Two Independently Comparative Transcriptome Analyses of Hemocytes Provide New Insights into Understanding the Disease-Resistant Characteristics of Shrimp against Vibrio Infection
Abstract
:Simple Summary
Abstract
1. Introduction
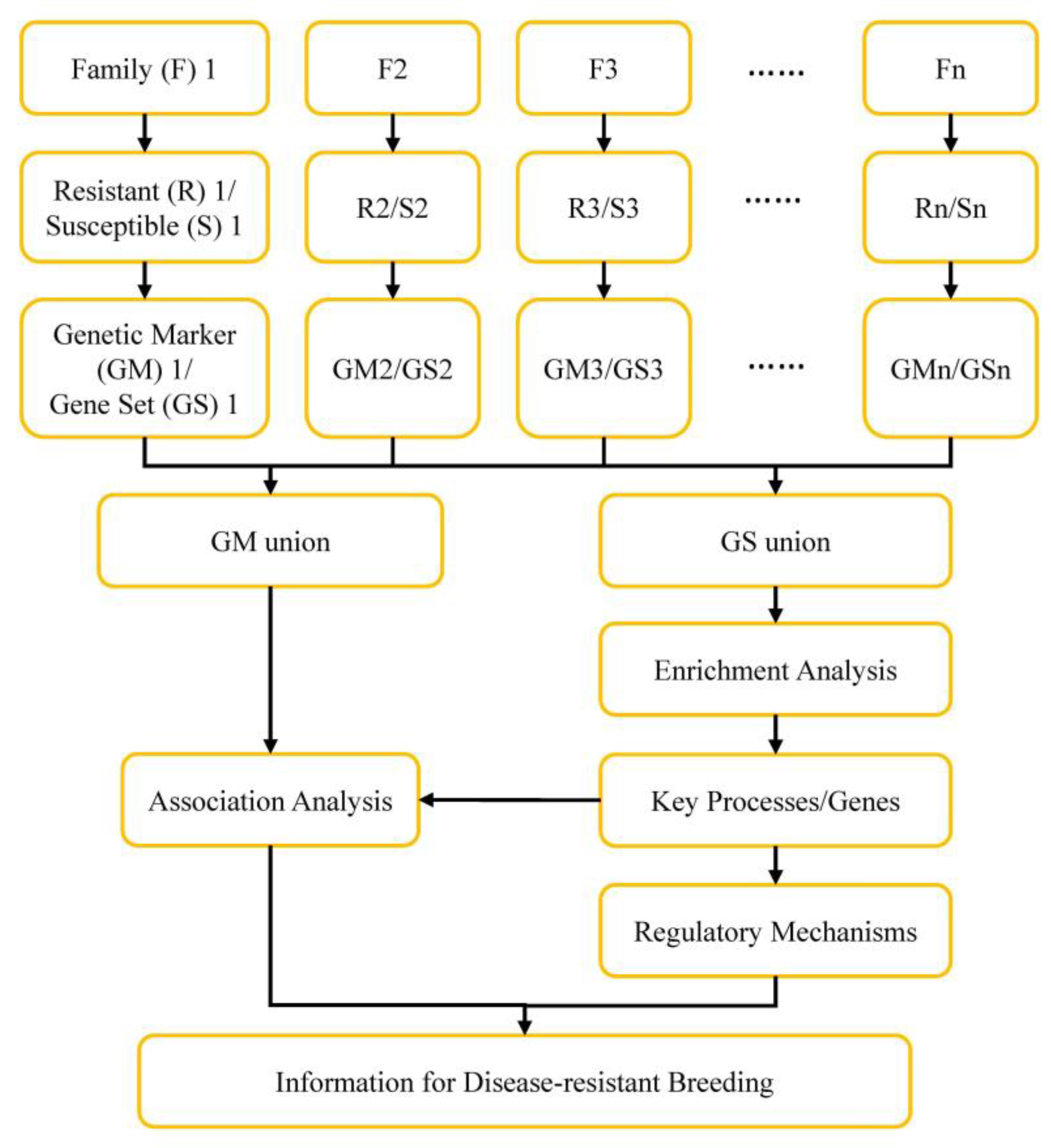
2. Materials and Methods
2.1. Experimental Animals
2.2. VIE Fluorescent Labeling and Hemolymph Collection
2.3. V. parahaemolyticus Immersion and Hepatopancreas Collection
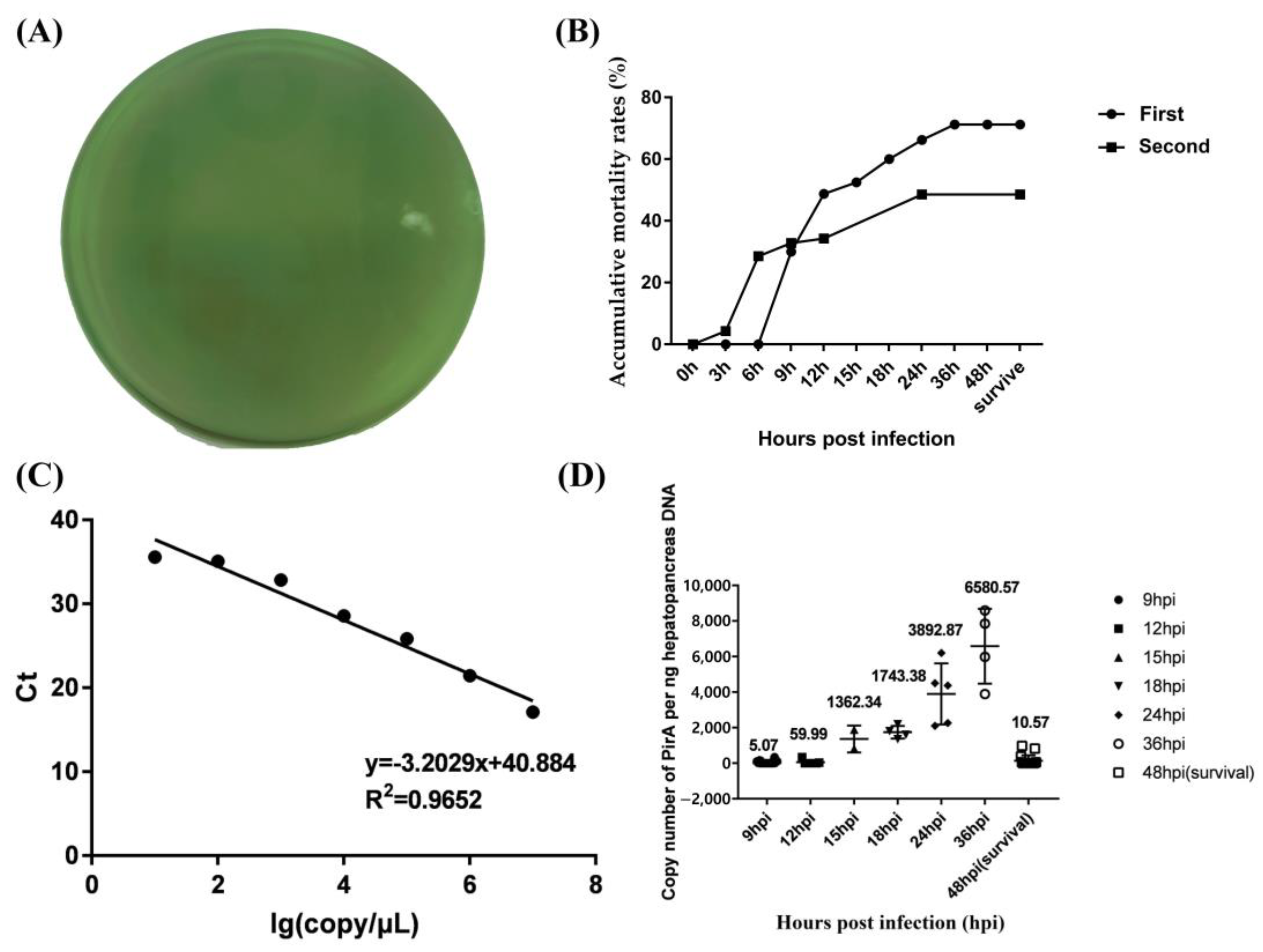
2.4. DNA Extraction and Bacteria Load Detection
2.5. RNA Extraction and Transcriptome Sequencing
2.6. Clean Data Mapping and Annotations
2.7. Differential Expression and Enrichment Analysis
2.8. Quantitative Real-Time PCR
3. Results
3.1. The Loads of V. parahaemolyticus in Hepatopancreas of Infected Shrimp
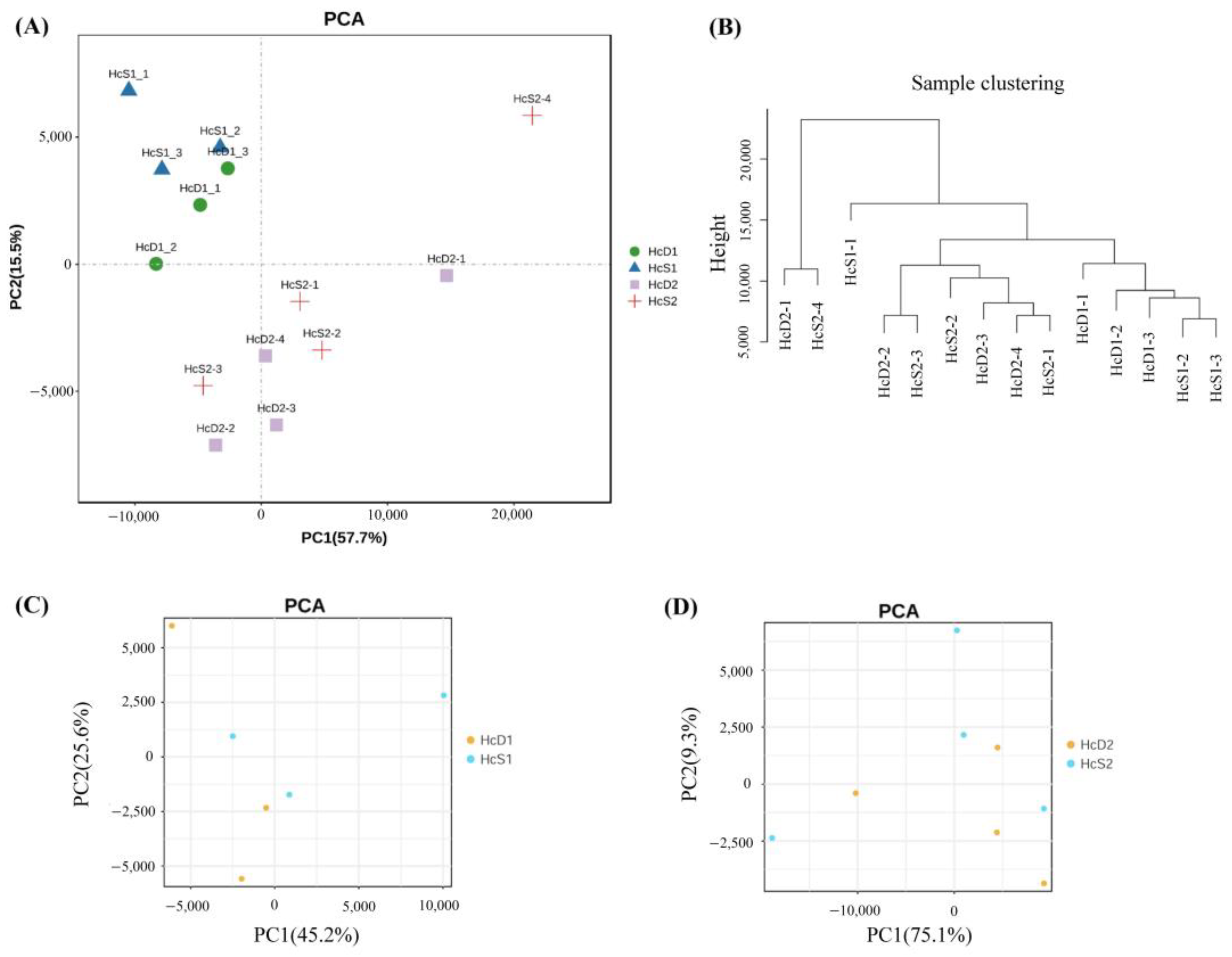
3.2. The Correlation of the Transcriptome Data from All Samples
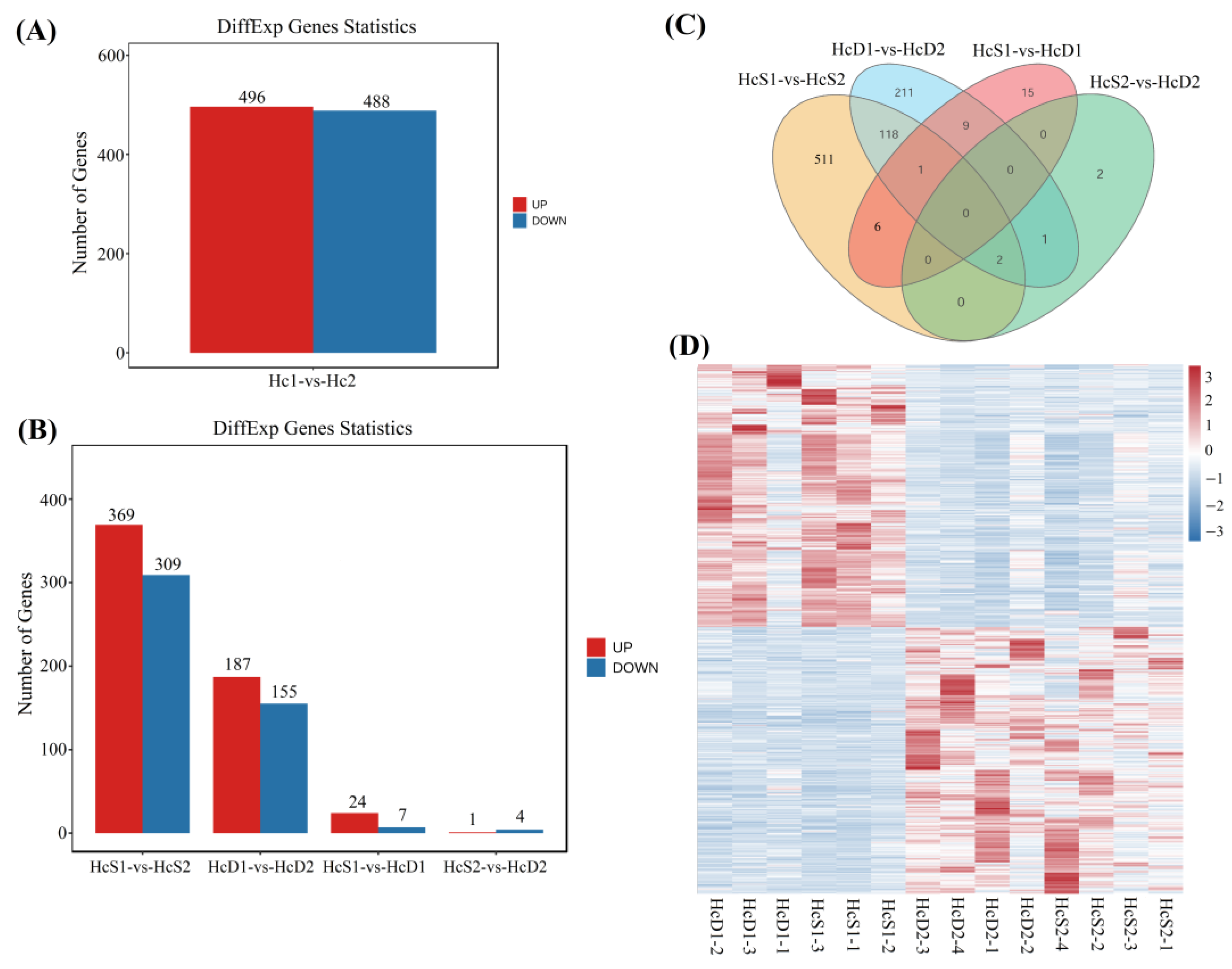
3.3. DEGs between Two Populations and between V. parahaemolyticus Resistant and Susceptible Shrimp
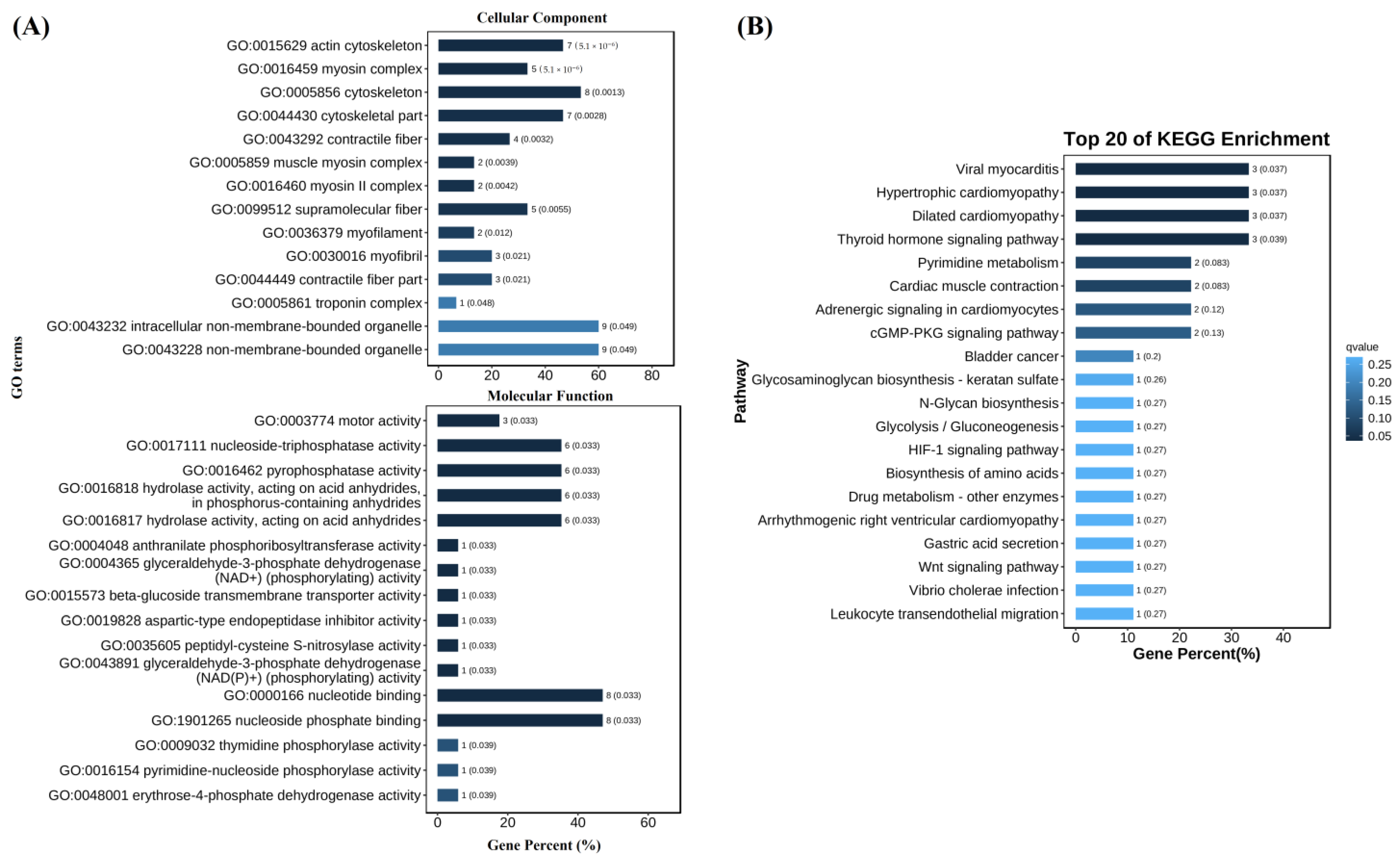
3.4. Enriched GO Items and KEGG Pathways between V. parahaemolyticus-Resistant and Susceptible Shrimp
3.5. Detailed Analysis of DEGs between V. parahaemolyticus-Resistant and Susceptible Shrimp
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHPND | acute hepatopancreatic necrosis disease |
