Effects of Chronic Stress from High Stocking Density in Mariculture: Evaluations of Growth Performance and Lipid Metabolism of Rainbow Trout (Oncorhychus mykiss)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experiment Design and Sample Collection
2.3. Growth and Feeding Performance
2.4. Organ Coefficients
2.5. Muscle Composition
2.6. Measurement of Lipids’ Biochemical Indicators
2.7. Statistical Analyses
3. Results
3.1. Growth Performance
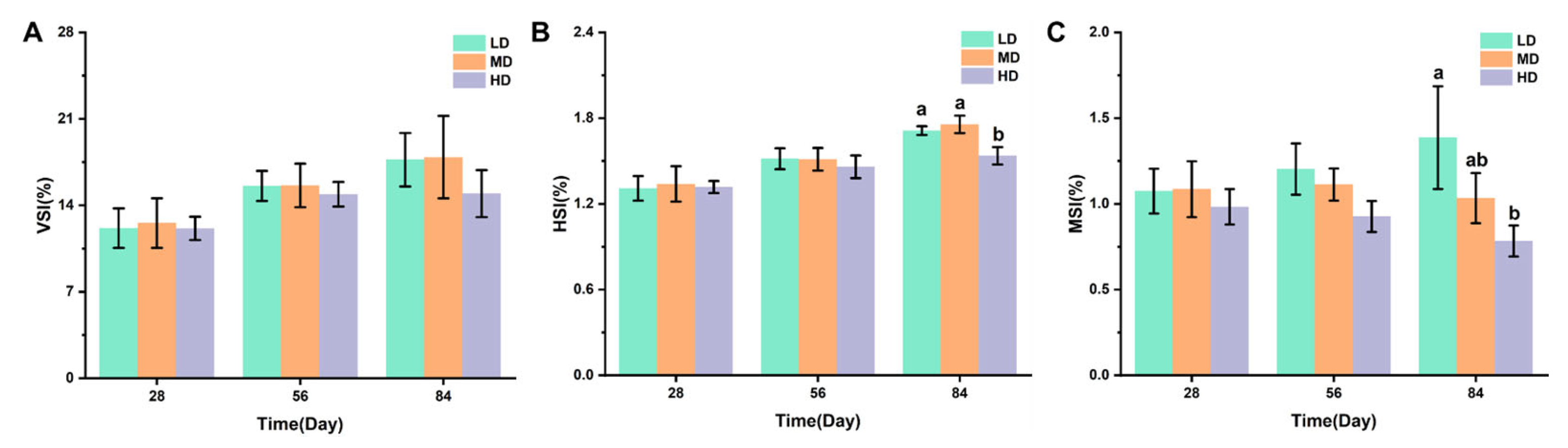
3.2. Organ Coefficients
3.3. Muscles’ Chemical Composition
3.4. Serum Lipid Metabolism
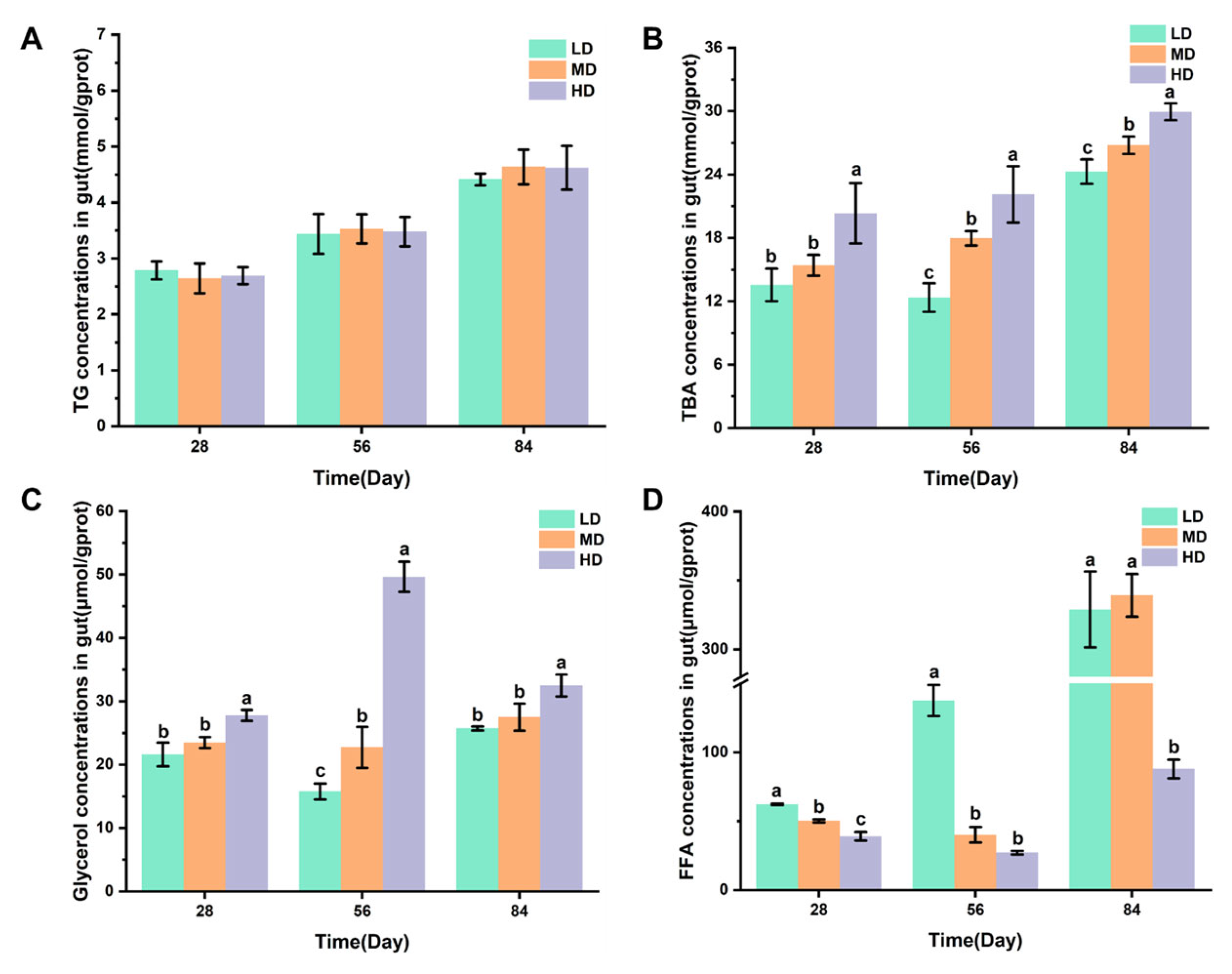
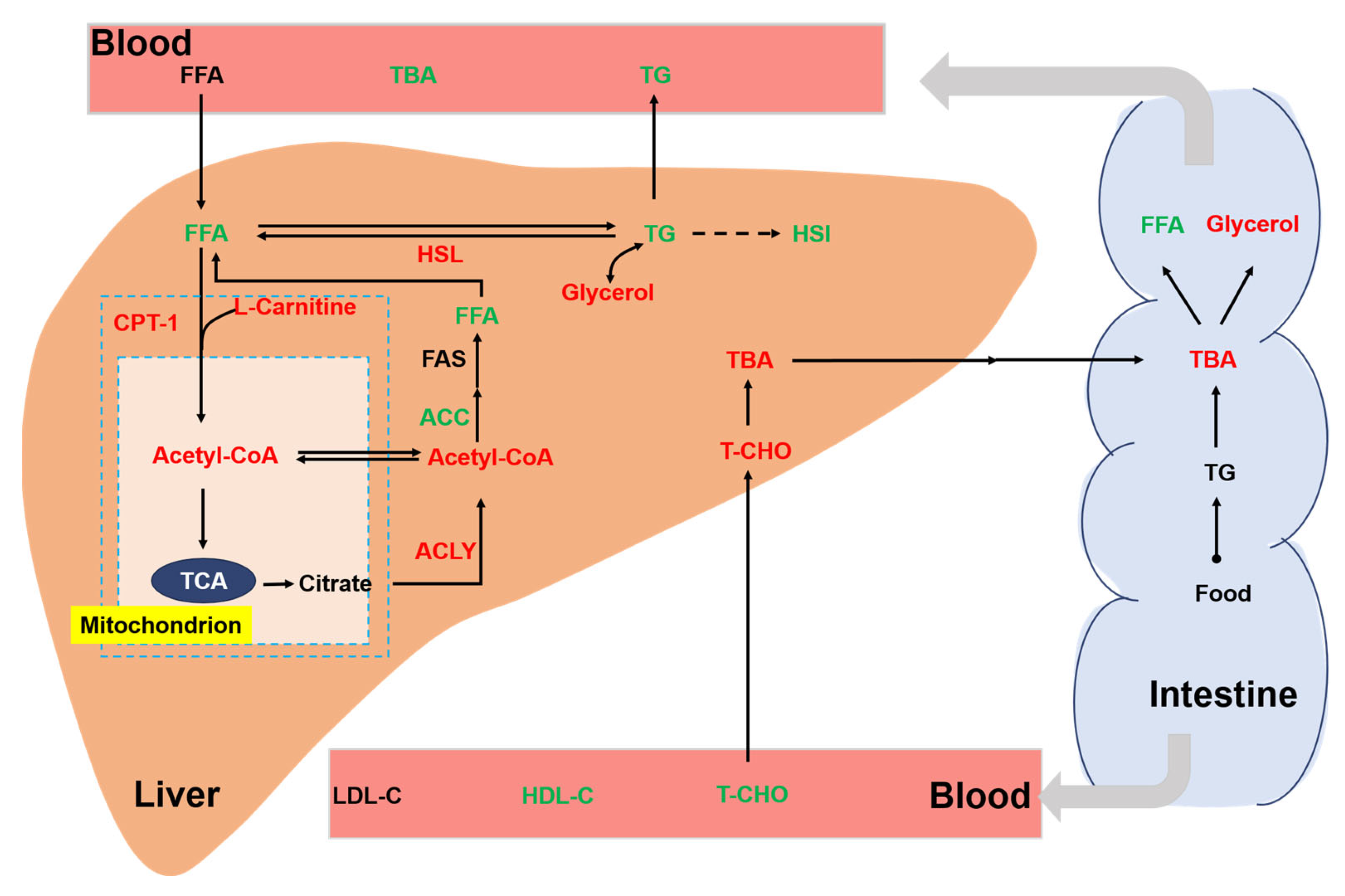
3.5. Intestinal Lipid Metabolism
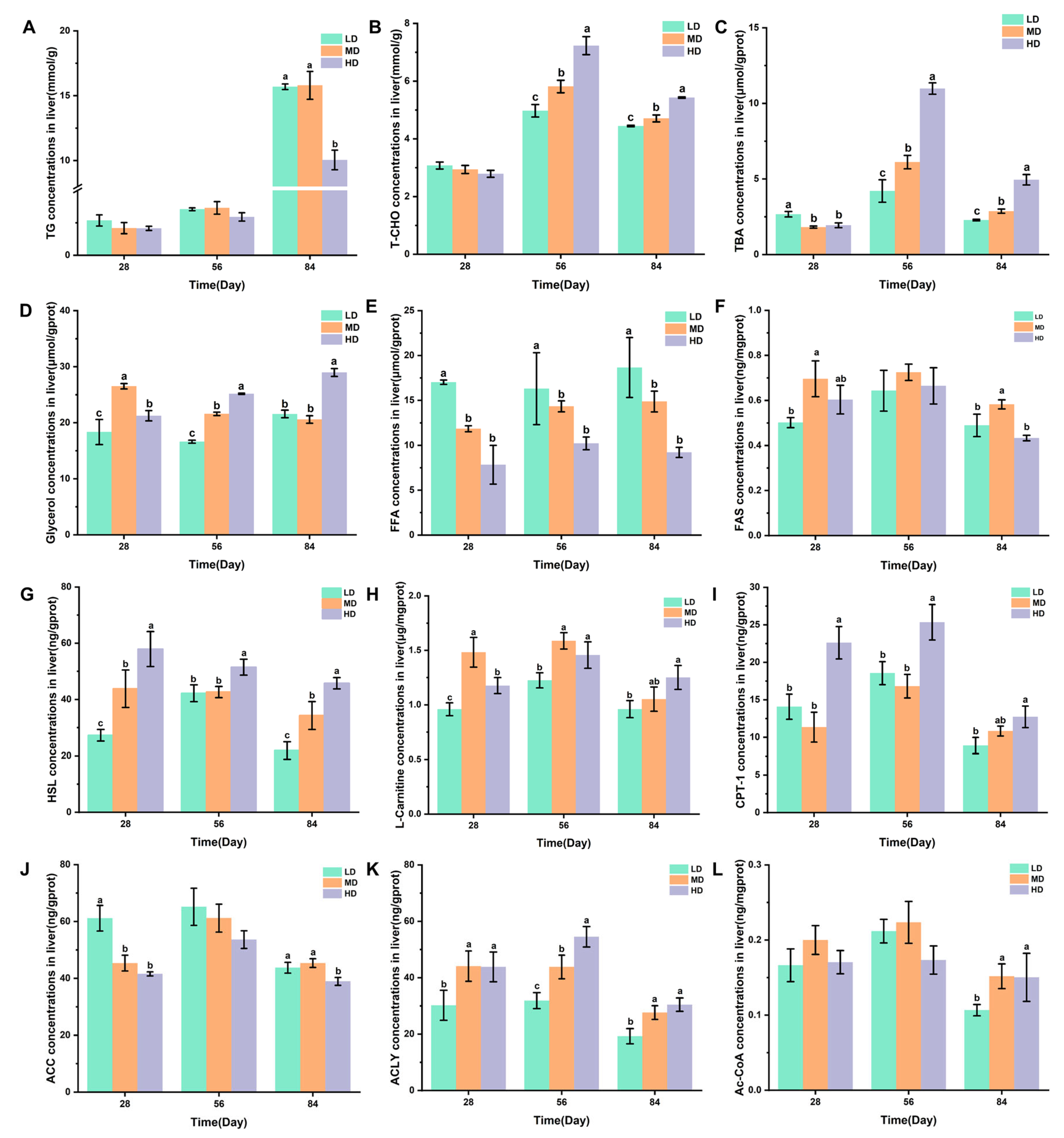
3.6. Liver Lipid Metabolism
3.7. Principal Component Analyses (PCA) and Redundancy Analysis (RDA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Items | Group | Day 0 | Day 42 | Day 70 |
|---|---|---|---|---|
| Body weight (g) | LD | 101.32 ± 6.72 | 141.78 ± 8.19 a | 190.75 ± 11.31 a |
| MD | 100.83 ± 6.27 | 135.89 ± 8.73 b | 182.58 ± 9.57 b | |
| HD | 101.24 ± 6.76 | 124.81 ± 7.80 c | 172.31 ± 13.47 c | |
| Fork length (cm) | LD | 18.95 ± 0.53 | 20.15 ± 0.61 | 22.53 ± 0.56 |
| MD | 19.04 ± 0.52 | 20.01 ± 0.66 | 22. 41 ± 0.62 | |
| HD | 18.97 ± 0.47 | 19.99 ± 0.57 | 21.98 ± 0.58 |
| Density Groups | LD | MD | HD | p Value |
|---|---|---|---|---|
| Survival rate (%) | 93.52 ± 4.24 | 94.44 ± 1.85 | 93.94 ± 2.92 | 0.938 |
Appendix B
References
- Tidwell, J.H.; Allan, G.L. Fish as food aquaculture’s contribution. EMBO Rep. 2001, 2, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, K.i.; Ogawa, K.; Nagae, M.; Ito, F. The influence of rearing density on stress response and disease susceptibility of ayu (Plecoglossus altivelis). Aquaculture 2003, 220, 515–523. [Google Scholar] [CrossRef]
- Rafatnezhad, S.; Falahatkar, B.; Gilani, M.H.T. Effects of stocking density on haematological parameters, growth and fin erosion of great sturgeon (Huso huso) juveniles. Aquac. Res. 2008, 39, 1506–1513. [Google Scholar] [CrossRef]
