Auditory Sensory Gating: Effects of Noise
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Auditory Gating Paradigm
2.3. Electroencephalography Analysis
2.4. Data Analysis
3. Results
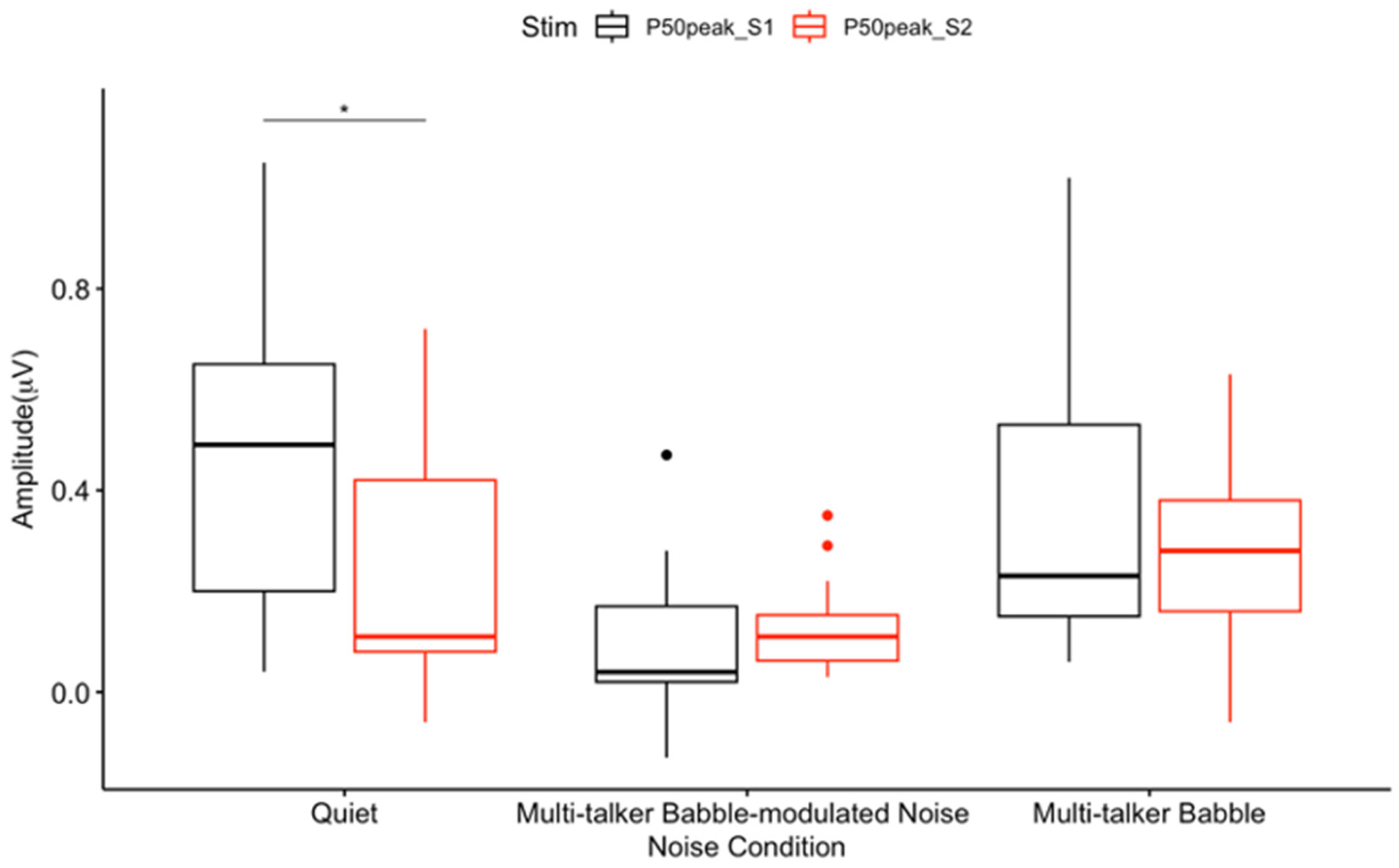
3.1. P50 Gating
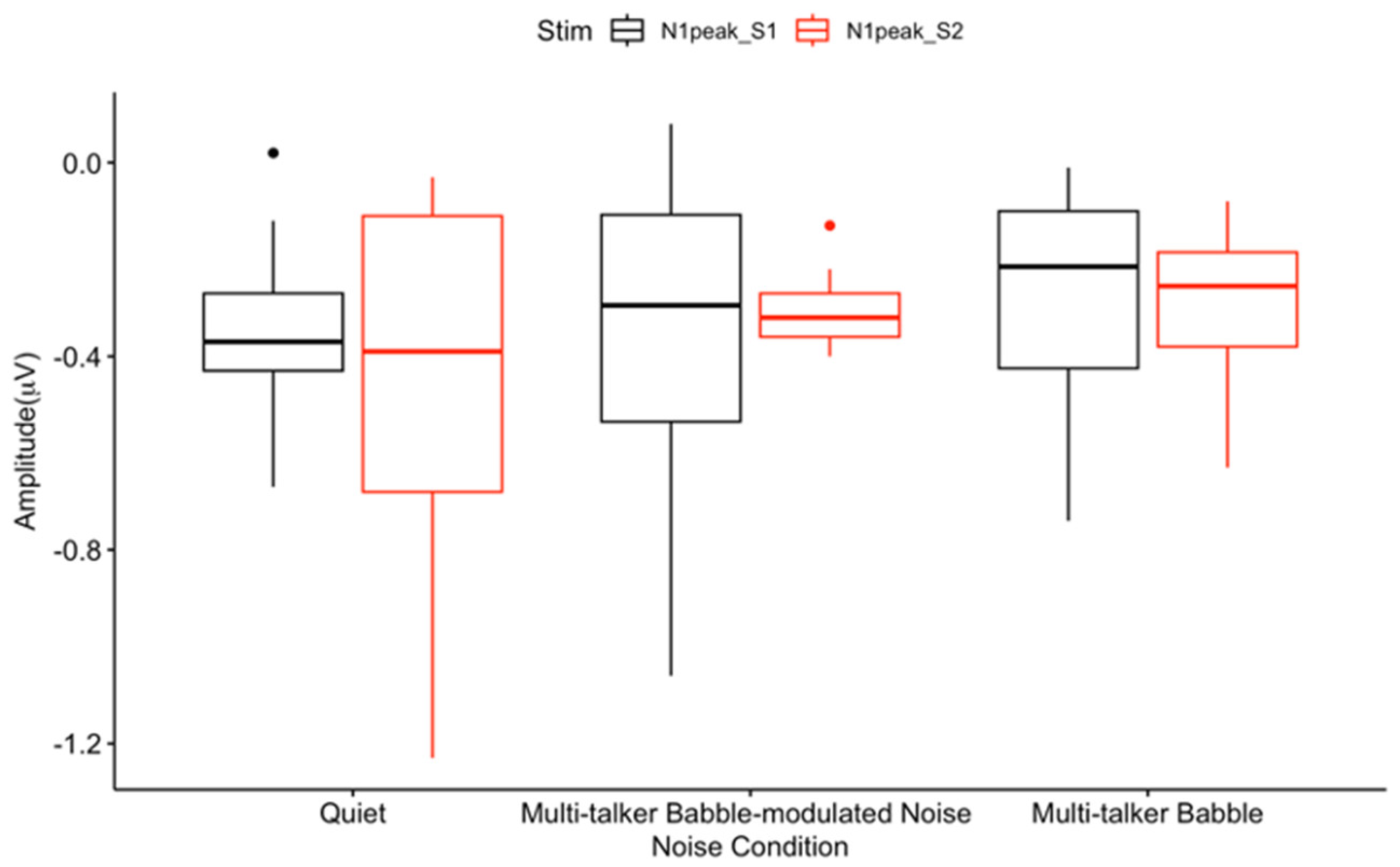
3.2. N1 Gating
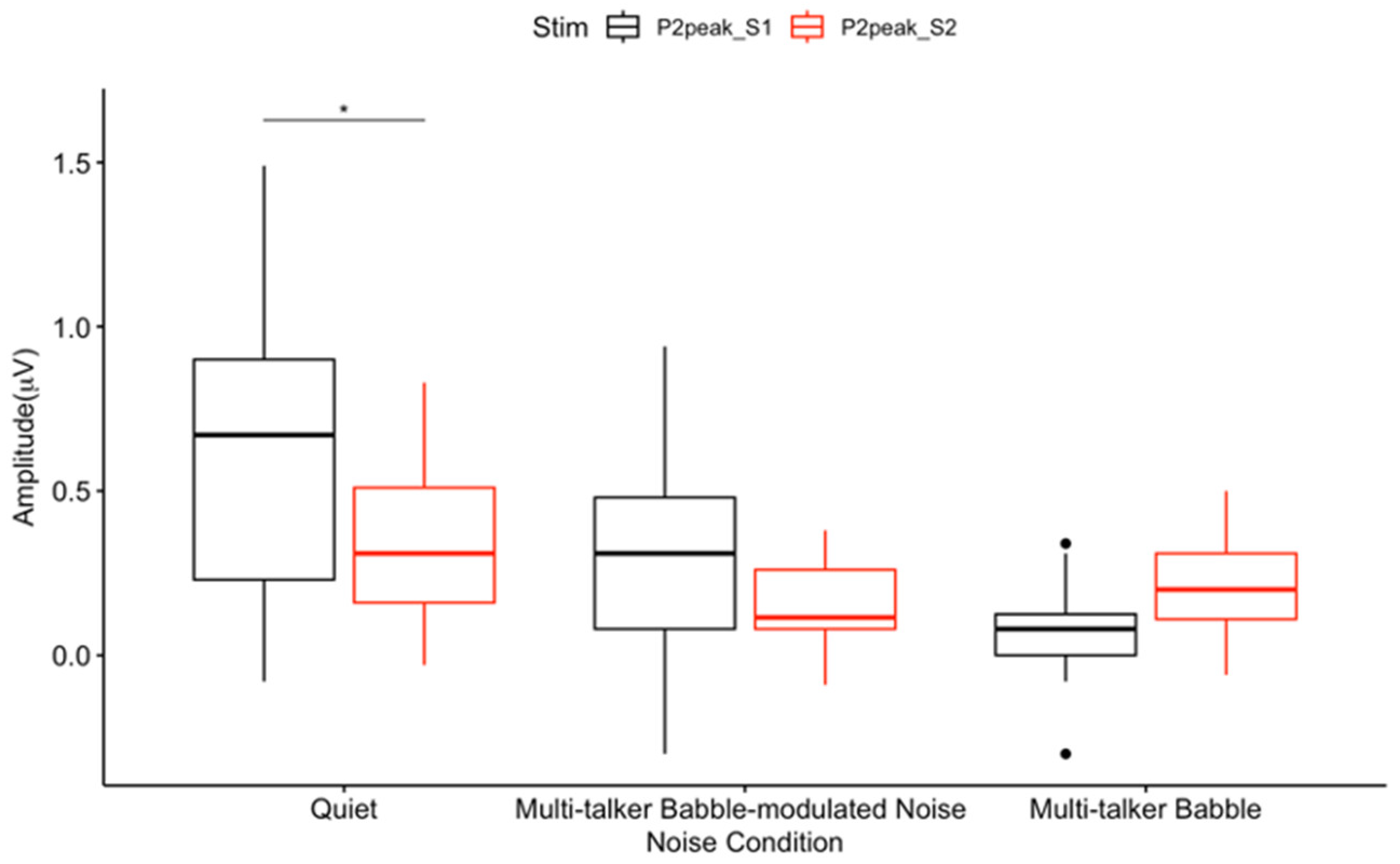
3.3. P2 Gating
3.4. P50 Gating Scalp Maps
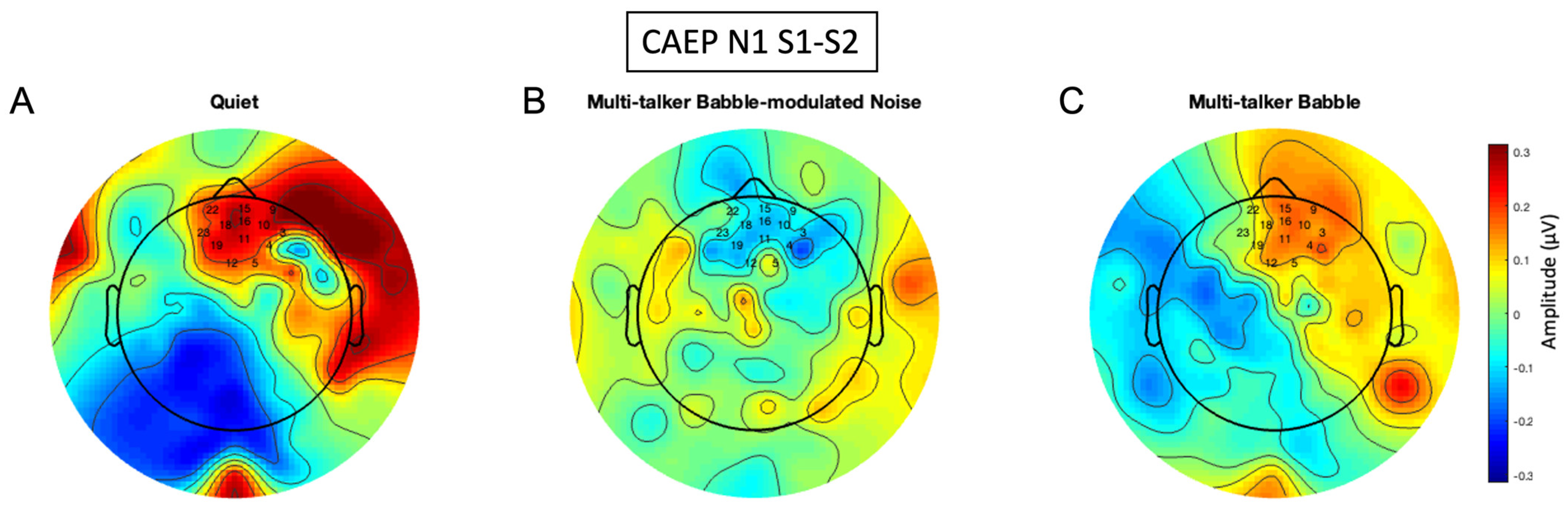
3.5. N1 Gating Scalp Maps
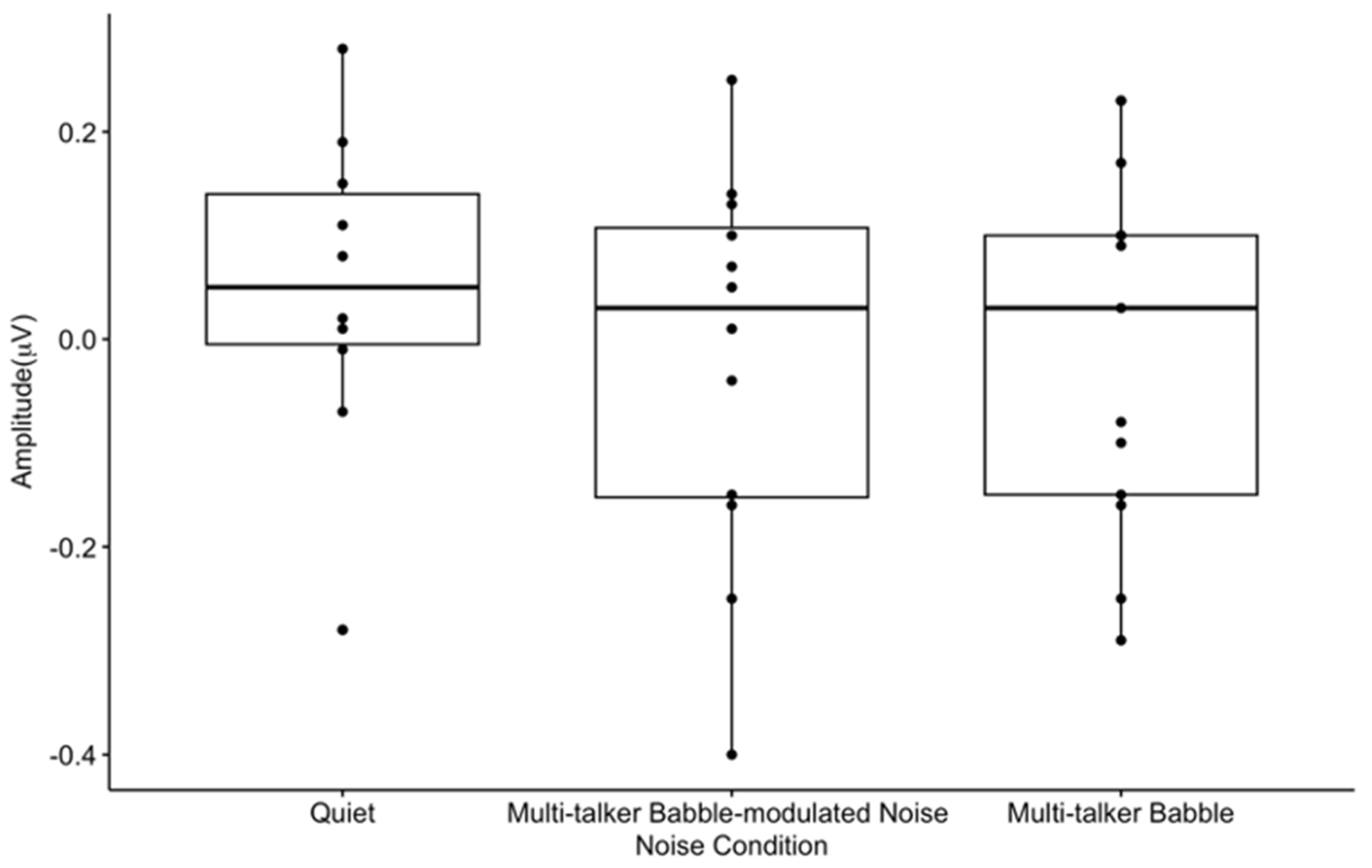
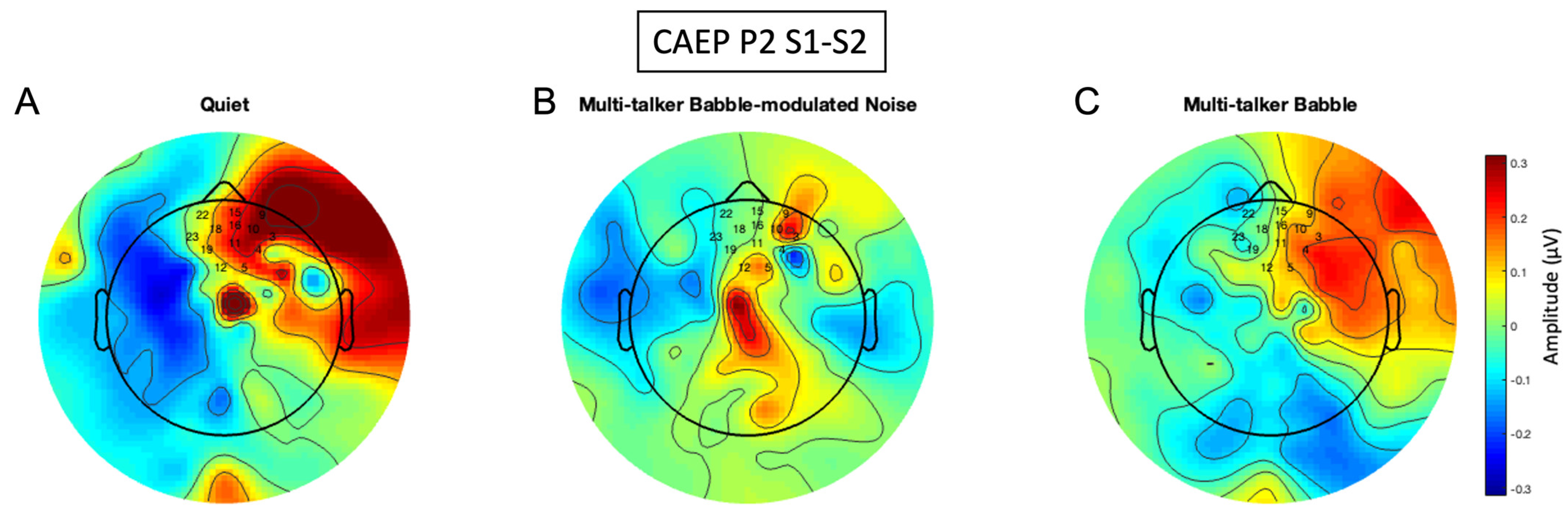
3.6. P2 Gating Scalp Maps
4. Discussion
4.1. General Noise Effects on Auditory Sensory Gating
4.2. Absent Differential Effects of Noise on Auditory Sensory Gating
4.3. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Androulidakis, A.; Jones, S. Detection of signals in modulated and unmodulated noise observed using auditory evoked potentials. Clin. Neurophysiol. 2006, 117, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, S.; Smurzynski, J.; Probst, R. Effects of Age, Age-related hearing loss, and contralateral cafeteria noise on the discrimination of small frequency changes: Psychoacoustic and electrophysiological measures. JARO 2005, 6, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Billings, C.J.; Tremblay, K.L.; Stecker, G.C.; Tolin, W.M. Human evoked cortical activity to signal-to-noise ratio and absolute signal level. Hear Res. 2009, 254, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Billings, C.J.; Bennett, K.O.; Molis, M.R.; Leek, M.R. Cortical encoding of signals in noise: Effects of stimulus type and recording paradigm. Ear Hear. 2011, 32, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Billings, C.J.; McMillan, G.P.; Penman, T.M.; Gille, S.M. Predicting perception in noise using cortical auditory evoked potentials. JARO 2013, 14, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Billings, C.J.; Grush, L.D.; Maamor, N. Acoustic change complex in background noise: Phoneme level and timing effects. Physiol. Rep. 2017, 5, e13464. [Google Scholar] [CrossRef] [PubMed]
- Billings, C.J.; Grush, L.D. Signal type and signal-to-noise ratio interact to affect cortical auditory evoked potentials. JASA 2016, 140, EL221–EL226. [Google Scholar] [CrossRef] [PubMed]