| DEGs | differentially expressed genes |
| HIF-1 | hypoxia-inducible factor 1 |
| PI3K/Akt | phosphoinositide 3-kinase/protein kinase B |
| NF-KappaB | nuclear factor kappa-B |
| VIE | visible implant elastomer |
| EDTA | ethylene diamine tetraacetic acid |
| PCR | polymerase chain reaction |
| cDNA | complementary DNA |
| NR | non-redundant protein |
| KEGG | kyoto encyclopedia of genes and genomes |
| COG | clusters of orthologous genes |
| FPKM | fragment per kliobase of transcript per million mapped reads |
| FDR | false discovery rate |
| GO | gene ontology |
| PCA | principal component analysis |
| WSSV | white spot syndrome virus |
References
- Zorriehzahra, J.; Banaederakhshan, R. Early Mortality Syndrome (EMS) as new Emerging Threat in Shrimp Industry. Adv. Anim. Vet. Sci. 2015, 3, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Liu, L.; Ke, Y.; Li, X.; Liu, Y.; Pan, Y.; Yan, S.; Wang, Y. Shrimp AHPND-causing plasmids encoding the PirAB toxins as mediated by pirAB-Tn903 are prevalent in various Vibrio species. Sci. Rep. 2017, 7, 42177. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-T.; Chen, I.-T.; Lee, C.-T.; Chen, C.-Y.; Lin, S.-S.; Hor, L.-I.; Tseng, T.-C.; Huang, Y.