- Schreck, C.B.; Tort, L. The Concept of Stress in Fish. In Biology of Stress in Fish—Fish Physiology; Academic Press: Cambridge, MA, USA, 2016; pp. 1–34. [Google Scholar] [CrossRef]
- Islam, M.J.; Slater, M.J.; Kunzmann, A. What metabolic, osmotic and molecular stress responses tell us about extreme ambient heatwave impacts in fish at low salinities: The case of European seabass, Dicentrarchus labrax. Sci. Total Environ. 2020, 749, 141458. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.M.; Glock, M.; Ryu, S. An optimized whole-body cortisol quantification method for assessing stress levels in larval zebrafish. PLoS ONE 2013, 8, e79406. [Google Scholar] [CrossRef] [PubMed]
- Fanouraki, E.; Mylonas, C.C.; Papandroulakis, N.; Pavlidis, M. Species specificity in the magnitude and duration of the acute stress response in Mediterranean marine fish in culture. Gen. Comp. Endocrinol. 2011, 173, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Ramos, F.M.; Abe, H.A.; Couto, M.V.S.D.; Paixão, P.E.G.; Martins, M.L.; Carneiro, P.C.F.; Maria, A.N.; Fujimoto, R.Y. Terminalia catappa improves growth performance and survival of the Amazon leaf fish (Monocirrhus polyacanthus) larvae submitted to handling stress. Aquac. Res. 2020, 51, 4805–4808. [Google Scholar] [CrossRef]
- Leal, E.; Fernández-Durán, B.; Guillot, R.; Ríos, D.; Cerdá-Reverter, J.M. Stress-induced effects on feeding behavior and growth performance of the sea bass (Dicentrarchus labrax): A self-feeding approach. J. Comp. Physiol. B 2011, 181, 1035–1044. [Google Scholar] [CrossRef]
- Glover, K.A. Genetic characterisation of farmed rainbow trout in Norway: Intra- and inter-strain variation reveals potential for identification of escapees. BMC Genet. 2008, 9, 87. [Google Scholar] [CrossRef]
- Dong, S. Researching progresses and prospects in large salmonidae farming in Clod Water of Yellow Sea. Period. Ocean Univ. China 2019, 49, 1–6. [Google Scholar]
