Adenylyl Cyclase in Ocular Health and Disease: A Comprehensive Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methodology
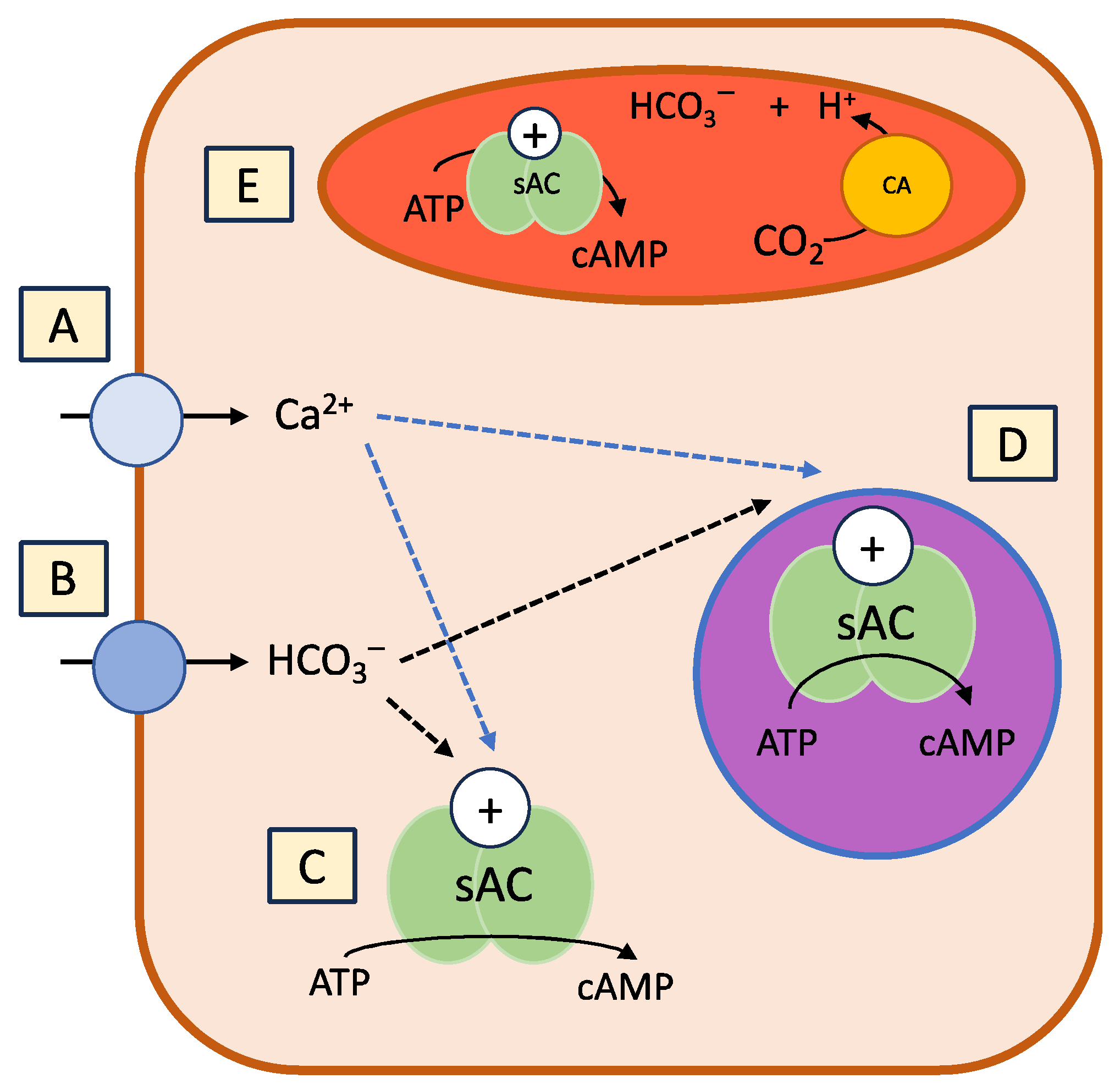
2.1. Soluble Adenylyl Cylase
2.2. The Cornea
2.3. The Crystalline Lens
2.4. The Ciliary Body
2.5. The Retina
2.6. Retinal Disease
2.7. Retinal Circadian Rhythm
2.8. The Lacrimal Gland
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Adenylyl cyclase |
| AH | Aqueous humour |
| AMPK | Adenosine monophosphate activated protein kinase A |
| ATP | Adenosine-5′-triphosphate |
| CA | Carbonic anhydrase |
| cAMP | Cyclic adenosine 3′,5′ monophosphate |
| CAP1 | Adenylyl-cyclase-associated protein 1 |
| CEC | Corneal endothelial cell |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| CNS | Central nervous system |
| D4R | Retinal dopamine receptor |
| DA | Dopamine |
| GPCRs | G-protein coupled receptors |
| IOP | Intraocular pressure |
| LG | Lacrimal gland |
| MS | Multiple sclerosis |
| PACAP | Pituitary adenylate cyclase-activating polypeptide |
| PKA | Protein kinase A |
| RGCs | Retinal ganglion cell |
| ROP | Retinopathy of prematurity |
| sAC | Soluble adenylyl cyclase |
| sACfl | Full-length mammalian Soluble adenylyl cyclase |
| TM | Trabecular meshwork |
| TRPV4 | Transient receptor potential cation channel subfamily 4 |
| tmAC | Transmembrane adenylyl cyclase |
| VEGF | Vascular endothelial growth factor |
| VIP | Vasoactive intestinal peptide |
References
- Smit, M.J.; Iyengar, R. Mammalian adenylyl cyclases. Adv. Second Messenger Phosphoprot. Res. 1998, 32, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Haber, N.; Stengel, D.; Defer, N.; Roeckel, N.; Mattei, M.G.; Hanoune, J. Chromosomal mapping of human adenylyl cyclase genes type III, type V and type VI. Hum. Genet. 1994, 94, 69–73. [Google Scholar] [CrossRef]
- Seifert, R.; Lushington, G.H.; Mou, T.C.; Gille, A.; Sprang, S.R. Inhibitors of Membranous Adenylyl Cyclases. Trends Pharmacol. Sci. 2012, 33, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Buck, J.; Sinclair, M.L.; Schapal, L.; Cann, M.J.; Levin, L.R. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc. Natl. Acad. Sci. USA 1999, 96, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Defer, N.; Best-Belpomme, M.; Hanoune, J. Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase. Am. J. Physiol. Ren. Physiol. 2000, 279, F400–F416. [Google Scholar] [CrossRef]
- Tang, W.J.; Gilman, A.G. Adenylyl cyclases. Cell 1992, 70, 869–872. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.M.F. Regulation and organization of adenylyl cyclases and cAMP. Biochem. J. 2003, 375 Pt 3, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Garbers, D.L.; Tubb, D.J.; Hyne, R.V. A requirement of bicarbonate for Ca2+-induced elevations of cyclic AMP in guinea pig spermatozoa. J. Biol. Chem. 1982, 257, 8980–8984. [Google Scholar] [CrossRef] [PubMed]
