Impacts of Strong ENSO Events on Fish Communities in an Overexploited Ecosystem in the South China Sea
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
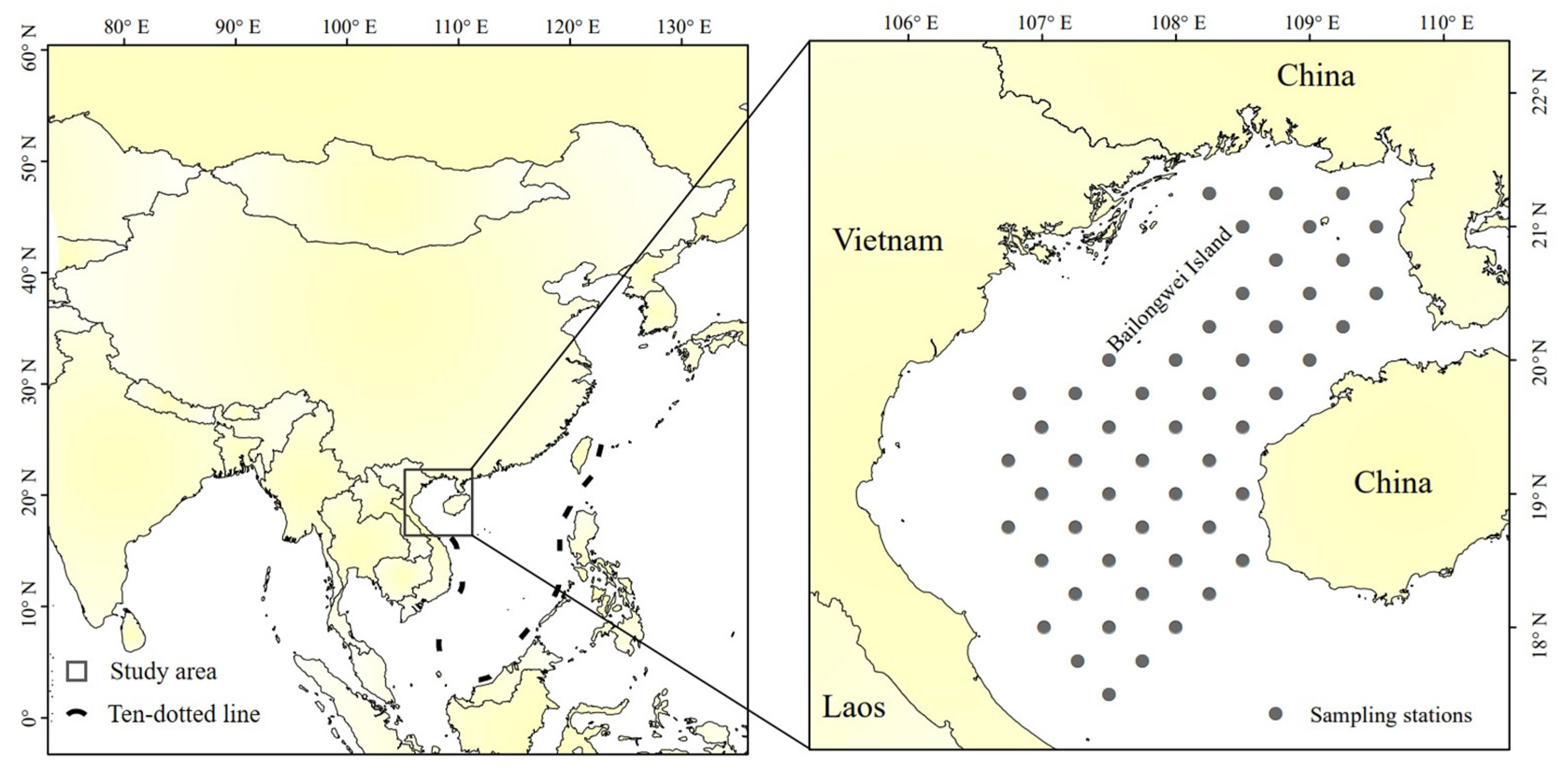
2.1. Data Collection
2.1.1. Oceanic Niño Index (ONI)
2.1.2. Survey Data
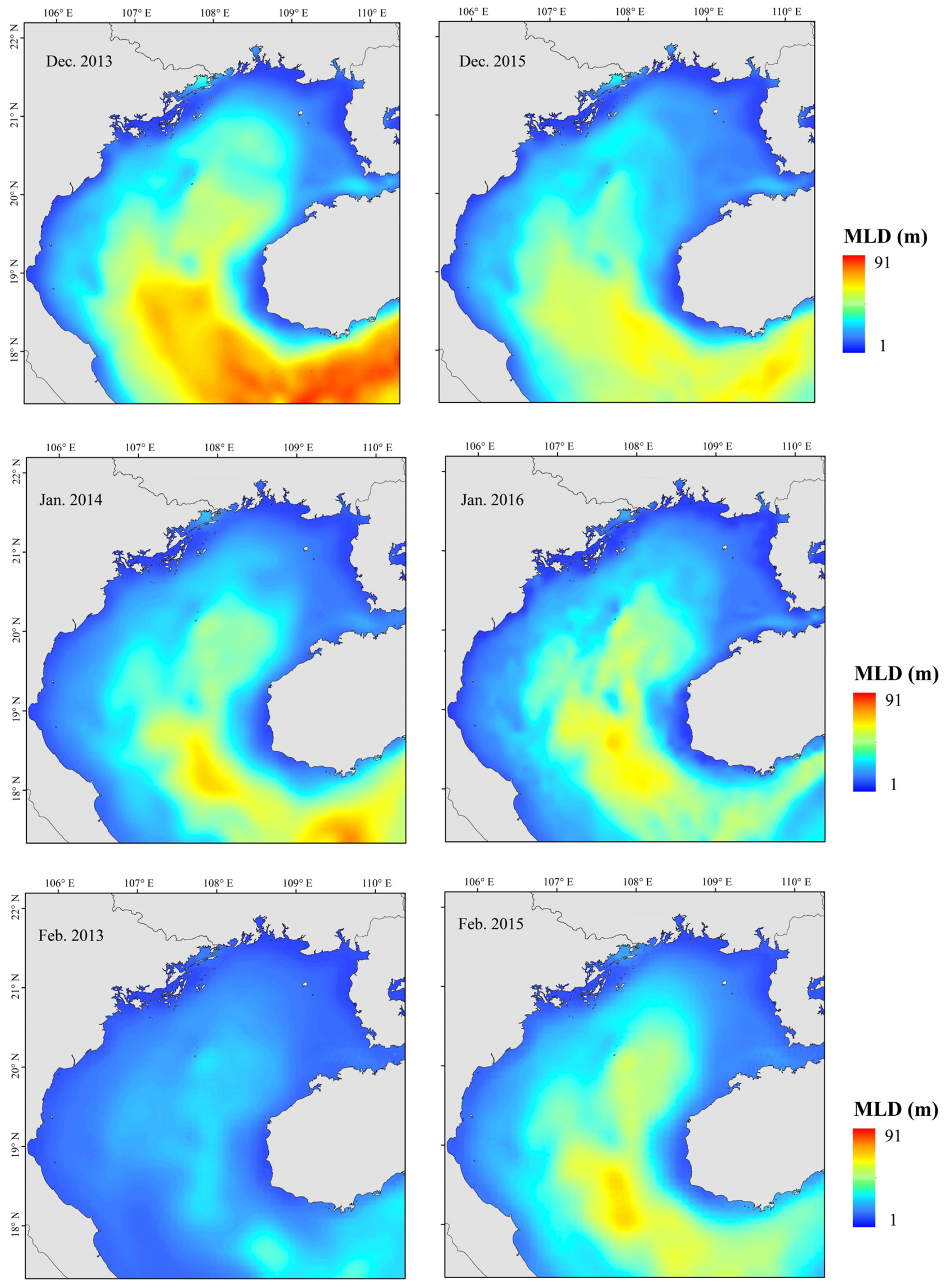
2.2. Environmental Data
2.3. Data Analyses
2.3.1. Dominant Fish Species
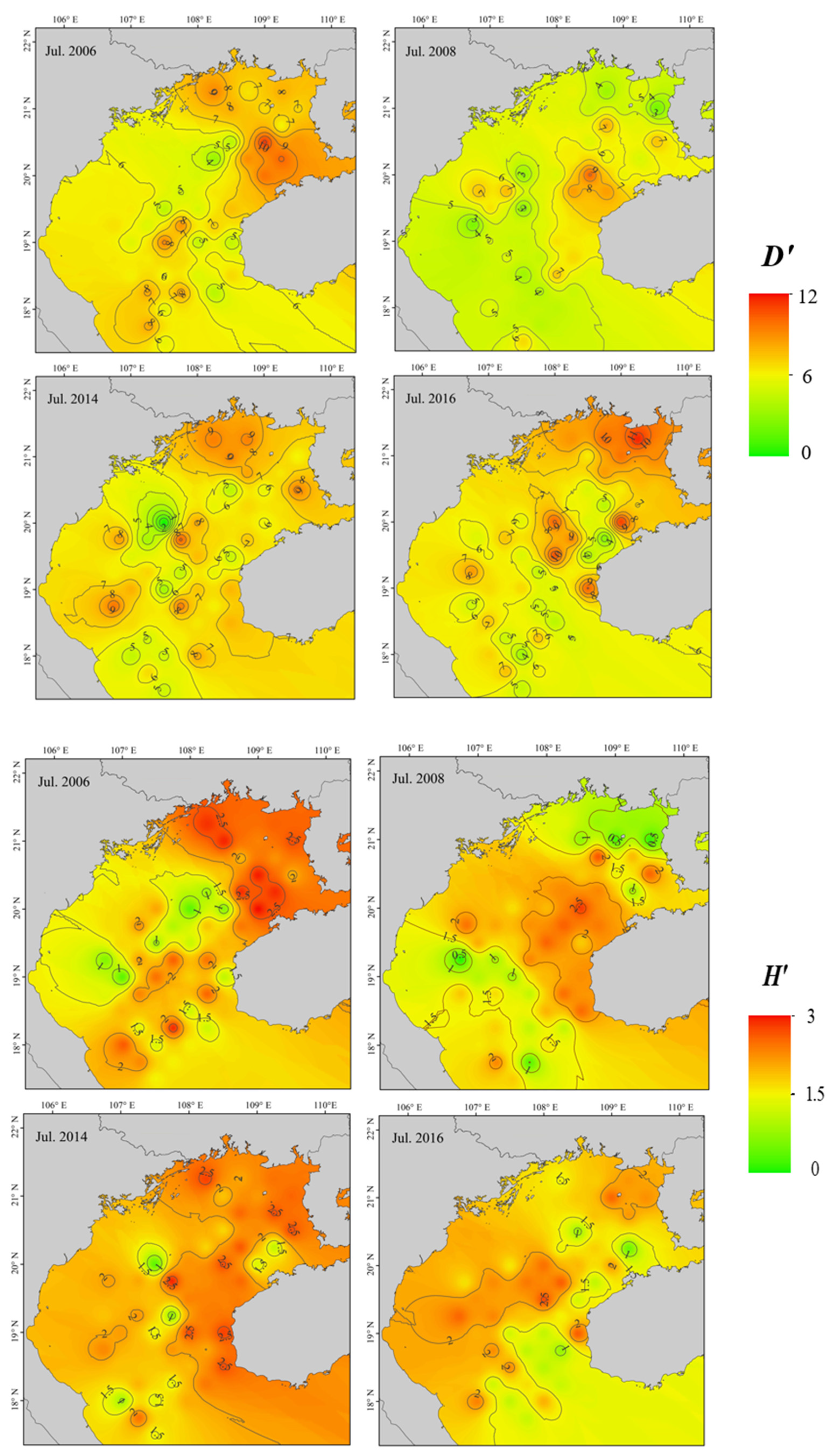
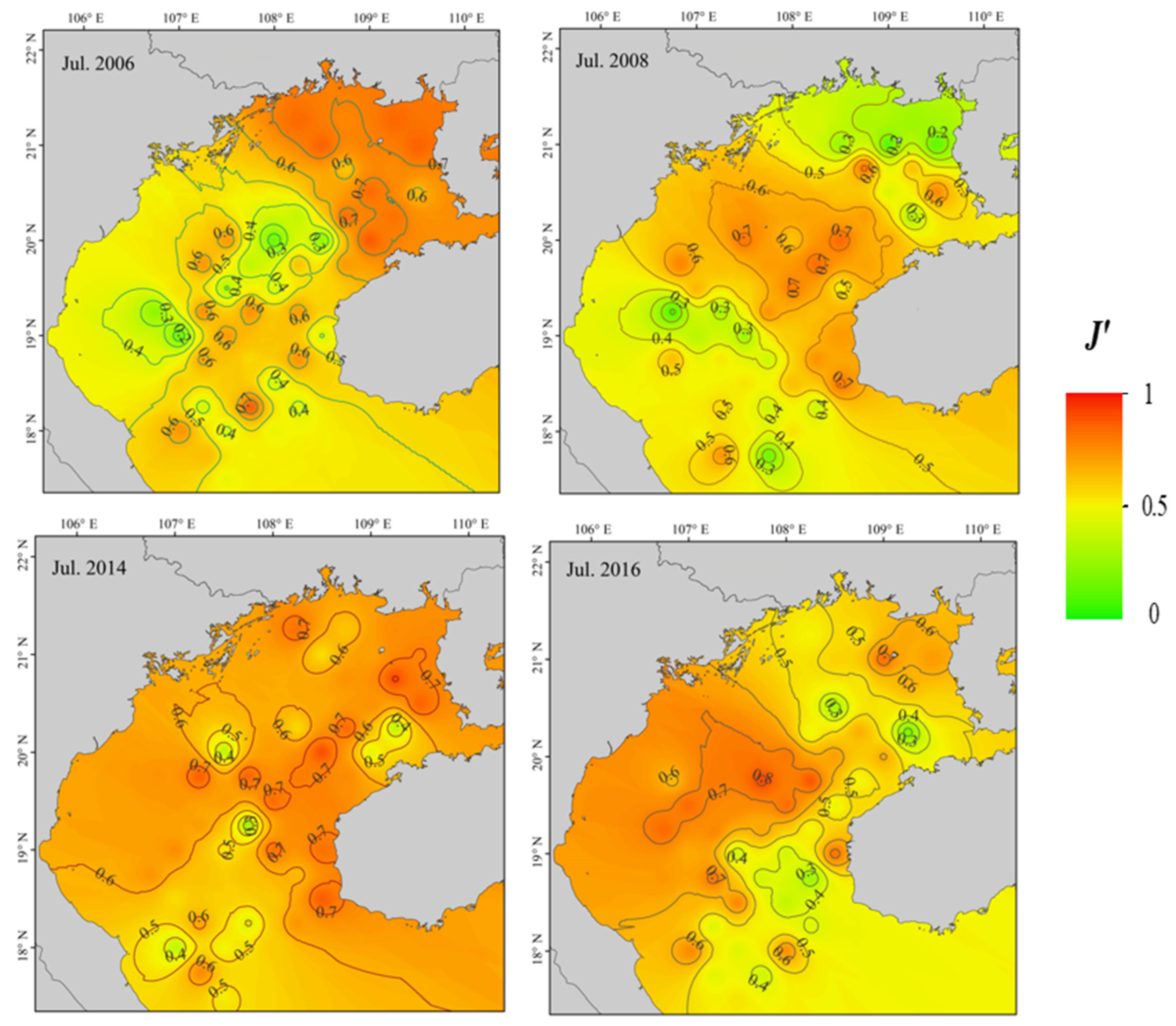
2.3.2. Diversity
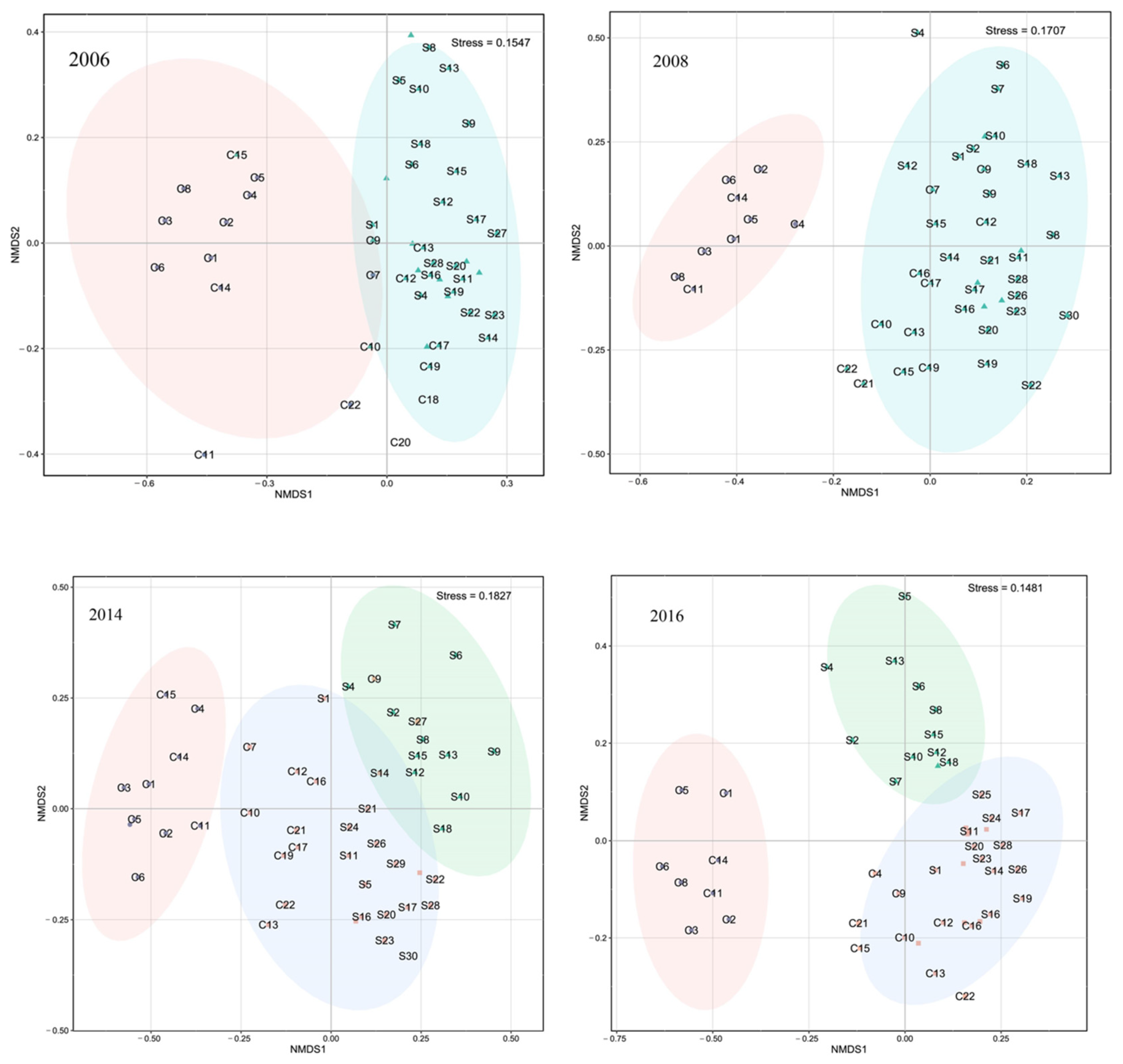
2.3.3. Fish Community Composition
2.3.4. GAM Model Development
3. Results
3.1. Fish Species Composition
3.2. Fish Diversity and Community Structure
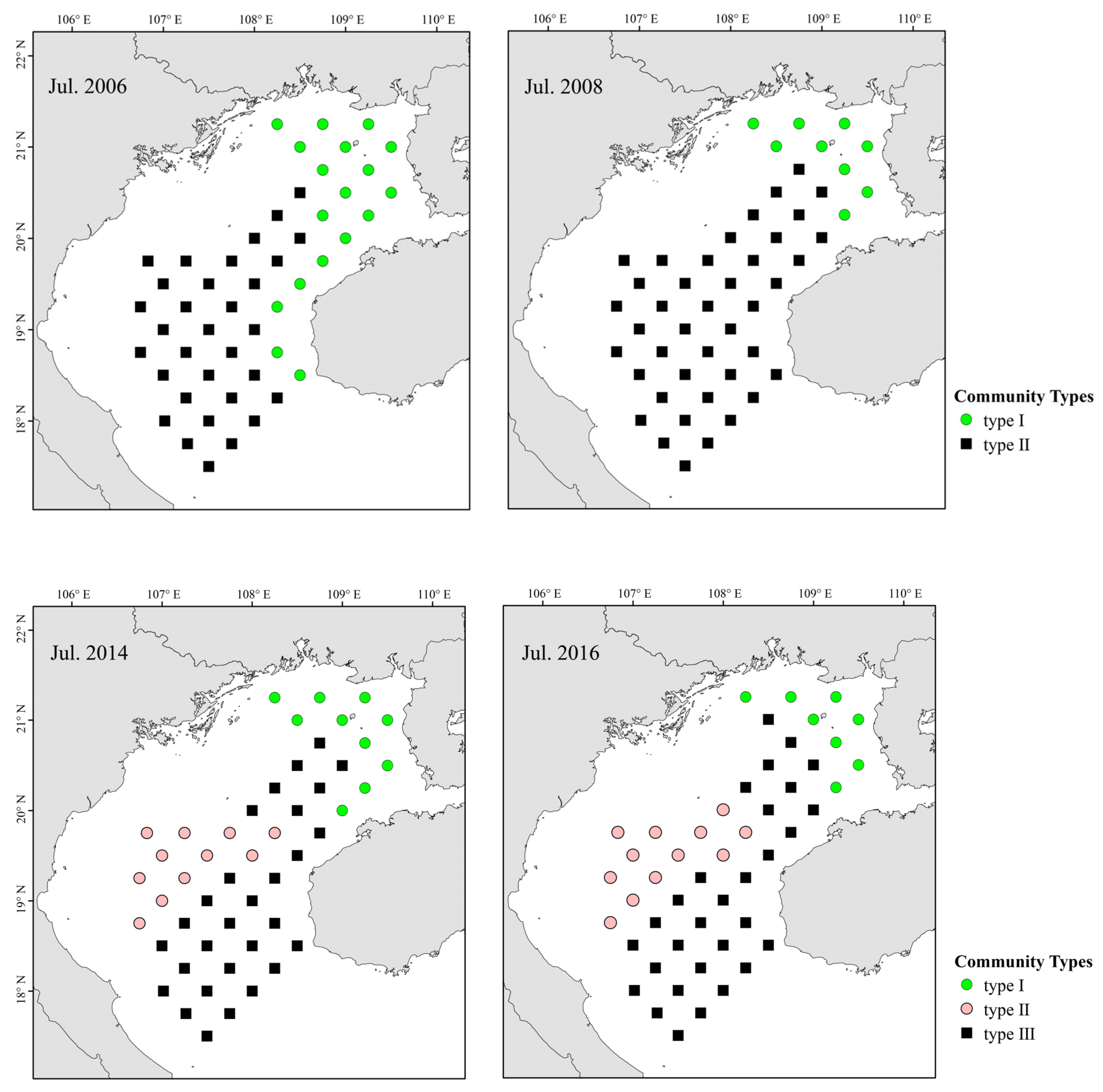
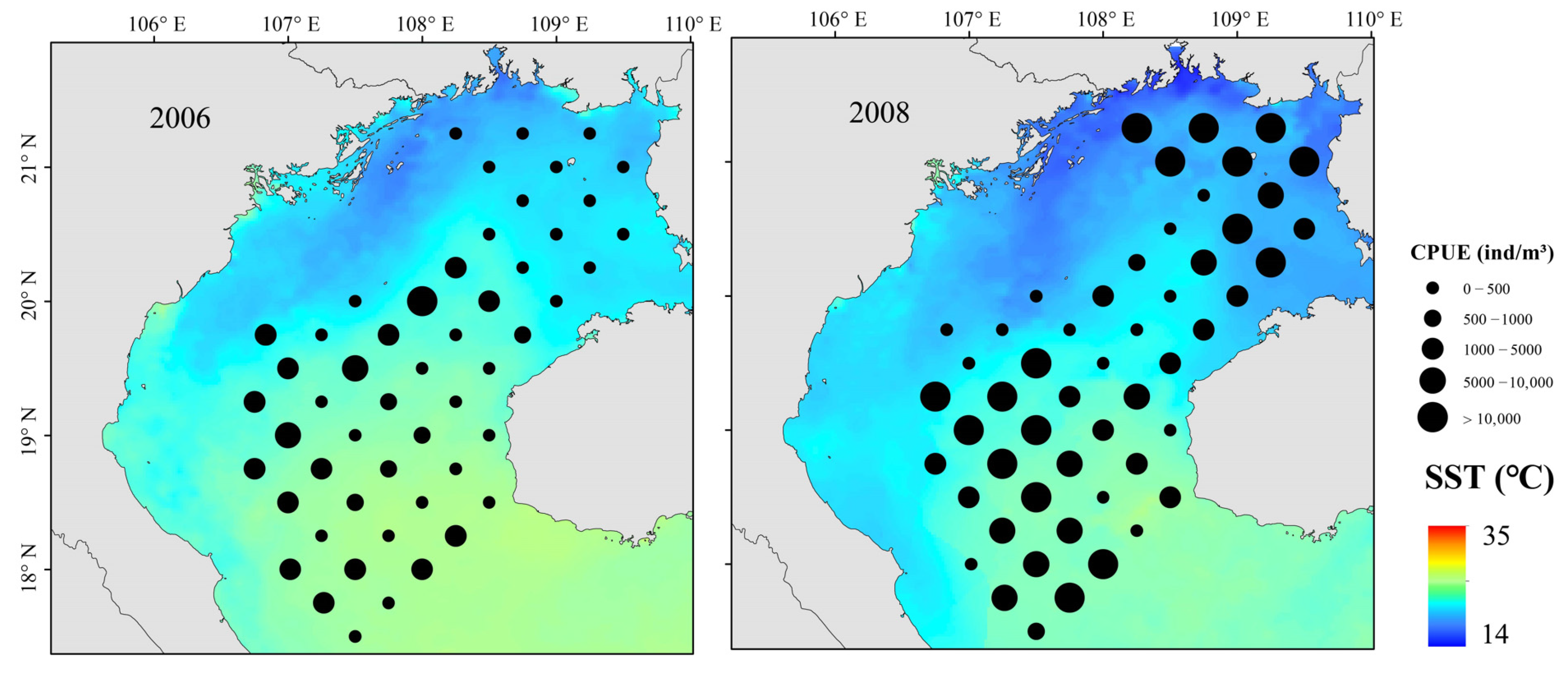
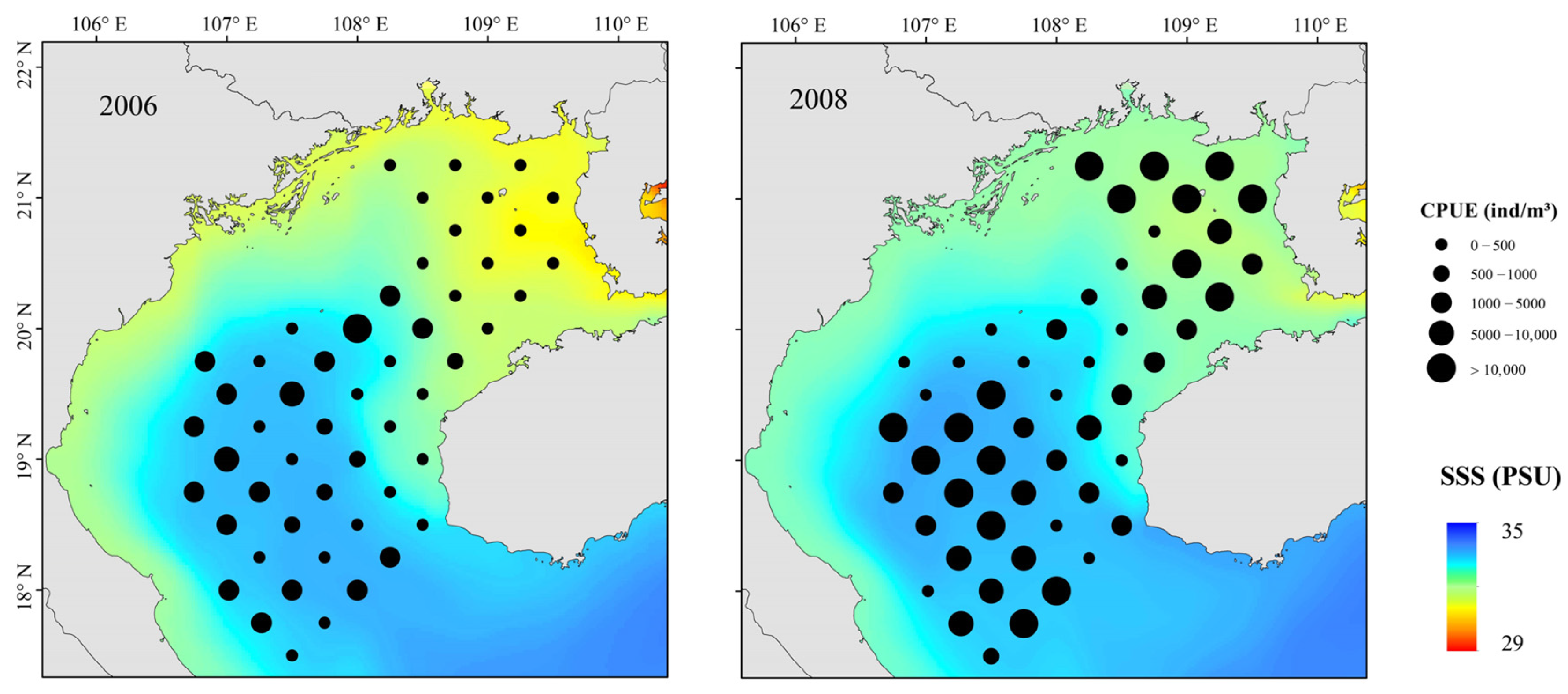
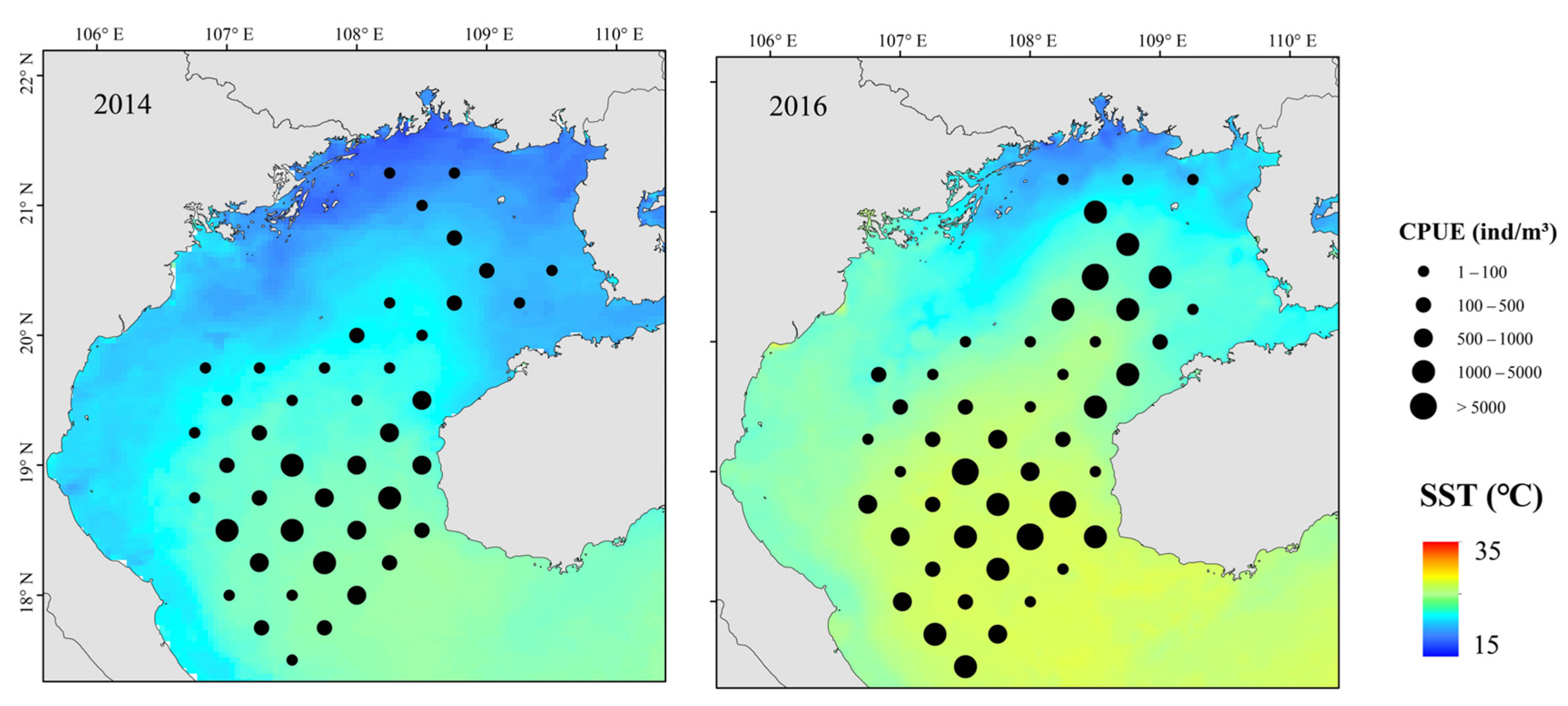
3.3. Distributions of Fish Assemblages
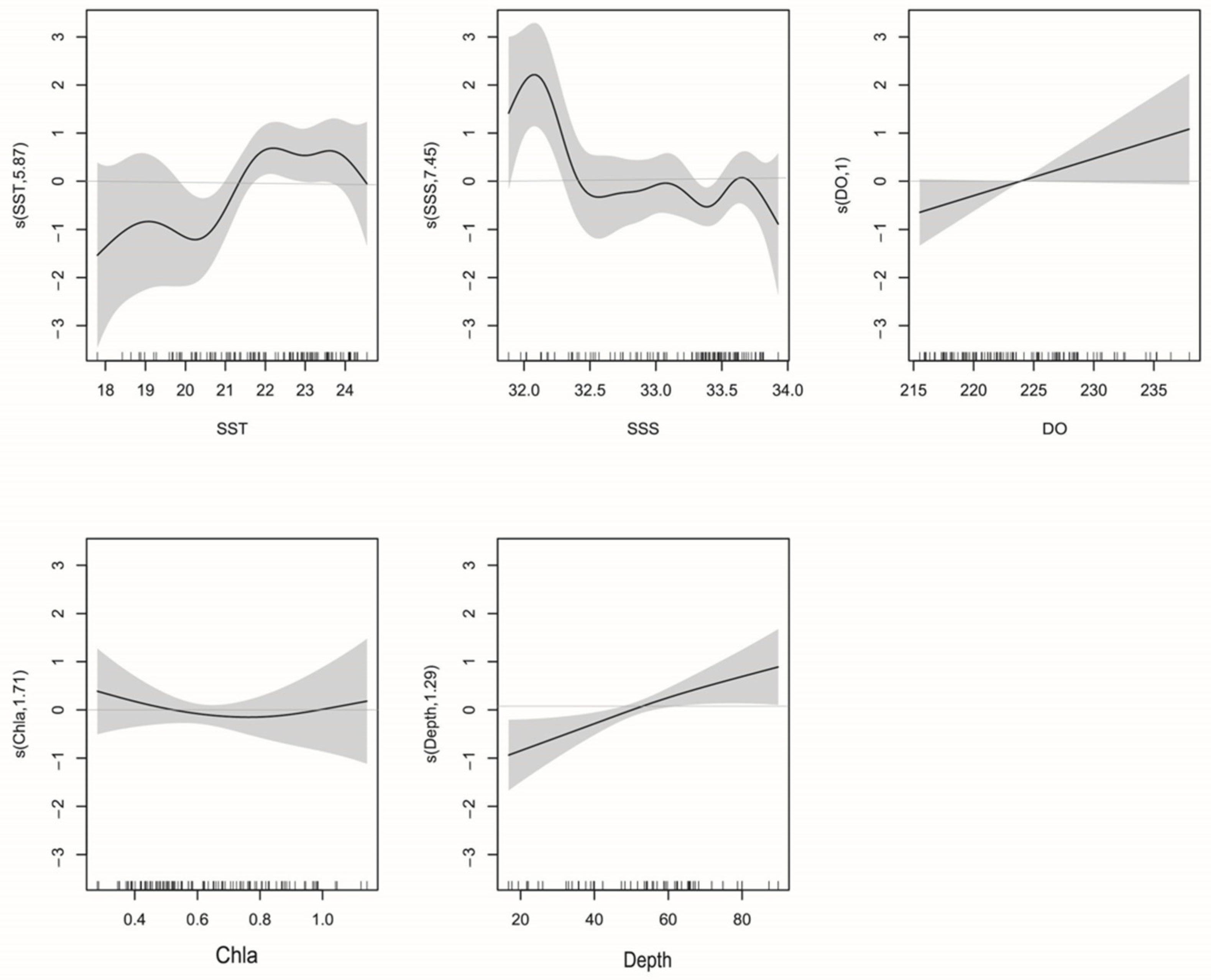
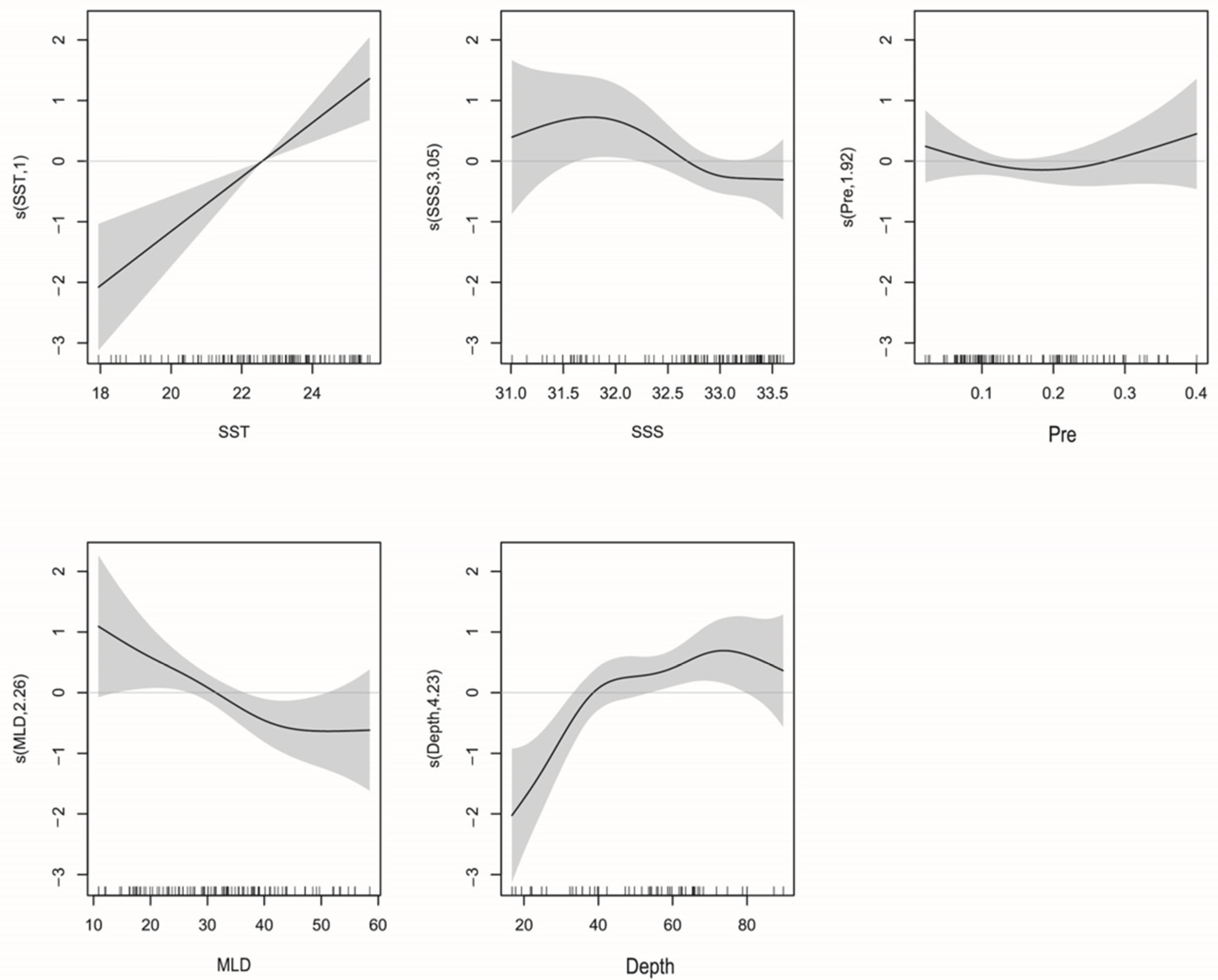
3.4. GAM Analysis
3.4.1. La Niña
3.4.2. El Niño
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Philander, S.G.H. El Niño southern oscillation phenomena. Nature 1983, 302, 295. [Google Scholar] [CrossRef]
- Alves, J.C.; Andreotti, G.F.; Agostinho, A.A.; Gomes, L.C. Effects of the El Niño Southern Oscillation (ENSO) on fish assemblages in a Neotropical floodplain. Hydrobiologia 2021, 848, 1811–1823. [Google Scholar] [CrossRef]
- Grothe, P.R.; Cobb, K.M.; Liguori, G.; Lorenzo, E.D.; Capotondi, A.; Lu, Y.B.; Cheng, H.; Edwards, R.L.; Southon, J.R.; Santos, J.M.; et al. Enhanced El Niño–Southern oscillation variability in recent decades. Geophys. Res. Lett. 2020, 47, e2019GL083906. [Google Scholar] [CrossRef]
- Pineda, A.; Pelaéz, Ó.; Dias, J.D.; Segovia, B.T.; Bonecker, C.C.; Velho, L.F.M.; Rodrigues, L.C. The El Niño Southern Oscillation (ENSO) is the main source of variation for the gamma diversity of plankton communities in subtropical shallow lakes. Aquat. Sci. 2019, 81, 49. [Google Scholar] [CrossRef]
- Lehodey, P.; Bertrand, A.; Hobday, A.J.; Kiyofuji, H.; McClatchie, S.; Menkès, C.E.; Pilling, G.; Polovina, J.; Tommasi, D. ENSO Impact on Marine Fisheries and Ecosystems; American Geophysical Union: Washington, DC, USA, 2020; pp. 1–244. [Google Scholar] [CrossRef]