- Santos, F.A.C.; Boaventura, T.P.; da Costa Julio, G.S.; Cortezzi, P.P.; Figueiredo, L.G.; Favero, G.C.; Palheta, G.D.A.; de Melo, N.F.A.C.; Luz, R.K. Growth performance and physiological parameters of Colossoma macropomum in a recirculating aquaculture system (RAS): Importance of stocking density and classification. Aquaculture 2021, 534, 736274. [Google Scholar] [CrossRef]
- Ellis, T. The relationships between stocking density and welfare in farmed rainbow trout. J. Fish Biol. 2002, 61, 493–531. [Google Scholar] [CrossRef]
- Yousefi, M.; Paktinat, M.; Mahmoudi, N.; Perez-Jimenez, A.; Hoseini, S.M. Serum biochemical and non-specific immune responses of rainbow trout (Oncorhynchus mykiss) to dietary nucleotide and chronic stress. Fish Physiol. Biochem. 2016, 42, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Naderi, M.; Keyvanshokooh, S.; Salati, A.P.; Ghaedi, A. Combined or individual effects of dietary vitamin E and selenium nanoparticles on humoral immune status and serum parameters of rainbow trout (Oncorhynchus mykiss) under high stocking density. Aquaculture 2017, 474, 40–47. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Taheri Mirghaed, A.; Ghelichpour, M.; Pagheh, E.; Iri, Y.; Kor, A. Effects of dietary tryptophan supplementation and stocking density on growth performance and stress responses in rainbow trout (Oncorhynchus mykiss). Aquaculture 2020, 519, 734908. [Google Scholar] [CrossRef]
- Sahin, T.; Okumus, I.; Çelikkale, M.S. Evaluation of rainbow trout (Oncorhynchus mykiss<) mariculture on the Turkish Black Sea coast. Isr. J. Aquac.-Bamidgeh 1999, 51, 17–25. [Google Scholar]