- Okamura, N.; Tajima, Y.; Soejima, A.; Masuda, H.; Sugita, Y. Sodium bicarbonate in seminal plasma stimulates the motility of mammalian spermatozoa through direct activation of adenylate cyclase. J. Biol. Chem. 1985, 260, 9699–9705. [Google Scholar] [CrossRef]
- Garty, N.B.; Salomon, Y. Stimulation of partially purified adenylate cyclase from bull sperm by bicarbonate. FEBS Lett. 1987, 218, 148–152. [Google Scholar] [CrossRef]
- Visconti, P.E.; Muschietti, J.P.; Flawia, M.M.; Tezon, J.G. Bicarbonate dependence of cAMP accumulation induced by phorbol esters in hamster spermatozoa. Biochim. Biophys. Acta 1990, 1054, 231–236. [Google Scholar] [CrossRef]
- Chen, Y.; Cann, M.J.; Litvin, T.N.; Iourgenko, V.; Sinclair, M.L.; Levin, L.R.; Buck, J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 2000, 289, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Tresguerres, M.; Levin, L.R.; Buck, J. Intracellular cAMP signaling by soluble adenylyl cyclase. Kidney Int. 2011, 79, 1277–1288. [Google Scholar] [CrossRef]
- Mittag, T.W.; Guo, W.B.; Kobayashi, K. Bicarbonate-activated adenylyl cyclase in fluid-transporting tissues. Am. J. Physiol. 1993, 264 Pt 2, F1060–F1064. [Google Scholar] [CrossRef]
- Schmid, A.; Sutto, Z.; Nlend, M.-C.; Horvath, G.; Schmid, N.; Buck, J.; Levin, L.R.; Conner, G.E.; Fregien, N.; Salathe, M. Soluble adenylyl cyclase is localized to cilia and contributes to ciliary beat frequency regulation via production of cAMP. J. Gen. Physiol. 2007, 130, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Dods, R.F. Development of a Mn-2+-sensitive, “soluble” adenylate cyclase in rat testis. Proc. Natl. Acad. Sci. USA 1975, 72, 1097–1101. [Google Scholar] [CrossRef]
- Chen, J.; Levin, L.R.; Buck, J. Role of soluble adenylyl cyclase in the heart. Am. J. Physiol.-Heart Circ. Physiol. 2012, 302, H538–H543. [Google Scholar] [CrossRef]
- Corredor, R.G.; Trakhtenberg, E.F.; Pita-Thomas, W.; Jin, X.; Hu, Y.; Goldberg, J.L. Soluble adenylyl cyclase activity is necessary for retinal ganglion cell survival and axon growth. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 7734–7744. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Tresguerres, M.; Hess, K.; Marmorstein, L.Y.; Levin, L.R.; Buck, J.; Marmorstein, A.D. Regulation of anterior chamber drainage by bicarbonate-sensitive soluble adenylyl cyclase in the ciliary body. J. Biol. Chem. 2011, 286, 41353–41358. [Google Scholar] [CrossRef]