- Faucette, S.P.; Stuart, A. Evidence of a speech evoked electrophysiological release from masking in noise. JASA 2017, 142, EL218–EL223. [Google Scholar] [CrossRef] [PubMed]
- Hiraumi, H.; Nagamine, T.; Morita, T.; Naito, Y.; Fukuyama, H.; Ito, J. Effect of amplitude modulation of background noise on auditory-evoked magnetic fields. Brain Res. 2008, 1239, 191–197. [Google Scholar] [CrossRef]
- Maamor, N.; Billings, C.J. Cortical signal-in-noise coding varies by noise type, signal-to-noise ratio, age, and hearing status. Neurosci. Lett. 2017, 636, 258–264. [Google Scholar] [CrossRef]
- Martin, B.A.; Stapells, D.R. Effects of low-pass noise masking on auditory event-related potentials to speech. Ear Hear. 2005, 26, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Niemczak, C.E.; Vander Werff, K.R. Informational Masking effects on neural encoding of stimulus onset and acoustic change. Ear Hear. 2019, 40, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Papesh, M.A.; Billings, C.J.; Baltzell, L.S. Background noise can enhance cortical auditory evoked potentials under certain conditions. Clin. Neurophysiol. 2015, 126, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Small, S.A.; Sharma, M.; Bradford, M.; Mandikal Vasuki, P.R. The Effect of signal to noise ratio on cortical auditory–evoked potentials elicited to speech stimuli in infants and adults with normal hearing. Ear Hear. 2018, 39, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, L.; Wu, X.; Li, L. Attentional modulation of the early cortical representation of speech signals in informational or energetic masking. Brain Lang. 2014, 135, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Kidd, G.; Mason, C.R.; Gallun, F.J. Combining energetic and informational masking for speech identification. JASA 2005, 118, 982–992. [Google Scholar] [CrossRef]
- Kidd, G.; Mason, C.R.; Richards, V.M.; Gallun, F.J.; Durlach, N.I. Informational masking. In Auditory Perception of Sound Sources; Springer: Boston, MA, USA, 2008; pp. 143–189. [Google Scholar] [CrossRef]