- Biton-Porsmoguer, S.; Bou, R.; Lloret, E.; Alcaide, M.; Lloret, J. Fatty acid composition and parasitism of European sardine (Sardina pilchardus) and anchovy (Engraulis encrasicolus) populations in the northern Catalan Sea in the context of changing environmental conditions. Conserv. Physiol. 2020, 8, coaa121. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Ruíz-Jarabo, I.; Hachero, I.; Vargas-Chacoff, L.; Amo, A.; Mancera, J.M. Stocking density affects growth and metabolic parameters in the brill (Scophthalmus rhombus). Aquac. Int. 2012, 20, 1041–1052. [Google Scholar] [CrossRef]
- Montero, D.; Izquierdo, M.S.; Tort, L.; Robaina, L.; Vergara, J.M. High stocking density produces crowding stress altering some physiological and biochemical parameters in gilthead seabream, Sparus aurata, juveniles. Fish Physiol. Biochem. 1999, 20, 53–60. [Google Scholar] [CrossRef]
- Son, T.; Jeong, I.; Park, J.; Jun, W.; Kim, A.; Kim, O.K. Adipose tissue-derived exosomes contribute to obesity-associated liver diseases in long-term high-fat diet-fed mice, but not in short-term. Front. Nutr. 2023, 10, 1162992. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, G.; Song, L.; Chai, M.; Wang, Y.; Shui, S.; Zhang, H.; Sha, Y.; Yao, Y. Effects of Dietary Protein Levels on Bamei Pig Intestinal Colony Compositional Traits. BioMed Res. Int. 2020, 2020, 2610431. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Wang, L.; Hou, Y.; Feng, W.; Li, B.; Zhu, J. Effects of Stocking Density on the Growth Performance, Physiological Parameters, Redox Status and Lipid Metabolism of Micropterus salmoides in Integrated Rice-Fish Farming Systems. Antioxidants 2022, 11, 1215. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Liu, M.; Lou, J.; Mi, G.; Yuan, J.; Gu, Z. Stocking density alters growth performance, serum biochemistry, digestive enzymes, immune response, and muscle quality of largemouth bass (Micropterus salmoides) in in-pond raceway system. Fish Physiol. Biochem. 