- Sunday, J.M.; Pecl, G.T.; Frusher, S.; Hobday, A.J.; Hill, N.; Holbrook, N.J.; Edgar, G.J.; Stuart-Smith, R.; Barrett, N.; Wernberg, T.; et al. Species traits and climate velocity explain geographic range shifts in an ocean-warming hotspot. Ecol. Lett. 2015, 18, 944–953. [Google Scholar] [CrossRef]
- Ash, J.D.; Givnish, T.J.; Waller, D.M. Tracking lags in historical plant species’ shifts in relation to regional climate change. Glob. Chang. Bio. 2017, 23, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- García Molinos, J.; Burrows, M.T.; Poloczanska, E.S. Ocean currents modify the coupling between climate change and biogeographical shifts. Sci. Rep. 2017, 7, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Alabia, I.D.; García Molinos, J.; Saitoh, S.I.; Hirawake, T.; Hirata, T.; Mueter, F.J. Distribution shifts of marine taxa in the Pacific Arctic under contemporary climate changes. Divers. Distrib. 2018, 24, 1583–1597. [Google Scholar] [CrossRef]
- Fredston-Hermann, A.; Selden, R.; Pinsky, M.; Gaines, S.D.; Halpern, B.S. Cold range edges of marine fishes track climate change better than warm edges. Glob. Chang. Biol. 2020, 26, 2908–2922. [Google Scholar] [CrossRef]
- Garcia, A. Dynamics of the shallow-water fish assemblage of the Patos Lagoon estuary (Brazil) during cold and warm ENSO episodes. J. Fish. Biol. 2001, 59, 1218–1238. [Google Scholar] [CrossRef]
- Syamsuddin, M.; Sunarto, Y.L. How do El Niño Southern Oscillation events impact on small pelagic fish catches in the west Java Sea. Earth Eev. Sci. 2018, 176, 012014. [Google Scholar] [CrossRef]
- Jaureguizar, A.J.; De Wysiecki, A.M.; Camiolo, M.D.; Clara, M.L. Inter-annual fluctuation in the population structure of an estuarine fish: Influence of environmental drivers. J. Mar. Syst. 2021, 218, 103526. [Google Scholar] [CrossRef]
- Barber, R.T.; Chávez, F.P. Ocean variability in relation to living resources during the 1982–1983 El Niño. Nature 1986, 319, 279–285. [Google Scholar] [CrossRef]