- Neff, D.L. Signal properties that reduce masking by simultaneous, random-frequency maskers. JASA 1995, 98, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Pollack, I. Auditory informational masking. JASA 1975, 57, S5. [Google Scholar] [CrossRef]
- Watson, C.S. Uncertainty, informational masking, and the capacity of immediate auditory memory. In Auditory Processing of Complex Sounds; Springer: Boston, MA, USA, 1987; pp. 267–277. [Google Scholar]
- Wightman, F.L.; Kistler, D.J. Informational masking of speech in children: Effects of ipsilateral and contralateral distracters. JASA 2005, 118, 3164–3176. [Google Scholar] [CrossRef] [PubMed]
- Brungart, D.S. Informational and energetic masking effects in the perception of two simultaneous talkers. JASA 2001, 109, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Sharma, A. Compensatory changes in cortical resource allocation in adults with hearing loss. Front. Syst. Neurosci. 2013, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, G. Summary of the N1-P2 cortical auditory evoked potential to estimate the auditory threshold in adults. Semin. Hear. 2016, 37, 001–008. [Google Scholar] [CrossRef] [PubMed]
- Helfer, K.S.; Freyman, R.L. Aging and speech-on-speech masking. Ear Hear. 2008, 29, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Shinn-Cunningham, B. Cortical and sensory causes of individual differences in selective attention ability among listeners with normal hearing thresholds. J. Speech Lang. Hear. Res. 2017, 60, 2976–2988. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Nielsen, M.; LaBrec, A.; Bean, C. Sensory inhibition is related to variable speech perception in noise in adults with normal hearing. J. Speech Lang. Hear. Res. 2020, 63, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Dryden, A.; Allen, H.A.; Henshaw, H.; Heinrich, A. The Association between cognitive performance and speech-in-noise perception for adult listeners: A systematic literature review and meta-analysis. Trends Hear. 2017, 21, 233121651774467. [Google Scholar] [CrossRef] [PubMed]