2021, 47, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Whittamore, J.M. Osmoregulation and epithelial water transport: Lessons from the intestine of marine teleost fish. J. Comp. Physiol. B 2012, 182, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Dong, S.; Huang, M.; Li, Y.; Wang, X.; Wang, F.; Ma, S.; Zhou, Y. Growth, osmoregulatory response, adenine nucleotide contents, and liver transcriptome analysis of steelhead trout (Oncorhynchus mykiss) under different salinity acclimation methods. Aquaculture 2020, 520, 734937. [Google Scholar] [CrossRef]
- Oxford University Press. Official Methods of Analysis of AOAC INTERNATIONAL; Oxford University Press: Oxford, UK, 2023. [Google Scholar] [CrossRef]
- Musie, W.; Gonfa, G. Fresh water resource, scarcity, water salinity challenges and possible remedies: A review. Heliyon 2023, 9, e18685. [Google Scholar] [CrossRef] [PubMed]
- Morro, B.; Balseiro, P.; Albalat, A.; MacKenzie, S.; Pedrosa, C.; Nilsen, T.O.; Suzuki, S.; Shimizu, M.; Sveier, H.; Gorissen, M.; et al. Effects of temperature and photoperiod on rainbow trout (Oncorhynchus mykiss) smoltification and haematopoiesis. Aquaculture 2020, 519, 734711. [Google Scholar] [CrossRef]
- Ellis, T.; Yildiz, H.Y.; Lopez-Olmeda, J.; Spedicato, M.T.; Tort, L.; Overli, O.; Martins, C.I. Cortisol and finfish welfare. Fish Physiol. Biochem. 2012, 38, 163–188. [Google Scholar] [CrossRef]
- Schjolden, J.; Backstrom, T.; Pulman, K.G.; Pottinger, T.G.; Winberg, S. Divergence in behavioural responses to stress in two strains of rainbow trout (Oncorhynchus mykiss) with contrasting stress responsiveness. Horm. Behav. 2005, 48, 537–544. [Google Scholar] [CrossRef]
- Van de Nieuwegiessen, P.G.; Ramli, N.M.; Knegtel, B.P.; Verreth, J.A.; Schrama, J.W. Coping strategies in farmed African catfish Clarias gariepinus. Does it affect their welfare? J. Fish Biol. 2010, 76, 2486–2501. [Google Scholar] [CrossRef] [PubMed]
- Larsen, B.K.; Skov, P.V.; McKenzie, D.J.; Jokumsen, A. The effects of stocking density and low level sustained exercise on the energetic efficiency of rainbow trout (Oncorhynchus mykiss) reared at 19 °C. Aquaculture 2012, 324–325, 226–233. [Google Scholar] [CrossRef]
