Predicting Boar Sperm Survival during Liquid Storage Using Vibrational Spectroscopic Techniques
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Semen Samples
2.2. Sperm Motility Analysis
2.3. Raman Spectroscopy Analysis
2.4. Near-Infrared Spectroscopy (NIRS) Analysis
2.5. Flow Cytometry Analyses
2.6. Total Antioxidant and Lipid Peroxidation
2.7. Data Processing and Statistical Analyses
3. Results
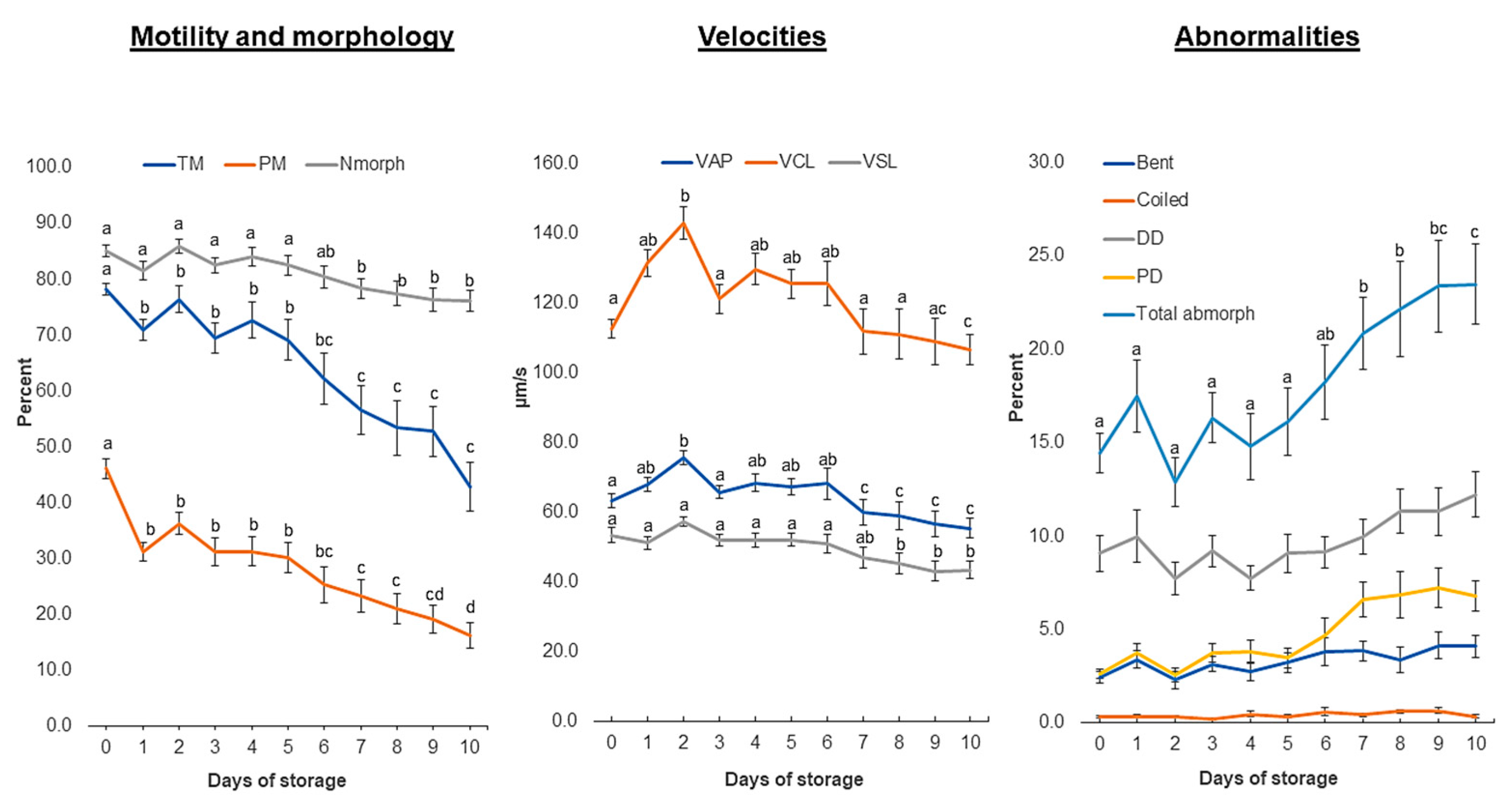
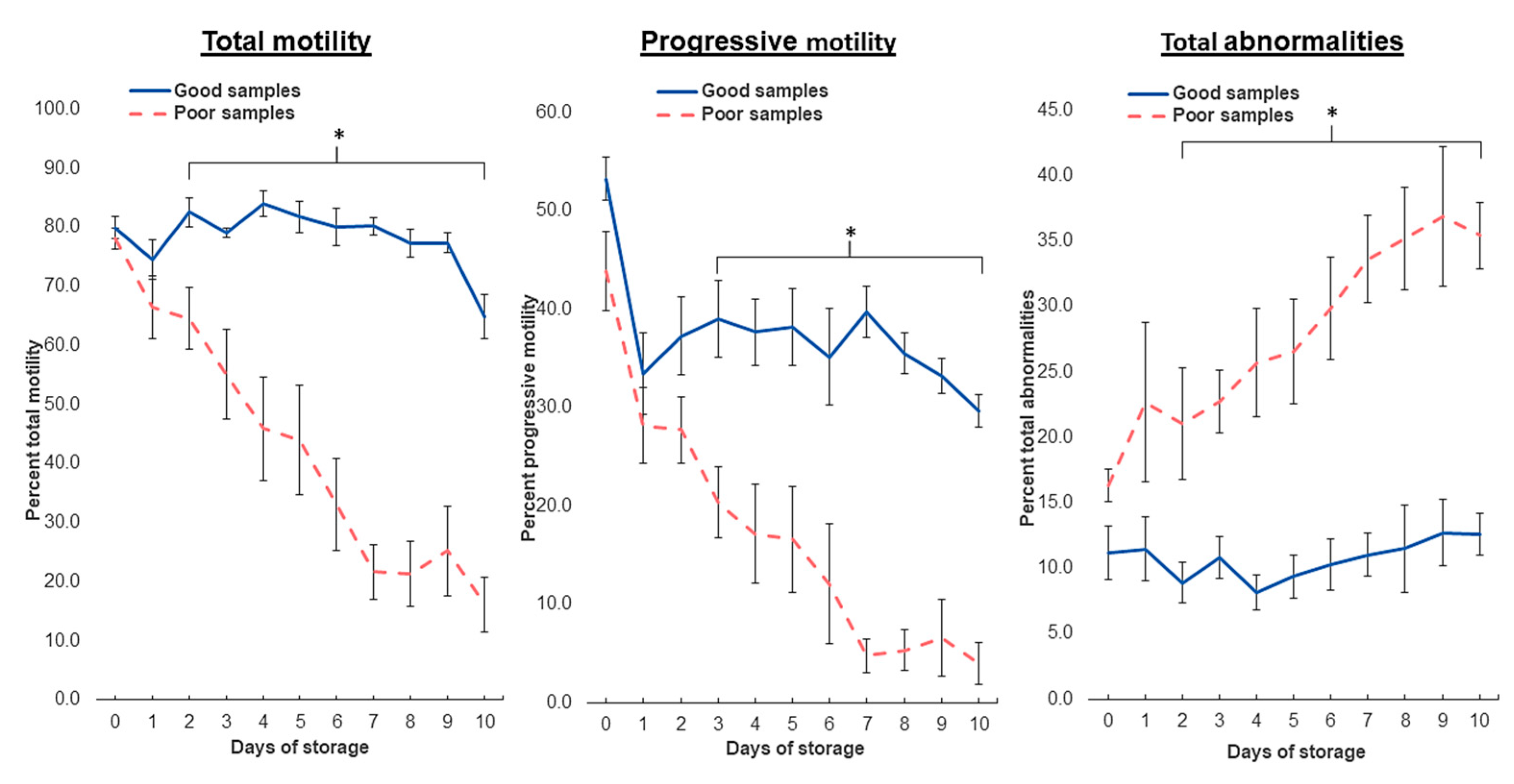
3.1. Sperm Motility: Differential Response of Semen Doses to Storage
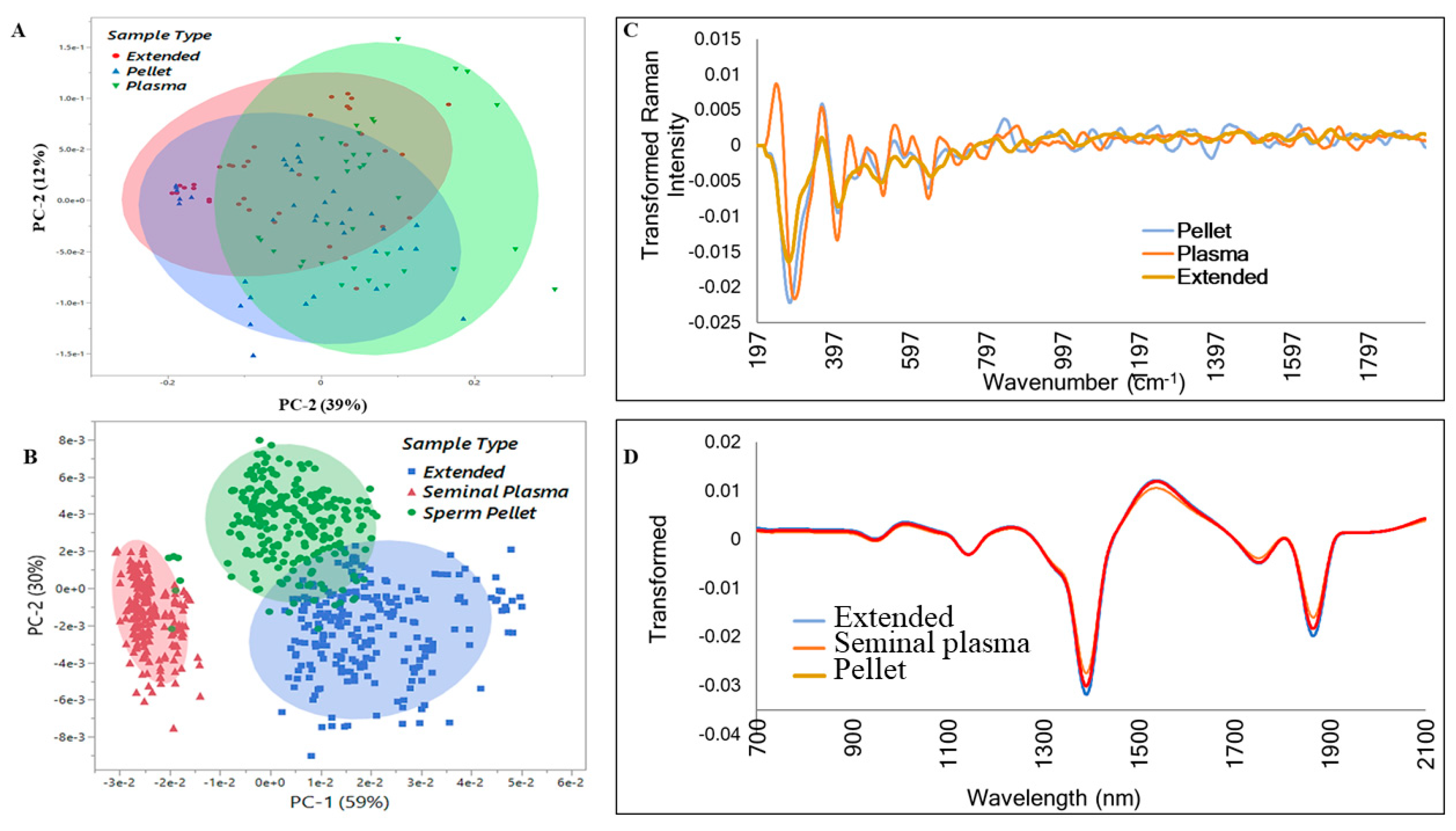
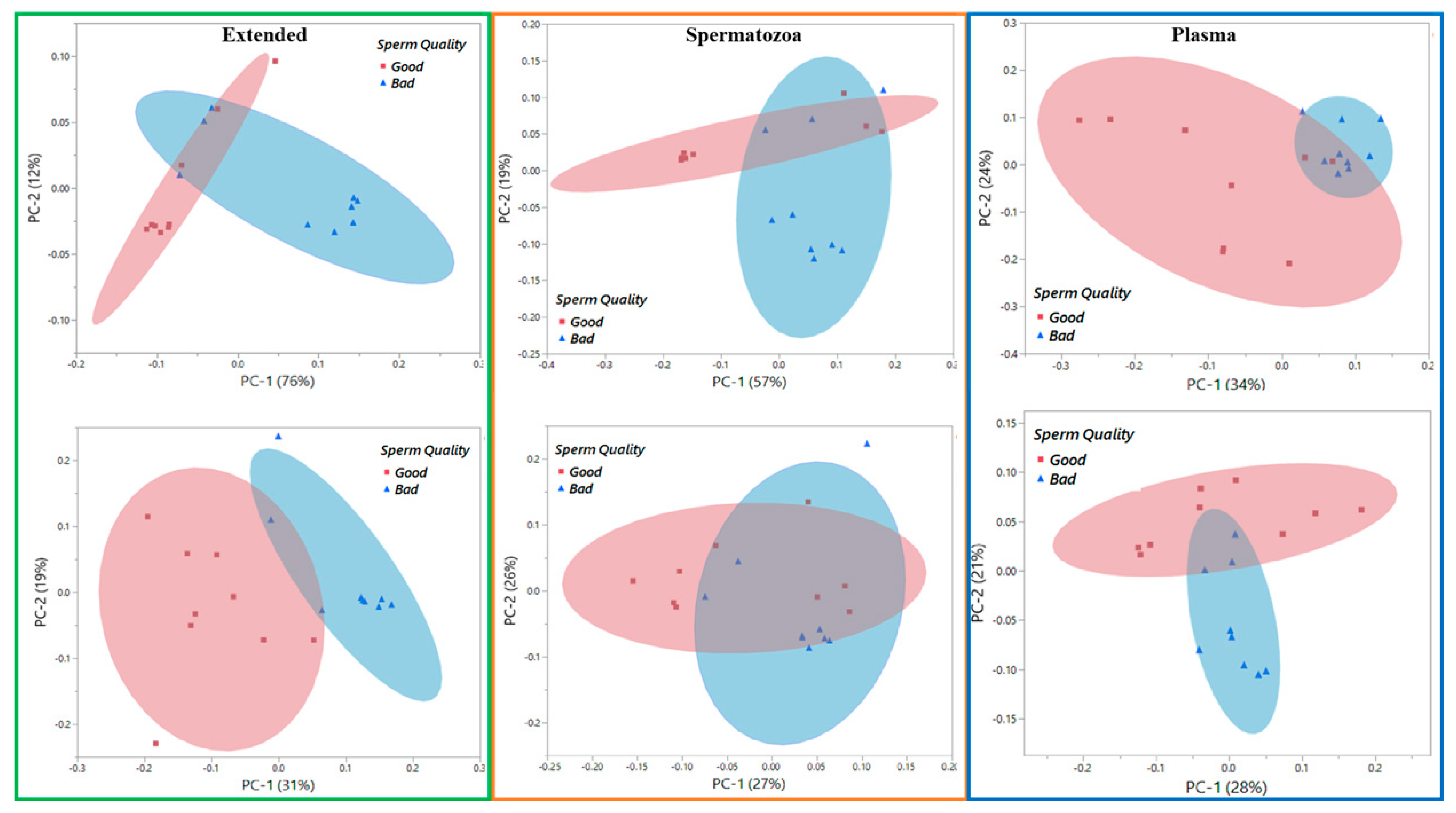
3.2. Vibrational Spectroscopy Fingerprints and Classification of Samples
3.3. Sperm Quality Attributes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeste, M.; Rodríguez-Gil, J.E.; Bonet, S. Artificial Insemination with Frozen-Thawed Boar Sperm. Mol. Reprod. Dev. 2017, 84, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Knox, R.V. Artificial Insemination in Pigs Today. Theriogenology 2016, 85, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Avendaño, C.; Franchi, A.; Taylor, S.; Morshedi, M.; Bocca, S.; Oehninger, S. Fragmentation of DNA in Morphologically Normal Human Spermatozoa. Fertil. Steril. 2009, 91, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Ferrigno, A.; Ruvolo, G.; Capra, G.; Serra, N.; Bosco, L. Correlation between the DNA Fragmentation Index (DFI) and Sperm Morphology of Infertile Patients. J. Assist. Reprod. Genet. 2021, 38, 979–986. [Google Scholar] [CrossRef]
- Hamidi, J.; Frainais, C.; Amar, E.; Bailly, E.; Clément, P.; Ménézo, Y. A Double-Blinded Comparison of in Situ TUNEL and Aniline Blue versus Flow Cytometry Acridine Orange for the Determination of Sperm DNA Fragmentation and Nucleus Decondensation State Index. Zygote 2015, 23, 556–562. [Google Scholar] [CrossRef]
- Vašiček, J.; Svoradová, A.; Baláži, A.; Jurčik, R.; Macháč, M.; Chrenek, P. Ram Semen Quality Can Be Assessed by Flow Cytometry Several Hours after Post-Fixation. Zygote 2021, 29, 130–137. [Google Scholar] [CrossRef]
- Hossain, M.S.; Johannisson, A.; Wallgren, M.; Nagy, S.; Siqueira, A.P.; Rodriguez-Martinez, H. Flow Cytometry for the Assessment of Animal Sperm Integrity and Functionality: State of the Art. Asian J. Androl. 2011, 13, 406–419. [Google Scholar] [CrossRef]
- Martínez-Pastor, F.; Mata-Campuzano, M.; Álvarez-Rodríguez, M.; Álvarez, M.; Anel, L.; de Paz, P. Probes and Techniques for Sperm Evaluation by Flow Cytometry. Reprod. Domest. Anim. 2010, 45, 67–78. [Google Scholar] [CrossRef]