- Janse, E. A non-auditory measure of interference predicts distraction by competing speech in older adults. Aging Neuropsychol. Cogn. 2012, 19, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Hills, P.; Dick, K.; Jones, S.; Bright, P. Cognitive mechanisms associated with auditory sensory gating. Brain Cogn. 2016, 102, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Knight, S.; Heinrich, A. Different measures of auditory and visual stroop interference and their relationship to speech intelligibility in noise. Front. Psychol. 2017, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Knight, S.; Heinrich, A. Visual inhibition measures predict speech-in-noise perception only in people with low levels of education. Front. Psychol. 2019, 9, 2779. [Google Scholar] [CrossRef]
- Prat, C.S.; Stocco, A.; Neuhaus, E.; Kleinhans, N.M. Basal ganglia impairments in autism spectrum disorder are related to abnormal signal gating to prefrontal cortex. Neuropsychologia 2016, 91, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Terada, H.; Kurayama, T.; Nakazawa, K.; Matsuzawa, D.; Shimizu, E. Transcranial direct current stimulation (tDCS) onthe dorsolateral prefrontal cortex alters P50 gating. Neurosci. Lett. 2015, 602, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Del Casale, A.; Kotzalidis, G.D.; Rapinesi, C.; Serata, D.; Ambrosi, E.; Simonetti, A.; Girardi, P. Functional neuroimaging in obsessive-compulsive disorder. Neuropsychobiology 2011, 64, 61–85. [Google Scholar] [CrossRef] [PubMed]
- Javitt, D.C.; Freedman, R. Sensory processing dysfunction in the personal experience and neuronal machinery of schizophrenia. AJP 2015, 172, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.T.; Richard Staines, W.; Swick, D.; Chao, L.L. Prefrontal cortex regulates inhibition and excitation in distributed neural networks. Acta Psychol. 1999, 101, 159–178. [Google Scholar] [CrossRef] [PubMed]
- Knott, V.; Millar, A.; Fisher, D. Sensory gating and source analysis of the auditory P50 in low and high suppressors. NeuroImage 2009, 44, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Knott, V.; Fisher, D.; Millar, A. Differential effects of nicotine on P50 amplitude, its gating, and their neural sources in low and high suppressors. Neuroscience 2010, 170, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Choueiry, J.; Blais, C.M.; Shah, D.; Smith, D.; Fisher, D.; Illivitsky, V.; Knott, V. Combining CDP-choline and galantamine: Effects of a selective α7 nicotinic acetylcholine receptor agonist strategy on P50 sensory gating of speech sounds in healthy volunteers. J. Psychopharmacol. 