Simple Summary
An in-depth study of the feeding habits characterizing bioinvaders may provide key information on the magnitude of their impacts on recipient communities. Specifically, if invaders’ trophic niche is superimposed on that of native species, interspecific competition may increase, resulting in negative consequences for the competing species; alternatively, trophic niche divergence may occur, facilitating the invaders’ integration into the community. In the present study, the analysis of carbon and nitrogen stable isotopes was used to investigate the trophic overlap of native and non-indigenous consumers. We found a generally low degree of isotopic overlap in both the invertebrate and fish assemblage, a condition that may facilitate coexistence and, in turn, limit the strength of invaders’ impact. The only exception was the Louisiana crayfish Procambarus clarkii, which was demonstrated to interact with a wide spectrum of native invertebrate species, confirming the necessity of guaranteeing appropriate measures of control and mitigation of its ecological impacts.
Abstract
An advanced characterization of the trophic niche of non-indigenous species (NIS) may provide useful information on their ecological impact on invaded communities. Here, we used carbon and nitrogen stable isotopes to estimate pairwise niche overlaps between non-indigenous and native consumers in the winter food web of Lake Trasimeno (central Italy). Overall, a relatively low pairwise overlap of isotopic niches was observed between NIS and native species. The only exception was the Louisiana crayfish Procambarus clarkii, which showed a relatively high and diffuse overlap with other native invertebrates. Our findings highlighted a high niche divergence between non-indigenous and native species in Lake Trasimeno, suggesting a potentially low degree of interspecific competition that may facilitate coexistence and, in turn, limit the strength of impacts. The divergent results obtained for the Louisiana crayfish indicate that additional control measures for this invasive species are needed to mitigate its impact on the Lake Trasimeno system.
1. Introduction
The introduction and establishment of non-indigenous species (NIS hereafter) represents one of the most important anthropogenic threats to the biodiversity, functioning, and integrity of freshwater ecosystems [1,2]. This is particularly evident when NIS establish themselves and become invasive, altering the structure and functions of recipient communities and whole ecosystems and ultimately causing environmental and economic harm [3,4,5,6].
A growing body of evidence is accumulating on the negative impacts of bioinvaders in both lentic and lotic environments, but an overwhelming majority of these investigations are focused on the effects of single species or single taxonomic groups [7]; see also the studies used for the meta-analyses performed by, e.g., [8,9,10,11]. In freshwater environments, however, repeated introductions may take place, resulting in a generalized occurrence of multispecific NIS assemblages [12,13]. Multiple invaders are recognized to exert a wide spectrum of cumulative effects, from synergistic to negligible to antagonistic [14,15,16,17]; yet, compared with plant species, multiple animal invasions have generally received less attention, in particular in freshwater ecosystems [18]. The establishment of invasive consumers necessarily implies a “rewiring” of trophic interactions directly through predation or indirectly through competition [19,20,21,22,23]. From this perspective, the study of interspecific feeding interactions at a whole-food-web scale may provide important insights into the combined ecological effects and impacts of multiple invaders on native species.
In the present study, we focused on Lake Trasimeno, a laminar basin in central Italy characterized by a diverse assemblage of non-indigenous species of fish and invertebrates [24]. The investigation was performed in winter with the aim of estimating the trophic overlap between NIS and native species and to assess their potential for competition. If trophic resources are limiting, NIS establishment within a recipient community can generally result in increased interspecific competition; this may ultimately be reflected in competitive exclusion and local extinction of the inferior competitor or, alternatively, in trophic niche divergence, which would facilitate the integration of invaders and their coexistence with indigenous species [25,26,27]. Here, we conjectured a generally high degree of trophic overlap and potential for competition between native and non-indigenous consumers, given the occurrence of highly invasive species in both the invertebrate and fish assemblages of the lake (see further in the next sections). However, in temperate lakes, the abundances of primary producers, intermediate consumers, and top predators undergo strong seasonal fluctuations with minima generally observed in winter; see, e.g., [28,29,30] for Lake Trasimeno. Accordingly, an alternative hypothesis was that the low abundances and metabolic requirements of native and non-indigenous consumers in the colder season should correspond to conditions of weak competitive interactions and reduced trophic overlap [31,32]. We tested these hypotheses using stable isotope analysis (SIA). This methodology has in the last decades gained huge popularity in the study of aquatic food webs and the assessment of the response of marine and freshwater ecosystems to anthropogenic pressures, including bioinvasions [33,34]. In recent years, methods for estimating trophic niche space—conventionally relying on direct observations and stomach content analysis—have improved by integrating SIA-based approaches (“isotopic niche” in [35,36]). The δ13C and δ15N values of living organisms vary at both an inter- and intraspecific level; accordingly, consumers will occupy a different isotopic space depending on the resources they exploit, making an organism’s isotopic niche a useful proxy of its trophic niche [37,38]. Carbon and nitrogen stable isotopes were measured in winter in fish, invertebrates, and basal resources collected from a littoral site of the lake. We generated Bayesian standard ellipse areas of each species representing relative niche widths in the isotopic space, and pairwise overlaps were computed. In addition, the mean proportional overlap between the native and non-indigenous assemblages (all species combined) was used as a metric for estimating the cumulative influence of the latter on the transfer of energy and matter in the lacustrine food web [39,40,41].
2. Materials and Methods
2.1. Site Description
This study was performed in Lake Trasimeno (43.133283° N, 12.100064° E, central Italy; Figure 1). The basin is the largest laminar lake in Italy (124 km2, average depth: 4.7 m, maximum depth: 6.3 m). It is located 257 m above sea level, it has a single artificial effluent, and it is fed by several ephemeral creeks. Given the relatively small extension of the watershed (396 km2), its hydrological regime is driven by precipitation, and strong seasonal and interannual oscillations in water level and chemistry are observed [42,43]. Further details on the lake’s morphometric and hydrological characteristics are provided in Ludovisi and Gaino [42] and in Bresciani et al. [44].
Figure 1.
Lake Trasimeno. The study location is highlighted in red.
The lake is included in a regional natural park within the Natura 2000 European network. The littoral zones are generally muddy and dominated by common reeds (Phragmites australis (Cav.) Trin. ex Steud.), with dense beds of aquatic macrophytes of the genera Myriophyllum, Stuckenia, and Vallisneria extending in summer especially along the southern coasts of the lake [45]. The native macroinvertebrate community includes a diverse assemblage of annelid, mollusk, insect, and crustacean taxa [46]. Conversely, the assemblage of native fish species currently comprises only five species, i.e., Esox cisalpinus Bianco & Delmastro 2011, Anguilla anguilla Linneus 1758, Tinca tinca Linnaeus 1758, Scardinius erythrophthalmus Linnaeus, 1758, and Squalius squalus Bonaparte 1837. Two additional species, Sarmarutilus rubilio Bonaparte 1837 and Cobitis bilineata Canestrini 1865, are considered to be extinct since the 1970s [46,47].
Phytoplankton are characterized by wide seasonal fluctuations and are dominated in summer by the cyanophyceans Cylindrospermopsis spp., whereas in winter, chlorophyceans of the genera Hyaloraphidium and Scenedesmus together with cyanophyceans of the genus Leptolyngbya represent the most abundant taxa [48,49]. Similarly, remarkable seasonal variations characterize the abundance and composition of the zooplankton, dominated in summer by the cladoceran Daphnia galeata Sars 1864 and by copepods of the genus Cyclops in winter [48,50,51]. During the last century, the lacustrine community has been drastically altered by the introduction of several NIS of different origins (Table 1). They comprise invertebrates (e.g., the decapods Procambarus clarkii and Dikerogammarus villosus, the bivalves Dreissena polymorpha and Sinanodonta woodiana, and the tubificid Branchiura sowerbyi) and 15 species of fish, including Ameiurus melas, Carassius auratus, Lepomis gibbosus, Micropterus salmoides, and Perca fluviatilis [24,47,52].

Table 1.
Non-indigenous invertebrate and fish species currently occurring in Lake Trasimeno. Information on the years of first record were collated from [24,53,54,55,56].
2.2. Sample Collection and Laboratory Procedures
In early February 2018, fish and crayfish were captured by fishers operating fyke nets in Sant’Arcangelo, a locality in the southern sector of the lake (43.089788° N, 12.156246° E). Nets were located at an approximate distance of 50 m from the coast at a depth of 2–2.5 m. Collected specimens were transferred to the laboratory in refrigerated containers (4 °C), where they were euthanized by thermal shock (−80 °C for 10 min), identified to the lowest taxonomic level possible, and enumerated. Subsequently, a ruler was used to determine to the nearest mm the total standard length of fish specimens (i.e., from the tip of the snout to the posterior end of the last vertebra); a caliper was used to measure in mm the total length of crustacean individuals from the tip of the rostrum to the end of the telson.
For stable isotope analysis, the dorsal muscle tissue of fish and the caudal muscle of crustaceans was dissected from each specimen using a ceramic scalpel, dried (60 °C, >1 week), and powdered using a mortar and a pestle. Subsequently, subsamples (0.8 ± 0.02 mg, mean ± 1 SE) were pressed into Ultra-Pure tin capsules (Costech Analytical Technologies, Valencia, CA, USA) and analyzed using an elemental analyzer connected to an isotope ratio mass spectrometer (Thermo Scientific Flash EA 1112, Waltham, MA, USA and Delta Plus XP, Suzhou, China, respectively). Isotopic contents were expressed in conventional δ unit notation as ‰ deviations from international standards:
where R = 13C/12C or 15N/14N. Pee Dee belemnite (PDB) limestone carbonate and atmospheric nitrogen (N2) were used as standards for carbon and nitrogen isotope ratios, respectively. Analytical precision based on the standard deviation of replicates of internal standards (International Atomic Energy Agency IAEA-NO-3 for δ15N and IAEA-CH-6 for δ13C) was 0.2‰ for both δ15N and δ13C.
Invertebrates were collected in a shallow embayment (approximate depth = 1 m) situated in front of the area where fish and crayfish were sampled. Details of the location are provided in Mancinelli et al. [57], while information on the sampling procedures are included in Mancini et al. [58] and Ludovisi et al. [59]. In brief, the embayment has muddy bottoms and artificial rocky shores, characterized in winter by accumulations of decaying plant material, including P. australis leaf litter and mixed detritus of macrophytes belonging to the genera Myriophyllum, Potamogeton, and Vallisneria. At the time of the sampling, in the embayment, the temperature and dissolved oxygen concentration of surficial water (depth = 10–12 cm) were 12.3 ± 0.2 °C and 9.8 ± 0.8 mg L−1, respectively (mean ± 1 SE, n = 3; YSI® 556 MPS, Yellow Springs, OH, USA). A handheld pond net (mesh size = 1 mm) was swept five times in floating leaf litter accumulations to capture macroinvertebrates; additional specimens were collected by hand from rocks and artificial hard substrata. Samples were subsequently placed in Falcon tubes or in other plastic buckets containing filtered lake water. Samples of the superficial sediment layer (3 replicates) were collected using a methacrylate core (400 mm length, 114 mm ∅) driven into the sediment to a depth of approximately 10 cm and were then transferred to plastic bags.
All samples were transported in refrigerated containers (4 °C) to the laboratory, where invertebrates were identified to the lowest taxonomic level, enumerated, kept in distilled water for 12 h to clear gut contents, and eventually euthanized by thermal shock (−80 °C for 10 min). The total length of each individual was determined to the nearest 0.1 mm either using a digital caliper for palaemonids (see Results (Section 3)) or a stereo microscope (Nikon® SMZ1270, Tokyo, Japan) equipped with a CCD camera for the remaining taxa. For gastropods, the maximum shell length was measured. Subsequently, the caudal muscle of each palaemonid was dissected and dried (60 °C, >1 week); all the remaining invertebrates were dried whole with the exclusion of gastropods, which had their shells removed. Sediment samples were wet-sieved on a 1 mm screen; invertebrates retained in the sieve were collected and processed according to the procedures already described.
The isotopic data obtained in the present investigation were complemented with those published in Mancini et al. [58] and Ludovisi et al. [59]. They were performed at the same location as this study and include δ13C and δ15N values of benthic invertebrates and zooplankton. The study by Ludovisi et al. [59] was carried out in 2018 and coincided with the present investigation, while that of Mancini et al. [58] was performed in February 2016. The isotopic characteristics of the components of the food web at the study site were assumed to have remained unchanged between the sampling years. The assumption was tested for a subset of invertebrate species (Appendix A; see the next section).
2.3. Data Analysis
In general, all statistical analyses were carried out in the R statistical environment v. 4.2.2 [60]. For univariate analyses, data were checked for normality and homoscedasticity (Shapiro–Wilks and Levene’s tests, respectively) and log- or square root-transformed if required. When significant effects were detected by ANOVA tests, post hoc bivariate comparisons were performed using Tukey HSD tests. Bivariate relationships were tested using Pearson’s coefficient of correlation, while F-tests were used to verify differences between slopes or intercepts.
To preliminarily verify whether the invertebrate taxa sampled in 2016 and 2018 (Appendix A) differed significantly between years in their isotopic values and CN contents, a Euclidean distance similarity matrix of Z-scaled δ13C, δ15N, and total carbon and nitrogen (both expressed as %) values was calculated. Subsequently, a type III (partial) permutational analysis of variance [61] (PERMANOVA hereafter) with 9999 permutations was performed using the function adonis in the vegan package v. 2.6-2 [62] with “species” and “year” as the fixed and random factors, respectively. Since no significant effects were detected (see Results), the two isotopic datasets were cumulated and treated in further analyses as one.
Independently from the sampling date, the tissues of several invertebrates and fish showed C:N ratios > 3.5 (Appendix A), indicating a significant contribution of lipids to the tissues’ carbon pool [63]. Since lipids are depleted in 13C compared to proteins and carbohydrates and can significantly bias δ13C estimations [64], samples with a C:N ratio > 3.5 had their δ13C values mathematically corrected for lipid content [63].