- Kamphuis, C.; Duenk, P.; Veerkamp, R.F.; Visser, B.; Singh, G.; Nigsch, A.; De Mol, R.M.; Broekhuijse, M.L.W.J. Machine Learning to Further Improve the Decision Which Boar Ejaculates to Process into Artificial Insemination Doses. Theriogenology 2020, 144, 112–121. [Google Scholar] [CrossRef]
- Amann, R.P.; Waberski, D. Computer-Assisted Sperm Analysis (CASA): Capabilities and Potential Developments. Theriogenology 2014, 81, 5–17.e3. [Google Scholar] [CrossRef]
- Tanga, B.M.; Qamar, A.Y.; Raza, S.; Bang, S.; Fang, X.; Yoon, K.; Cho, J. Semen Evaluation: Methodological Advancements in Sperm Quality-Specific Fertility Assessment—A Review. Anim. Biosci. 2021, 34, 1253–1270. [Google Scholar] [CrossRef] [PubMed]
- Huser, T.; Orme, C.A.; Hollars, C.W.; Corzett, M.H.; Balhorn, R. Raman Spectroscopy of DNA Packaging in Individual Human Sperm Cells Distinguishes Normal from Abnormal Cells. J. Biophotonics 2009, 2, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Durfey, C.L.; Swistek, S.E.; Liao, S.F.; Crenshaw, M.A.; Clemente, H.J.; Thirumalai, R.V.K.G.; Steadman, C.S.; Ryan, P.L.; Willard, S.T.; Feugang, J.M. Nanotechnology-Based Approach for Safer Enrichment of Semen with Best Spermatozoa. J. Anim. Sci. Biotechnol. 2019, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Feugang, J.M.; Liao, S.F.; Crenshaw, M.A.; Clemente, H.; Willard, S.T.; Ryan, P.L. Lectin-Functionalized Magnetic Iron Oxide Nanoparticles for Reproductive Improvement. JFIV Reprod. Med. Genet. 2015, 3, 1000145. [Google Scholar] [CrossRef]
- Muro, C.K.; Doty, K.C.; de Souza Fernandes, L.; Lednev, I.K. Forensic Body Fluid Identification and Differentiation by Raman Spectroscopy. Forensic Chem. 2016, 1, 31–38. [Google Scholar] [CrossRef]
- Butler, H.J.; Ashton, L.; Bird, B.; Cinque, G.; Curtis, K.; Dorney, J.; Esmonde-White, K.; Fullwood, N.J.; Gardner, B.; Martin-Hirsch, P.L.; et al. Using Raman Spectroscopy to Characterize Biological Materials. Nat. Protoc. 2016, 11, 664–687. [Google Scholar] [CrossRef]
- Mallidis, C.; Sanchez, V.; Wistuba, J.; Wuebbeling, F.; Burger, M.; Fallnich, C.; Schlatt, S. Raman Microspectroscopy: Shining a New Light on Reproductive Medicine. Hum. Reprod. Update 2014, 20, 403–414. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Huck, C.W. Near-Infrared Spectroscopy in Bio-Applications. Molecules 2020, 25, 2948. [Google Scholar] [CrossRef]
- Das, T.; Ammal, A.; Harshey, A.; Mishra, V.; Srivastava, A. Vibrational Spectroscopic Approaches for Semen Analysis in Forensic Investigation: State of the Art and Way Forward. Microchem. J. 2021, 171, 106810. [Google Scholar] [CrossRef]
