The Influence of Environmental Factors on Site Selection Augment Breeding Success in Honey Bees: An Insight of Honey Bee Genetic Resource Conservation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
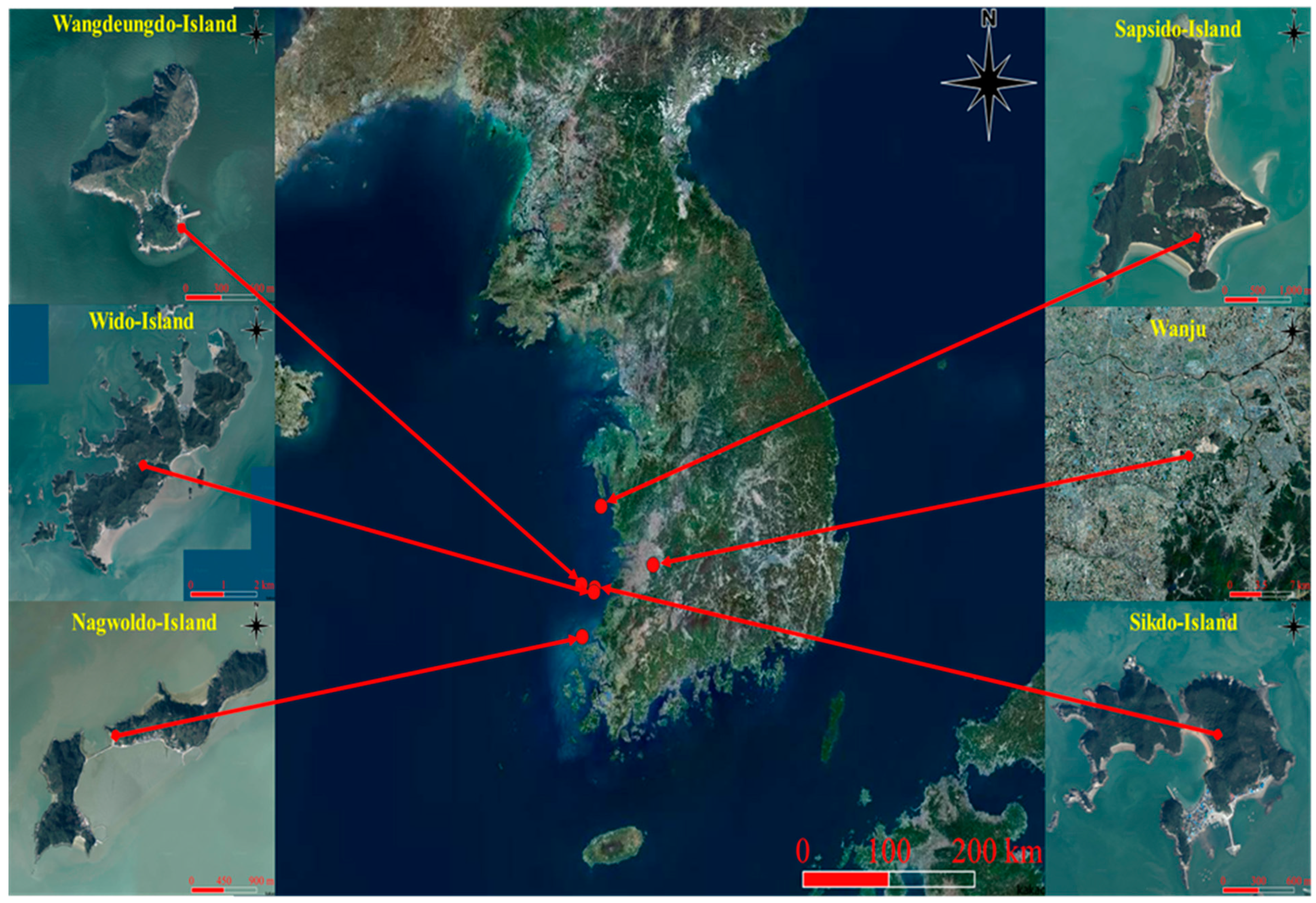
2.1. Location of Mating Stations and Determination of Landscape Factors
2.2. Drone and Queen Rearing for Breeding
2.3. Description of Breeding Colonies and Breeding Lines
2.4. Preparation, Transportation, and Placement of Drone Colonies and Mating Hives at Mating Stations
2.5. Evaluation of Mating Success Rate and Transportation of Mating Hives to the Mainland
2.6. Data Analysis
3. Results
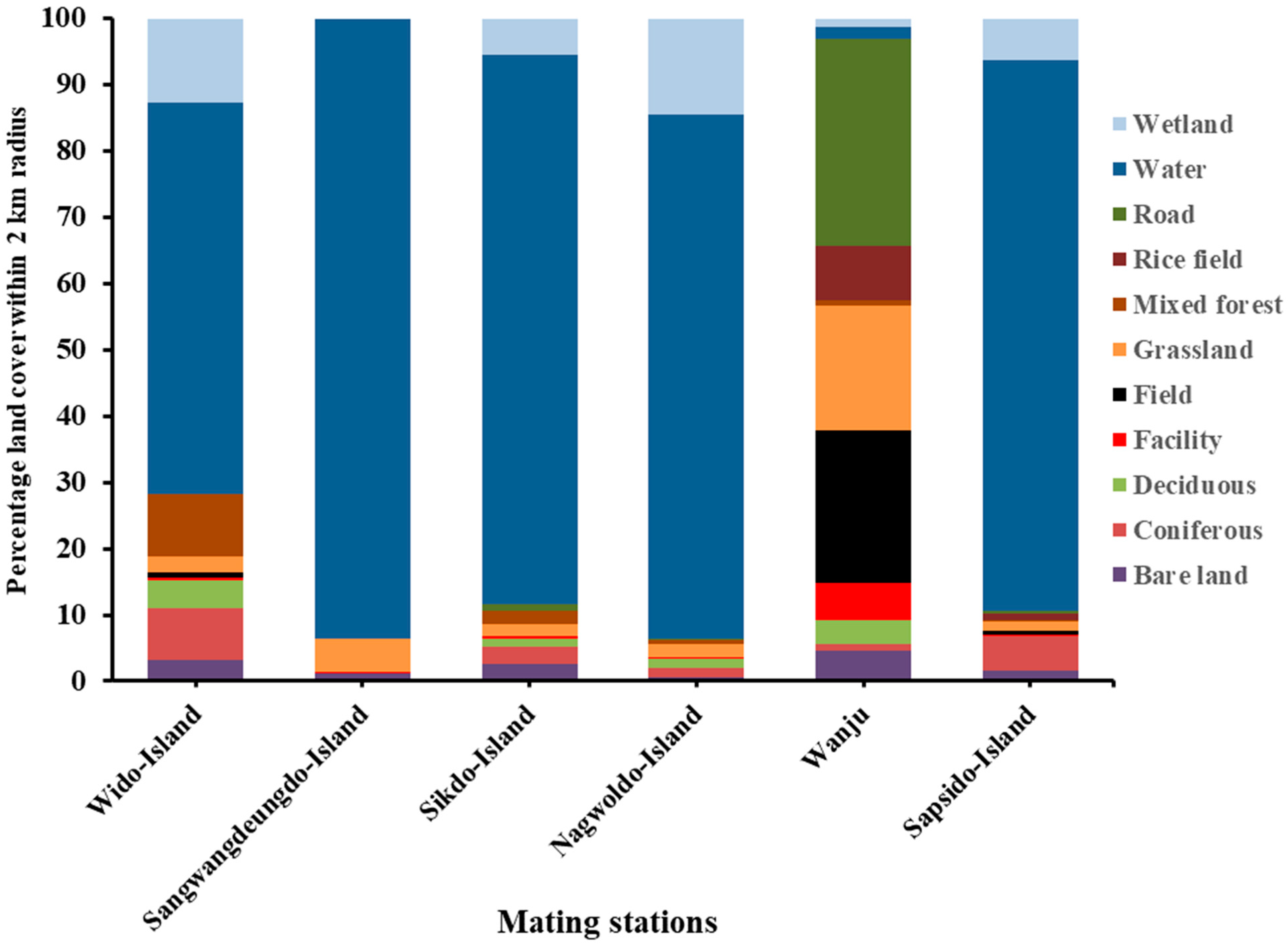
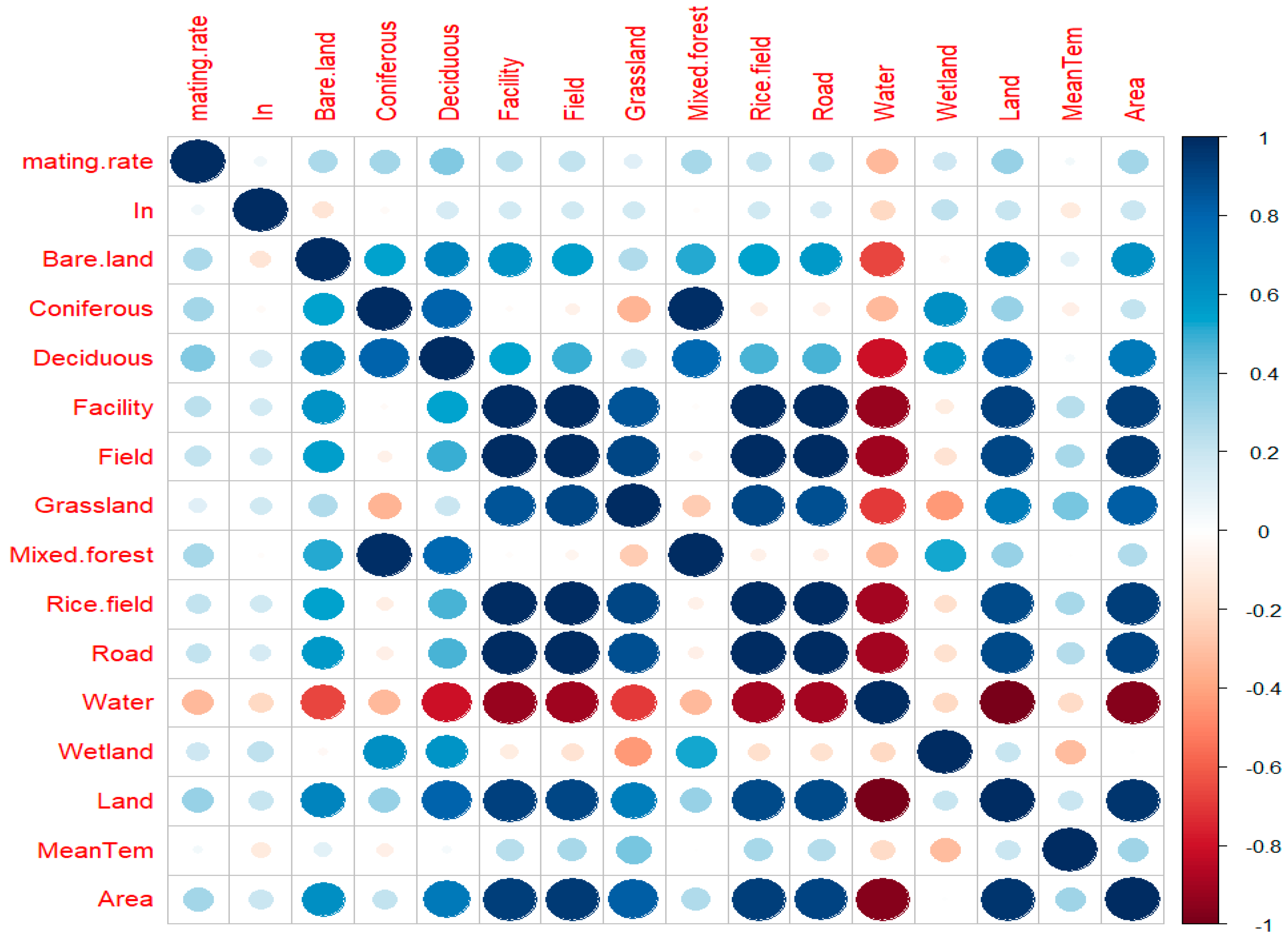
3.1. Characterization of Landscape Factors of the Breeding Stations
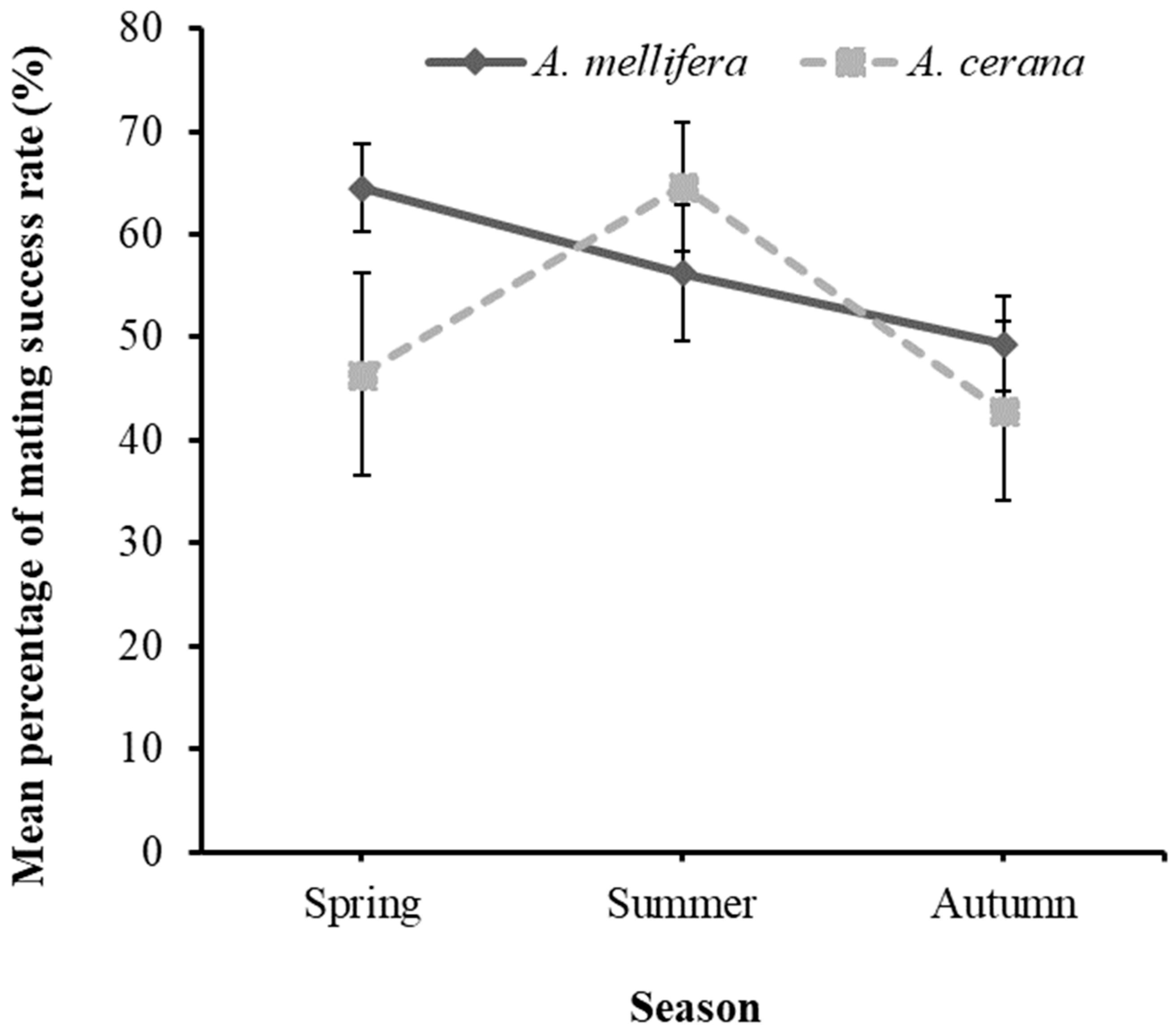
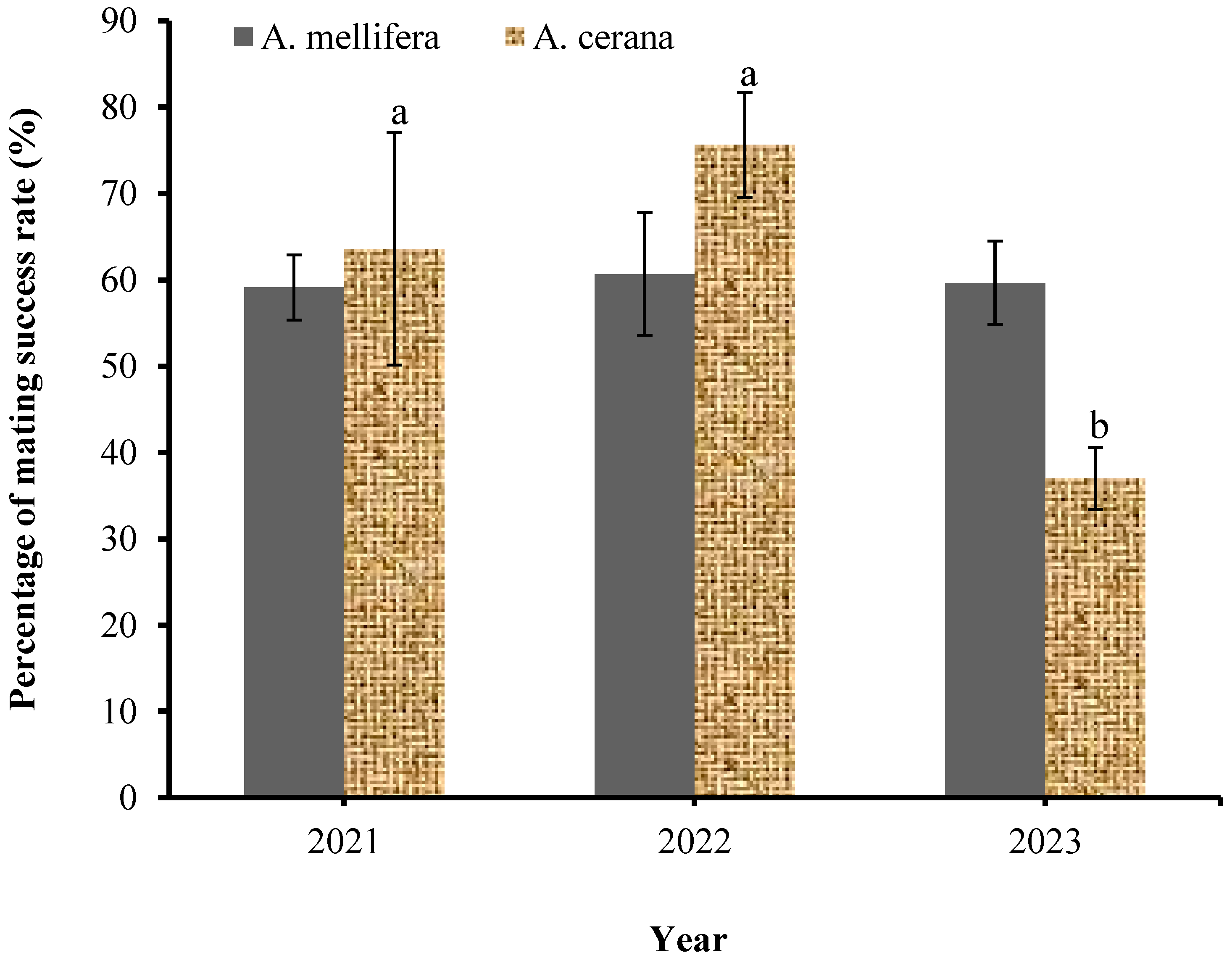
3.2. Seasonal and Annual Variation in the Mating Success Rate of Honey Bees
3.3. Comparison of the Mating Success Rate of Honey Bees at Different Mating Stations
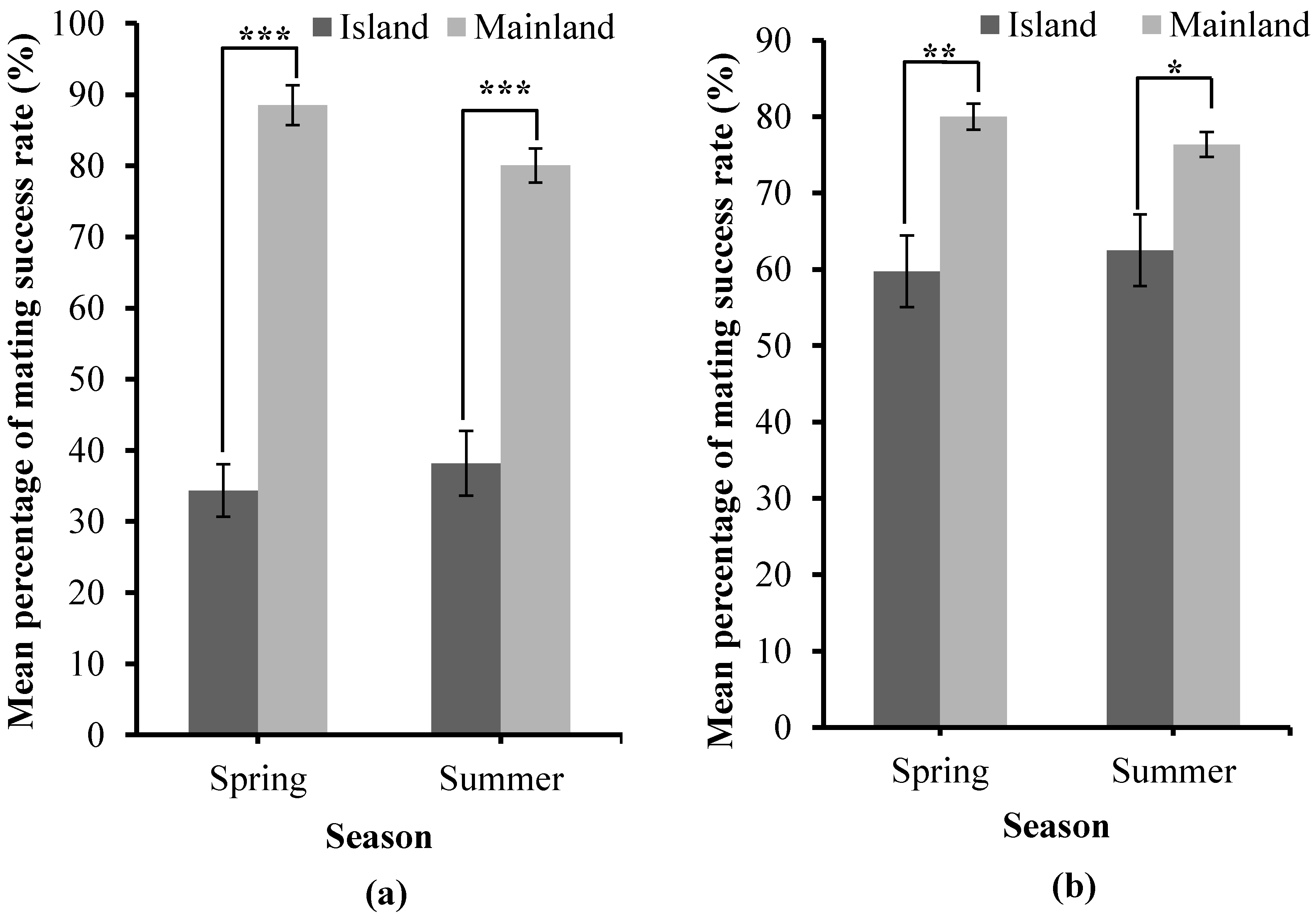
3.4. Variation in the Mating Success Rate in the Mainland and Island
3.5. Comparison of the Mating Success Rate between Purebred and Crossbred Lines of Honey Bees
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rousseau, A.; Fournier, V.; Giovenazzo, P. Apis mellifera (Hymenoptera: Apidae) drone sperm quality in relation to age, genetic line, and time of breeding. Can. Entomol. 2015, 147, 702–711. [Google Scholar] [CrossRef]