- Gomez, F.A.; Lee, S.K.; Hernandez, F.J.; Chiaverano, L.M.; Muller-Karger, F.E.; Liu, Y.Y.; Lamkin, J.T. ENSO-induced co-variability of Salinity, Plankton Biomass and Coastal Currents in the Northern Gulf of Mexico. Sci. Rep. 2019, 9, 178. [Google Scholar] [CrossRef]
- Feng, Z.; Zhan, Y.; Yu, W.; Chen, X. Differences in habitat pattern response to various ENSO events in Trachurus murphyi and Dosidicus gigas located outside the exclusive economic zones of Chile. J. Fish. Sci. China 2021, 28, 1195–1207. [Google Scholar]
- Sumaila, U.R.; Tai, T.C.; Lam, V.W.Y.; Cheung, W.W.L.; Bailey, M.; Chen, O.L. Benefits of the Paris Agreement to ocean life, economies, and people. Sci. Adv. 2019, 5, eaau3855. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Qou, Y.; Xu, S. Changes in trophic flows and ecosystem properties of the Beibu Gulf ecosystem before and after the collapse of fish stocks. Ocean. Coast. Manag. 2011, 54, 601–611. [Google Scholar] [CrossRef]
- The, L.S.L.; Witter, A.; Cheung, W.W.L.; Sumaila, U.R.; Yin, X.Y. What is at stake? Status and threats to South China Sea marine fisheries. Ambio 2017, 46, 57–72. [Google Scholar] [CrossRef]
- Pauly, D.; Liang, C. The fisheries of the South China Sea: Major trends since 1950. Mar. Policy 2020, 121, 103584. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, C.; Liu, Y.; Zhou, W.; Shan, B.; Wang, L.; Wang, S.; Sun, D.; Jia, C. Pattern of fish community and its relationship with environmental factors in Fangchenggang (Qinzhou coastal area of Beibu Gulf). South China Fish. Sci. 2022, 18, 20–33. [Google Scholar]
- Yuan, H.; Chen, P.; Yu, J.; Li, X. Assessment of Quality of Fishery Resources in the Northeastern South China Sea. J. Mar. Sci. Eng. 2022, 10, 930. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, J.; Xu, Y.; Fan, J.; Xu, S.; Chen, Z. Long-term variations in fish community structure under multiple stressors in a semi-closed marine ecosystem in the South China Sea. Sci. Total Environ. 2020, 745, 140892. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Chen, Z.; Zhang, K.; Xu, Y.; Xu, S.; Wang, K. Decadal-Scale Variation in Mean Trophic Level in Beibu Gulf Based on Bottom-Trawl Survey Data. Mar. Coast. Fish 2021, 13, 174–182. [Google Scholar] [CrossRef]