- Alves, R.N.; Cordeiro, O.; Silva, T.S.; Richard, N.; de Vareilles, M.; Marino, G.; Di Marco, P.; Rodrigues, P.M.; Conceição, L.E.C. Metabolic molecular indicators of chronic stress in gilthead seabream (Sparus aurata) using comparative proteomics. Aquaculture 2010, 299, 57–66. [Google Scholar] [CrossRef]
- Costábile, A.; Castellano, M.; Aversa-Marnai, M.; Quartiani, I.; Conijeski, D.; Perretta, A.; Villarino, A.; Silva-Álvarez, V.; Ferreira, A.M. A different transcriptional landscape sheds light on Russian sturgeon (Acipenser gueldenstaedtii) mechanisms to cope with bacterial infection and chronic heat stress. Fish Shellfish Immunol. 2022, 128, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Bodeman, C.E.; Dzierlenga, A.L.; Tally, C.M.; Mulligan, R.M.; Lake, A.D.; Cherrington, N.J.; McKarns, S.C. Differential regulation of hepatic organic cation transporter 1, organic anion-transporting polypeptide 1a4, bile-salt export pump, and multidrug resistance-associated protein 2 transporter expression in lymphocyte-deficient mice associates with interleukin-6 production. J. Pharmacol. Exp. Ther. 2013, 347, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Ren, S.; Cui, G.; Ni, Q.; Li, X.; Meng, Y.; Meng, Z.; Zhang, J.; Su, X.; Chen, H.; et al. Short-term stress due to dietary pectin induces cholestasis, and chronic stress induces hepatic steatosis and fibrosis in yellow catfish, Pelteobagrus fulvidraco. Aquaculture 2020, 516, 734607. [Google Scholar] [CrossRef]
- Yi, K.W.; Yuk, J.S.; Shin, J.H.; Kim, T.; Hur, J.Y.; Kim, S.H. The ratio of estrogen receptor α to β is associated with the expression of lipoprotein lipase and hormone-sensitive lipase in human adipose tissue. Fertil. Steril. 2013, 100, S341. [Google Scholar] [CrossRef]