- Cobey, S.W. An introduction to instrumental insemination of honey bee queens. Bee World 2016, 93, 33–36. [Google Scholar] [CrossRef]
- Gençer, H.; Kahya, Y.; Woyke, J. Why the viability of spermatozoa diminishes in the honeybee (Apis mellifera) within short time during natural mating and preparation for instrumental insemination. Apidologie 2014, 45, 757–770. [Google Scholar] [CrossRef]
- Koeniger, N.; Koeniger, G. Mating flight duration of Apis mellifera queens: As short as possible, as long as necessary. Apidologie 2007, 38, 606–611. [Google Scholar] [CrossRef]
- Morse, R.A. Rearing Queen Honeybees; Wicwas Press: Ithaca, NY, USA, 1979; p. 128. [Google Scholar]
- Winston, M.L.; Slessor, K.N. Honey bee primer pheromones and colony organization: Gaps in our knowledge. Apidologie 1998, 29, 81–95. [Google Scholar] [CrossRef]
- Rhodes, J.W. Semen Production in Drone Honeybees; Publication No. 08/130; Rural Industries Research and Development Corporation: Barton, Australia, 2008; Available online: https://rirdc.infoservices.com.au/items/08-130 (accessed on 19 October 2023).
- Koeniger, G. Reproduction and mating behaviour. In Bee Genetics and Breeding; Rinderer, T.E., Ed.; Academic Press Inc.: London, UK, 1986; pp. 255–280. [Google Scholar]
- Hartfelder, K.; Guidugli-Lazzarini, K.R.; Cervoni, M.S.; Santos, D.E.; Humann, F.C. Old threads make new tapestry—Rewiring of signalling pathways underlies caste phenotypic plasticity in the honey bee, Apis mellifera L. In Advances in Insect Physiology; Academic Press: Cambridge, MA, USA, 2015; Volume 48, pp. 1–36. [Google Scholar]