-T.; Sritunyalucksana, K.; Thitamadee, S.; et al. Draft Genome Sequences of Four Strains of Vibrio parahaemolyticus, Three of Which Cause Early Mortality Syndrome/Acute Hepatopancreatic Necrosis Disease in Shrimp in China and Thailand. Genome Announc. 2014, 2, e00816-14. [Google Scholar] [CrossRef] [Green Version]
- Velázquez-Lizárraga, A.E.; Juárez-Morales, J.L.; Racotta, I.S.; Villarreal-Colmenares, H.; Valdes-Lopez, O.; Luna-González, A.; Rodríguez-Jaramillo, C.; Estrada, N.; Ascencio, F. Transcriptomic analysis of Pacific white shrimp (Litopenaeus vannamei, Boone 1931) in response to acute hepatopancreatic necrosis disease caused by Vibrio parahaemolyticus. PLoS ONE 2019, 14, e0220993. [Google Scholar] [CrossRef] [Green Version]
- Soonthornchai, W.; Chaiyapechara, S.; Klinbunga, S.; Thongda, W.; Tangphatsornruang, S.; Yoocha, T.; Jarayabhand, P.; Jiravanichpaisal, P. Differentially expressed transcripts in stomach of Penaeus monodon in response to AHPND infection analyzed by ion torrent sequencing. Dev. Comp. Immunol. 2016, 65, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Li, S.; Liu, Y.; Yu, Y.; Li, F. Transcriptome Analysis on Hepatopancreas Reveals the Metabolic Dysregulation Caused by Vibrio parahaemolyticus Infection in Litopenaeus vannamei. Biology 2023, 12, 417. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, F.; Aweya, J.J.; Li, R.; Yao, D.; Zhong, M.; Li, S.; Zhang, Y. Comparative transcriptomic analysis of shrimp hemocytes in response to acute hepatopancreas necrosis disease (AHPND) causing Vibrio parahemolyticus infection. Fish Shellfish. Immunol. 2018, 74, 10–18. [Google Scholar] [CrossRef]
- Maralit, B.A.; Jaree, P.; Boonchuen, P.; Tassanakajon, A.; Somboonwiwat, K. Differentially expressed genes in hemocytes of Litopenaeus vannamei challenged with Vibrio parahaemolyticus AHPND (VPAHPND) and VPAHPND toxin. Fish Shellfish. Immunol. 2018, 81, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.N.; Caro, L.F.A.; Cruz-Flores, R.; White, B.N.; Dhar, A.K. Differentially Expressed Genes in Hepatopancreas of Acute Hepatopancreatic Necrosis Disease Tolerant and Susceptible Shrimp (Penaeus vannamei). Front. Immunol. 2021, 12, 634152. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, Y.; Luo, Z.; Xiang, J.; Li, F. Comparison of Gene Expression Between Resistant and Susceptible Families Against VPAHPND and Identification of Biomarkers Used for Resistance Evaluation in Litopenaeus vannamei. Front. Genet. 2021, 12, 772442. [Google Scholar] [CrossRef]
- Liu, F.; Li, S.; Yu, Y.; Yuan, J.; Yu, K.; Li, F. Pathogenicity of a Vibrio owensii strain isolated from Fenneropenaeus chinensis carrying pirAB genes and causing AHPND. Aquaculture 2021, 530, 735747. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Mostowy, S.; Shenoy, A.R. The cytoskeleton in cell-autonomous immunity: Structural determinants of host defence. Nat. Rev. Immunol. 2015, 15, 559–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Lu, K.-C.; Chen, G.-L.; Li, M.; Zhang, C.-Z.; Chen, Y.-H. A Litopenaeus vannamei TRIM32 gene is involved in oxidative stress response and innate immunity. Fish Shellfish. Immunol. 2020, 107, 547–555. [Google Scholar] [CrossRef]
- Reue, K.; Lee, J.M.; Vergnes, L. Regulation of bile acid homeostasis by the intestinal Diet1-FGF15/19 axis. Curr. Opin. Lipidol. 2014, 25, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.L.; Takeda, K.; Sundrud, M.S. Emerging roles of bile acids in mucosal immunity and inflammation. Mucosal Immunol. 2019, 12, 851–861. [Google Scholar] [CrossRef] [Green Version]
- Ng, Y.S.; Lee, D.-Y.; Liu, C.-H.; Tung, C.-Y.; He, S.-T.; Wang, H.-C. White Spot Syndrome Virus Triggers a Glycolytic Pathway in Shrimp Immune Cells (Hemocytes) to Benefit Its Replication. Front. Immunol. 2022, 13, 901111. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, J.; Tay, S.; Ling, E.-A.; Dheen, S. Dihydropyrimidinase-like 3 regulates the inflammatory response of activated microglia. Neuroscience 2013, 253, 40–54. [Google Scholar] [CrossRef]