2019, 33, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Choueiry, J.; Blais, C.M.; Shah, D.; Smith, D.; Fisher, D.; Labelle, A.; Knott, V. Combining CDP-choline and galantamine, an optimized α7 nicotinic strategy, to ameliorate sensory gating to speech stimuli in schizophrenia. Int. J. Psychophysiol. 2019, 145, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Hirano, S.; Maekawa, T.; Obayashi, C.; Oribe, N.; Monji, A.; Kasai, K.; Kanba, S.; Onitsuka, T. Auditory gating deficit to human voices in schizophrenia: A MEG study. Schizophr. Res. 2010, 117, 61–67. [Google Scholar] [CrossRef] [PubMed]
- de la Salle, S.; Choueiry, J.; McIntosh, J.; Bowers, H.; Ilivitsky, V.; Knott, V. N-methyl-D-aspartate receptor antagonism impairs sensory gating in the auditory cortex in response to speech stimuli. Psychopharmacology 2022, 239, 2155–2169. [Google Scholar] [CrossRef] [PubMed]
- Gafoor, S.A.; Uppunda, A.K. Sensory gating to speech and nonspeech stimulus and its relationship to speech perception in noise. Am. J. Audiol. 2023, 32, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.E.; Graham, J.; Schafer, E. Auditory sensory gating of speech and nonspeech stimuli. J. Speech Lang. Hear. Res. 2021, 64, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Fuerst, D.R.; Gallinat, J.; Boutros, N.N. Range of sensory gating values and test-retest reliability in normal subjects. Psychophysiology 2007, 44, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.M.; Grant, B.; Fisher, D.J.; Borracci, G.; Labelle, A.; Knott, V.J. Auditory verbal hallucinations in schizophrenia correlate with P50 gating. Clin. Neurophysiol. 2013, 124, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Lijffijt, M.; Moeller, F.G.; Boutros, N.N.; Steinberg, J.L.; Meier, S.L.; Lane, S.D.; Swann, A.C. Diminished P50, N100 and P200 auditory sensory gating in bipolar I disorder. Psychiatry Res. 2009, 167, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Lijffijt, M.; Moeller, F.G.; Boutros, N.N.; Burroughs, S.; Lane, S.D.; Steinberg, J.L.; Swann, A.C. The role of age, gender, education, and intelligence in P50, N100, and P200 auditory sensory gating. J. Psychophysiol. 2009, 23, 52–62. [Google Scholar] [CrossRef]