- Kairo, G.; Provost, B.; Tchamitchian, S.; Abdelkader, F.B.; Bonnet, M.; Cousin, M.; Sénéchal, J.; Benet, P.; Kretzschmar, A.; Belzunces, L.P. Drone exposure to the systemic insecticide Fipronil indirectly impairs queen reproductive potential. Sci. Rep. 2016, 6, 31904. [Google Scholar] [CrossRef] [PubMed]
- Kairo, G.; Poquet, Y.; Haji, H.; Tchamitchian, S.; Cousin, M.; Bonnet, M.; Pelissier, M.; Kretzschmar, A.; Belzunces, L.P.; Brunet, J. Assessment of the toxic effect of pesticides on honey bee drone fertility using laboratory and semi-field approaches: A case study of fipronil. Environ. Toxicol. Chem. 2017, 36, 2345–2351. [Google Scholar] [CrossRef] [PubMed]
- Akongte, P.N.; Frunze, O.; Kim, D.W.; Kang, E.J.; Park, B.S.; Kim, K.M.; Choi, Y.S. Pupa (Apis mellifera L.) rearing conditions to improve queen weight at emergence. J. Apic. 2022, 37, 365–371. [Google Scholar] [CrossRef]
- Locke, S.J.; Peng, Y.S. The effects of drone age, semen storage and contamination on semen quality in the honeybee (Apis mellifera). Physiol. Entomol. 1993, 18, 144–148. [Google Scholar] [CrossRef]
- Cobey, S.W. Comparison studies of instrumentally inseminated and naturally mated honey bee queens and factors affecting their performance. Apidologie 2007, 38, 390–410. [Google Scholar] [CrossRef]
- Rhodes, J.W.; Harden, S.; Spooner-Hart, R.; Andersen, D.L.; Wheen, G. Effects of age, season and genetics on semen and sperm production in Apis mellifera drones. Apidologie 2011, 42, 29–38. [Google Scholar] [CrossRef]