- Abramczyk, H.; Sobkiewicz, B.; Walczack-Jędrzejowska, R.; Marchlewska, K.; Surmacki, J. Decoding the Role of Cytochrome c in Metabolism of Human Spermatozoa by Raman Imaging. Front. Cell Dev. Biol. 2022, 10, 983993. [Google Scholar] [CrossRef]
- Sánchez, V.; Redmann, K.; Wistuba, J.; Wübbeling, F.; Burger, M.; Olenhof, H.; Wolkers, W.F.; Kliesch, S.; Schlatt, S.; Mallidis, C. Oxidative DNA Damage in Human Sperm Can Be Detected by Raman Microspectroscopy. Fertil. Steril. 2012, 98, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, M.A.; Di Caprio, G.; Managò, S.; De Angelis, A.; Sirleto, L.; Coppola, G.; De Luca, A.C. Label-Free Imaging and Biochemical Characterization of Bovine Sperm Cells. Biosensors 2015, 5, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.M.; Bruno, C.; Parachalil, D.R.; Baker, M.J.; Bonnier, F.; Butler, H.J.; Byrne, H.J. Vibrational Spectroscopic Analysis and Quantification of Proteins in Human Blood Plasma and Serum. In Vibrational Spectroscopy in Protein Research: From Purified Proteins to Aggregates and Assemblies; Academic Press: Cambridge, MA, USA, 2020; pp. 269–314. ISBN 9780128186107. [Google Scholar]
- Barčot, O.; Balarin, M.; Gamulin, O.; Ježek, P.; Romac, P.; Brnjas-kraljević, J. Investigation of Spermatozoa and Seminal Plasma by Fourier Transform Infrared Spectroscopy. Appl. Spectrosc. 2007, 61, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, F. Near-Infrared Laboratory Spectroscopy of Mineral Chemistry: A Review. Int. J. Appl. Earth Obs. Geoinfor. 2018, 65, 71–78. [Google Scholar]
- De Luca, A.C.; Managó, S.; Ferrara, M.A.; Rendina, I.; Sirleto, L.; Puglisi, R.; Balduzzi, D.; Galli, A.; Ferraro, P.; Coppola, G. Non-Invasive Sex Assessment in Bovine Semen by Raman Spectroscopy. Laser Phys. Lett. 2014, 11, 055604. [Google Scholar] [CrossRef]
- Kumar Yata, V.; Kumar Gangwar, D.; Sharma, V.; Kumar Dubey, S.; Kumar Yadav, S.; Choudhary, S.; Kumar, S.; Kumar Mohanty, T.; Kumar Mohanty, A. Semen Analysis and Sperm Characteristics of Karan Fries Cattle. Anim. Reprod. Sci. 2020, 212, 106250. [Google Scholar] [CrossRef]
- Li, C.; Wang, D.; Zhou, X. Sperm Proteome and Reproductive Technologies in Mammals. Anim. Reprod. Sci. 2016, 173, 1–7. [Google Scholar] [CrossRef]
- Du, S.; Zhang, Q.; Guan, H.; Chen, G.; Wang, S.; Sun, Y.; Li, Y.; Chen, R.; He, Y.; Huang, Z. Micro-Raman Analysis of Sperm Cells on Glass Slide: Potential Label-Free Assessment of Sperm DNA toward Clinical Applications. Biosensors 2022, 12, 1051. [Google Scholar] [CrossRef]