- Wertheimer, E.; Krapf, D.; de la Vega-Beltran, J.L.; Sánchez-Cárdenas, C.; Navarrete, F.; Haddad, D.; Escoffier, J.; Salicioni, A.M.; Levin, L.R.; Buck, J.; et al. Compartmentalization of distinct cAMP signaling pathways in mammalian sperm. J. Biol. Chem. 2013, 288, 35307–35320. [Google Scholar] [CrossRef]
- Sun, X.C.; Cui, M.; Bonanno, J.A. [HCO3−]-regulated expression and activity of soluble adenylyl cyclase in corneal endothelial and Calu-3 cells. BMC Physiol. 2004, 4, 8. [Google Scholar] [CrossRef]
- Roa, J.N.; Tresguerres, M. Bicarbonate-sensing soluble adenylyl cyclase is present in the cell cytoplasm and nucleus of multiple shark tissues. Physiol. Rep. 2017, 5, e13090. [Google Scholar] [CrossRef] [PubMed]
- Tavares-Silva, M.; Ferreira, D.; Cardoso, S.; Raimundo, A.R.; Barbosa-Breda, J.; Leite-Moreira, A.; Rocha-Sousa, A. Modulation of iris sphincter and ciliary muscles by urocortin 2. Physiol. Res. 2018, 67, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Yousufzai, S.Y.; Ye, Z.; Abdel-Latif, A.A. Prostaglandins mediate the stimulatory effects of endothelin-1 on cAMP accumulation and inositol-1,4,5-trisphosphate production and contraction in cat iris sphincter. J. Pharmacol. Exp. Ther. 1995, 275, 1280–1287. [Google Scholar] [PubMed]
- Abdel-Latif, A.A.; Yousufzai, S.Y.; De, S.; Tachado, S.D. Carbachol stimulates adenylate cyclase and phospholipase C and muscle contraction-relaxation in a reciprocal manner in dog iris sphincter smooth muscle. Eur. J. Pharmacol. 1992, 226, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Mittag, T.W.; Tormay, A.; Severin, C.; Taniguchi, T.; Lee, P.Y.; Wang, R.F.; Podos, S.M. Effects of Al3+ and Be2+ ions combined with NaF on ciliary process adenylyl cyclase activity and aqueous humor dynamics in the rabbit eye. Investig. Ophthalmol. Vis. Sci. 1993, 34, 606–612. [Google Scholar]
- Dunn, T.A.; Storm, D.R.; Feller, M.B. Calcium-dependent increases in protein kinase-A activity in mouse retinal ganglion cells are mediated by multiple adenylate cyclases. PLoS ONE 2009, 4, e7877. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.-G.; Geyer, O.; Mittag, T.W. Adenylyl and guanylyl cyclase activity in the choroid. Exp. Eye Res. 2004, 78, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Pan, M.; Liu, S.; Fang, F.; Lu, R.; Lu, C.; Zheng, M.; An, J.; Xu, H.; Zhao, F.; et al. cAMP Level Modulates Scleral Collagen Remodeling, a Critical Step in the Development of Myopia. PLoS ONE 2013, 8, e71441. [Google Scholar] [CrossRef]
- Berczeli, O.; Szarka, D.; Elekes, G.; Vizvári, E.; Szalay, L.; Almássy, J.; Tálosi, L.; Ding, C.; Tóth-Molnár, E. The regulatory role of vasoactive intestinal peptide in lacrimal gland ductal fluid secretion: A new piece of the puzzle in tear production. Mol. Vis. 2020, 26, 780–788. [Google Scholar]