- Czekońska, K.; Chuda-Mickiewicz, B.; Chorbiski, P. The influence of age of honey bee (Apis mellifera) drones on volume of semen and viability of spermatozoa. J. Apic. Sci. 2013, 57, 61–66. [Google Scholar] [CrossRef]
- Stürup, M.; Baer-Inhoof, B.; Nash, D.R.; Boomsma, J.J.; Baer, B. When every sperm counts: Factors affecting male fertility in the honey bee Apis mellifera. Behav. Ecol. 2013, 24, 1192–1198. [Google Scholar] [CrossRef]
- Rangel, J.; Fisher, A. Factors affecting the reproductive health of honey bee (Apis mellifera) drones—A review. Apidologie 2019, 50, 759–778. [Google Scholar] [CrossRef]
- Metz, B.N.; Tarpy, D.R. Reproductive senescence in drones of the honey bee (Apis mellifera). Insects 2019, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Lensky, Y.; Demter, M. Mating flights of the queen honeybee (Apis mellifera) in a subtropical climate. Comp. Biochem. Physiol. 1985, 81, 229–241. [Google Scholar] [CrossRef]
- Heidinger, I.M.M.; Meixner, M.D.; Berg, S.; Büchler, R. Observation of the mating behavior of honey bee (Apis mellifera L.) queens using Radio-Frequency Identification (RFID): Factors influencing the duration and frequency of nuptial flights. Insects 2014, 5, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.J.; Orel, V. Scientific breeding in Central Europe during the early nineteenth century: Background to Mendel’s later work. J. Hist. Biol. 2005, 38, 239–272. [Google Scholar] [CrossRef]