- Cui, C.; Zhu, L.; Tang, X.; Xing, J.; Sheng, X.; Zhan, W. Molecular characterization of prohibitins and their differential responses to WSSV infection in hemocyte subpopulations of Fenneropenaeus chinensis. Fish Shellfish. Immunol. 2020, 106, 296–306. [Google Scholar] [CrossRef]
- Li, J.; Shen, Y.; Chen, Y.; Zhang, Z.; Ma, S.; Wan, Q.; Tong, Q.; Glaubitz, C.; Liu, M.; Yang, J. Structure of membrane diacylglycerol kinase in lipid bilayers. Commun. Biol. 2021, 4, 282. [Google Scholar] [CrossRef] [PubMed]
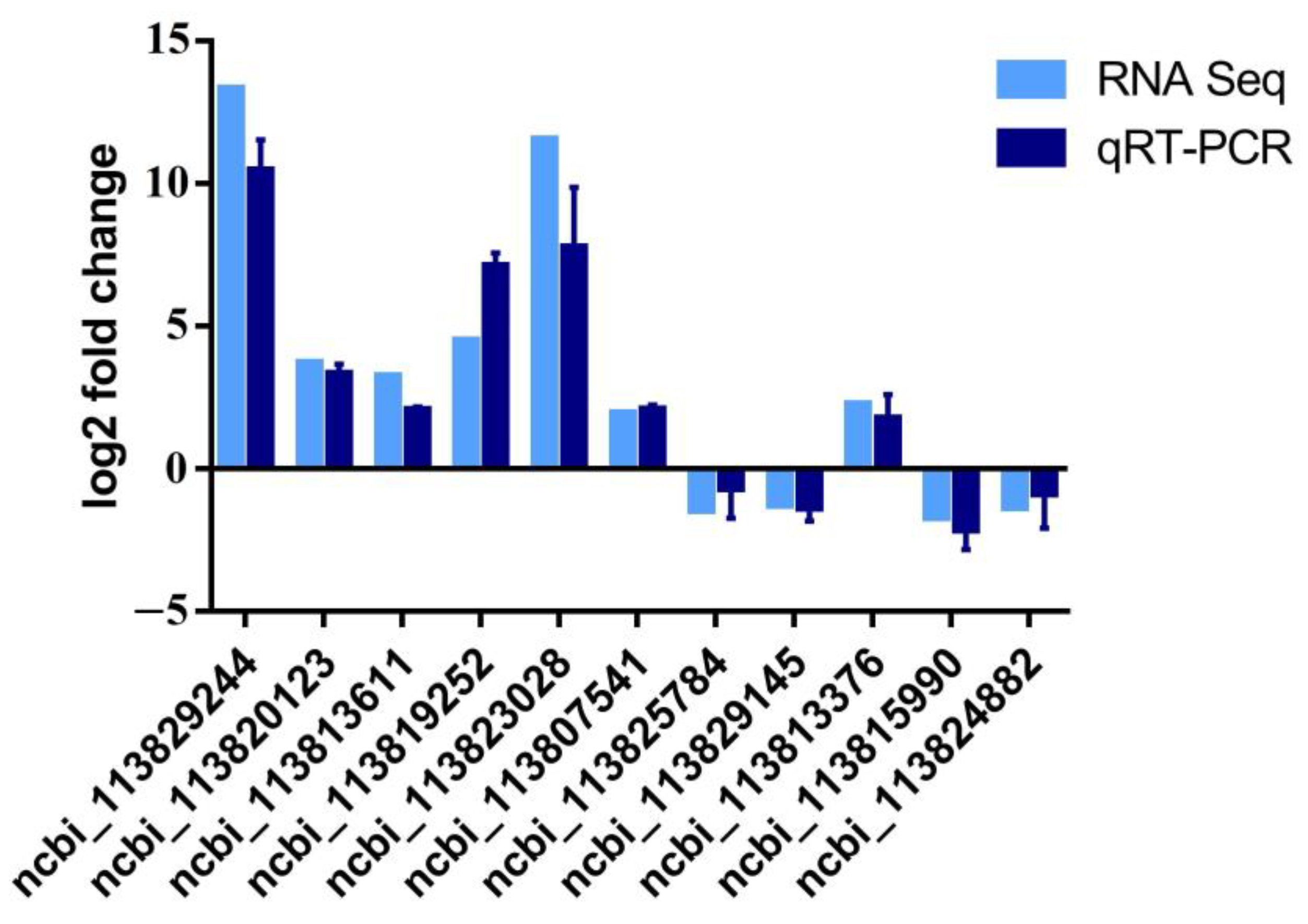
| Gene ID | HcS1_fpkm | HcD1_fpkm | log2(HcD1/HcS1) | FDR | Description |
|---|---|---|---|---|---|
| ncbi_113820920 | 0.40 | 0.00 | −8.63 | 0.0376 | structural maintenance of chromosomes protein 2-like |
| ncbi_113815624 | 0.71 | 0.08 | −3.13 | 0.0154 | vang-like protein 2 |
| MSTRG.1662 | 5.77 | 1.38 | −2.07 | 0.0017 | APC membrane recruitment protein 1-like |
| ncbi_113812130 | 67.07 | 109.46 | 0.71 | 0.0499 | 4-coumarate—CoA ligase 1 isoform X1 |
| ncbi_113824615 | 186.25 | 373.56 | 1.00 | 0.0001 | spermatogonial stem-cell renewal factor |
| MSTRG.4583 | 11.36 | 42.55 | 1.91 | 0.0154 | arasin-like protein |
| ncbi_113807541 | 51.35 | 197.85 | 1.95 | 0.0397 | immune-associated nucleotide-binding protein 13-like |
| ncbi_113817613 | 5.39 | 25.35 | 2.23 | 0.0027 | E3 ubiquitin-protein ligase TRIM32 |