- Kryter, K.D. Evaluation of Hearing Handicap. 2004. Available online: https://www.semanticscholar.org/paper/Evaluation-of-Hearing-Handicap-Kryter/2f8136a5eed1a6c19cf251376495408d0ba4b94c#citing-papers (accessed on 28 March 2024).
- Campbell, J.; Bean, C.; LaBrec, A. Normal hearing young adults with mild tinnitus: Reduced inhibition as measured through sensory gating. Audiol. Res. 2018, 8, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; LaBrec, A.; Bean, C.; Nielsen, M.; So, W. Auditory gating and extended high-frequency thresholds in normal-hearing adults with minimal tinnitus. Am. J. Audiol. 2019, 28, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, N.; Sugiyama, S.; Inui, K.; Kanemoto, K.; Nishihara, M. New paradigm for auditory paired pulse suppression. PLoS ONE 2017, 12, e0177747. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.W.S.; Jolicoeur, P.; Alain, C. Attentional capacity limits gap detection during concurrent sound segregation. J. Cogn. Neurosci. 2015, 27, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Minagawa-Kawai, Y.; Mori, K.; Furuya, I.; Hayashi, R.; Sato, Y. Assessing cerebral representations of short and long vowel categories by NIRS. NeuroReport 2002, 13, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Liu, C. Intelligibility of american English vowels and consonants spoken by international students in the United States. J. Speech Lang. Hear. Res. 2014, 57, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, W.; Tao, S.; Dong, Q.; Guan, J.; Liu, C. Mandarin Chinese vowel-plus-tone identification in noise: Effects of language experience. Hear Res. 2016, 331, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Bennett, K.O.; Billings, C.J.; Molis, M.R.; Leek, M.R. Neural encoding and perception of speech signals in informational masking. Ear Hear. 2012, 33, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.A.; Cooke, M. Consonant identification in N-talker babble is a nonmonotonic function of N. JASA 2005, 118, 2775–2778. [Google Scholar] [CrossRef] [PubMed]