- Theunissen, B. Darwin and his pigeons. The analogy between artificial and natural selection revisited. J. Hist. Biol. 2012, 45, 179–212. [Google Scholar] [CrossRef]
- Armbruster, L. Bienenzüchtungskunde; T. Fisher: Leipzig, Gemrany, 1919. [Google Scholar]
- Uzunov, A.; Brascamp, E.W.; Büchler, R. The basic concept of honey bee breeding programs. Bee World 2017, 94, 84–87. [Google Scholar] [CrossRef]
- Plate, M.; Bernstein, R.; Hoppe, A.; Bienefeld, K. The importance of controlled mating in honeybee breeding. Genet. Sel. Evol. 2019, 51, 74. [Google Scholar] [CrossRef] [PubMed]
- Olmo, L.; Ashley, K.; Young, J.R.; Suon, S.; Thomson, P.C.; Windsor, P.A.; Bush, R.D. Improving smallholder cattle reproductive efficiency in Cambodia to address expanding regional beef demand. Trop. Anim. Health Prod. 2017, 49, 163–172. [Google Scholar] [CrossRef]
- Abdel-Salam, S.A.; Sayed, A.I.; Elsayed, M.; Abou-Bakr, S. Genetic gain in open nucleus breeding scheme to improve milk production in Egyptian Buffalo. Livest. Sci. 2010, 131, 162–167. [Google Scholar] [CrossRef]
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Decourtye, A.; Mader, E.; Desneux, N. Landscape enhancement of floral resources for honey bees in agro-ecosystems. Apidologie 2010, 41, 264–277. [Google Scholar] [CrossRef]