Isotopic niche overlaps for non-indigenous and native consumers were estimated using the SIBER package v. 2.1.6 [65]; consumers’ standard ellipse area (SEA, expressed in ‰2) was used as a measure of the core population isotopic niche [66]. Given the different number of specimens per taxon included in the analyses (Appendix A), we calculated a sample-size-corrected version (SEAc hereafter) [66] representing the core (40%) isotopic niche area and allowing for robust comparisons across species of varying sample sizes. SEAc estimations were used for illustrative purposes; for interspecific statistical comparisons, we calculated the Bayesian equivalent SEAB of SEAc [66] using 100,000 posterior iterations of SEAc to compute credible intervals. Specifically, pairwise contrasts were performed by calculating the probability that the SEAB of one species was different from that of a second one with a probability of at least 95% [66].
SEAc estimations were further used to calculate interspecific isotopic niche overlaps. They were determined assuming negligible competitive interactions between invertebrate and fish consumers; in addition, for each of the two groups, overlaps were calculated between NIS and native species as well within NIS and native assemblages separately.
Overlaps between two species were expressed as the % ratio of the estimated overlap itself and the sum of the nonoverlapping area of the ellipses for each species [66], a measure conceptually consistent with other classical symmetric indices, such as Pianka’s niche overlap index O [67]. Overlaps were considered significant when the shared isotopic space between species was >60%, a criterion identical to that used by Schoener for his dietary overlap index [68].
3. Results
3.1. Invertebrates
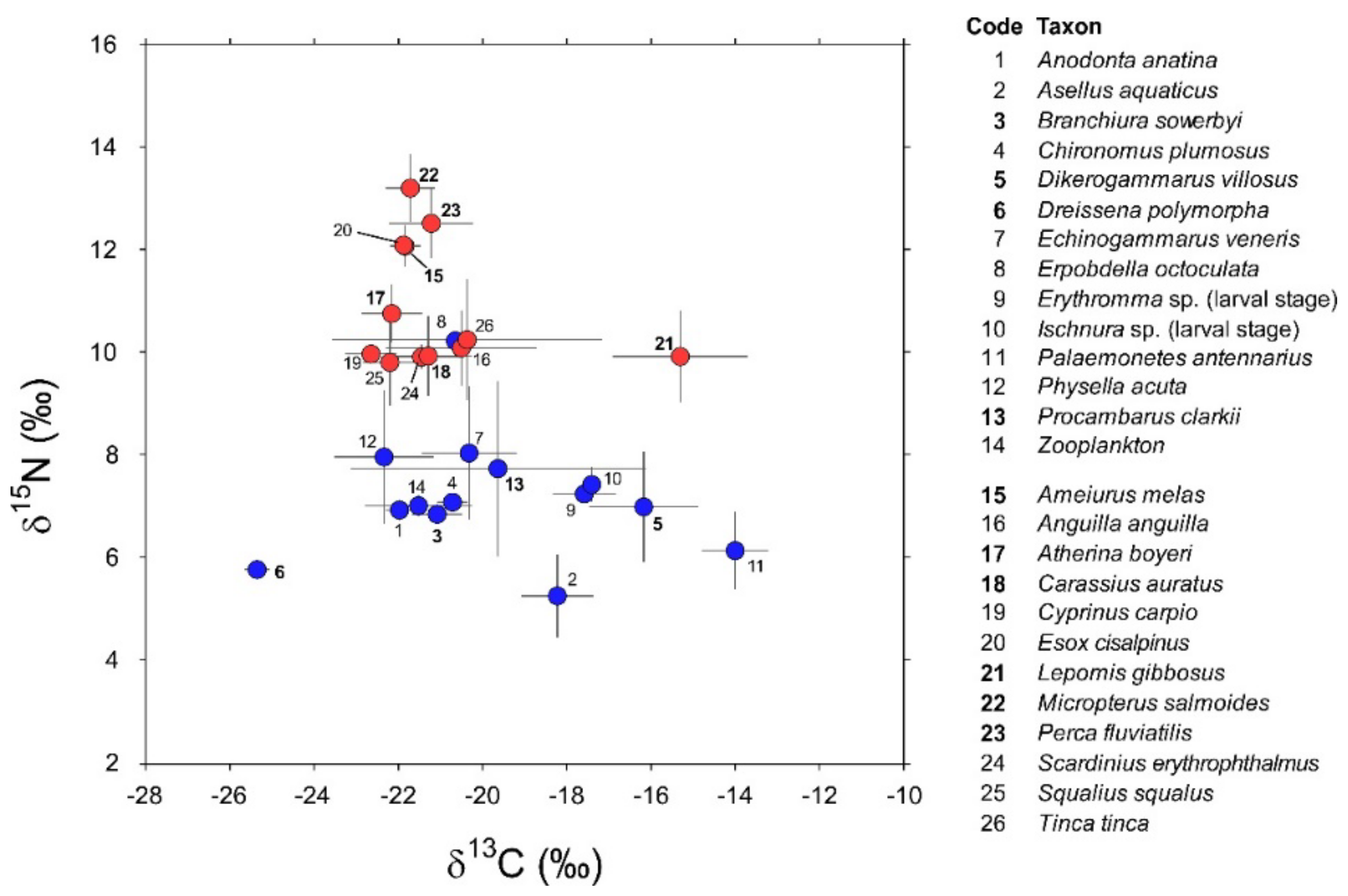
Invertebrate taxa sampled in 2016 and 2018 (Appendix A) showed negligible between-year variations in their isotopic values (PERMANOVA, factor “year”: Pseudo-F1,72 = 0.79, PMonte Carlo = 0.44; interaction factor “species × year”: Pseudo-F5,61 = 0.3, PMonte Carlo = 0.97). Additionally, post hoc taxon-specific comparisons highlighted negligible temporal differences (min t = 1.16, PMonte Carlo = 0.27 for P. clarkii). Accordingly, the data collected during the two sampling occasions were cumulated and treated as a single dataset containing isotopic measurements for a total of 163 specimens belonging to 14 invertebrate taxa (Appendix A). Their mean δ13C values varied considerably between −25.6 in the zebra mussel D. polymorpha and −14.1 in the pond shrimp P. antennarius (Figure 2).
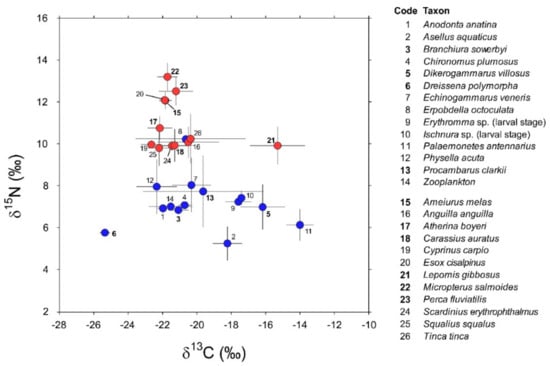
Figure 2.
δ13C and δ15N values (Mean ± 1 SD) of consumers (invertebrates: codes 1–14; fish: codes 15–26; blue and red circles, respectively) characterizing the food web of Lake Trasimeno in winter. Non-indigenous species are indicated in bold.
The isopod Asellus aquaticus and the leech Erpobdella octoculata were the most depleted and enriched in 15N, respectively (Figure 2; 5.2 ± 0.8 vs. 10.2 ± 0.2, mean ± 1 SD). Overall, invertebrates showed significant interspecific differences in isotopic composition (PERMANOVA, Pseudo-F13,162 = 26.3, PMonte Carlo = 0.0001); this result was generally confirmed by further post hoc bivariate comparisons (Table A2 in Appendix B). Noticeable exceptions were Chironomus plumosus vs. Branchiura sowerbyi and Echinogammarus veneris and, most importantly, Procambarus clarkii vs. a taxonomically heterogeneous assemblage including most of the invertebrates with the exclusion of Dikerogammarus villosus, Dreissena polymorpha, and Physella acuta (Table A2 in Appendix B).
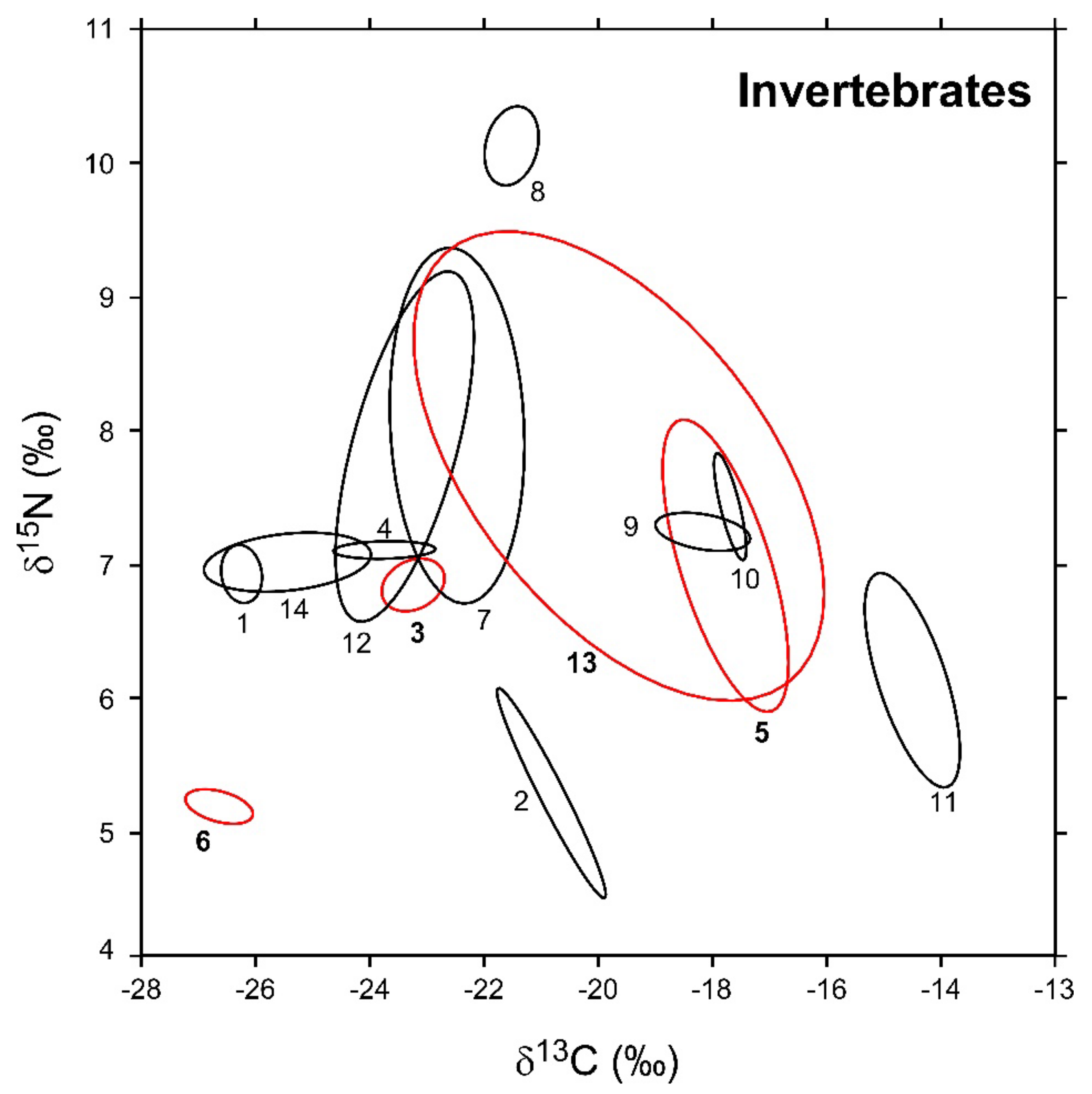
In Figure 3, the sample-size-corrected standard ellipse areas (SEAc) of invertebrate consumers are illustrated, while numerical values are reported in Table 2 together with the respective Bayesian estimates (SEAB). Overall, modal SEAB and SEAc values were in good agreement, and the latter always fell within SEAB 95% confidence intervals. The robustness of Bayesian estimations against potential biases related to variations in sample size was corroborated by the negligible relationship observed between SEAB values and the number of specimens analyzed for each taxa (Pearson r = 0.27, p = 0.35, d.f. = 12).