- Locke, D. The New Children’s Encyclopedia; DK Publishing: New York, NY, USA, 2009; pp. 8–78. [Google Scholar]
- Tang, W.; Wang, X.; Li, J.; Liu, C.; Dong, Q.; Nan, Y. Vowel and tone recognition in quiet and in noise among Mandarin-speaking amusics. Hear Res. 2018, 363, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, C.; Wang, Y.; Zhang, H.; Xu, L.; Yang, X. Mandarin Chinese vowel and tone perception in six-talker babble: Informational masking and aging effect. Am. J. Audiol. 2021, 30, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Tao, S.; Wang, W.; Dong, Q.; Dong, B.; Li, M.; Liu, C. Training non-native vowel perception: In quiet or noise. JASA 2021, 149, 4607–4619. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Nielsen, M.; Bean, C.; LaBrec, A. Auditory gating in hearing loss. J. Am. Acad. Audiol. 2020, 31, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.M.; Murray, M.M.; Lantz, G.; Gonzalez, S.; Spinelli, L.; de Peralta, R.G. EEG source imaging. Clin. Neurophysiol. 2004, 115, 2195–2222. [Google Scholar] [CrossRef] [PubMed]
- Makeig, S.; Debener, S.; Onton, J.; Delorme, A. Mining event-related brain dynamics. TiCS 2004, 8, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Nash-Kille, A.; Sharma, A. Inter-trial coherence as a marker of cortical phase synchrony in children with sensorineural hearing loss and auditory neuropathy spectrum disorder fitted with hearing aids and cochlear implants. Clin. Neurophysiol. 2014, 125, 1459–1470. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Campbell, J.; Rouse, R.; Nielsen, M.; Potter, S. Sensory inhibition and speech perception-in-noise performance in children with normal hearing. J. Speech Lang. Hear. Res. 2023, 66, 382–399. [Google Scholar] [CrossRef] [PubMed]
- Ralston, L.; Campbell, J.; Gilley, P.; Nielson, M.; Brown, K. Sensory gating netowrks in normal-hearing adults with minimal tinnitus. Am. J. Audiol. 2024, 23, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Dalecki, A.; Croft, R.J.; Johnstone, S.J. An evaluation of P50 paired-click methodologies. Psychophysiology 2011, 48, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- Major, S.; Carpenter, K.; Beyer, L.; Kwak, H.; Dawson, G.; Murias, M. The influence of background auditory noise on P50 and N100 suppression elicited by the paired-click paradigm. J. Psychophysiol. 2020, 34, 171–178. [Google Scholar] [CrossRef]