- Urcan, A.; Marghitas, L.; Dezmirean, D.S.; Bobis, O.; Bonta, V.; Muresan, C.; Margaoan, R. Chemical composition and biological activities of beebread—Review. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Anim. Sci. Biotechnol. 2017, 74, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, M.; Emsen, B.; Dodologlu, A. Some Characteristics of queenbees (Apis mellifera L.) rearing in queenright and queenless colonies. J. Anim. Vet. Adv. 2009, 8, 1083–1085. [Google Scholar]
- Önk, K.; Cengiz, M.M.; Yazici, K.; Kirmizibayrak, T. Effects of rearing periods on some reproductive characteristics of Caucasian (Apis mellifera caucasica) queen bees. Vet. Bilim. Derg. 2016, 11, 259–266. [Google Scholar] [CrossRef]
- Bienefeld, K. Breeding success or genetic diversity in honey bees? Bee World 2016, 99, 40–44. [Google Scholar] [CrossRef]
- Alattal, Y.; Al-Sarhan, R.; Al-Ghamdi, A.; Adgaba, N.; Migdadi, H. Mating frequency of Apis mellifera jemenitica under desert conditions of Saudi Arabia. Saudi J. Biol. Sci. 2020, 28, 578–581. [Google Scholar] [CrossRef]
- Zhao, H.; Mashilingi, S.K.; Liu, Y.; An, J. Factors Influencing the Reproductive Ability of Male Bees: Current Knowledge and Further Directions. Insects 2021, 12, 529. [Google Scholar] [CrossRef]
- Henderson, C.R. Best linear unbiased estimation and prediction under a selection model. Biometrics 1975, 31, 423–447. [Google Scholar] [CrossRef] [PubMed]
- QGIS Development Team. QGIS Geographic Information Systems. 2023. Available online: https://www.qgis.org (accessed on 15 September 2023).
- National Oceanic and Atmospheric Administration (NOAA). Assessing the Global Climate. 2023. Available online: www.ncei.noaa.gov (accessed on 21 December 2023).