- Meister, K.; Schmidt, D.A.; Bründermann, E.; Havenith, M. Confocal Raman Microspectroscopy as an Analytical Tool to Assess the Mitochondrial Status in Human Spermatozoa. Analyst 2010, 135, 1370–1374. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, G.; Chen, X.; Wang, J.; Chen, J.; Lu, P.; Chen, R. Rapid and Label-Free Identification of Normal Spermatozoa Based on Image Analysis and Micro-Raman Spectroscopy. J. Biophotonics 2014, 7, 671–675. [Google Scholar] [CrossRef]
- Li, N.; Chen, D.; Xu, Y.; Liu, S.; Zhang, H.; Evenson, D.P.; Jost, L.K.; Marshall, D.; Zinaman, M.J.; Clegg, E.; et al. Confocal Raman Micro-Spectroscopy for Rapid and Label-Free Detection of Maleic Acid-Induced Variations in Human Sperm. Reprod. Update 2004, 10, 2681–2687. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Dechant, M.; Sanchez, V.; Wistuba, J.; Boiani, M.; Pilatz, A.; Stammler, A.; Middendorff, R.; Schuler, G.; Bhushan, S.; et al. Structural and Functional Integrity of Spermatozoa Is Compromised as a Consequence of Acute Uropathogenic E. coli-Associated Epididymitis. Biol. Reprod. 2013, 89, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oldenhof, H.; Schütze, S.; Wolkers, W.F.; Sieme, H. Fourier Transform Infrared Spectroscopic Analysis of Sperm Chromatin Structure and DNA Stability. Andrology 2016, 4, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, N.H.; Nguyen, T.; Gad, A.; Tesfaye, D.; Liao, S.F.; Willard, S.T.; Ryan, P.L.; Feugang, J.M. Characterization of Extracellular Vesicle-Coupled MiRNA Profiles in Seminal Plasma of Boars with Divergent Semen Quality Status. Int. J. Mol. Sci. 2023, 24, 3194. [Google Scholar] [CrossRef]
- Sheng, Q.; Santos-Rivera, M.; Ouyang, X.; Kouba, A.J.; Vance, C.K. Near-Infrared Spectroscopy and Mode Cloning (NIR-MC) for In-Situ Analysis of Crude Protein in Bamboo. Remote Sens. 2022, 14, 1302. [Google Scholar] [CrossRef]
- Llavanera, M.; Delgado-bermúdez, A.; Olives, S.; Mateo-otero, Y.; Recuero, S.; Bonet, S.; Fernández-Fuertes, B.; Yeste, M.; Barranco, I. Glutathione S-Transferases Play a Crucial Role in Mitochondrial Function, Plasma Membrane Stability and Oxidative Regulation of Mammalian Sperm. Antioxidants 2020, 9, 100. [Google Scholar] [CrossRef]
- Stevens, A.; Ramirez-Lopez, L. An Introduction to the Prospectr Package. Available online: https://cran.r-project.org/web/packages/prospectr/vignettes/prospectr.html (accessed on 8 November 2023).