- Zhang, K.; Li, J.; Hou, G.; Huang, Z.; Shi, D.; Chen, Z.; Qiu, Y. Length-based assessment of fish stocks in a data-poor, jointly exploited (China and Vietnam) fishing ground, northern South China Sea. Front. Mar. Sci. 2021, 8, 718052. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, Y.; Du, F. Roles of fishing and climate change in long-term fish species succession and population dynamics in the outer Beibu Gulf, South China Sea. Acta. Oceanol. Sin. 2019, 38, 1–8. [Google Scholar] [CrossRef]
- Yan, H.; Sun, L.; Liu, X.; Qiu, S. Relationship between ENSO events and regional climate anomalies around the Xisha Islands during the last 50 years. J. Trop. Oceanogr. 2010, 29, 29–35. [Google Scholar]
- Hu, P.; Chen, W. The Relationship between the East Asian Winter Monsoon Anomaly and the Subsequent Summer Monsoon Onset over the South China Sea and the Impact of ENSO. Clim. Environ. Res. 2018, 23, 401–412. [Google Scholar]
- Zou, W.; Xu, F.; Tu, S.; Zhang, X.; Ji, Q.; Chen, S.; Zhang, Y. Study on the variation characteristics of near-surface wind field over the South China Sea and its correlation with ENSO. J. Mar. Meteor. 2018, 38, 83–91. [Google Scholar]
- Niu, M.; Li, X.; Xu, Y. Effects of spatiotemporal and environmental factors on the fishing ground of Trachurus murphyi in Southeast Pacific Ocean based on generalized additive model. Chin. J. Appl. Ecol. 2010, 21, 1049–1055. [Google Scholar] [CrossRef]
- Planka, E.R. Ecology of the agamid lizard Amphibolurus isolepis in Western Australia. Copeia 1971, 3, 527–536. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, Y.; Du, F.; Lin, Z.; Sun, D. Spatio-temporal variability of fish diversity and dominant species in the Beibu Gulf. J. Fish. Sci. China 2011, 18, 427–436. [Google Scholar] [CrossRef]
- Margalef, R. Information theory in ecology. Gen. Syst. 1958, 3, 36–71. [Google Scholar] [CrossRef]