| ncbi_113824399 | 82.06 | 388.92 | 2.24 | 0.0023 | Septin-4-like protein |
| ncbi_113813611 | 0.34 | 3.21 | 3.24 | 0.0373 | troponin I |
| ncbi_113805465 | 0.66 | 6.27 | 3.25 | 0.0017 | myosin light chain 2 |
| ncbi_113822686 | 0.65 | 7.58 | 3.55 | 0.0001 | myosin light chain |
| ncbi_113820123 | 5.27 | 68.89 | 3.71 | 0.0000 | glyceraldehyde-3-phosphate-dehydrogenase |
| ncbi_113819252 | 0.12 | 2.74 | 4.50 | 0.0154 | actin 2 |
| ncbi_113816511 | 0.04 | 0.95 | 4.64 | 0.0035 | myosin heavy chain, muscle-like isoform X4 |
| MSTRG.15220 | 0.18 | 7.35 | 5.34 | 0.0002 | Retrovirus-related Pol polyprotein from transposon 297 |
| ncbi_113807016 | 0.01 | 0.43 | 6.44 | 0.0411 | myosin heavy chain, muscle-like isoform X8 |
| ncbi_113823028 | 0.00 | 2.97 | 11.53 | 0.0000 | alpha-(1,6)-fucosyltransferase-like |
| ncbi_113829244 | 0.00 | 10.32 | 13.33 | 0.0000 | MAM and LDL-receptor class A domain-containing protein 2-like |
| Gene ID | HcS2_fpkm | HcD2_fpkm | log2(HcD2/HcS2) | FDR | Description |
|---|---|---|---|---|---|
| ncbi_113815990 | 11.16 | 3.46 | −1.69 | 0.0184 | dihydropyrimidinase-like isoform X3 |
| ncbi_113825784 | 13.80 | 5.06 | −1.45 | 0.0473 | prohibitin |
| ncbi_113824882 | 5.22 | 2.06 | −1.34 | 0.0184 | iroquois-class homeodomain protein IRX-2-like |
| ncbi_113829145 | 6.34 | 2.66 | −1.25 | 0.0402 | diacylglycerol kinase 1 |