- Meteorological Administration of Korea. 2023. Available online: www.wether.go.kr/data.kma.go.kr (accessed on 11 January 2024).
- Winston, M.L. The Biology of the Honey Bee; Harvard University Press: Cambridge, MA, USA, 1987; p. 294. [Google Scholar]
- Flottum, K. The Backyard Beekeeper: An Absolute Beginner’s Guide to Keeping Bees in Your Yard and Garden, 4th ed.; Quarry Books: Beverly, MA, USA, 2018. [Google Scholar]
- Doolittle, G.M. Scientific Queen-Rearing as Practically Applied; Being a Method by Which the Best of Queen-Bees Are Reared in Perfect Accord with Nature’s Ways: For the Amateur and Veteran in Beekeeping, 6th ed.; American Bee Journal: Hamilton, IL, USA, 1915; p. 126. [Google Scholar]
- Büchler, R.; Andonov, S.; Bienefeld, K.; Costa, C.; Hatjina, F.; Kezic, N.; Kryger, P.; Spivak, M.; Uzunov, A.; Wilde, J. Standard methods for rearing and selection of Apis mellifera queens. J. Apic. Res. 2013, 52, 1–30. [Google Scholar] [CrossRef]
- Frunze, O.; Akongte, P.N.; Kim, D.W.; Kang, E.J.; Kim, K.M.; Park, B.S.; Choi, Y.S. Remote honey bee breeding center in the Wido Island, the Republic of Korea. J. Apic. 2022, 37, 175–183. [Google Scholar] [CrossRef]
- Johnstone, M. Rearing Queen Bees. 2008. Available online: https://apiardeal.ro/biblioteca/carti/Straine/EN_-_Rearing-queen-bees_-_11_pag.pdf (accessed on 12 November 2023).
- Meyer-Rochow, V.B.; Jung, C. Remote honey bee breeding centre: A case study of heligoland island in Germany. J. Apic. 2019, 34, 285–293. [Google Scholar] [CrossRef]
- Ilyasov, R.A.; Park, J.; Takahashi, J.; Kwon, H.W. Phylogenetic uniqueness of honeybee Apis cerana from the korean peninsula inferred from the mitochondrial, nuclear, and morphological data. J. Apic. Res. 2018, 62, 189–214. [Google Scholar] [CrossRef]
- Ruttner, F. Reproductive behaviour in honey bees. In Experimental Behavioural Ecology and Sociobiology; Hölldobler, B., Lindauer, M., Eds.; Gustav Fischer Verlag: Stuttgart, Germany; New York, NY, USA, 1985; pp. 225–236. [Google Scholar]
- Schlüns, H.; Moritz, R.F.A.; Neumann, P.; Kryger, P.; Koeniger, G. Multiple nuptial flights, sperm transfer and the evolution of extreme polyandry in honeybee queens. Anim. Behav. 2005, 70, 125–131. [Google Scholar] [CrossRef]
- Tarpy, D.R.; Page, R.E. No behavioral control over mating frequency in queen honey bees (Apis mellifera L.): Implications for the evolution of extreme polyandry. Am. Nat. 2000, 155, 820–827. [Google Scholar] [CrossRef]
- Alber, W.; Jordan, R.; Ruttner, F.; Ruttner, H. Von der paarung der honigbiene. Z. Bienenforsch 1955, 3, 1–28. [Google Scholar]
- Verbeek, B. Investigation of the flight activity of young honeybee queens under continental and insular conditions by means of photoelectronic control. Apidologie 1976, 7, 151–168. [Google Scholar] [CrossRef]
- Taber, S. Factors influencing the circadian flight rhythm of drone honeybees. Ann. Entomol. Soc. Am. 1964, 57, 769–775. [Google Scholar] [CrossRef]
- Witherell, P.C. Flight activity and natural mortality of normal and mutant drone honeybees. J. Apic. Res. 1972, 11, 65–75. [Google Scholar] [CrossRef]
- Bol’Shakova, M.D. The flight of honey bee drones, Apis mellifera L. (hymenoptera, apidae) to the queen in relation to various ecological factors. Entomol. Rev. 1978, 56, 53–56. [Google Scholar]
- National Oceanic and Atmospheric Administration (NOAA). Daily wind changes at the lower level of the atmosphere. Front 2014, 3, 1–4. Available online: https://www.weather.gov/media/publications/front/14feb-front.pdf (accessed on 12 November 2023).