- Pielou, E.C. The use of information theory in the study of ecological succession. J. Theor. Biol. 1966, 10, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Wilhm, J.L. Use of biomass units in Shannon’s formula. Ecology 1968, 49, 153. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, Z.; Xiong, P.; Cai, Y.; Li, J.; Zhang, P.; Zhang, J.; Li, M.; Fan, J. Exploring the Spatial and Temporal Distribution of Frigate Tuna (Auxis thazard) Habitat in the South China Sea in Spring and Summer during 2015–2019 Using Fishery and Remote Sensing Data. Fishes 2022, 7, 218. [Google Scholar] [CrossRef]
- Antoine, G.; Thomas, C.E.; Trevor, H. Generalized linear and generalized additive models in studies of species distributions: Setting the scene. Ecol. Modelg. 2002, 157, 89–100. [Google Scholar] [CrossRef]
- Venables, W.N.; Dichmont, C.M. GLMs, GAMs and GLMMs: An overview of theory for applications in fisheries research. Fish. Res. 2004, 70, 319–337. [Google Scholar] [CrossRef]
- Berg, D. Bankruptcy prediction by generalized additive models. Appl. Stoch. Model Bus. 2007, 23, 129–143. [Google Scholar] [CrossRef]
- Beier, P. Model selection and inference: A practical information-theoretic approach by Kenneth P. Burnham, David R. Anderson. J. Wildl. Manag. 2001, 65, 606–608. [Google Scholar] [CrossRef]
- Geng, T.; Cai, W.; Wu, L.; Santoso, A.; Wang, G.; Jing, Z.; Gan, B.; Yang, Y.; Li, S.; Wang, S.; et al. Emergence of changing Central-Pacific and Eastern-Pacific El Niño-Southern Oscillation in a warming climate. Nat. Commun. 2022, 13, 6616. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhang, R.; Song, P.; Zhong, Z.; Wang, Y.; Lin, L. Fish diversity in southwestern seas of Nansha Islands and the mouth of Beibu Bay. Chin. Biodivers. 2016, 24, 166–174. [Google Scholar] [CrossRef]
- Sun, D.; Lin, Z. Variations of major commercial fish stocksand strategies for fishery management in beibu gulf. J. Trop. Oceanogr. 2004, 4, 62–68. [Google Scholar] [CrossRef]
- Glantz, M.H.; Ebrary, I. La Niña and Its Impacts: Facts and Speculation; United Nations University Press: Tokyo, Japan, 2002; pp. 1–290. [Google Scholar]
