- Shimmi, O.; Matsuda, S.; Hatakeyama, M. Insights into the Molecular Mechanisms Underlying Diversified Wing Venation among Insects. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140264. [Google Scholar] [CrossRef]
- Lawrence, J.F.; Zhou, Y.L.Z.; Lemann, C.; Sinclair, B.; Ślipiński, A. The Hind Wing of Coleoptera (Insecta): Morphology, Nomenclature and Phylogenetic Significance. Part 1. General Discussion and Archostemata–Elateroidea. Ann. Zool. 2021, 71, 421–606. [Google Scholar] [CrossRef]
- Lawrence, J.F.; Zhou, Y.L.Z.; Lemann, C.; Sinclair, B.; Ślipiński, A. The Hind Wing of Coleoptera (Insecta): Morphology, Nomenclature and Phylogenetic Significance: Part 2. Further Discussion, Histeroidea, Bostrichoidea to Curculionoidea. Ann. Zool. 2022, 72, 433–755. [Google Scholar] [CrossRef]
- Soule, A.J.; Decker, L.E.; Hunter, M.D. Effects of Diet and Temperature on Monarch Butterfly Wing Morphology and Flight Ability. J. Insect. Conserv. 2020, 24, 961–975. [Google Scholar] [CrossRef]
- Henriques, D.; Chávez-Galarza, J.; Teixeira, J.S.G.; Ferreira, H.; J. Neves, C.; Francoy, T.M.; Pinto, M.A. Wing Geometric Morphometrics of Workers and Drones and Single Nucleotide Polymorphisms Provide Similar Genetic Structure in the Iberian Honey Bee (Apis Mellifera Iberiensis). Insects 2020, 11, 89. [Google Scholar] [CrossRef]
- Song, F.; Yan, Y.; Sun, J. Review of Insect-Inspired Wing Micro Air Vehicle. Arthropod Struct. Dev. 2023, 72, 101225. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.V.; Park, H.C. Mechanisms of Collision Recovery in Flying Beetles and Flapping-Wing Robots. Science 2020, 370, 1214–1219. [Google Scholar] [CrossRef]
- Zeng, Y.; O’Malley, C.; Singhal, S.; Rahim, F.; Park, S.; Chen, X.; Dudley, R. A Tale of Winglets: Evolution of Flight Morphology in Stick Insects. Front. Ecol. Evol. 2020, 8, 121. [Google Scholar] [CrossRef]
- Dudley, R. The Biomechanics of Insect Flight: Form, Function, Evolution; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Sun, J.; Wu, W.; Ling, M.; Tong, J.; Ren, L. Fluid Analysis of Vein of Beetle Hindwing during Unfolding Action. Int. J. Heat Mass Transf. 2016, 101, 379–386. [Google Scholar] [CrossRef]
- Nakata, T.; Liu, H. Aerodynamic Performance of a Hovering Hawkmoth with Flexible Wings: A Computational Approach. Proc. R. Soc. B Biol. Sci. 2011, 279, 722–731. [Google Scholar] [CrossRef]
- Song, Z.; Tong, J.; Pfleging, W.; Sun, J. A Review: Learning from the Flight of Beetles. Comput. Biol. Med. 2021, 133, 104397. [Google Scholar] [CrossRef]
- Cannet, A.; Simon-Chane, C.; Akhoundi, M.; Histace, A.; Romain, O.; Souchaud, M.; Jacob, P.; Delaunay, P.; Sereno, D.; Bousses, P.; et al. Wing Interferential Patterns (WIPs) and Machine Learning, a Step toward Automatized Tsetse (Glossina spp.) Identification. Sci. Rep. 2022, 12, 20086. [Google Scholar] [CrossRef]
- Park, J.; Kim, D.I.; Choi, B.; Kang, W.; Kwon, H.W. Classification and Morphological Analysis of Vector Mosquitoes Using Deep Convolutional Neural Networks. Sci. Rep. 2020, 10, 1012. [Google Scholar] [CrossRef]
- Buschbacher, K.; Ahrens, D.; Espeland, M.; Steinhage, V. Image-Based Species Identification of Wild Bees Using Convolutional Neural Networks. Ecol. Inform. 2020, 55, 101017. [Google Scholar] [CrossRef]
- Yosinski, J.; Clune, J.; Bengio, Y.; Lipson, H. How transferable are features in deep neural networks? In Proceedings of the Advances in Neural Information Processing Systems, Montreal, QC, Canada, 8–13 December 2014; Curran Associates, Inc.: New York, NY, USA, 2014; Volume 27. [Google Scholar]
- Zhu, X.; Lei, Z.; Liu, X.; Shi, H.; Li, S.Z. Face alignment across large poses: A 3D solution. In Proceedings of the Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 26 June–1 July 2016; pp. 146–155.
- Murty, N.A.R.; Bashivan, P.; Abate, A.; DiCarlo, J.J.; Kanwisher, N. Computational Models of Category-Selective Brain Regions Enable High-Throughput Tests of Selectivity. Nat. Commun. 2021, 12, 5540. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P.; McIntyre, G.S. Geometric Morphometrics of Developmental Instability: Analyzing Patterns of Fluctuating Asymmetry with Procrustes Methods. Evolution 1998, 52, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P.; Zaklan, S.D. Morphological Integration between Developmental Compartments in the Drosophila Wing. Evolution 2000, 54, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).