| ncbi_113813376 | 1.43 | 6.81 | 2.26 | 0.0142 | tubulin alpha-3 chain-like |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zhang, K.; Du, W.; Li, F. Two Independently Comparative Transcriptome Analyses of Hemocytes Provide New Insights into Understanding the Disease-Resistant Characteristics of Shrimp against Vibrio Infection. Biology 2023, 12, 977. https://doi.org/10.3390/biology12070977
Li S, Zhang K, Du W, Li F. Two Independently Comparative Transcriptome Analyses of Hemocytes Provide New Insights into Understanding the Disease-Resistant Characteristics of Shrimp against Vibrio Infection. Biology. 2023; 12(7):977. https://doi.org/10.3390/biology12070977
Chicago/Turabian StyleLi, Shihao, Keke Zhang, Wenran Du, and Fuhua Li. 2023. "Two Independently Comparative Transcriptome Analyses of Hemocytes Provide New Insights into Understanding the Disease-Resistant Characteristics of Shrimp against Vibrio Infection" Biology 12, no. 7: 977. https://doi.org/10.3390/biology12070977
APA StyleLi, S., Zhang, K., Du, W., & Li, F. (2023). Two Independently Comparative Transcriptome Analyses of Hemocytes Provide New Insights into Understanding the Disease-Resistant Characteristics of Shrimp against Vibrio Infection. Biology, 12(7), 977. https://doi.org/10.3390/biology12070977