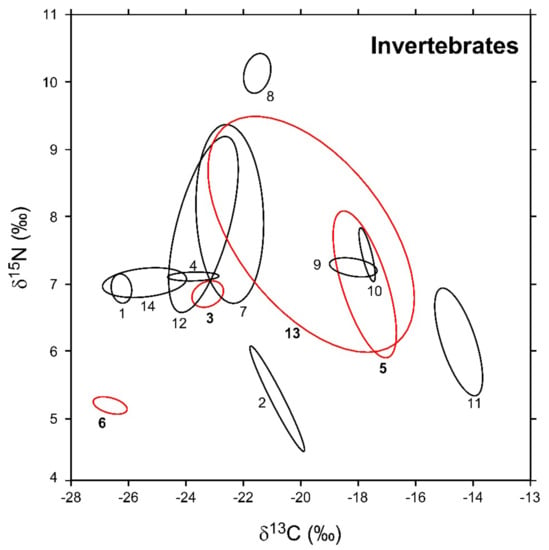
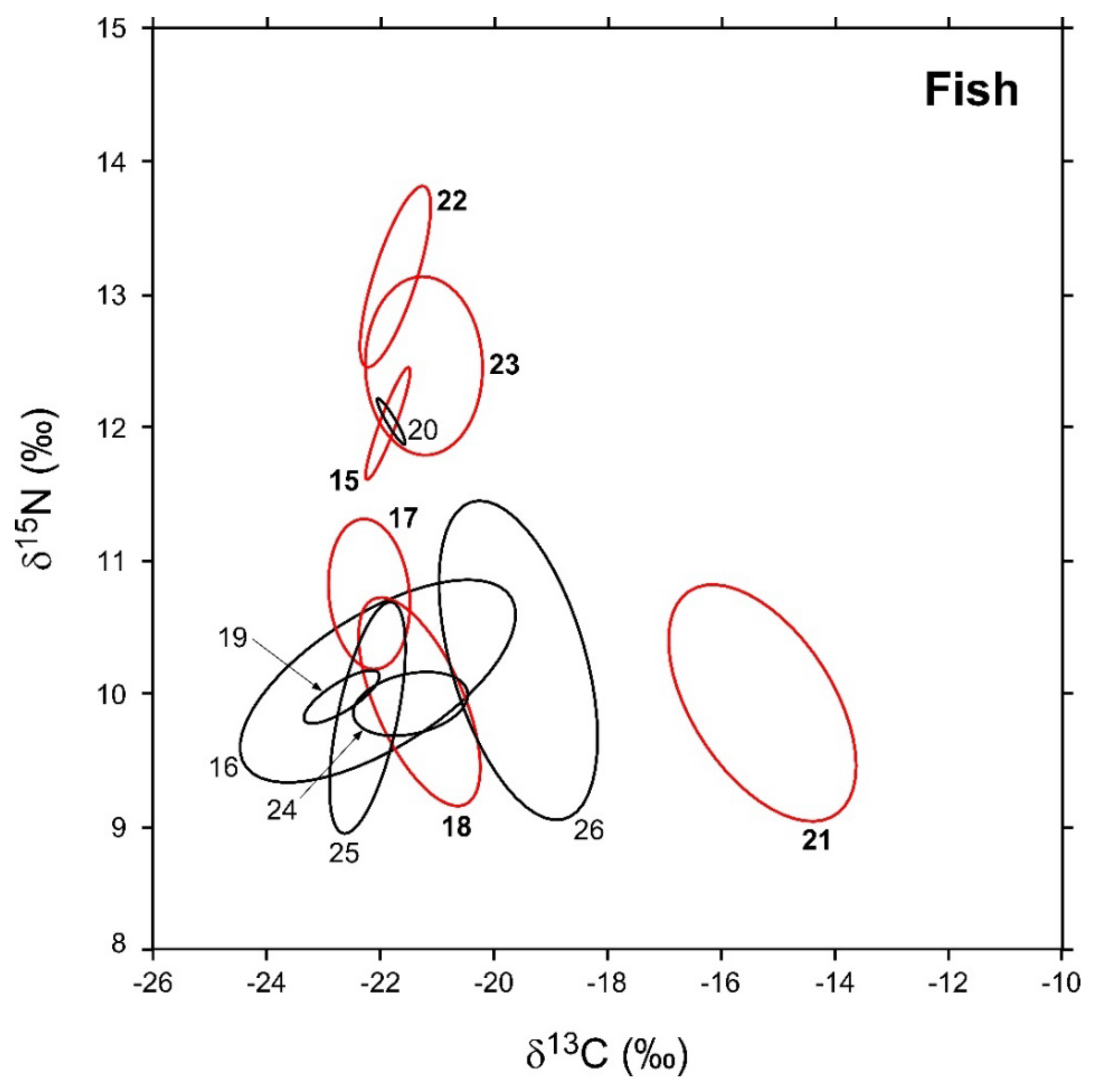
Figure 3.
Sample-size-corrected Bayesian standard ellipse areas (SEAc) calculated from δ13C and δ15N values of invertebrate consumers. Black and red ellipses refer to native and non-indigenous taxa, respectively; see Figure 2 for the corresponding identification codes.

Table 2.
SEAc and SEAB of invertebrate consumers expressed as ‰2. For SEAB, modal values and the corresponding 95% confidence intervals (in italics) are included.
Isotopic niche areas varied across the different taxa by a factor > 100 (Table 2). Among NIS, P. clarkii showed the highest SEAB value (16.9‰2), one order of magnitude larger than D. villosus (2.8‰2). B. sowerbyi and D. polymorpha, conversely, were characterized by the lowest SEAB estimations (0.3 and 0.2‰2, respectively). Bivariate comparisons indicated significant differences in isotopic niche areas among all the taxa with the exclusion of B. sowerbyi and D. polymorpha (probability tests, B. sowerbyi ≠ D. polymorpha: p = 0.48; p > 0.95 for all the remaining comparisons). Similar to what was observed for NIS, native invertebrates showed a high heterogeneity in their modal SEAB estimations (Table 2); P. acuta showed the largest value, close to that of E. veneris (4.7 and 4.5‰2, respectively; probability test p = 0.62). In turn, they were significantly different from SEAB values determined for both P. clarkii and D. villosus (probability tests, p always > 0.95). In contrast, Ischnura sp. and C. plumosus showed the smallest SEAB (0.1‰2). Erythromma sp., A. anatina, and E. octoculata showed areas ranging between 0.3 and 0.2‰2 (p < 0.95 for all bivariate comparisons), whereas for a second taxonomically heterogeneous group including P. antennarius, zooplanktonic crustaceans, and A. aquaticus, the estimated areas ranged between 1.6 and 1‰2 (p < 0.95 for all bivariate comparisons).
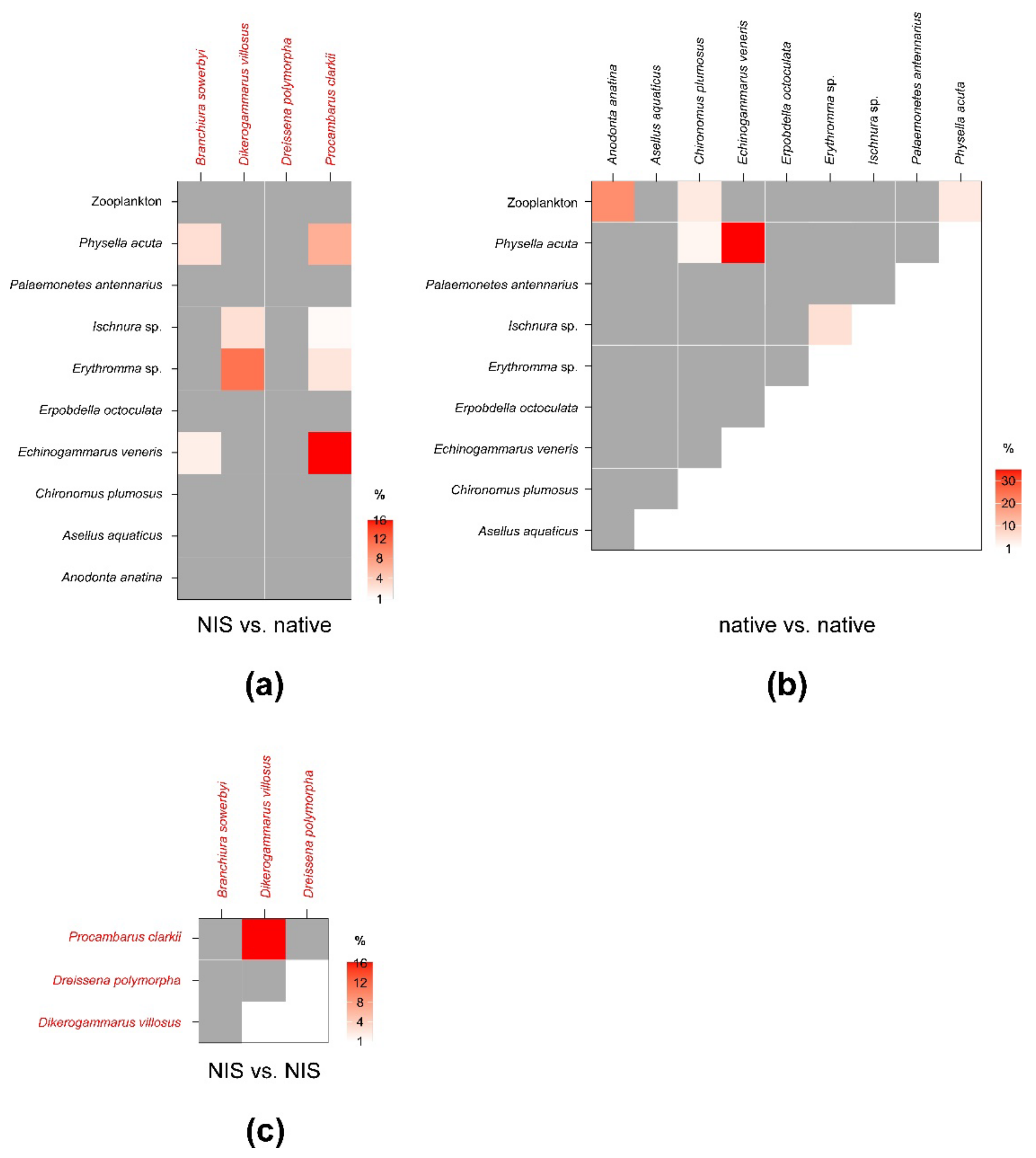
SEAc and SEAB percent overlaps between NIS and native invertebrate species were generally low and well below the critical limit of 60% (Figure 4; see Table A4 in Appendix C for modal SEAB values and the respective 95% confidence intervals). The mean of nonzero SEAc and SEAB % overlaps was 3.3% and 3.4%, respectively, with values ranging between a minimum approximating 0% for P. clarkii vs. A. aquaticus and a maximum of 16 (SEAc)–18% (SEAB) for P. clarkii vs. E. veneris. Noticeably, the mean % overlap among native species was 7.1% and 6.4% (SEAc and SEAB % overlaps, respectively), with values ranging between a minimum approximating 0% for E. veneris vs. A. aquaticus and a maximum of 35.2 (SEAc)–30.8% (SEAB) for E. veneris vs. P. acuta (Figure 4; Table A5 in Appendix C).
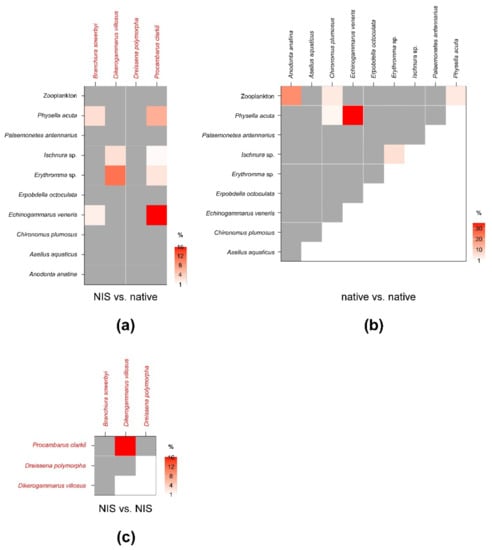
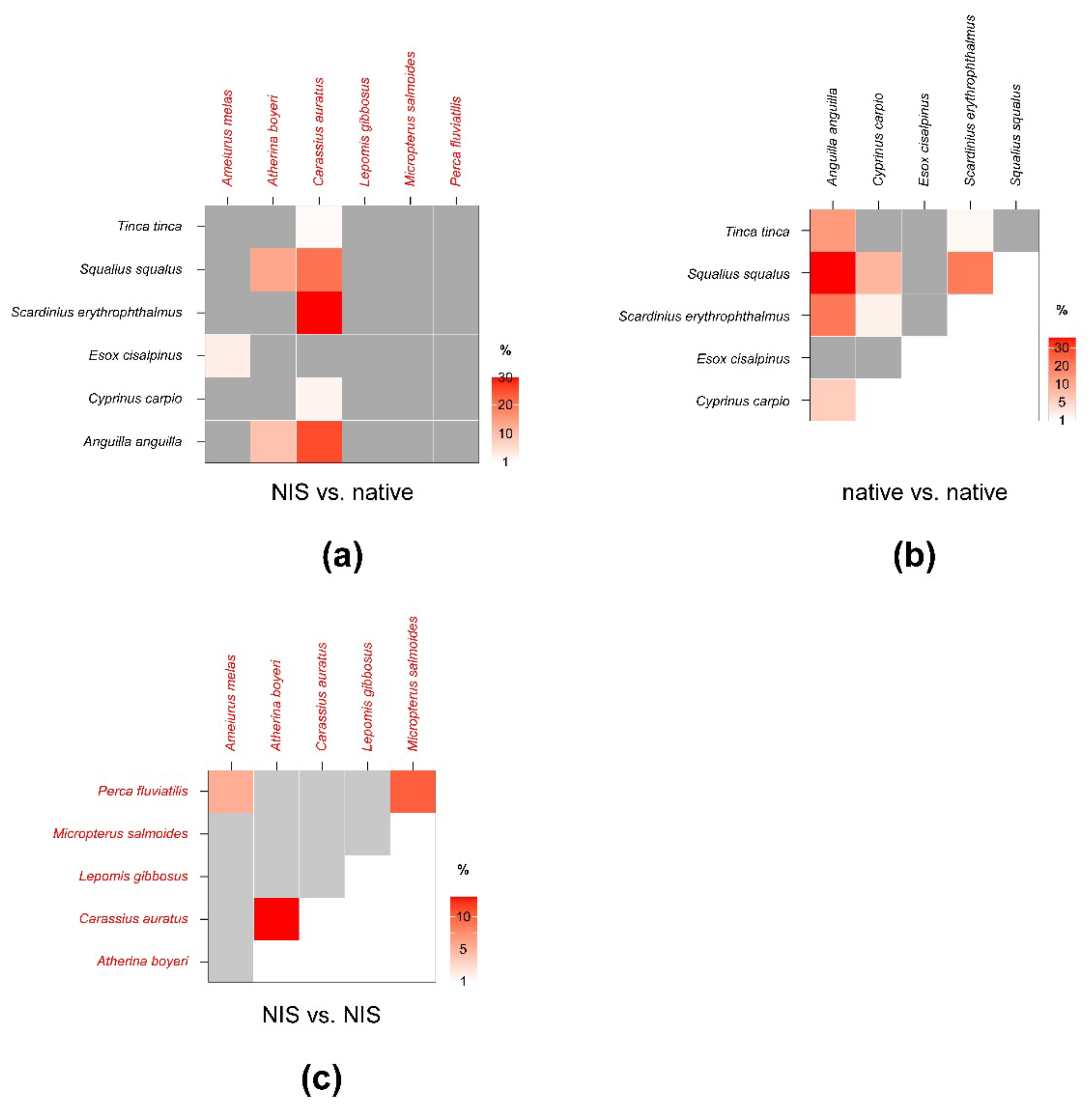
Figure 4.