- Ruttner, F. The mating of the honeybee. Bee World 1956, 37, 3–15. [Google Scholar] [CrossRef]
- Jung, G. Klima und Begattung. Bienenvater 1981, 102, 71–74. [Google Scholar]
- Etelvina, C.A.S.; Ronaldo, M.B.S.; José, C.N.; Augusta, C.C.M.; Ivani, P.O. Influence of management and environmental factors on mating success of Africanized queen honey bees. J. Apic. Res. 1995, 34, 169–175. [Google Scholar] [CrossRef]
- Neumann, P.; Moritz, R.F.A.; Van Praagh, J. Queen mating frequency in different types of honey bee mating apiaries. J. Apic. Res. 1999, 38, 11–18. [Google Scholar] [CrossRef]
- Woyke, J. Not the honeybee (Apis mellifera) queen, but the drone determines the termination of the nuptial flight and the onset of oviposition—Polemics, abnegations, corrections and supplement. J. Apic. Sci. 2016, 60, 25–40. [Google Scholar] [CrossRef]
- Koeniger, N.; Koeniger, G.; Pechhacker, H. The nearer the better? Drones (Apis mellifera) prefer nearer drone congregation areas. Insectes Soc. 2005, 52, 31–35. [Google Scholar]
- Muñoz, I.; Dall’Olio, R.; Lodesani, M.; De La Rúa, P. Estimating introgression in Apis mellifera siciliana populations: Are the conservation islands really effective? Insect Conserv. Divers. 2014, 7, 563–571. [Google Scholar] [CrossRef]
- Pinto, M.A.; Henriques, D.; Chávez-Galarza, J.; Kryger, P.; Garnery, L.; Van der Zee, R.; Dahle, B.; Soland-Reckeweg, G.; De la Rúa, P.; Dall’Olio, R.; et al. Genetic integrity of the dark European honey bee (Apis mellifera mellifera) from protected populations: A genome-wide assessment using SNPs and mtDNA sequence data. J. Apic. Res. 2014, 53, 269–278. [Google Scholar] [CrossRef]
- Moore, W.S.; Price, J.T. Nature of selection in the Northern flicker hybrid zone and its implications for speciation theory. In Hybrid Zones and the Evolutionary Process; Harrison, R.G., Ed.; Oxford University Press: Oxford, UK, 1993; pp. 196–225. [Google Scholar]
- Beekman, M.; Allsopp, M.H.; Wossler, T.C.; Oldroyd, B.P. Factors affecting the dynamics of the honeybee (Apis mellifera) hybrid zone of South Africa. Heredity 2008, 100, 13–18. [Google Scholar] [CrossRef][Green Version]
- Keil, A.; Sachser, A. Reproductive benefits from female promiscuous mating in a small mammal. Ethology 1998, 104, 897–903. [Google Scholar] [CrossRef]
- Oldroyd, B.P.; Moran, C.; Nicolas, F.W. Diallele crosses of honey bees. A note presenting the heritability of honey production under Australian conditions. Aust. Agric. Res. 1987, 38, 651–654. [Google Scholar] [CrossRef]
- Bienefeld, K.; Pirchner, F. Heritabilities for several colony traits in the honeybee Apis mellifera carnica. Apidologie 1990, 21, 175–184. [Google Scholar]
- Oxley, P.R.; Hinhumpatch, P.; Gloag, R.; Oldroyd, B.P. Genetic evaluation of a novel system for controlled mating of the honey bee, Apis mellifera. J. Hered. 2010, 101, 334–338. [Google Scholar] [CrossRef] [PubMed]

| Mating Stations | Weather Factors/Year | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Average Temperature (°C) | Total Precipitation (mm) | Average Relative Humidity (%) | |||||||
| 2021 | 2022 | 2023 | 2021 | 2022 | 2023 | 2021 | 2022 | 2023 | |
| Wido | 14 | 13.5 | 14.3 | 1354 | 971.2 | 1853.6 | 76.3 | 73 | 74.8 |
| Wangdeungdo | 14 | 13.5 | 14.3 | 1354 | 971.2 | 1853.6 | 76.3 | 73 | 74.8 |
| Sikdo | 14 | 13.5 | 14.3 | 1354 | 971.2 | 1853.6 | 76.3 | 73 | 74.8 |
| Nagwaldo | 14 | 13.4 | 14.1 | 1202.6 | 802.7 | 1496.1 | 73.8 | 72 | 74.3 |
| Sapsido | 13.8 | 13.1 | 13.9 | 1107.9 | 1348.4 | 1726.7 | 71.6 | 70.3 | 71.8 |
| Wanju | 14.6 | 14 | 14.8 | 1496.6 | 1071.5 | 1986.6 | 68.4 | 66.2 | 68.2 |
| Breeding Stations | Mating Success Rate (%) | ||
|---|---|---|---|
| A. cerana | A. mellifera | ||
| Island | Wido | 59.59 ± 6.2 b | 61.25 ± 9.44 ab |
| Wangdeungdo | - | 57.26 ± 11.44 b | |
| Nakwoldo | 45.11 ± 10.23 bc | 60.27 ± 6.76 ab | |
| Sikdo | - | 60.26 ± 4.03 b | |
| Sapsido | 29.58 ± 1.72 c | - | |
| Mainland | Wanju | 84.28 ± 2.10 a | 78.17 ± 1.22 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akongte, P.N.; Park, B.-S.; Son, M.; Lee, C.-h.; Oh, D.; Choi, Y.-S.; Kim, D. The Influence of Environmental Factors on Site Selection Augment Breeding Success in Honey Bees: An Insight of Honey Bee Genetic Resource Conservation. Biology 2024, 13, 444. https://doi.org/10.3390/biology13060444
Akongte PN, Park B-S, Son M, Lee C-h, Oh D, Choi Y-S, Kim D. The Influence of Environmental Factors on Site Selection Augment Breeding Success in Honey Bees: An Insight of Honey Bee Genetic Resource Conservation. Biology. 2024; 13(6):444. https://doi.org/10.3390/biology13060444
Chicago/Turabian StyleAkongte, Peter Njukang, Bo-Sun Park, Minwoong Son, Chang-hoon Lee, Daegeun Oh, Yong-Soo Choi, and Dongwon Kim. 2024. "The Influence of Environmental Factors on Site Selection Augment Breeding Success in Honey Bees: An Insight of Honey Bee Genetic Resource Conservation" Biology 13, no. 6: 444. https://doi.org/10.3390/biology13060444
APA StyleAkongte, P. N., Park, B.-S., Son, M., Lee, C.-h., Oh, D., Choi, Y.-S., & Kim, D. (2024). The Influence of Environmental Factors on Site Selection Augment Breeding Success in Honey Bees: An Insight of Honey Bee Genetic Resource Conservation. Biology, 13(6), 444. https://doi.org/10.3390/biology13060444