- Li, M.; Xu, Y.; Sun, M.; Fang, J.; Li, J.; Zhang, K.; Chen, Z. Variations in fish community structure before and after La Niña event in the Beibu Gulf. South China Fish. Sci. 2022, 19, 1–11. [Google Scholar] [CrossRef]
- Jiang, R.; Wang, Y. A preliminary ecological modeling study on response of the East Guangdong coastal ecosystem to summer coastal upwelling. Ecol. Sci. 2017, 36, 25–34. [Google Scholar] [CrossRef]
- Qiu, Y.; Lin, Z.; Wang, Y. Responses of fish production to fishing and climate variability in the northern South China Sea. Prog. Oceanogr. 2010, 85, 197–212. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Reiss, C.; Watson, W.; Allen, M.J.; Hunter, J.R.; Lea, R.N.; Rosenblatt, R.H.; Smith, P.E.; Sugihara, G. A comparison of long-term trends and variability in populations of larvae of exploited and unexploited fishes in the Southern California region: A community approach. Prog. Oceanogr. 2007, 67, 160–185. [Google Scholar] [CrossRef]
- Planque, B.; Bellier, E.; Lazure, P. Modelling potential spawning habitat of sardine (Sardina pilchardus) and anchovy (Engraulis encrasicolus) in the Bay of Biscay. Fish. Oceanogr. 2007, 16, 16–30. [Google Scholar] [CrossRef]
- Hsieh, H.Y.; Lo, W.T.; Liao, C.C.; Meng, P.J. Shifts in the Assemblage of Summer Mesopelagic Fish Larvae in the Gaoping Waters of Southwestern Taiwan: A Comparison between El Niño Events and Regular Years. J. Mar. Sci. Eng. 2021, 9, 1065. [Google Scholar] [CrossRef]
- Lehodey, P.; Bertignac, M.; Hampton, J.; Lewis, A.; Picaut, J. El Niño Southern Oscillation and tuna in the western Pacific. Nature 1997, 389, 715–718. [Google Scholar] [CrossRef]
- Tian, Y.; Akamine, T.; Suda, M. Variations in the abundance of Pacific saury (Cololabis saira) from the northwestern Pacific in relation to oceanic-climate changes. Fish. Res. 2003, 60, 439–454. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, Y.; Chen, X.; Yi, Q.; Qian, W. Response of winter cohort abundance of Japanese common squid Todarodes pacificus to the ENSO events. Acta. Oceanol. Sin. 2018, 37, 61–71. [Google Scholar] [CrossRef]
- Vermeer, M.; Rahmstore, S. Global sea level linked to global temperature. Proc. Natl. Acad. Sci. USA 2009, 106, 21527–21532. [Google Scholar] [CrossRef]