Percent isotopic niche overlaps of Bayesian standard ellipse areas (SEAB) of non-indigenous vs. native (a), native vs. native (b), and non-indigenous vs. non-indigenous invertebrate consumers (c). Heat maps were built using modal overlaps; the reader should refer to Table A4, Table A5 and Table A6 in Appendix C for 95% confidence intervals of modal values and the corresponding SEAc estimations. Please note the different % scales.
Beside E. veneris, P. clarkii showed overlaps > 1% also with P. acuta (8.4%) and with Erythromma sp. (1.8%), while D. villosus overlapped with dragonfly nymphs (i.e., 9.4% with Ischnura sp. and 2.9% with Erythromma sp.). Other NIS showed negligible overlaps with native taxa; however, P. clarkii showed a relatively high SEAB % overlap with D. villosus of 15.8% (8.4–21.3%, 95% CI; Figure 4; Table A6 in Appendix C), indicating a potential interaction.
3.2. Fish
Isotopic analyses were performed on a total of 140 specimens belonging to 12 fish taxa (Table A1, Appendix A). In general, they showed significant interspecific differences in isotopic composition (PERMANOVA, Pseudo-F11,139 = 50.9, PMonte Carlo = 0.001). The highest δ15N values were observed in Micropterus salmoides together with Perca fluviatilis, Esox cisalpinus, and Ameiurus melas, the latter two species showing negligible isotopic differences (Table A3, Appendix B). The remaining species clustered in a group characterized by a lower enrichment in 15N and generally showing significant interspecific variations, with some notable exceptions represented by Carassius auratus vs. Cyprinus carpio, Scardinius erythrophthalmus, and Anguilla anguilla, the latter two characterized by negligible isotopic differences (Table A3, Appendix B). Lepomis gibbosus had δ15N values consistent with those characterizing this second group but with δ13C values significantly more enriched (Figure 2 and Table A3, Appendix B).
In Figure 5, the sample-size-corrected standard ellipse areas (SEAc) of invertebrate consumers are illustrated, while numerical values are reported in Table 3 together with the respective Bayesian estimates (SEAB). As observed for invertebrates, modal SEAB and SEAc estimations were in good agreement, and the latter were always included within SEAB 95% confidence intervals. The robustness of Bayesian estimations against potential biases related with variations in sample size was confirmed by the negligible relationship observed between SEAB values and the number of specimens analyzed for each taxa (Pearson r = 0.38, p = 0.21, d.f. = 10).
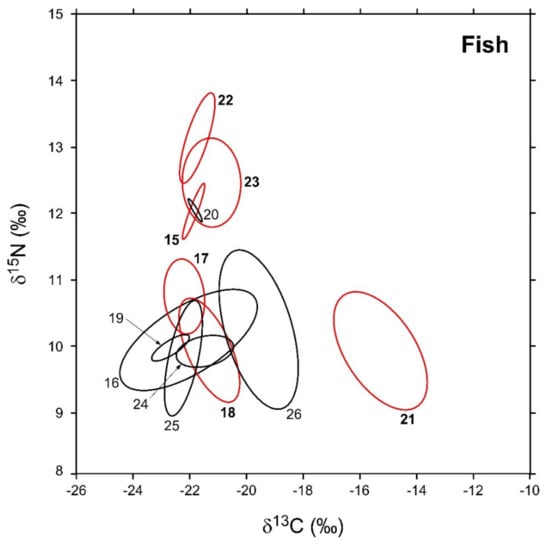
Figure 5.
Sample-size-corrected Bayesian standard ellipse areas (SEAc) calculated from δ13C and δ15N values of fish consumers. Black and red ellipses refer to native and non-indigenous taxa, respectively; see Figure 2 for the corresponding identification codes.

Table 3.
SEAc and SEAB of fish consumers expressed as ‰2. For SEAB, modal values and the corresponding 95% confidence intervals (in italics) are included.
In general, SEAB values varied across the different taxa by one order of magnitude (Table 3). Among NIS, L. gibbosus and A. melas were characterized by the largest and smallest areas (3.8 and 0.3‰2, respectively), while P. fluviatilis, C. auratus, A. boyeri, and M. salmoides showed intermediate values ranging between 2 and 0.8‰2. Bivariate comparisons indicated significant differences with a probability > 95% in the SEAB of all the species with the exception of P. fluviatilis vs. C. auratus and A. boyeri vs. M. salmoides (probability tests, max p = 0.73 for the comparison P. fluviatilis ≠ C. auratus). Native fish varied in SEAB values by a factor > 100, ranging between maxima of 4.2 and 3.8‰2 observed for Tinca tinca and A. anguilla, respectively, and a minimum of 0.02‰2 characterizing E. cisalpinus. S. squalus, S. erythrophthalmus, and C. carpio showed intermediate values ranging between 1.4 and 0.2‰2; all fish taxa showed significant interspecific differences in SEAB values with the exception of T. tinca and A. anguilla (probability test, p = 0.56).
SEAc and SEAB percent overlaps between NIS and native fish species were generally higher than those observed for invertebrates (Figure 6, see Table A7 in Appendix D for modal SEAB values and the respective 95% confidence intervals), yet they were always below the 60% threshold. The mean of nonzero SEAc and SEAB % overlaps was 3.8% and 3.6%, respectively, with values ranging between a minimum approximating 0% for M. salmoides vs. E. cisalpinus and a maximum of 25.1 (SEAB)–29% (SEAc) for C. auratus vs. S. erythrophthalmus. Similar to what was observed for invertebrates, the mean % overlap among native species was higher than that determined between NIS and native taxa, i.e., 7.5% and 6.2% (SEAc and SEAB % overlaps, respectively), with values ranging between a minimum approximating 0% for E. cisalpinus vs. S. erythrophthalmus, S. squalus, and A. anguilla and a maximum of 18.1 (SEAB)–22.9% (SEAc) for A. anguilla vs. S. squalus (Figure 6; Table A8 in Appendix D). Noticeably, the mean of nonzero SEAc and SEAB % overlaps among NIS was 2.9% and 3.7%, respectively, with values ranging between a minimum approximating 0% for A. melas vs. C. auratus and A. boyeri and a maximum of 13.1 (SEAc)–18.8% (SEAB) for C. auratus vs. A. boyeri (Figure 6; Table A9 in Appendix D).
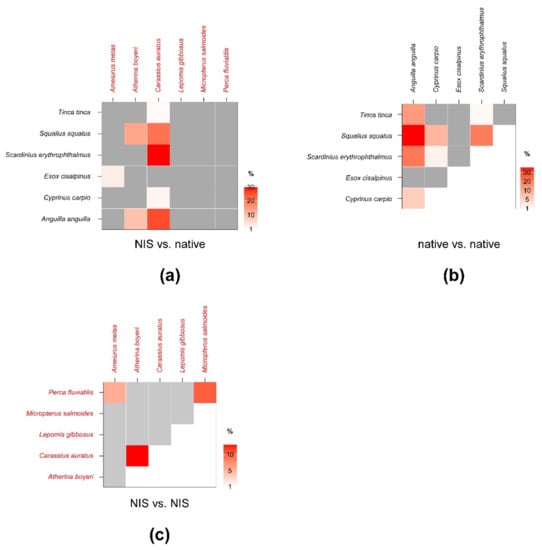
Figure 6.
Percent isotopic niche overlaps of Bayesian standard ellipse areas (SEAB) of non-indigenous vs. native (a), native vs. native (b), and non-indigenous vs. non-indigenous fish consumers (c). Heat maps were built using modal overlaps; the reader should refer to Table A7, Table A8 and Table A9 in Appendix D for 95% confidence intervals of modal values and the corresponding SEAc estimations. Please note the different % scales.
4. Discussion
Of the two contrasting hypotheses originally formulated in this study, only the second received support from the data, as the results indicated that a low potential trophic overlap occurs between non-indigenous and native consumers in the winter food web of Lake Trasimeno. The average percent overlap, measured using either conventional sample-size-corrected standard ellipse areas (SEAc) or Bayesian standard ellipse areas (SEAB), was below 4% for both the invertebrate and the fish assemblage. Additionally, pairwise % overlaps estimated between NIS and native species were below 16% for invertebrates and 30% for fish, far from the 60% critical threshold generally acknowledged to be related to active competitive interactions, e.g., [68,69]. The highest overlaps were observed among invertebrates for the Louisiana crayfish P. clarkii and to a minor extent for the killer shrimp D. villosus, while the goldfish C. auratus was characterized by the highest and most diffuse overlaps with native fish species. These findings have general theoretical implications but deserve to be discussed while focusing also on a species-specific perspective.
4.1. General Considerations
The low overlap indices observed between NIS and native consumers indicates that low potential competition occurs and that a stable coexistence equilibrium takes place in both the invertebrate and fish assemblages. In addition, it suggests that NIS play important functional roles in the flux of energy and matter from basal to higher trophic levels and have become pivotal in supporting the whole food web of Lake Trasimeno.
Mutual coevolution shapes competitors’ niches in natural communities [70]; accordingly, coexisting species should exhibit relatively low overlap in resource utilization; alternatively, if competition for limited resources is ongoing, a high niche overlap should occur [70,71,72]. In the context of bioinvasions, this latter scenario generally characterizes non-indigenous populations in their post-introduction and early-establishment phases, generally coupled with anomalously high abundances, e.g., [73]. The duration of coexistence with native species in the recipient food web is considered a key factor driving relevant ecological and evolutionary processes [74,75], as short-term coexistence between NIS and native species with a similar trophic ecology may induce high niche overlaps [76,77]. In contrast, long-term coexistence (>10 years) has been shown to be paralleled by shifts in diet and habitat segregations with important consequences for competitors’ trophic interactions [78,79,80,81]. Here, no significant relationships were observed in, e.g., the time since the introduction of NIS in Lake Trasimeno and their mean overlaps with native species (Pearson r = −0.21, p = 0.59, d.f. = 8). Indeed, for invertebrates as well as for fish, the observed low overlaps can be partially explained by differences in trophic habits; however, even focusing on species belonging to the same trophic guild, such as filter-feeding bivalves (D. polymorpha vs. A. anatina), detritivorous amphipods and isopods (D. villosus vs. E. veneris and A. aquaticus), or predatory fish (M. salmoides vs. E. cisalpinus), negligible or very limited overlaps have been observed, with larger overlaps determined within native- or NIS-only assemblages. The relatively long invasion history experienced by the Lake Trasimeno community can be hypothesized as the main determinant of the limited isotopic overlaps observed here. Among invertebrates, D. villosus can be considered an exception, as it appeared in 2017, whereas the remaining species were introduced around 2000 or earlier (Table 1); among fish, the most recent introductions were C. auratus and M. salmoides in 1990. Thus, it is plausible that the low overlap observed in the isotopic space may result from the peculiar conditions of relatively low abundance and metabolic requirements of both native and non-indigenous consumers taking place in Lake Trasimeno during the cold season as well as from long-term phenomena of adaptation and partitioning of the available resources in order to reduce competition and promote coexistence. Other factors related with, e.g., life history and geographic origin of NIS, cannot be excluded, as they have been indicated to play a role in freshwater communities characterized by multiple invasions [82].
Interestingly, the low overlap between NIS and native consumers suggests a high diversification in the contribution of both invertebrate and fish species to the channelling of matter and energy from basal resources to higher trophic levels. In other words, NIS appear to have acquired in the years after their introduction and establishement a structural as well as a functional role in the food web of Lake Trasimeno. For island ecosystems, the eradication of invasive species has been indicated to exert unquestionable benefits to the extant native biota, but empirical observations have also shown that these benefits can vary widely and unpredictably, and that adverse consequences may take place due to the disruption of the novel functional relationships generated by the invaders’ “surprise effects” in [83,84,85,86]. Thus, for Lake Trasimeno, this may have important implications from a management perspective: with the exclusion of P. clarkii and C. auratus, both showing low but diffuse overlaps with native species, any mitigation strategy based, e.g., on the reduction in the abundance of a NIS should also take into consideration a variation in the functioning of the food web.
4.2. Species-Specific Issues
Procambarus clarkii was shown to have the largest nichecluding among the invertebrate species included in this study, confirming the results of other isotopic investigations performed in both lentic and lotic environments [82,87]. Current information on the trophic ecology of this omnivorous species mostly pertains to food selection and dietary overlap with other crayfish species [88,89,90,91]; only recently, Wu and colleagues [92] verified in a Chinese reservoir a substantial resource overlap between P. clarkii and native crustaceans and gastropods, indicating the potential for the crayfish to exert negative impacts through competition. Here, P. clarkii overlapped with a number of native species, including the amphipod E. veneris and the gastropod P. acuta. Further comparative studies on the trophic niche of the crayfish are needed, specifically addressing potential competitive interactions with representatives of the detritivorus guild, native as well non-indigenous; here, P. clarkii showed a relatively high overlap also with the killer shrimp D. villosus. Indeed, while investigations based on conventional gut content analysis suggest similarity in dietary habits [93,94], the results of the only isotopic study including these two species, does not lend support to this view [82]; thus, to date, the nature and strength of the interaction between P. clarkii and D. villosus remain virtually unexplored.
Among fish taxa, the pumpkinseed Lepomis gibbosus was characterized by a large niche, yet no overlaps occurred with other fish species, either native or non-indigenous. The species was characterized by δ13C values far more enriched than those characterizing other fish species (Figure 2) yet are fully consistent with a group of potential invertebrate prey including D. villosus, P. antennarius, and the dragonfly nymphs Erithromma sp. and Ischnura sp. L. gibbosus is known to have paleomonids, Odonata larval stages, and D. villosus as common items in its diet, yet it is also recognized as an active predator of the zebra mussel D. polymorpha [95,96,97,98,99]. Here, isotopic values and niche position suggest that the invasive bivalve does not contribute significantly to the diet of L. gibbosus, at least in winter. It is worth noting that the specimens of L. gibbosus analyzed in this study had a relatively small size, with a standard length ranging between 45 and 95 mm (Appendix A, Table A1). Mollusks become a significant component of the diet of the pumpkinseed only for individuals larger than 80–90 mm, e.g., [99]; thus, it is plausible that the pattern observed here might testify to a size-related dietary shift associated with a number of additional factors linked to, e.g., ontogeny or prey availability.