- Lijffijt, M.; Cox, B.; Acas, M.D.; Lane, S.D.; Moeller, F.G.; Swann, A.C. Differential relationships of impulsivity or antisocial symptoms on P50, N100, or P200 auditory sensory gating in controls and antisocial personality disorder. J. Psychiatr. Res. 2012, 46, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Baillet, S.; Lin, Y. Region-specific reduction of auditory sensory gating in older adults. Brain Cogn. 2015, 101, 64–72. [Google Scholar] [CrossRef]
- Lamminmäki, S.; Parkkonen, L.; Hari, R. Human neuromagnetic steady-state responses to amplitude-modulated tones, speech, and music. Ear Hear. 2014, 35, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Dalecki, A.; Johnstone, S.J.; Croft, R.J. Clarifying the functional process represented by P50 suppression. Int. J. Psychophysiol. 2015, 96, 149–154. [Google Scholar] [CrossRef] [PubMed]




| P50 | N1 | P2 | ||||
|---|---|---|---|---|---|---|
| S1 (s.d.) | S2 | S1 | S2 | S1 | S2 | |
| Quiet | 58 ms (11 ms) | 58 ms (7 ms) | 85 ms (10 ms) | 83 ms (21 ms) | 116 ms (17 ms) | 118 ms (14 ms) |
| Multi-talker babble-modulated noise | 57 ms (14 ms) | 51 ms (14 ms) | 114 ms (24 ms) | 125 ms (14 ms) | 185 ms (25 ms) | 208 ms (19 ms) |
| Multi-talker babble | 81 ms (5 ms) | 86 ms (9 ms) | 171 ms (16 ms) | 188 ms (15 ms) | 204 ms (18 ms) | 228 ms (18 ms) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, F.-Y.; Campbell, J.; Liu, C. Auditory Sensory Gating: Effects of Noise. Biology 2024, 13, 443. https://doi.org/10.3390/biology13060443
Cheng F-Y, Campbell J, Liu C. Auditory Sensory Gating: Effects of Noise. Biology. 2024; 13(6):443. https://doi.org/10.3390/biology13060443
Chicago/Turabian StyleCheng, Fan-Yin, Julia Campbell, and Chang Liu. 2024. "Auditory Sensory Gating: Effects of Noise" Biology 13, no. 6: 443. https://doi.org/10.3390/biology13060443
APA StyleCheng, F.-Y., Campbell, J., & Liu, C. (2024). Auditory Sensory Gating: Effects of Noise. Biology, 13(6), 443. https://doi.org/10.3390/biology13060443