- Cazenave, A.; Llovel, W. Contemporary sea level rise. Annu. Rev. Mar. Sci. 2010, 2, 145–173. [Google Scholar] [CrossRef] [PubMed]
- Giffard-Mena, I.; Hernández-Montiel, Á.H.; Pérez-Robles, J.; David-True, C. Effects of salinity on survival and plasma osmolarity of Totoaba macdonaldi eggs, larvae, and juveniles. J. Exp. Mar. Biol. Ecol. 2020, 526, 151339. [Google Scholar] [CrossRef]
- Yan, R.; Fan, J.; Chen, Z.; Cai, Y.; Zhang, K.; Xu, Y.; Xu, S. The probability distribution of the jack mackerel (Trachurus japonicus) density in the offshore of the northern South China Sea. J. Fish. Sci. China 2019, 26, 91–98. [Google Scholar] [CrossRef]
- Ya, H.; Gao, J.; Dong, D. Analysis of variation characteristics and driving factors of sea surface temperature in Beibu Guif. Guangxi Sci. 2015, 3, 26–31. [Google Scholar] [CrossRef]

| Variables (Unit) | Data Sources | Spatial Resolution | Download Website |
|---|---|---|---|
| SSS (PSU) | CMEMS | 1/12° × 1/12° | https://Marine.Copernicus.eu (accessed on 10 July 2022) |
| DO (mg·m−3) | CMEMS | 1/12° × 1/12° | https://Marine.Copernicus.eu (accessed on 10 July 2022) |
| MLD (m) | CMEMS | 1/12° × 1/12° | https://Marine.Copernicus.eu (accessed on 10 July 2022) |
| Chl-a (mg·m−3) | CMEMS | 4 km | https://Marine.Copernicus.eu (accessed on 10 July 2022) |
| SST (°C) | MODIS-Aqua | 4 km | http://www.cpc.ncep.noaa.gov/ (accessed on 20 July 2022) |
| Pre (mm·h−1) | TRMM | 5 km | https://disc.gsfc.nasa.gov/ (accessed on 20 July 2022) |
| Month Year | Species | IRI | pIRI (%) |
|---|---|---|---|
| Summer 2006 | Acropoma japonicum | 4341.33 | 38.46 |
| Trachurus japonicus | 3550.31 | 31.45 | |
| Summer 2008 | Trachurus japonicus | 7745.79 | 49.97 |
| Decapterus maruadsi | 4457.01 | 28.75 | |
| Acropoma japonicum | 1607.03 | 10.37 | |
| Summer 2014 | Acropoma japonicum | 3918.11 | 33.08 |
| Evynnis cardinalis | 2531.58 | 21.37 | |
| Trachurus japonicus | 2022.55 | 17.07 | |
| Summer 2016 | Trachurus japonicus | 5365.45 | 40.66 |
| Acropoma japonicum | 2272.35 | 17.22 | |
| Psenopsis anomala | 1799.63 | 13.64 | |