Carassius auratus showed a relatively high and diffuse overlap with a number of native species, such as S. erythrophthalmus and S. squalus, and to a lower extent with A. anguilla, all known to feed mainly on benthic invertebrates [100,101,102]. C. auratus has zooplanktivorous habits yet is able to shift to a benthivorous diet depending on resource availability [103,104,105]. In Lake Trasimeno, given the low abundance of zooplankton in winter months [29,106], it is likely that C. auratus modified its trophic habits, converging towards those characterizing native benthivorous species. A similar trophic shift may have occurred also for Atherina boyeri, showing a diffuse overlap with S. squalus, S. erythrophthalmus, C. auratus, and other benthivorous predators such as C. carpio and T. tinca [107,108]. Freshwater populations of A. boyeri are known to feed mainly on zooplankton, yet depending on size, prey availability, and local conditions, an important component of their diet in freshwater and transitional environments may be represented by benthic invertebrates such as amphipods and chironomids [109,110] see also [111,112] for confirmative examples from transitional environments.
Noticeably, E. cisalpinus, the only native piscivorous predator occurring in Lake Trasimeno, showed a negligible overlap with other introduced predators such as P. fluviatilis and M. salmoides, both showing significantly higher δ15N values than E. cisalpinus. In contrast, a relatively high overlap was observed between E. cisalpinus and A. melas. In winter months, the benthivorous A. melas may shift to piscivory, e.g., [113], while Crustacea may become a significant component of the diet of pikes, in particular for small-sized specimens [114,115,116]. Thus, given the relatively small size of E. cisalpinus individuals analyzed in the present study (222–412 mm standard length range; Appendix A, Table A1), it can be hypothesized that its diet converged towards that of A. melas, resulting in the observed high overlap.
5. Conclusions
The present study was carried out in winter, and additional analyses may provide a more advanced resolution of species interactions as affected by seasonal variation in, e.g., abundance and trophic habits. In addition, the location where invertebrate and fish consumers were sampled can be considered representative of the littoral benthic environments of the southern sectors of Lake Trasimeno [45]. However, given the 53 km long coastline of the basin [43], future analyses should include multiple locations to account for, e.g., local variations in the availability of basal resources such as primary producers [117,118] and how these influence the isotopic niches of consumers. Nonetheless, this investigation provided a first assessment of the potential for competition between non-indigenous and native invertebrates and fish currently occurring in Lake Trasimeno, indicating that isotopic approaches may represent a powerful tool for disentangling the complexity of the trophic interactions characterizing the food web of the lake, providing at the same time useful information for future actions of management and mitigation of the impact of non-indigenous species.
Author Contributions
Conceptualization, D.C., A.L. and G.M.; investigation, G.M. and A.L.; methodology, G.M., A.L., R.B. and S.V.; formal analysis, D.C., M.T.G., R.B. and G.M.; data curation, G.M., R.B., C.D.M. and M.T.G.; writing—original draft preparation, D.C. and G.M.; writing—review and editing, all authors; funding acquisition, G.M. and A.L. All authors have read and agreed to the published version of the manuscript.
Funding
Funding from the Italian Ministry of Instruction, University, and Research (Law n. 232 11 December 2016) to G.M. and from the project “Tratti bio-ecologici chiave in una specie invasiva: Procambarus clarkii” (Fundamental Research Funding), Department of Chemistry, Biology, and Biotechnology, University of Perugia to A.L. are acknowledged.
Institutional Review Board Statement
No ethical issues related with the use of animals in the performed analyses were involved.
Informed Consent Statement
Not applicable.
Data Availability Statement
Isotopic data used in this study are available upon request from the corresponding author. The data are not publicly available due to ongoing comparative analyses.
Acknowledgments
The authors wish to thank for their help during field operations the fishermen from the “Cooperativa Pescatori del Trasimeno”, town of Sant’Arcangelo (Perugia, Italy). D.A. dedicates this paper to Bianca.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Table A1.
List of invertebrate and fish consumers sampled in the present study and in Mancini et al. [58]. The number of collected specimens is reported; for each taxon, the mean C:N ratio is included (±1 SE), together with the C:N ratio range, the mean length of individuals in mm (±1 SE), and the individual length range in mm. * = Shell length of bivalves.
Table A1.
List of invertebrate and fish consumers sampled in the present study and in Mancini et al. [58]. The number of collected specimens is reported; for each taxon, the mean C:N ratio is included (±1 SE), together with the C:N ratio range, the mean length of individuals in mm (±1 SE), and the individual length range in mm. * = Shell length of bivalves.
| Taxon | 2018 | 2016 | Total | Mean C:N | C:N Range | Mean Length | Length Range |
|---|---|---|---|---|---|---|---|
| Invertebrates | |||||||
| Anodonta anatina * | --- | 5 | 5 | 7.66 ± 0.61 | 7.04–8.32 | 99.7 ± 13.88 | 87–116 |
| Asellus aquaticus | 5 | --- | 5 | 3.99 ± 1.12 | 2.77–5.78 | 8.47 ± 2.06 | 6.7–11.8 |
| Branchiura sowerbyi | 3 | 3 | 6 | 5.53 ± 0.23 | 5.22–5.75 | 46.83 ± 19.05 | 29–77 |
| Chironomus plumosus | 3 | 3 | 6 | 6.58 ± 0.39 | 6.14–7.09 | 5.8 ± 1.89 | 3.3–8.2 |
| Dikerogammarus villosus | 54 | --- | 54 | 4.96 ± 0.45 | 4.31–5.9 | 12.71 ± 4.64 | 5.4–20.2 |
| Dreissena polymorpha * | --- | 8 | 8 | 4.65 ± 0.36 | 4.28–5.21 | 17.63 ± 5.93 | 10–26 |
| Echinogammarus veneris | 16 | 4 | 20 | 5.52 ± 0.9 | 4.39–8.03 | 7.37 ± 1.14 | 5.3–9.3 |
| Erpobdella octoculata | 3 | 3 | 6 | 4.25 ± 0.24 | 3.89–4.51 | 14.17 ± 4.49 | 7–19 |
| Erythromma sp. | 12 | --- | 12 | 3.94 ± 0.17 | 3.66–4.18 | 14.52 ± 1.59 | 12.4–16.5 |
| Ischnura sp. | 6 | --- | 6 | 3.74 ± 0.09 | 3.61–3.86 | 9.11 ± 1.34 | 7.5–11.5 |
| Palaemonetes antennarius | 4 | 2 | 6 | 3.85 ± 0.15 | 3.67–4.08 | 17.33 ± 3.88 | 12–22 |
| Physella acuta * | 6 | --- | 6 | 4.41 ± 0.33 | 4.04–4.87 | 9.5 ± 3.08 | 6–14 |
| Procambarus clarkii | 12 | 6 | 18 | 3.18 ± 0.25 | 2.33–3.46 | 97.26 ± 24.84 | 15–125 |
| Zooplankton | --- | 5 | 5 | 7.49 ± 0.32 | 7.23–8.01 | --- | --- |
| Fish | |||||||
| Ameiurus melas | 6 | --- | 6 | 3.15 ± 0.05 | 3.09–3.24 | 188.75 ± 6.18 | 184–197 |
| Anguilla anguilla | 6 | --- | 6 | 4.89 ± 1.92 | 3.75–8.44 | 401.25 ± 40.49 | 370–460 |
| Atherina boyeri | 21 | --- | 21 | 3.13 ± 0.32 | 2.45–3.65 | 62.95 ± 13.95 | 35–80 |
| Carassius auratus | 15 | --- | 15 | 3.16 ± 0.13 | 2.94–3.39 | 203.87 ± 94.52 | 91–312 |
| Cyprinus carpio | 6 | --- | 6 | 3.2 ± 0.2 | 2.93–3.43 | 326.83 ± 100.75 | 240–480 |
| Esox cisalpinus | 8 | --- | 8 | 3.12 ± 0.02 | 3.07–3.14 | 308.38 ± 74.41 | 222–412 |
| Lepomis gibbosus | 26 | --- | 26 | 3.3 ± 0.05 | 3.21–3.45 | 76.5 ± 13.37 | 45–95 |
| Micropterus salmoides | 7 | --- | 7 | 3.14 ± 0.1 | 3.06–3.29 | 183.86 ± 17.14 | 165–210 |
| Perca fluviatilis | 19 | --- | 19 | 3.19 ± 0.08 | 3.03–3.33 | 145.16 ± 32.06 | 58–205 |
| Scardinius erythrophthalmus | 8 | --- | 8 | 3.11 ± 0.1 | 2.96–3.24 | 143.43 ± 22.45 | 120–182 |
| Squalius squalus | 8 | --- | 8 | 3.29 ± 0.12 | 3.04–3.48 | 99.67 ± 46.65 | 53–150 |
| Tinca tinca | 10 | --- | 10 | 3.23 ± 0.1 | 3.18–3.46 | 243.25 ± 45.96 | 160–300 |
Appendix B

Table A2.
PERMANOVA post hoc bivariate comparisons performed on δ13C and δ15N values of invertebrate consumers. Monte Carlo-corrected probability values (PMonte Carlo) are reported. Significant PMonte Carlo values are indicated in bold. Codes in parentheses refer to those reported in column headings.
Table A2.
PERMANOVA post hoc bivariate comparisons performed on δ13C and δ15N values of invertebrate consumers. Monte Carlo-corrected probability values (PMonte Carlo) are reported. Significant PMonte Carlo values are indicated in bold. Codes in parentheses refer to those reported in column headings.
| Taxon | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anodonta anatina (1) | |||||||||||||
| Asellus aquaticus (2) | 0.0002 | ||||||||||||
| Branchiura sowerbyi (3) | 0.02 | 0.0009 | |||||||||||
| Chironomus plumosus (4) | 0.0003 | 0.0003 | 0.13 | ||||||||||
| Dikerogammarus villosus (5) | 0.0001 | 0.0001 | 0.0001 | 0.0001 | |||||||||
| Dreissena polymorpha (6) | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | ||||||||
| Echinogammarus veneris (7) | 0.01 | 0.0004 | 0.03 | 0.12 | 0.0001 | 0.0001 | |||||||
| Erpobdella octoculata (8) | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0007 | ||||||
| Erythromma sp. (9) | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.09 | 0.0001 | 0.0001 | 0.0001 | |||||
| Ischnura sp. (10) | 0.0001 | 0.0002 | 0.0001 | 0.0001 | 0.08 | 0.0001 | 0.0004 | 0.0001 | 0.45 | ||||
| Palaemonetes antennarius (11) | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.002 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | |||
| Physella acuta (12) | 0.15 | 0.001 | 0.02 | 0.02 | 0.0001 | 0.0001 | 0.02 | 0.0013 | 0.0001 | 0.0001 | 0.0001 | ||
| Procambarus clarkii (13) | 0.17 | 0.07 | 0.31 | 0.49 | 0.0001 | 0.0001 | 0.51 | 0.03 | 0.07 | 0.19 | 0.001 | 0.12 | |
| Zooplankton (14) | 0.49 | 0.002 | 0.44 | 0.17 | 0.0001 | 0.0001 | 0.05 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.18 | 0.29 |

Table A3.
PERMANOVA post hoc bivariate comparisons performed on δ13C and δ15N values of fish consumers. Monte Carlo-corrected probability values (PMonte Carlo) are reported. Significant PMonte Carlo values are indicated in bold. Codes in parentheses refer to those reported in column headings.
Table A3.
PERMANOVA post hoc bivariate comparisons performed on δ13C and δ15N values of fish consumers. Monte Carlo-corrected probability values (PMonte Carlo) are reported. Significant PMonte Carlo values are indicated in bold. Codes in parentheses refer to those reported in column headings.
| Taxon | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ameiurus melas (1) | |||||||||||
| Anguilla anguilla (2) | 0.002 | ||||||||||
| Atherina boyeri (3) | 0.001 | 0.001 | |||||||||
| Carassius auratus (4) | 0.001 | 0.35 | 0.002 | ||||||||
| Cyprinus carpio (5) | 0.001 | 0.02 | 0.005 | 0.03 | |||||||
| Esox cisalpinus (6) | 0.94 | 0.001 | 0.001 | 0.001 | 0.001 | ||||||
| Lepomis gibbosus (7) | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |||||
| Micropterus salmoides (8) | 0.01 | 0.001 | 0.001 | 0.001 | 0.001 | 0.003 | 0.001 | ||||
| Perca fluviatilis (9) | 0.05 | 0.001 | 0.001 | 0.001 | 0.001 | 0.05 | 0.001 | 0.04 | |||
| Scardinius erythrophthalmus (10) | 0.001 | 0.25 | 0.002 | 0.91 | 0.02 | 0.001 | 0.001 | 0.001 | 0.001 | ||
| Squalius squalus (11) | 0.001 | 0.04 | 0.002 | 0.11 | 0.47 | 0.001 | 0.001 | 0.001 | 0.001 | 0.17 | |
| Tinca tinca (12) | 0.001 | 0.43 | 0.001 | 0.007 | 0.002 | 0.001 | 0.001 | 0.001 | 0.001 | 0.01 | 0.002 |
Appendix C

Table A4.
Interspecific overlaps in SEAc and modal SEAB values of non-indigenous and native invertebrate taxa. For the sake of succinctness, values lower than 0.01‰2 are reported as “<0.01”. Percent overlaps are reported for both metrics; those lower than 1% are reported as “<1%”. For SEAB modal values, 95% confidence intervals are included in italics.
Table A4.