- Bischl, B.; Lang, M.; Kotthoff, L.; Schiffner, J.; Richter, J.; Studerus, E.; Casalicchio, G.; Jones, Z.M. Mlr: Machine Learning in R. J. Mach. Learn. Res. 2016, 17, 1–5. [Google Scholar]
- Kursa, M.B.; Rudnicki, W.R. Feature Selection with the Boruta Package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef]
- Chen, L.D.; Santos-Rivera, M.; Burger, I.J.; Kouba, A.J.; Barber, D.M.; Vance, C.K. Near-Infrared Spectroscopy (NIRS) as a Method for Biological Sex Discrimination in the Endangered Houston Toad (Anaxyrus houstonensis). Methods Protoc. 2022, 5, 4. [Google Scholar] [CrossRef]
- Holt, W.V. Fundamental Aspects of Sperm Cryobiology: The Importance of Species and Individual Differences. Theriogenology 2000, 53, 47–58. [Google Scholar] [CrossRef]
- Yeste, M. Recent Advances in Boar Sperm Cryopreservation: State of the Art and Current Perspectives. Reprod. Domest. Anim. 2015, 50, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Szymanowicz, J.; Schwarz, T.; Murawski, M.; Małopolska, M.; Oszczęda, Z.; Tuz, R.; Nowicki, J.; Mieczyslaw Bartlewski, P. Storage of Boar Semen at 16–18 °C in the Long-Term Commercial Extender Prepared with Deionized Water or Nanowater. Anim. Reprod. 2019, 16, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Wysokińska, A.; Szablicka, D.; Dziekońska, A.; Wójcik, E. Analysis of Changes in the Morphological Structures of Sperm during Preservation of Liquid Boar Semen in Two Different Seasons of the Year. Anim. Reprod. Sci. 2023, 256, 107297. [Google Scholar] [CrossRef] [PubMed]
- Wiebke, M.; Hensel, B.; Nitsche-Melkus, E.; Jung, M.; Schulze, M. Cooled Storage of Semen from Livestock Animals (Part I): Boar, Bull, and Stallion. Anim. Reprod. Sci. 2022, 246, 106822. [Google Scholar] [CrossRef]
- Johnson, L.A.; Weitze, K.F.; Fiser, P.; Maxwell, W.M.C. Storage of Boar Semen. Anim. Reprod. Sci. 2000, 62, 143–172. [Google Scholar] [CrossRef]
- Gadea, J. Sperm Factors Related to in Vitro and in Vivo Porcine Fertility. Theriogenology 2005, 63, 431–444. [Google Scholar] [CrossRef]
- Premrov Bajuk, B.; Zrimšek, P.; Zakošek Pipan, M.; Tilocca, B.; Soggiu, A.; Bonizzi, L.; Roncada, P. Proteomic Analysis of Fresh and Liquid-Stored Boar Spermatozoa. Animals 2020, 10, 553. [Google Scholar] [CrossRef]
- Khoi, H.X.; Shimizu, K.; Yoneda, Y.; Minagawa, I.; Abe, Y.; Kuwabara, Y.; Sasanami, T.; Kohsaka, T. Monitoring the Reactive Oxygen Species in Spermatozoa during Liquid Storage of Boar Semen and Its Correlation with Sperm Motility, Free Thiol Content and Seasonality. Andrologia 2021, 53, e14237. [Google Scholar] [CrossRef]
- Lucca, M.S.; Gianluppi, R.D.F.; Mellagi, A.P.G.; Bortolozzo, F.P.; Wentz, I.; Ulguim, R. da R. Effects of the Classification of Boars According to Progressive Sperm Motility and the Extender Type on the Reproductive Performance of a Single Fixed-Time Insemination. Theriogenology 2021, 161, 120–125. [Google Scholar] [CrossRef]
- George, N.; Singh, H.; Jotaniya, R.; Pandya, S.R. Raman Spectroscopy for the Determination of Forensically Important Bio-Fluids. Forensic Sci. Int. 2022, 340, 111441. [Google Scholar] [CrossRef]
- Vyas, B.; Halámková, L.; Lednev, I.K. A Universal Test for the Forensic Identification of All Main Body Fluids Including Urine. Forensic Chem. 2020, 20, 100247. [Google Scholar] [CrossRef]