| Evynnis cardinalis | 1587.05 | 12.03 | |
| Decapterus maruadsi | 1153.2 | 8.74 |
| Event | Model Variables | R2 | Deviance Explained/% | AIC | GCV |
|---|---|---|---|---|---|
| La Niña | log(CPUE + 1) = s(Year) + s(SST) | 0.353 | 39.7 | 317.50 | 1.22 |
| log(CPUE + 1) = s(Year) + s(SST) + s(Chl-a) | 0.371 | 42.4 | 315.89 | 1.20 | |
| log(CPUE + 1) = s(Year) + s(SST) + s(Chl-a) + s(Depth) | 0.379 | 42.8 | 314.00 | 1.19 | |
| log(CPUE + 1) = s(Year) + s(SST) + s(Chl-a) + s(Depth) + s(DO) | 0.442 | 51.2 | 307.05 | 1.12 | |
| log(CPUE + 1) = s(Year) + s(SST) + s(Chl-a) + s(Depth) + s(DO) + s(SSS) | 0.553 | 63.2 | 288.58 | 0.96 | |
| El Niño | log(CPUE + 1) = s(Year) + s(SSS) | 0.244 | 25.8 | 301.55 | 1.04 |
| log(CPUE + 1) = s(Year) + s(SSS) + s(Pre) | 0.336 | 36.8 | 290.93 | 0.95 | |
| log(CPUE + 1) = s(Year) + s(SSS) + s(Pre) + s(MLD) | 0.387 | 43.1 | 284.89 | 0.90 | |
| log(CPUE + 1) = s(Year) + s(SSS) + s(Pre) + s(MLD) + s(Depth) | 0.522 | 57.9 | 263.15 | 0.73 | |
| log(CPUE + 1) = s(Year) + s(SSS) + s(Pre) + s(MLD) + s(Depth) + s(SST) | 0.571 | 62.7 | 253.05 | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Xu, Y.; Sun, M.; Li, J.; Zhou, X.; Chen, Z.; Zhang, K. Impacts of Strong ENSO Events on Fish Communities in an Overexploited Ecosystem in the South China Sea. Biology 2023, 12, 946. https://doi.org/10.3390/biology12070946
Li M, Xu Y, Sun M, Li J, Zhou X, Chen Z, Zhang K. Impacts of Strong ENSO Events on Fish Communities in an Overexploited Ecosystem in the South China Sea. Biology. 2023; 12(7):946. https://doi.org/10.3390/biology12070946
Chicago/Turabian StyleLi, Miao, Youwei Xu, Mingshuai Sun, Jiajun Li, Xingxing Zhou, Zuozhi Chen, and Kui Zhang. 2023. "Impacts of Strong ENSO Events on Fish Communities in an Overexploited Ecosystem in the South China Sea" Biology 12, no. 7: 946. https://doi.org/10.3390/biology12070946
APA StyleLi, M., Xu, Y., Sun, M., Li, J., Zhou, X., Chen, Z., & Zhang, K. (2023). Impacts of Strong ENSO Events on Fish Communities in an Overexploited Ecosystem in the South China Sea. Biology, 12(7), 946. https://doi.org/10.3390/biology12070946