Interspecific overlaps in SEAc and modal SEAB values of non-indigenous and native invertebrate taxa. For the sake of succinctness, values lower than 0.01‰2 are reported as “<0.01”. Percent overlaps are reported for both metrics; those lower than 1% are reported as “<1%”. For SEAB modal values, 95% confidence intervals are included in italics.
| NIS | Native | Overlap (SEAc) | % Overlap (SEAc) | Overlap (SEAB) | % Overlap (SEAB) | 95% CI | |
|---|---|---|---|---|---|---|---|
| Branchiura sowerbyi | Chironomus plumosus | <0.01 | <1% | <0.01 | <1% | <1% | <1% |
| Echinogammarus veneris | 0.07 | 1.36 | 0.08 | 1.37 | 1.19 | 1.54 | |
| Physella acuta | 0.13 | 2.75 | 0.08 | 1.96 | <1% | 4.35 | |
| Dikerogammarus villosus | Erythromma sp. | 0.32 | 11.36 | 0.39 | 9.44 | 9.43 | 9.46 |
| Ischnura sp. | 0.07 | 2.66 | 0.11 | 2.87 | 2.62 | 2.93 | |
| Dreissena polymorpha | Anodonta anatina | <0.01 | <1% | <0.01 | <1% | <1% | <1% |
| Zooplankton | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |
| Procambarus clarkii | Asellus aquaticus | <0.01 | <1% | <0.01 | <1% | <1% | <1% |
| Echinogammarus veneris | 3.02 | 16.15 | 4.82 | 17.94 | 11.99 | 24.58 | |
| Erpobdella octoculata | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |
| Erythromma sp. | 0.36 | 2.12 | 0.44 | 1.77 | 1.74 | 1.81 | |
| Ischnura sp. | 0.07 | <1% | 0.13 | <1% | <1% | <1% | |
| Physella acuta | 1.35 | 6.74 | 2.26 | 8.45 | 8.34 | 8.57 | |

Table A5.
Interspecific overlaps in SEAc and modal SEAB values of native invertebrate taxa. For the sake of succinctness, values lower than 0.01‰2 are reported as “<0.01”. Percent overlaps are reported for both metrics; those lower than 1% are reported as “<1%”. For SEAB modal values, 95% confidence intervals are included in italics.
Table A5.
Interspecific overlaps in SEAc and modal SEAB values of native invertebrate taxa. For the sake of succinctness, values lower than 0.01‰2 are reported as “<0.01”. Percent overlaps are reported for both metrics; those lower than 1% are reported as “<1%”. For SEAB modal values, 95% confidence intervals are included in italics.
| Native | Native | Overlap (SEAc) | % Overlap (SEAc) | Overlap (SEAB) | % Overlap (SEAB) | 95% CI | |
|---|---|---|---|---|---|---|---|
| Echinogammarus veneris | Asellus aquaticus | <0.01 | <1% | <0.01 | <1% | <1% | <1% |
| Chironomus plumosus | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |
| Erpobdella octoculata | Asellus aquaticus | <0.01 | <1% | <0.01 | <1% | <1% | <1% |
| Echinogammarus veneris | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |
| Ischnura sp. | Erythromma sp. | 0.02 | 5.79 | 0.03 | 6.02 | 3.31 | 8.45 |
| Physella acuta | Chironomus plumosus | 0.08 | 1.86 | 0.07 | 1.15 | 0.05 | 3.49 |
| Echinogammarus veneris | 2.47 | 35.21 | 3.16 | 30.95 | 22.84 | 42.12 | |
| Zooplankton | Anodonta anatina | 0.24 | 20.38 | 0.27 | 18.12 | 10.22 | 26.04 |
| Chironomus plumosus | 0.05 | 3.88 | 0.05 | 4.01 | 2.33 | 5.86 | |
| Physella acuta | 0.22 | 4 | 0.14 | 3.74 | 2.72 | 5.15 | |

Table A6.
Interspecific overlaps in SEAc and modal SEAB values of non-indigenous invertebrate taxa. For the sake of succinctness, values lower than 0.01‰2 are reported as “<0.01”. Percent overlaps are reported for both metrics; those lower than 1% are reported as “<1%”. For SEAB modal values, 95% confidence intervals are included in italics.
Table A6.
Interspecific overlaps in SEAc and modal SEAB values of non-indigenous invertebrate taxa. For the sake of succinctness, values lower than 0.01‰2 are reported as “<0.01”. Percent overlaps are reported for both metrics; those lower than 1% are reported as “<1%”. For SEAB modal values, 95% confidence intervals are included in italics.
| NIS | NIS | Overlap (SEAc) | % Overlap (SEAc) | Overlap (SEAB) | % Overlap (SEAB) | 95% CI | |
|---|---|---|---|---|---|---|---|
| Procambarus clarkii | Branchiura sowerbyi | <0.01 | <1% | <0.01 | <1% | <1% | <1% |
| Dikerogammarus villosus | 2.73 | 16.22 | 3.59 | 15.83 | 10.38 | 19.28 | |
Appendix D

Table A7.
Interspecific overlaps in SEAc and modal SEAB values of non-indigenous and native fish taxa. For the sake of succinctness, values lower than 0.01‰2 are reported as “<0.01”. Percent overlaps are reported for both metrics; those lower than 1% are reported as “<1%”. For SEAB modal values, 95% confidence intervals are included in italics.
Table A7.
Interspecific overlaps in SEAc and modal SEAB values of non-indigenous and native fish taxa. For the sake of succinctness, values lower than 0.01‰2 are reported as “<0.01”. Percent overlaps are reported for both metrics; those lower than 1% are reported as “<1%”. For SEAB modal values, 95% confidence intervals are included in italics.
| NIS | Native | Overlap (SEAc) | % Overlap (SEAc) | Overlap (SEAB) | % Overlap (SEAB) | 95% CI | |
|---|---|---|---|---|---|---|---|
| Ameiurus melas | Anguilla anguilla | <0.01 | <1% | <0.01 | <1% | <1% | <1% |
| Cyprinus carpio | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |
| Esox cisalpinus | <0.01 | 2.73 | <0.01 | 2.32 | 1.54 | 3.03 | |
| Scardinius erythrophthalmus | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |
| Squalius squalus | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |
| Atherina boyeri | Anguilla anguilla | 0.53 | 9.24 | 0.89 | 10.44 | 8.09 | 12.81 |
| Cyprinus carpio | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |
| Esox cisalpinus | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |
| Scardinius erythrophthalmus | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |
| Squalius squalus | 0.34 | 13.28 | 0.29 | 12.59 | 7.82 | 14.52 | |
| Carassius auratus | Anguilla anguilla | 1.4 | 24.79 | 1.67 | 23.7 | 21.27 | 26.23 |
| Cyprinus carpio | 0.04 | 1.69 | 0.05 | 2.21 | 1.06 | 3.51 | |
| Esox cisalpinus | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |
| Scardinius erythrophthalmus | 0.65 | 29.02 | 0.63 | 25.1 | 22 | 32.17 | |
| Squalius squalus | 0.62 | 20.32 | 0.75 | 18.46 | 17.66 | 22.54 | |
| Tinca tinca | 0.06 | <1% | 0.08 | <1% | <1% | 1.31 | |
| Micropterus salmoides | Anguilla anguilla | <0.01 | <1% | <0.01 | <1% | <1% | <1% |
| Cyprinus carpio | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |
| Esox cisalpinus | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |
| Scardinius erythrophthalmus | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |
| Squalius squalus | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |
| Perca fluviatilis | Anguilla anguilla | <0.01 | <1% | <0.01 | <1% | <1% | <1% |
| Cyprinus carpio | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |
| Esox cisalpinus | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |
| Scardinius erythrophthalmus | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |
| Squalius squalus | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |
| Tinca tinca | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |

Table A8.
Interspecific overlaps in SEAc and modal SEAB values of native fish taxa. For the sake of succinctness, values lower than 0.01‰2 are reported as “<0.01”. Percent overlaps are reported for both metrics; those lower than 1% are reported as “<1%”. For SEAB modal values, 95% confidence intervals are included in italics.
Table A8.
Interspecific overlaps in SEAc and modal SEAB values of native fish taxa. For the sake of succinctness, values lower than 0.01‰2 are reported as “<0.01”. Percent overlaps are reported for both metrics; those lower than 1% are reported as “<1%”. For SEAB modal values, 95% confidence intervals are included in italics.
| Native | Native | Overlap (SEAc) | % Overlap (SEAc) | Overlap (SEAB) | % Overlap (SEAB) | 95% CI | |
|---|---|---|---|---|---|---|---|
| Anguilla anguilla | Cyprinus carpio | 0.29 | 5.89 | 0.31 | 5.05 | 3.56 | 6.36 |
| Esox cisalpinus | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |
| Scardinius erythrophthalmus | 0.77 | 15.54 | 0.69 | 10.71 | 8.02 | 16.25 | |
| Squalius squalus | 1.21 | 22.88 | 1.28 | 18.14 | 16.07 | 23.24 | |
| Tinca tinca | 1.04 | 11.85 | 0.97 | 11.33 | 8.12 | 12.38 | |
| Scardinius erythrophthalmus | Esox lucius | <0.01 | <1% | <0.01 | <1% | <1% | <1% |
| Cyprinus carpio | 0.02 | 1.72 | <0.01 | 1.6 | 1.25 | 1.93 | |
| Squalius squalus | Esox lucius | <0.01 | <1% | <0.01 | <1% | <1% | <1% |
| Cyprinus carpio | 0.15 | 8.66 | 0.08 | 7.21 | 5.53 | 9.43 | |
| Scardinius erythrophthalmus | 0.31 | 15.12 | 0.37 | 12.62 | 11.72 | 14.89 | |
| Tinca tinca | Scardinius erythrophthalmus | 0.04 | <1% | 0.07 | 1.05 | <1% | 1.16 |

Table A9.
Interspecific overlaps in SEAc and modal SEAB values of non-indigenous fish taxa. For the sake of succinctness, values lower than 0.01‰2 are reported as “<0.01”. Percent overlaps are reported for both metrics; those lower than 1% are reported as “<1%”. For SEAB modal values, 95% confidence intervals are included in italics.
Table A9.
Interspecific overlaps in SEAc and modal SEAB values of non-indigenous fish taxa. For the sake of succinctness, values lower than 0.01‰2 are reported as “<0.01”. Percent overlaps are reported for both metrics; those lower than 1% are reported as “<1%”. For SEAB modal values, 95% confidence intervals are included in italics.