- Yeaman, S.J.; Smith, G.M.; Jepson, C.A.; Wood, S.L.; Emmison, N. The multifunctional role of hormone-sensitive lipase in lipid metabolism. Adv. Enzyme Regul. 1994, 34, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xie, C.; Fan, X.-X.; Jiang, Z.-B.; Wong, V.K.-W.; Xu, J.-H.; Yao, X.-J.; Liu, L.; Leung, E.L.-H. Novel direct AMPK activator suppresses non-small cell lung cancer through inhibition of lipid metabolism. Oncotarget 2017, 8, 96089–96102. [Google Scholar] [CrossRef]
- Ewart, K.V.; Blanchard, B.; Johnson, S.C.; Bailey, W.L.; Martin-Robichaud, D.J.; Buzeta, M.I. Freeze susceptibility in haddock (Melanogrammus aeglefinus). Aquaculture 2000, 188, 91–101. [Google Scholar] [CrossRef]
- Tian, J.; Wen, H.; Zeng, L.-B.; Jiang, M.; Wu, F.; Liu, W.; Yang, C.-G. Changes in the activities and mRNA expression levels of lipoprotein lipase (LPL), hormone-sensitive lipase (HSL) and fatty acid synthetase (FAS) of Nile tilapia (Oreochromis niloticus) during fasting and re-feeding. Aquaculture 2013, 400–401, 29–35. [Google Scholar] [CrossRef]



| Time | Indices | Density Groups | ||
|---|---|---|---|---|
| LD | MD | HD | ||
| 0–14 days | Body weight (g) | 111.25 ± 11.67 | 109.18 ± 6.70 | 107.60 ± 7.72 |
| Fork length (cm) | 19.86 ± 0.69 | 19.93 ± 0.62 | 19.84 ± 0.72 | |
| Condition factor | 1.42 ± 0.06 | 1.38 ± 0.07 | 1.38 ± 0.07 | |
| FCR | 2.05 ± 0.45 | 2.64 ± 0.91 | 3.17 ± 0.58 | |
| WG (%) | 10.90 ± 3.19 | 9.05 ± 3.19 | 7.70 ± 0.60 | |
| 0–28 days | Body weight (g) | 128.66 ± 12.82 a | 123.31 ± 15.45 a | 113.26 ± 9.71 b |
| Fork length (cm) | 20.10 ± 0.78 | 19.95 ± 0.95 | 19.66 ± 0.70 | |
| Condition factor | 1.54 ± 0.11 | 1.54 ± 0.07 | 1.51 ± 0.07 | |
| FCR | 1.46 ± 0.14 b | 1.80 ± 0.0.24 b | 3.29 ± 0.40 a | |
| WG (%) | 28.25 ± 3.79 a | 23.18 ± 3.79 a | 13.35 ± 1.89 b | |
| 0–56 days | Body weight (g) | 174.43 ± 12.09 a | 166.92 ± 10.88 a | 157.54 ± 9.66 b |
| Fork length (cm) | 21.61 ± 0.71 | 21.40 ± 0.65 | 21.23 ± 0.52 | |
| Condition factor | 1.73 ± 0.10 | 1.71 ± 0.12 | 1.71 ± 0.10 | |
| FCR | 1.50 ± 0.01 b | 1.60 ± 0.17 ab | 1.75 ± 0.05 a | |
| WG (%) | 73.85 ± 2.20 a | 66.72 ± 2.20 b | 57.68 ± 1.97 c | |