- Rivera, J.M.S. Novel Strategies in Near Infrared Spectroscopy (NIRS) and Multivariate Analysis (MVA) for Detecting and Profiling Pathogens and Diseases of Agricultural Importance; Mississippi State University: Starkville, MS, USA, 2022. [Google Scholar]
- Fikiet, M.A.; Lednev, I.K. Raman Spectroscopic Method for Semen Identification: Azoospermia. Talanta 2019, 194, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Chang, L.; Wang, B.; Zhang, Z.; Wei, Y.; Dou, Y.; Qi, K.; Yang, F.; Li, X.; Li, X.; et al. Seminal Plasma Metabolomics Analysis of Differences in Liquid Preservation Ability of Boar Sperm. J. Anim. Sci. 2023, 101, skad392. [Google Scholar] [CrossRef] [PubMed]
- Yánez-Ortiz, I.; Catalán, J.; Rodríguez-Gil, J.E.; Miró, J.; Yeste, M. Advances in Sperm Cryopreservation in Farm Animals: Cattle, Horse, Pig and Sheep. Anim. Reprod. Sci. 2022, 246, 106904. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Martinez, E.A.; Calvete, J.J.; Peña Vega, F.J.; Roca, J. Seminal Plasma: Relevant for Fertility? Int. J. Mol. Sci. 2021, 22, 4368. [Google Scholar] [CrossRef]
- Gerwert, K.; Hess, B.; Michel, H.; Buchanan, S. FTIR Studies on Crystals of Photosynthetic Reaction Centers. FEBS Lett. 1988, 232, 303–307. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, Q.; Li, B.; Zhao, X. Raman Spectroscopy: A Novel Technology for Gastric Cancer Diagnosis. Front. Bioeng. Biotechnol. 2022, 10, 856591. [Google Scholar] [CrossRef]
- Amaral, S.; Da Costa, R.; Wübbeling, F.; Redmann, K.; Schlatt, S. Raman Micro-Spectroscopy Analysis of Different Sperm Regions: A Species Comparison. Mol. Hum. Reprod. 2018, 24, 185–202. [Google Scholar] [CrossRef]
- Song, C.; Zhang, Z.; Wei, Y.; Dou, Y.; Qi, K.; Li, X.; Yang, F.; Li, X.; Wang, K.; Qiao, R.; et al. Proteomic Analysis of Boar Sperm with Differential Ability of Liquid Preservation at 17 °C. Theriogenology 2024, 215, 103–112. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, W.; Liu, Y.; Liu, Y.; Liang, H.; Xu, Q.; Liu, Z.; Weng, X. Plasma Membrane Lipid Composition and Metabolomics Analysis of Yorkshire Boar Sperms with High and Low Resistance to Cryopreservation. Theriogenology 2023, 206, 28–39. [Google Scholar] [CrossRef]
- Li, J.; Zhao, W.; Zhu, J.; Ju, H.; Liang, M.; Wang, S.; Chen, S.; Ferreira-Dias, G.; Liu, Z. Antioxidants and Oxidants in Boar Spermatozoa and Their Surrounding Environment Are Associated with AMPK Activation during Liquid Storage. Vet. Sci. 2023, 10, 214. [Google Scholar] [CrossRef]
- Pascolo, L.; Bedolla, D.E.; Vaccari, L.; Venturin, I.; Cammisuli, F.; Gianoncelli, A.; Mitri, E.; Giolo, E.; Luppi, S.; Martinelli, M.; et al. Pitfalls and Promises in FTIR Spectromicroscopy Analyses to Monitor Iron-Mediated DNA Damage in Sperm. Reprod. Toxicol. 2016, 61, 39–46. [Google Scholar] [CrossRef]
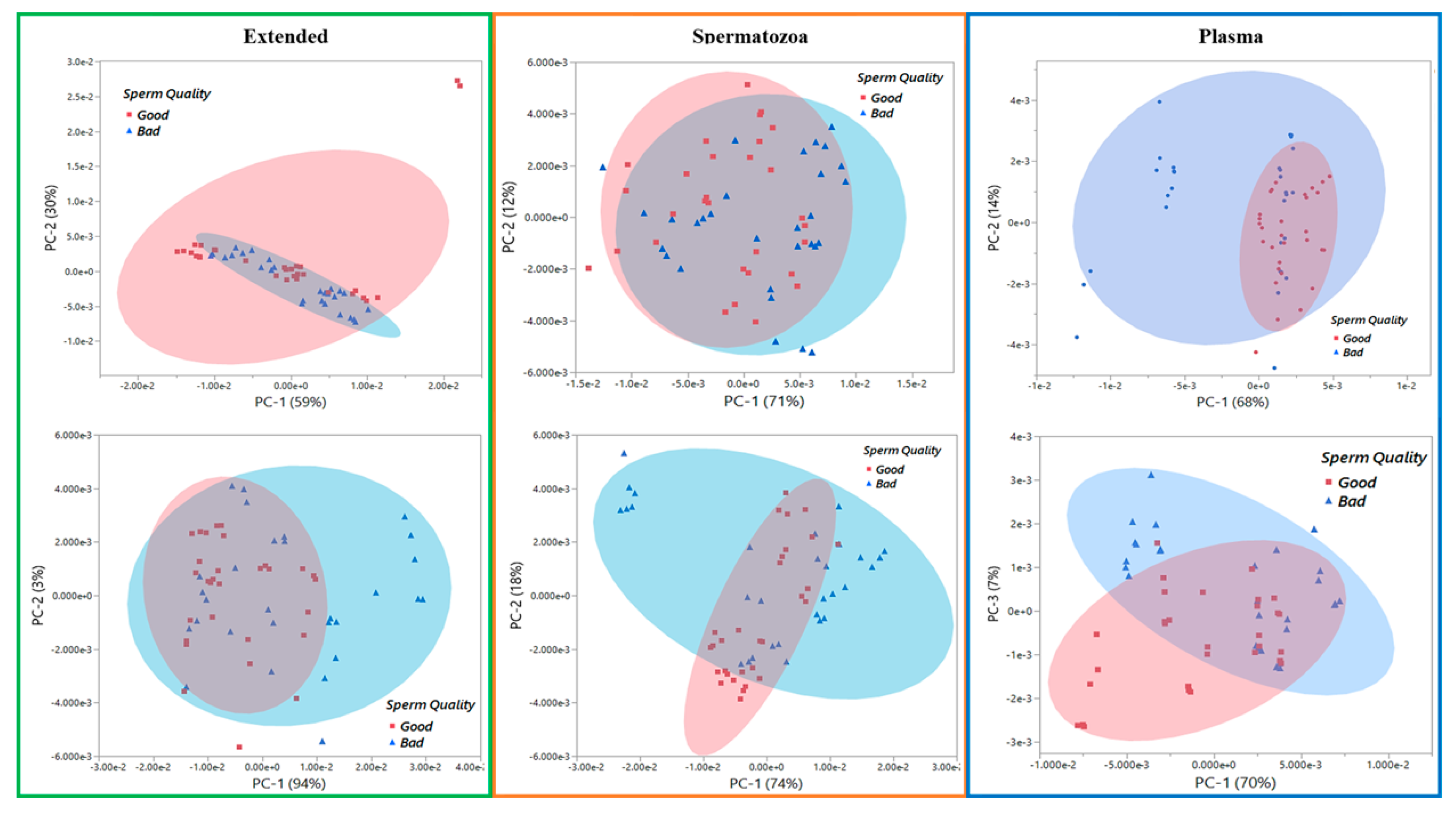
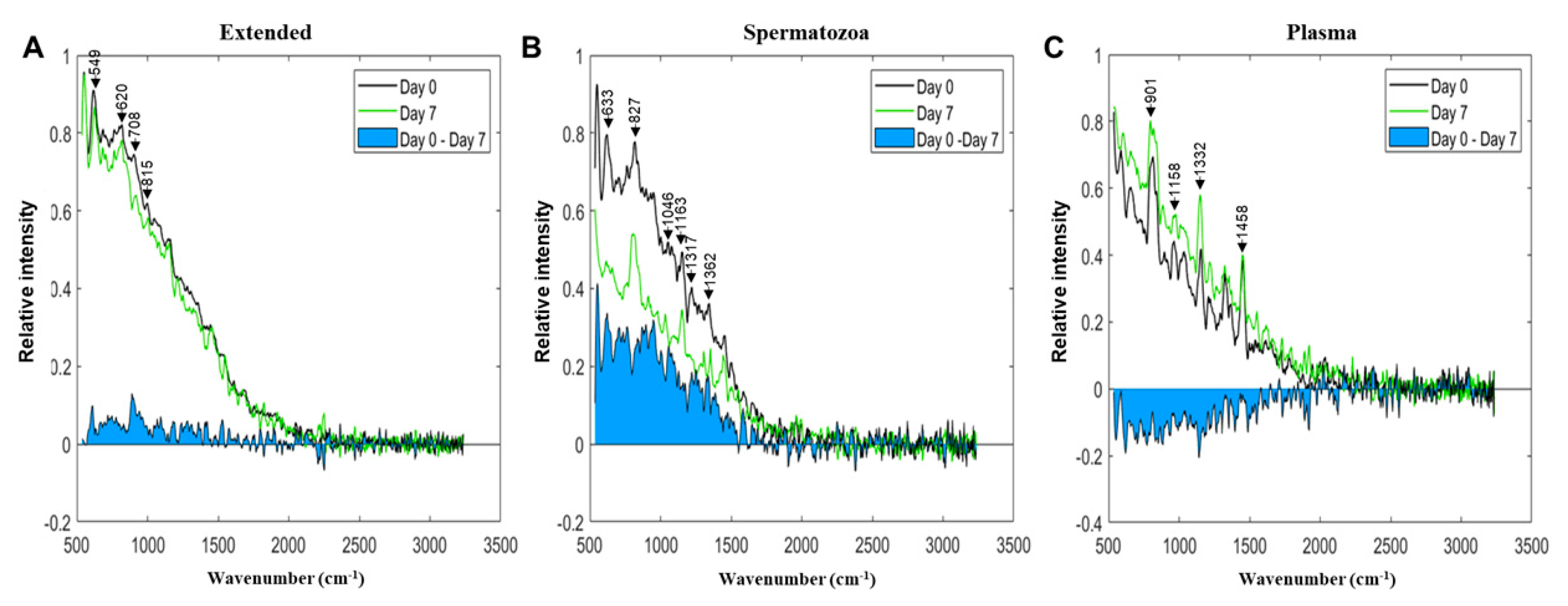
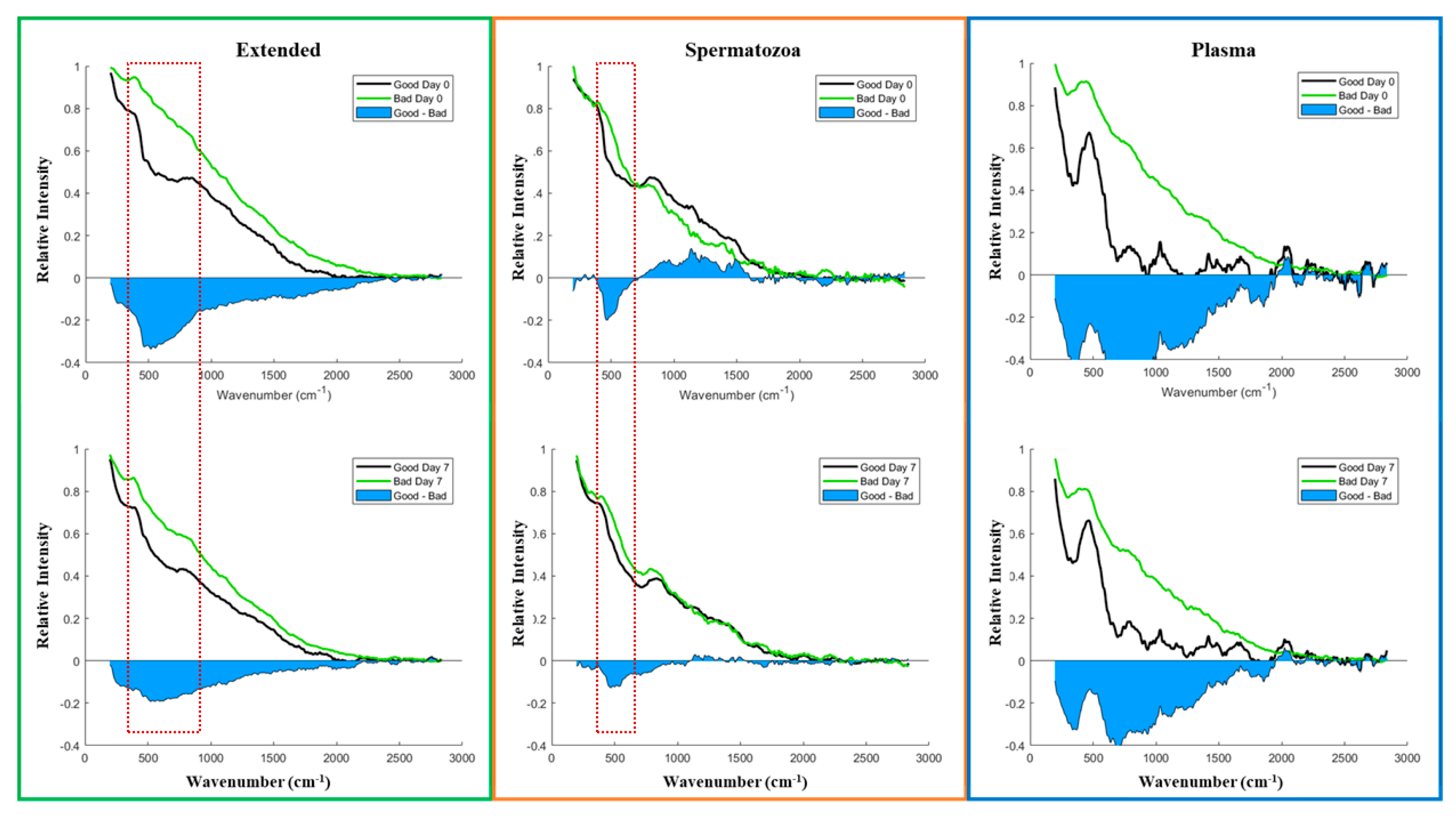
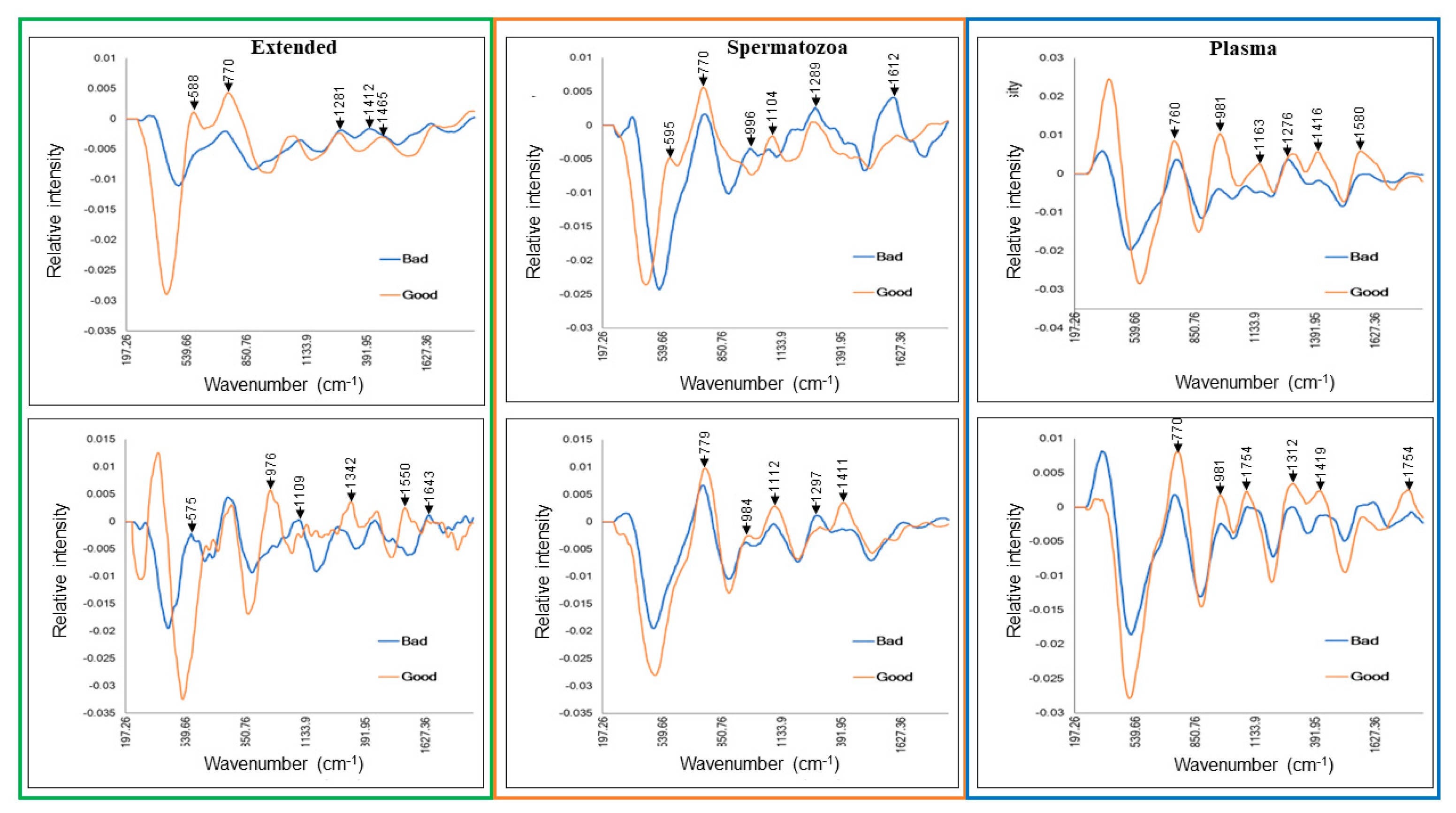
| Sample and Size | Models | Extended | Spermatozoa | Plasma | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Accuracy | Sensitivity | Specificity | Accuracy | Sensitivity | Specificity | Accuracy | Sensitivity | Specificity | ||