| NIS | NIS | Overlap (SEAc) | % Overlap (SEAc) | Overlap (SEAB) | % Overlap (SEAB) | 95% CI | |
|---|---|---|---|---|---|---|---|
| Atherina boyeri | Ameiurus melas | <0.01 | <1% | <0.01 | <1% | <1% | <1% |
| Carassius auratus | Ameiurus melas | <0.01 | <1% | <0.01 | <1% | <1% | <1% |
| Atherina boyeri | 0.39 | 13.1 | 0.61 | 18.77 | 12.02 | 20.58 | |
| Micropterus salmoides | Ameiurus melas | <0.01 | <1% | <0.01 | <1% | <1% | <1% |
| Atherina boyeri | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |
| Carassius auratus | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |
| Perca fluviatilis | Ameiurus melas | 0.13 | 5.56 | 0.27 | 7.59 | 4.54 | 7.66 |
| Micropterus salmoides | 0.29 | 10.26 | 0.34 | 10.69 | 8.09 | 12.33 | |
| Atherina boyeri | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |
| Carassius auratus | <0.01 | <1% | <0.01 | <1% | <1% | <1% | |
References
- Gherardi, F. Biological Invaders in Inland Waters: Profiles, Distribution, and Threats; Springer: Dordrecht, The Netherlands, 2007; Chapter 1; pp. 3–25. [Google Scholar]
- Dudgeon, D. Freshwater Biodiversity; Cambridge University Press: Cambridge, UK, 2020. [Google Scholar]
- UNEP. Convention on Biological Diversity; UNEP: Nairobi, Kenya, 1992. [Google Scholar]
- IUCN. IUCN Guidelines for the Prevention of Biodiversity Loss Caused by Alien Invasive Species; Species Survival Commission: Gland, CH, USA, 2000. [Google Scholar]
- Blackburn, T.M.; Pyšek, P.; Bacher, S.; Carlton, J.T.; Duncan, R.P.; Jarošík, V.; Wilson, J.R.U.; Richardson, D.M. A proposed unified framework for biological invasions. Trends Ecol. Evol. 2011, 26, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef] [PubMed]
- Crystal-Ornelas, R.; Lockwood, J.L. The ‘known unknowns’ of invasive species impact measurement. Biol. Invasions 2020, 22, 1513–1525. [Google Scholar] [CrossRef]
- Higgins, S.N.; Vander Zanden, M.J. What a difference a species makes: A meta–analysis of dreissenid mussel impacts on freshwater ecosystems. Ecol. Monogr. 2010, 80, 179–196. [Google Scholar] [CrossRef]
- Twardochleb, L.A.; Olden, J.D.; Larson, E.R. A global meta-analysis of the ecological impacts of nonnative crayfish. Freshw. Sci. 2013, 32, 1367–1382. [Google Scholar] [CrossRef]
- Gallardo, B.; Clavero, M.; Sánchez, M.I.; Vilà, M. Global ecological impacts of invasive species in aquatic ecosystems. Glob. Chang. Biol. 2016, 22, 151–163. [Google Scholar] [CrossRef]
- Mollot, G.; Pantel, J.H.; Romanuk, T.N. The effects of invasive species on the decline in species richness: A global meta-analysis. Adv. Ecol. Res. 2017, 56, 61–83. [Google Scholar]
- Strayer, D.L. Alien species in fresh waters: Ecological effects, interactions with other stressors, and prospects for the future. Freshwat. Biol. 2010, 55, 152–174. [Google Scholar] [CrossRef]
- Guareschi, S.; Laini, A.; England, J.; Barrett, J.; Wood, P.J. Multiple co-occurrent alien invaders constrain aquatic biodiversity in rivers. Ecol. Appl. 2021, 31, e02385. [Google Scholar] [CrossRef]
- Simberloff, D.; Von Holle, B. Positive interactions of non-indigenous species: Invasional meltdown? Biol. Invasions 1999, 1, 21–32. [Google Scholar] [CrossRef]
- Preston, D.L.; Henderson, J.S.; Johnson, P.T.J. Community ecology of invasions: Direct and indirect effects of multiple invasive species on aquatic communities. Ecology 2012, 93, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Kuebbing, S.E.; Nuñez, M.A.; Simberloff, D. Current mismatch between research and conservation efforts: The need to study co-occurring invasive plant species. Biol. Conserv. 2013, 160, 121–129. [Google Scholar] [CrossRef]
- Liversage, K.; Kotta, J.; Kuprijanov, I.; Rätsep, M.; Nõomaa, K. A trophic cascade facilitates native habitat providers within assemblages of multiple invasive marine species. Ecosphere 2021, 12, e03621. [Google Scholar] [CrossRef]
- Jackson, M.C. Interactions among multiple invasive animals. Ecology 2015, 96, 2035–2041. [Google Scholar] [CrossRef]
- Elton, C.S. The Ecology of Invasions by Animals and Plants; Chapman & Hall: New York, NY, USA, 1958; p. 181. [Google Scholar]
- David, P.; Thébault, E.; Anneville, O.; Duyck, P.F.; Chapuis, E.; Loeuille, N. Impacts of Invasive Species on Food Webs: A Review of Empirical Data. Adv. Ecol. Res. 2017, 56, 1–60. [Google Scholar]
- Jackson, M.C.; Wasserman, R.J.; Grey, J.; Ricciardi, A.; Dick, J.T.A.; Alexander, M.E. Novel and disrupted trophic links following invasion in freshwater ecosystems. Adv. Ecol. Res. 2017, 57, 55–97. [Google Scholar]
- Bartley, T.J.; McCann, K.S.; Bieg, C.; Cazelles, K.; Granados, M.; Guzzo, M.M.; MacDougall, A.S.; Tunney, T.D.; McMeans, B.C. Food web rewiring in a changing world. Nat. Ecol. Evol. 2019, 3, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.; Richardson, D.M. How to invade an ecological network. Trends Ecol. Evol. 2019, 34, 121–131. [Google Scholar] [CrossRef]
- Della Bella, V. (Ed.) Caratterizzazione e Diffusione delle Specie Aliene Acquatiche e di Ambienti Umidi in Umbria; ARPA Umbria: Perugia, Italy, 2020; p. 288. [Google Scholar]
- Tran, T.N.Q.; Jackson, M.C.; Sheath, D.; Verreycken, H.; Britton, J.R. Patterns of trophic niche divergence between invasive and native fishes in wild communities are predictable from mesocosm studies. J. Anim. Ecol. 2015, 84, 1071–1080. [Google Scholar] [CrossRef]
- Britton, J.R.; Gutmann Roberts, C.; Amat Trigo, F.; Nolan, E.T.; De Santis, V. Predicting the ecological impacts of an alien invader: Experimental approaches reveal the trophic consequences of competition. J. Anim. Ecol. 2019, 88, 1066–1078. [Google Scholar] [CrossRef]
- Dominguez Almela, V.; South, J.; Britton, J.R. Predicting the competitive interactions and trophic niche consequences of a globally invasive fish with threatened native species. J. Anim. Ecol. 2021, 90, 2651–2662. [Google Scholar] [CrossRef] [PubMed]
- Lorenzoni, M.; Corboli, M.; Dörr, A.J.M.; Giovinazzo, G.; Selvi, S.; Mearelli, M. Diets of Micropterus salmoides Lac. and Esox lucius L. in Lake Trasimeno (Umbria, Italy) and their diet overlap. Bull. Fr. Peche Piscic. 2002, 365–366, 537–547. [Google Scholar] [CrossRef]
- Havens, K.E.; Elia, A.C.; Taticchi, M.I.; Fulton, R.S. Zooplankton–phytoplankton relationships in shallow subtropical versus temperate lakes Apopka (Florida, USA) and Trasimeno (Umbria, Italy). Hydrobiologia 2009, 628, 165–175. [Google Scholar] [CrossRef]
- Pallottini, M.; Pagliarini, S.; Catasti, M.; La Porta, G.; Selvaggi, R.; Gaino, E.; Spacone, L.; Di Giulio, A.M.; Ali, A.; Goretti, E. Population dynamics and seasonal patterns of Chironomus plumosus (Diptera, Chironomidae) in the shallow Lake Trasimeno, Central Italy. Sustainability 2023, 15, 851. [Google Scholar] [CrossRef]
- McMeans, B.C.; McCann, K.S.; Humphries, M.; Rooney, N.; Fisk, A.T. Food web structure in temporally-forced ecosystems. Trends Ecol. Evol. 2015, 30, 662–672. [Google Scholar] [CrossRef]
- McMeans, B.C.; McCann, K.S.; Guzzo, M.M.; Bartley, T.J.; Bieg, C.; Blanchfield, P.J.; Fernandes, T.; Giacomini, H.C.; Middel, T.; Rennie, M.D.; et al. Winter in water: Differential responses and the maintenance of biodiversity. Ecol. Lett. 2020, 23, 922–938. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, G.; Vizzini, S. Assessing anthropogenic pressures on coastal marine ecosystems using stable CNS isotopes: State of the art, knowledge gaps, and community-scale perspectives. Estuar. Coast. Shelf Sci. 2015, 156, 195–204. [Google Scholar] [CrossRef]
- McCue, M.D.; Javal, M.; Clusella-Trullas, S.; Le Roux, J.J.; Jackson, M.C.; Ellis, A.G.; Richardson, D.M.; Valentine, A.J.; Terblanche, J.S. Using stable isotope analysis to answer fundamental questions in invasion ecology: Progress and prospects. Methods Ecol. Evol. 2020, 11, 196–214. [Google Scholar] [CrossRef]
- Bearhop, S.; Adams, C.E.; Waldron, S.; Fuller, R.A.; Macleod, H. Determining trophic niche width: A novel approach using stable isotope analysis. J. Anim. Ecol. 2004, 73, 1007–1012. [Google Scholar] [CrossRef]
- Newsome, S.D.; Martinez del Rio, C.; Bearhop, S.; Phillips, D.L. A niche for isotopic ecology. Front. Ecol. Environ. 2007, 5, 429–436. [Google Scholar] [CrossRef]
- Rodríguez-Malagón, M.A.; Herrera-Montalvo, L.G. Isotopic niche mirrors trophic niche in a vertebrate island invader. Oecologia 2013, 171, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, C.E.; Inger, R.; McDonald, R.A.; Barker, S.; Jackson, A.L.; Thompson, F.J.; Vitikainen, E.I.K.; Cant, M.A.; Marshall, H.H. Intragroup competition predicts individual foraging specialisation in a group-living mammal. Ecol. Lett. 2018, 21, 665–673. [Google Scholar] [CrossRef]
- Stewart, J.D.; Rohner, C.A.; Araujo, G.; Avila, J.; Fernando, D.; Forsberg, K.; Ponzo, A.; Rambahiniarison, J.M.; Kurle, C.M.; Semmens, B.X. Trophic overlap in mobulid rays: Insights from stable isotope analysis. Mar. Ecol. Prog. Ser. 2017, 580, 131–151. [Google Scholar] [CrossRef]
- De Santis, V.; Cicala, D.; Baneschi, I.; Boschi, C.; Brignone, S.; Iaia, M.; Zaupa, S.; Volta, P. Non-native fish assemblages display potential competitive advantages in two protected small and shallow lakes of northern Italy. Glob. Ecol. Conserv. 2022, 35, e02082. [Google Scholar]
- Cicala, D.; Polgar, G.; Mor, J.R.; Piscia, R.; Brignone, S.; Zaupa, S.; Volta, P. Trophic niches, trophic positions, and niche overlaps between non-native and native fish species in a subalpine lake. Water 2020, 12, 3475. [Google Scholar] [CrossRef]
- Ludovisi, A.; Gaino, E. Meteorological and water quality changes in Lake Trasimeno (Umbria, Italy) during the last fifty years. J. Limnol. 2010, 69, 174–188. [Google Scholar] [CrossRef]
- Ludovisi, A.; Poletti, A. Use of thermodynamic indices as ecological indicators of the development state of lake ecosystems. 1. Entropy production indices. Ecol. Modell. 2003, 159, 203–222. [Google Scholar] [CrossRef]
- Bresciani, M.; Pinardi, M.; Free, G.; Luciani, G.; Ghebrehiwot, S.; Laanen, M.; Peters, S.; Della Bella, V.; Padula, R.; Giardino, C. The use of multisource optical sensors to study phytoplankton spatio-temporal variation in a Shallow Turbid Lake. Water 2020, 12, 284. [Google Scholar] [CrossRef]
- Marchegiano, M.; Gliozzi, E.; Ceschin, S.; Mazzini, I.; Adatte, T.; Mazza, R.; Ariztegui, D. Ecology and distribution of living ostracod assemblages in a shallow endorheic lake: The example of the Lake Trasimeno (Umbria, central Italy). J. Limnol. 2017, 76, 469–487. [Google Scholar] [CrossRef][Green Version]
- Parco Regionale del Lago Trasimeno. Aspetti Faunistici–Anfibi Rettili Pesci e Invertebrati. Regione Umbria-Servizio Sistemi Naturalistici e Zootecnia. 2015, p. 55. Available online: https://www.regione.umbria.it/documents/18/2512711/Trasimeno_anfibi_rettili_pesci_inv_ott_15.pdf/359f3c6e-6c97-45b3-9cb9-5b6a57443f7e (accessed on 8 August 2023).
- Carosi, A.; Ghetti, L.; Padula, R.; Lorenzoni, M. Potential effects of global climate change on fisheries in the Trasimeno Lake (Italy), with special reference to the goldfish Carassius auratus invasion and the endemic southern pike Esox cisalpinus decline. Fish. Manag. Ecol. 2019, 26, 500–511. [Google Scholar] [CrossRef]
- Ludovisi, A.; Pandolfi, P.; Illuminata Taticchi, M. The strategy of ecosystem development: Specific dissipation as an indicator of ecosystem maturity. J. Theor. Biol. 2005, 235, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, A. Tutela Ambientale del Lago Trasimeno; ARPA Umbria: Perugia, Italy, 2012. [Google Scholar]
- Ludovisi, A.; Minozzo, M.; Pandolfi, P.; Taticchi, M.I. Modelling the horizontal spatial structure of planktonic community in Lake Trasimeno (Umbria, Italy) using multivariate geostatistical methods. Ecol. Modell. 2005, 181, 247–262. [Google Scholar] [CrossRef]
- Ludovisi, A.; Todini, C.; Pandolfi, P.; Taticchi, M.I. Scale patterns of diel distribution of the copepod Cyclops abyssorum Sars in a regulated lake: The relative importance of physical and biological factors. J. Plankton. Res. 2008, 30, 495–509. [Google Scholar] [CrossRef][Green Version]
- Mancinelli, G.; Goretti, E.; Vizzini, S.; Pallottini, M.; Ludovisi, A. Caratterizzazione funzionale delle specie aliene nella rete trofica del lago Trasimeno. In Caratterizzazione e Diffusione delle Specie Aliene Acquatiche e di Ambienti Umidi in Umbria; Della Bella, V., Ed.; ARPA Umbria: Perugia, Italy, 2020; pp. 151–159. [Google Scholar]
- Bianchini, M.L. Species introductions in the aquatic environment: Changes in biodiversity and economics of exploitation. Proc. World Fish. Congr. 1995, 3, 213–222. [Google Scholar]
- Goretti, E.; Marcucci, C.; Di Veroli, A.; Fabrizi, A.; Gaino, E. The tubificids (Annelida, Oligochaeta) of Lake Trasimeno and Lake Piediluco in Central Italy, with a study of SEM morphology of some species. Turk. J. Zool. 2014, 38, 334–341. [Google Scholar] [CrossRef]
- Cianfanelli, S.; Lori, E.; Bodon, M. Non-indigenous freshwater molluscs and their distribution in Italy. In Biological Invaders in Inland Waters: Profiles, Distribution, and Threats; Gherardi, F., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2007; pp. 103–121. [Google Scholar]
- Natali, M. La carpa erbivora (Ctenopharyngodon idella Val.) nel lago Trasimeno. Risultati a quattro anni dall’immissione sperimentale. Relev. Idrobiol. 1991, 30, 347–356. [Google Scholar]
- Mancinelli, G.; Papadia, P.; Ludovisi, A.; Migoni, D.; Bardelli, R.; Fanizzi, F.P.; Vizzini, S. Beyond the mean: A comparison of trace- and macroelement correlation profiles of two lacustrine populations of the crayfish Procambarus clarkii. Sci. Total Environ. 2018, 624, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.; De Giorgi, R.; Ludovisi, A.; Vizzini, S.; Mancinelli, G. Ontogenetic shift in the trophic role of the invasive killer shrimp Dikerogammarus villosus: A stable isotope study. Biol. Invasions 2021, 23, 1803–1817. [Google Scholar] [CrossRef]
- Ludovisi, A.; Goretti, E.; Pallottini, M.; Lucentini, L.; Pizzirani, C.; Vizzini, S.; Mancinelli, G. Stable isotope analysis reveals trophic segregation between the invasive zebra mussel Dreissena polymorpha and the native duck mussel Anodonta anatina in Lake Trasimeno (Italy). Hydrobiologia 2022, 849, 2091–2108. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. Available online: http://www.R-project.org/ (accessed on 1 July 2023).
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar]
- Gotelli, N.; Hart, E.; Ellison, A. EcoSimR-Null Model Analysis for Ecological Data. R Package Version 0.1.0. 2015. Available online: https://cran.microsoft.com/snapshot/2017-03-22/web/packages/EcoSimR/EcoSimR.pdf (accessed on 1 July 2023).
- Post, D.M.; Layman, C.A.; Arrington, D.A.; Takimoto, G.; Quattrochi, J.; Montana, C.G. Getting to the fat of the matter: Models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 2007, 152, 179–189. [Google Scholar] [CrossRef]
- Logan, J.M.; Jardine, T.D.; Miller, T.J.; Bunn, S.E.; Cunjak, R.A.; Lutcavage, M.E. Lipid corrections in carbon and nitrogen stable isotope analyses: Comparison of chemical extraction and modelling methods. J. Anim. Ecol. 2008, 77, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.L.; Parnell, A.C. SIBER: Stable Isotope Bayesian Ellipses in R. R Package Version 2.1.7. 2023. Available online: http://cran.r-project.org/web/packages/SIBER (accessed on 1 July 2023).