| 0–84 days | Body weight (g) | 244.40 ± 25.62 a | 237.44 ± 20.25 a | 189.95 ± 20.75 b |
| Fork length (cm) | 23.70 ± 1.19 a | 23.30 ± 1.09 b | 22.04 ± 1.17 c | |
| Condition factor | 1.85 ± 0.26 b | 1.91 ± 0.28 a | 1.78 ± 0.20 c | |
| FCR | 1.41 ± 0.05 b | 1.38 ± 0.04 b | 1.95 ± 0.10 a | |
| WG (%) | 143.63 ± 3.25 a | 137.13 ± 3.25 b | 90.31 ± 3.03 c | |
| Density (kg/m3) | 22.00 ± 0.11 | 32.05 ± 0.29 | 51.34 ± 0.81 | |
| Item (%) | Density Groups | ||
|---|---|---|---|
| LD | MD | HD | |
| Moisture | 74.62 ± 0.36 b | 75.91 ± 0.50 a | 75.97 ± 0.20 a |
| Crude protein | 18.71 ± 0.76 | 18.63 ± 0.42 | 18.96 ± 0.38 |
| Crude lipid | 21.99 ± 1.89 a | 17.06 ± 2.66 b | 17.34 ± 0.78 b |
| Ash | 6.00 ± 0.85 | 5.68 ± 0.63 | 5.59 ± 1.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Gao, Q.; Dong, S.; Dong, K.; Xu, Y.; Mei, Y.; Hou, Z. Effects of Chronic Stress from High Stocking Density in Mariculture: Evaluations of Growth Performance and Lipid Metabolism of Rainbow Trout (Oncorhychus mykiss). Biology 2024, 13, 263. https://doi.org/10.3390/biology13040263
Li Z, Gao Q, Dong S, Dong K, Xu Y, Mei Y, Hou Z. Effects of Chronic Stress from High Stocking Density in Mariculture: Evaluations of Growth Performance and Lipid Metabolism of Rainbow Trout (Oncorhychus mykiss). Biology. 2024; 13(4):263. https://doi.org/10.3390/biology13040263
Chicago/Turabian StyleLi, Zhao, Qinfeng Gao, Shuanglin Dong, Kang Dong, Yuling Xu, Yaoping Mei, and Zhishuai Hou. 2024. "Effects of Chronic Stress from High Stocking Density in Mariculture: Evaluations of Growth Performance and Lipid Metabolism of Rainbow Trout (Oncorhychus mykiss)" Biology 13, no. 4: 263. https://doi.org/10.3390/biology13040263
APA StyleLi, Z., Gao, Q., Dong, S., Dong, K., Xu, Y., Mei, Y., & Hou, Z. (2024). Effects of Chronic Stress from High Stocking Density in Mariculture: Evaluations of Growth Performance and Lipid Metabolism of Rainbow Trout (Oncorhychus mykiss). Biology, 13(4), 263. https://doi.org/10.3390/biology13040263