| Raman * (n = 36) | SVM | 72.2% | 61.1% | 83.3% | 75.0% | 72.2% | 77.8% | 97.2% | 100.0% | 94.4% |
| LDA | 75.0% | 72.2% | 77.8% | 77.8% | 72.2% | 83.3% | 91.7% | 94.4% | 88.9% | |
| PLS | 69.4% | 66.7% | 72.2% | 77.8% | 77.8% | 77.8% | 86.1% | 94.4% | 77.8% | |
| NIR Day 0 (n = 30) | SVM | 63.3% | 80.0% | 46.7% | 60.0% | 50.0% | 70.0% | 80.0% | 86.7% | 73.3% |
| LDA | 63.3% | 56.7% | 63.6% | 83.3% | 66.7% | 100.0% | 81.7% | 70.0% | 93.3% | |
| PLS | 55.0% | 66.7% | 43.3% | 73.3% | 56.7% | 90.0% | 55.0% | 40.0% | 70.0% | |
| NIR Day 7 (n = 30) | SVM | 88.3% | 86.7% | 90.0% | 61.7% | 46.7% | 76.7% | 90.0% | 80.0% | 100.0% |
| LDA | 75.0% | 70.0% | 80.0% | 55.0% | 43.3% | 66.7% | 75.0% | 80.0% | 70.0% | |
| PLS | 63.3% | 46.7% | 80.0% | 41.7% | 33.3% | 50.0% | 80.0% | 80.0% | 80.0% | |
| Sample Types | Peak Wavenumber (cm−1) | Assignments | Compounds | |
|---|---|---|---|---|
| Detected * | Reported ** | |||
| Extended | 328 | 379 | Not assigned | Not assigned |
| 549 | 536 | S-S stretch, symmetric skeletal vibration | Lysozyme, lactate, urea | |
| 620 | 620 | CC aliphatic stretch | Ascorbic acid | |
| 708 | 709 | C-N stretching | Albumin | |
| 815 | 829 | Ring breathing | Tyrosine | |
| Pellet | 331 | 379 | Not assigned | Not assigned |
| 396 | 431 | Not assigned | Not assigned | |
| 559 | 536 | S-S stretch, symmetric skeletal vibration | Lysozyme, lactate, urea | |
| 633 | 640; 638 | Ring deformation | Cytosine | |
| 827 | 829 | Ring breathing | n/a | |
| 1046 | 1055 | CN symmetric stretching | n/a | |
| 1163 | 1178; 1179; 1180 | CH3/NH3 rocking | Tyrosine | |
| 1317 | 1317 | C-H vibration | n/a | |
| 1362 | 1367 | Not assigned | Thymine, adenine, cytosine | |
| Plasma | 355 | 379 | Not assigned | Not assigned |
| 403 | 488; 497 | N-C-O bending | Urea | |
| 591 | 536 | S-S stretch, symmetric skeletal vibration | Lysozyme, lactate, urea | |
| 901 | 955; 956; 957; 958; 959 | PO43− symmetric stretching | SPH | |
| 1158 | 1178; 1179; 1180 | CH3/NH3 rocking | Tyrosine | |
| 1332 | 1326; 1327; 1329 | Ring stretching | Tyrosine | |
| 1458 | 1447; 1448; 1451 | CH3, CH2 bending | Tryptophan, lactate | |
| 1559 | 1572; 1577 | Not assigned | Guanine, adenine | |
| 1737 | 1666; 1667; 1668; 1670; 1671 | Polypeptide backbone | Amide I | |
| Sample Types | Storage Duration | Peak Wavenumber (cm−1) | Detected in | Assignments | Compounds | ||
|---|---|---|---|---|---|---|---|
| Detected * | Reported ** | Good | Poor | ||||
| Extended | Day 0 | 588 | 536 | P | A | S-S stretch, symmetric skeletal vibration | Lysozyme, lactate, urea |
| 770 | 768 | P | P | Ring vibrations | Albumin | ||
| 1281 | 1267 | P | P | Symmetric ring deformation/breathing | Tyrosine | ||
| 1412 | 1418 | A | P | CH2 scissoring band | Lipids | ||
| 1465 | 1451 | P | A | CH2, CH3 bend | Tryptophan, lactate | ||
| Day 7 | 575 | 536 | A | P | S-S stretch, symmetric skeletal vibration | Lysozyme, lactate, urea | |
| 976 | 1001 | P | A | Aromatic ring breathing | Phenylalanine | ||
| 1109 | 1127 | A | P | CN asymmetric stretching | Tyrosine | ||
| 1342 | 1342 | P | A | C-H bend | Tryptophan | ||
| 1550 | 1572 | P | A | Not assigned | Adenine, guanine | ||