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Pianka, E.R. The structure of lizard communities. Annu. Rev. Ecol. Syst. 1973, 4, 53–74. [Google Scholar] [CrossRef]
- Schoener, T.W. The Anolis lizards of Bimini: Resource partitioning in a complex fauna. Ecology 1968, 49, 704–726. [Google Scholar] [CrossRef]
- Layman, C.A.; Allgeier, J.E. Characterizing trophic ecology of generalist consumers: A case study of the invasive lionfish in The Bahamas. Mar. Ecol. Prog. Ser. 2012, 448, 131–141. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity and the coevolution of competitors, or the ghost of competition past. Oikos 1980, 35, 131–138. [Google Scholar] [CrossRef]
- Chase, J.; Leibold, M. Ecological Niches: Linking Classical and Contemporary Approaches; University of Chicago Press: Chicago, IL, USA, 2003. [Google Scholar]
- Gotelli, N.J.; Graves, G.R. Null Models in Ecology; Smithsonian Institution Press: Washington, DC, USA, 1996; p. 368. [Google Scholar]
- Soto, I.; Ahmed, D.A.; Balzani, P.; Cuthbert, R.N.; Haubrock, P.J. Sigmoidal curves reflect impacts and dynamics of aquatic invasive species. Sci. Total Environ. 2023, 872, 161818. [Google Scholar] [CrossRef]
- Bøhn, T.; Amundsen, P.-A.; Sparrow, A. Competitive exclusion after invasion? Biol. Invasions 2008, 10, 359–368. [Google Scholar] [CrossRef]
- Lang, I.; Evangelista, C.; Everts, R.M.; Loot, G.; Cucherousset, J. Stable resource polymorphism along the benthic littoral–pelagic axis in an invasive crayfish. Ecol. Evol. 2020, 10, 2650–2660. [Google Scholar] [CrossRef]
- Bøhn, T.; Amundsen, P.-A. The competitive edge of an invading specialist. Ecology 2001, 82, 2150–2163. [Google Scholar] [CrossRef]
- Olsson, K.; Stenroth, P.; Nyström, P.; Granéli, W. Invasions and niche width: Does niche width of an introduced crayfish differ from a native crayfish? Freshwat. Biol. 2009, 54, 1731–1740. [Google Scholar] [CrossRef]
- Brabrand, Å.; Faafeng, B. Habitat shift in roach (Rutilus rutilus) induced by pikeperch (Stizostedion lucioperca) introduction: Predation risk versus pelagic behaviour. Oecologia 1993, 95, 38–46. [Google Scholar] [CrossRef]
- Sharma, C.M.; Borgstrøm, R. Shift in density, habitat use, and diet of perch and roach: An effect of changed predation pressure after manipulation of pike. Fish Res. 2008, 91, 98–106. [Google Scholar] [CrossRef]
- Eloranta, A.P.; Knudsen, R.; Amundsen, P.A. Niche segregation of coexisting Arctic charr (Salvelinus alpinus) and brown trout (Salmo trutta) constrains food web coupling in subarctic lakes. Freshwat. Biol. 2013, 58, 207–221. [Google Scholar] [CrossRef]
- Pacioglu, O.; Zubrod, J.P.; Schulz, R.; Jones, J.I.; Pârvulescu, L. Two is better than one: Combining gut content and stable isotope analyses to infer trophic interactions between native and invasive species. Hydrobiologia 2019, 839, 25–35. [Google Scholar] [CrossRef]
- Haubrock, P.J.; Balzani, P.; Azzini, M.; Inghilesi, A.F.; Veselý, L.; Guo, W.; Tricarico, E. Shared histories of co-evolution may affect trophic interactions in a freshwater community dominated by alien species. Front. Ecol. Evol. 2019, 7, 355. [Google Scholar] [CrossRef]
- Courchamp, F.; Caut, S.; Bonnaud, E.; Bourgeois, K.; Angulo, E.; Watari, Y. Eradication of Alien Invasive Species: Surprise Effects and Conservation Successes. In Island Invasives: Eradication and Management; Veitch, C.R., Clout, M.N., Towns, D.R., Eds.; IUCN: Gland, Switzerland, 2011; pp. 285–289. [Google Scholar]
- Courchamp, F.; Chapuis, J.-L.; Pascal, M. Mammal invaders on islands: Impact, control and control impact. Biol. Rev. 2003, 78, 347–383. [Google Scholar] [CrossRef]
- Bergstrom, D.M.; Lucieer, A.; Kiefer, K.; Wasley, J.; Belbin, L.; Pedersen, T.K.; Chown, S.L. Indirect effects of invasive species removal devastate World Heritage Island. J. Appl. Ecol. 2009, 46, 73–81. [Google Scholar] [CrossRef]
- Russell, J.C.; Kaiser-Bunbury, C.N. Consequences of multispecies introductions on island ecosystems. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 169–190. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, W.; Li, B.; Wen, L.; Lei, G. Habitat alteration facilitates the dominance of invasive species through disrupting niche partitioning in floodplain wetlands. Divers. Distrib. 2021, 27, 1861–1871. [Google Scholar] [CrossRef]
- Alcorlo, P.; Geiger, W.; Otero, M. Feeding preferences and food selection of the red swamp crayfish, Procambarus clarkii, in habitats differing in food item diversity. Crustaceana 2004, 77, 435–453. [Google Scholar]
- Jackson, M.C.; Jones, T.; Milligan, M.; Sheath, D.; Taylor, J.; Ellis, A.; England, J.; Grey, J. Niche differentiation among invasive crayfish and their impacts on ecosystem structure and functioning. Freshw. Biol. 2014, 59, 1123–1135. [Google Scholar] [CrossRef]
- Larson, E.R.; Twardochleb, L.A.; Olden, J.D. Comparison of trophic function between the globally invasive crayfishes Pacifastacus leniusculus and Procambarus clarkii. Limnology 2016, 18, 275–286. [Google Scholar] [CrossRef]
- Chucholl, F.; Chucholl, C. Differences in the functional responses of four invasive and one native crayfish species suggest invader-specific ecological impacts. Freshw. Biol. 2021, 66, 2051–2063. [Google Scholar] [CrossRef]
- Wu, J.; Chen, H.; Jin, B.; Winemiller, K.O.; Wu, S.; Xu, W.; Zhang, H.; Wu, X. Seasonal variation in resource overlap between red swamp crayfish (Procambarus clarkii) and native species in Poyang Lake wetland, China. Front. Environ. Sci. 2022, 10, 923962. [Google Scholar] [CrossRef]
- Gherardi, F.; Barbaresi, S. Feeding opportunism of the red swamp crayfish Procambarus clarkii, an invasive species. Freshw. Crayfish 2008, 16, 77–85. [Google Scholar]
- Hellmann, C.; Worischka, S.; Mehler, E.; Becker, J.; Gergs, R.; Winkelmann, C. The trophic function of Dikerogammarus villosus (Sowinsky, 1894) in invaded rivers: A case study in the Elbe and Rhine. Aquat. Invasions 2015, 10, 385–397. [Google Scholar] [CrossRef]
- Locke, S.A.; Bulté, G.; Forbes, M.R.; Marcogliese, D.J. Estimating diet in individual pumpkinseed sunfish Lepomis gibbosus using stomach contents, stable isotopes and parasites. J. Fish Biol. 2013, 82, 522–537. [Google Scholar] [CrossRef]
- Nikolova, M.; Uzunova, E.; Studenkov, S.; Georgieva, M.; Pehlivanov, L.; Velkov, B. Fееding patterns and seasonal variation in the diet of non-indigenous fish species Lepomis gibbosus L. from shallow eutrophic lakes along River Vit, Bulgaria. Nat. Montenegrina 2008, 7, 71–85. [Google Scholar]
- Godinho, F.; Ferreira, M.T.; Cortes, R.V. The environmental basis of diet variation in pumpkinseed sunfish, Lepomis gibbosus, and largemouth bass, Micropterus salmoides, along an Iberian river basin. Environ. Biol. Fishes 1997, 50, 105–115. [Google Scholar] [CrossRef]
- Andraso, G.M. Summer food habits of pumpkinseeds (Lepomis gibbosus) and bluegills (Lepomis macrochirus) in Presque Isle Bay, Lake Erie. J. Great Lakes Res. 2005, 31, 397–404. [Google Scholar] [CrossRef]
- Rezsu, E.; Specziár, A. Ontogenetic diet profiles and size-dependent diet partitioning of ruffe Gymnocephalus cernuus, perch Perca fluviatilis and pumpkinseed Lepomis gibbosus in Lake Balaton. Ecol. Freshw. Fish 2006, 15, 339–349. [Google Scholar] [CrossRef]
- García-Berthou, E.; Moreno-Amich, R. Rudd (Scardinius erythrophthalmus) introduced to the Iberian peninsula: Feeding ecology in Lake Banyoles. Hydrobiologia 2000, 436, 159–164. [Google Scholar] [CrossRef]
- Ünver, B.; Erk’akan, F. Diet composition of chub, Squalius cephalus (Teleostei: Cyprinidae), in Lake Tödürge, Sivas, Turkey. J. Appl. Ichthyol. 2011, 27, 1350–1355. [Google Scholar] [CrossRef]
- Dörner, H.; Skov, C.; Berg, S.; Schulze, T.; Beare, D.J.; Van der Velde, G. Piscivory and trophic position of Anguilla anguilla in two lakes: Importance of macrozoobenthos density. J. Fish Biol. 2009, 74, 2115–2131. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.J.; Whoriskey, F.G.; Roy, L.H. Turbidity generation and biological impacts of an exotic fish Carassius auratus, introduced into shallow seasonally anoxic ponds. J. Fish Biol. 1995, 47, 576–585. [Google Scholar] [CrossRef]
- Marić, D. Feeding of Carassius auratus gibelio (Bloch) in Skadar Lake (Montenegro) and competetive relations with autochthonus cyprinid species. Montenegrin Acad. Sci. Arts Glas. Sect. Nat. Sci. 2000, 13, 237–258. [Google Scholar]
- Bissattini, A.M.; Haubrock, P.J.; Buono, V.; Balzani, P.; Borgianni, N.; Stellati, L.; Inghilesi, A.F.; Tancioni, L.; Martinoli, M.; Tricarico, E.; et al. Trophic structure of a pond community dominated by an invasive alien species: Insights from stomach content and stable isotope analyses. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 948–963. [Google Scholar] [CrossRef]
- Gaino, E.; Scoccia, F.; Piersanti, S.; Rebora, M.; Bellucci, L.G.; Ludovisi, A. Spicule records of Ephydatia fluviatilis as a proxy for hydrological and environmental changes in the shallow Lake Trasimeno (Umbria, Italy). Hydrobiologia 2012, 679, 139–153. [Google Scholar] [CrossRef]
- Alaş, A.; Altındağ, A.; Yılmaz, M.; Kırpık, M.A.; Ak, A. Feeding habits of tench (Tinca tinca L., 1758) in Beyşehir Lake (Turkey). Turk. J. Fish Aquat. Sci. 2010, 10, 187–194. [Google Scholar] [CrossRef]
- García-Berthou, E. Size- and depth-dependent variation in habitat and diet of the common carp (Cyprinus carpio). Aquat. Sci. 2001, 63, 466–476. [Google Scholar] [CrossRef]
- Rosecchi, E.; Crivelli, A.J. Study of a sand smelt (Atherina boyeri Risso 1810) population reproducing in fresh water. Ecol. Freshw. Fish 1992, 1, 77–85. [Google Scholar] [CrossRef]
- Chrisafi, E.; Kaspiris, P.; Katselis, G. Feeding habits of sand smelt (Atherina boyeri, Risso 1810) in Trichonis Lake (Western Greece). J. Appl. Ichthyol. 2007, 23, 209–214. [Google Scholar] [CrossRef]
- Vizzini, S.; Mazzola, A. Feeding ecology of the sand smelt Atherina boyeri (Risso 1810) (Osteichthyes, Atherinidae) in the western Mediterranean: Evidence for spatial variability based on stable carbon and nitrogen isotopes. Environ. Biol. Fishes 2005, 72, 259–266. [Google Scholar] [CrossRef]
- Vizzini, S.; Mazzola, A. Stable carbon and nitrogen ratios in the sand smelt from a Mediterranean coastal area: Feeding habits and effect of season and size. J. Fish Biol. 2002, 60, 1498–1510. [Google Scholar] [CrossRef]
- Jaćimović, M.; Krpo-Cetković, J.; Skorić, S.; Smederevac-Lalić, M.; Hegedis, A. Seasonal feeding habits and ontogenetic diet shift of black bullhead (Ameiurus melas) in Lake Sava (Serbia). Arch. Biol. Sci. 2021, 73, 513–521. [Google Scholar] [CrossRef]
- Alp, A.; Yeğen, V.; Apaydin Yağci, M.; Uysal, R.; Biçen, E.; Yağci, A. Diet composition and prey selection of the pike, Esox lucius, in Çivril Lake, Turkey. J. Appl. Ichthyol. 2008, 24, 670–677. [Google Scholar] [CrossRef]
- Yazicioglu, O.; Polat, N.; Yilmaz, S. Feeding biology of pike, Esox lucius L., 1758 inhabiting Lake Ladik, Turkey. Turk. J. Fish Aquat. Sci. 2018, 18, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, C.P.; Tonn, W.M.; Prepas, E.E.; Wassenaar, L.I. Individual specialization and trophic adaptability of northern pike (Esox lucius): An isotope and dietary analysis. Oecologia 1999, 120, 386–396. [Google Scholar] [CrossRef]
- Landucci, F.; Gigante, D.; Venanzoni, R. An application of the Cocktail method for the classification of the hydrophytic vegetation at Lake Trasimeno (Central Italy). Fitosociologia 2011, 48, 3–22. [Google Scholar]
- Villa, P.; Bresciani, M.; Bolpagni, R.; Pinardi, M.; Giardino, C. A rule-based approach for mapping macrophyte communities using multi-temporal aquatic vegetation indices. Remote Sens. Environ. 2015, 171, 218–233. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).