| 1643 | 1662 | A | P | Amide I bend | Lysozyme | ||
| Spermatozoa | Day 0 | 595 | 536 | P | A | S-S stretch, symmetric skeletal vibration | Lysozyme, lactate, urea |
| 770 | 768 | P | P | Ring vibrations | Albumin | ||
| 996 | 1001 | A | P | Aromatic ring breathing | Phenylalanine | ||
| 1104 | 1096 | P | A | PO4− stretching | PO4− backbone | ||
| 1289 | 1268 | P | P | Symmetric ring deformation | n/a | ||
| 1612 | 1616 | A | P | C-C stretching | Tyrosine | ||
| Day 7 | 779 | 780 | P | P | ν1 symmetric stretching | Thymine, cytosine | |
| 984 | 984 | P | P | CH2 wagging | n/a | ||
| 1112 | 1125 | P | P | CN asymmetric stretching | Tyrosine | ||
| 1297 | 1268 | A | P | Symmetric ring deformation | n/a | ||
| 1411 | 1418 | P | A | CH2 scissoring band | Lipids | ||
| Plasma | Day 0 | 760 | 762 | P | P | Symmetric ring breathing | Tryptophan |
| 981 | 983 | P | P | CH2 wagging | n/a | ||
| 1163 | 1178 | P | A | CH2/NH3 rocking | Tyrosine | ||
| 1276 | 1268 | A | P | Symmetric ring deformation | n/a | ||
| 1416 | 1418 | P | A | CH2 scissoring | Lipids | ||
| 1580 | 1577 | P | A | Not assigned | Adenine, guanine | ||
| Day 7 | 770 | 768 | P | P | Ring vibrations | Albumin | |
| 981 | 983 | P | P | CH2 wagging | n/a | ||
| 1312 | 1316 | P | P | CH2 CH3 twisting or bending | Guanine | ||
| 1101 | 1096 | P | P | PO4− stretching | PO4− backbone | ||
| 1419 | 1418 | P | A | CH2 scissoring | Lipids | ||
| 1754 | 1754 | P | A | C9=O ester carbonyl | Protein | ||
| Analyzed Parameters | Storage (Day) | Proportions (and AFI) of: | |
|---|---|---|---|
| Good Samples (n = 6) | Poor Samples (n = 6) | ||
| Apoptotic cells (%) | 0 | 2.79 ± 2.46 aA (10,480) | 38.67 ± 6.51 aB (16,310) |
| 7 | 27.86 ± 25.40 aA (12,833) | 14.09 ± 6.38 bA (19,185) | |
| MD cells (%) | 0 | 4.73 ± 4.65 aA (430) | 38.41 ± 3.71 aB (398) |
| 7 | 0.48 ± 0.30 aA (1347) | 60.95 ± 5.04 bB (1272) | |
| Intracellular ROS (%) | 0 | 5.34 ± 4.91 aA (6292) | 36.21 ± 3.62 aB (6693) |
| 7 | 5.43 ± 5.42 aA (8559) | 6.27 ± 2.99 bA (10,116) | |
| MDA (µM) | 0 | 1.58 ± 0.16 aA | 2.59 ± 0.50 aA |
| 7 | 1.26 ± 0.00 aA | 1.95 ± 0.26 aB | |
| TAOC (mM) | 0 | 2.53 ± 0.14 aA | 2.17 ± 0.07 aB |
| 7 | 3.56 ± 0.05 bA | 3.17 ± 0.23 bA | |
| TAOC/MDA (×103) | 0 | 1.68 ± 0.17 aA | 0.98 ± 0.16 aB |
| 7 | 2.82 ± 0.44 bA | 1.80 ± 0.29 aB | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kameni, S.L.; Semon, B.; Chen, L.-D.; Dlamini, N.H.; Ariunbold, G.O.; Vance-Kouba, C.K.; Feugang, J.M. Predicting Boar Sperm Survival during Liquid Storage Using Vibrational Spectroscopic Techniques. Biology 2024, 13, 763. https://doi.org/10.3390/biology13100763
Kameni SL, Semon B, Chen L-D, Dlamini NH, Ariunbold GO, Vance-Kouba CK, Feugang JM. Predicting Boar Sperm Survival during Liquid Storage Using Vibrational Spectroscopic Techniques. Biology. 2024; 13(10):763. https://doi.org/10.3390/biology13100763
Chicago/Turabian StyleKameni, Serge L., Bryan Semon, Li-Dunn Chen, Notsile H. Dlamini, Gombojav O. Ariunbold, Carrie K. Vance-Kouba, and Jean M. Feugang. 2024. "Predicting Boar Sperm Survival during Liquid Storage Using Vibrational Spectroscopic Techniques" Biology 13, no. 10: 763. https://doi.org/10.3390/biology13100763