- Pavan, B.; Frigato, E.; Pozzati, S.; Prasad, P.D.; Bertolucci, C.; Biondi, C. Circadian clocks regulate adenylyl cyclase activity rhythms in human RPE cells. Biochem. Biophys. Res. Commun. 2006, 350, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.C.; Zhai, C.-B.; Cui, M.; Chen, Y.; Levin, L.R.; Buck, J.; Bonanno, J.A. HCO3−-dependent soluble adenylyl cyclase activates cystic fibrosis transmembrane conductance regulator in corneal endothelium. Am. J. Physiol. Cell Physiol. 2003, 284, C1114–C1122. [Google Scholar] [CrossRef] [PubMed]
- Kuang, K.Y.; Xu, M.; Koniarek, J.P.; Fischbarg, J. Effects of ambient bicarbonate, phosphate and carbonic anhydrase inhibitors on fluid transport across rabbit corneal endothelium. Exp. Eye Res. 1990, 50, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.C.; Bonanno, J.A. Expression, localization, and functional evaluation of CFTR in bovine corneal endothelial cells. Am. J. Physiol. Cell Physiol. 2002, 282, C673–C683. [Google Scholar] [CrossRef] [PubMed]
- Steegborn, C.; Litvin, T.N.; Levin, L.R.; Buck, J.; Wu, H. Bicarbonate activation of adenylyl cyclase via promotion of catalytic active site closure and metal recruitment. Nat. Struct. Mol. Biol. 2005, 12, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Tresguerres, M.; Buck, J.; Levin, L.R. Physiological carbon dioxide, bicarbonate, and pH sensing. Pflug. Arch. 2010, 460, 953–964. [Google Scholar] [CrossRef]
- Edelhauser, H.F. The balance between corneal transparency and edema: The Proctor Lecture. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1754–1767. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.V.; Winkler, B.S.; Starnes, C.A.; Peters, M.I. Adenosine promotes regulation of corneal hydration through cyclic adenosine monophosphate. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1–10. [Google Scholar]
- Li, S.; Allen, K.T.; Bonanno, J.A. Soluble adenylyl cyclase mediates bicarbonate-dependent corneal endothelial cell protection. Am. J. Physiol.-Cell Physiol. 2011, 300, C368–C374. [Google Scholar] [CrossRef]
- Grueb, M.; Bartz-Schmidt, K.U.; Rohrbach, J.M. Adrenergic regulation of cAMP/protein kinase A pathway in corneal epithelium and endothelium. Ophthalmic Res. 2008, 40, 322–328. [Google Scholar] [CrossRef]
- Lass, J.H.; Spurney, R.V.; Dutt, R.M.; Andersson, H.; Kochar, H.; Rodman, H.M.; Stern, R.C.; Doershuk, C.F. A morphologic and fluorophotometric analysis of the corneal endothelium in type I diabetes mellitus and cystic fibrosis. Am. J. Ophthalmol. 1985, 100, 783–788. [Google Scholar] [CrossRef]
- Michael, R.; Bron, A.J. The ageing lens and cataract: A model of normal and pathological ageing. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1278–1292. [Google Scholar] [CrossRef] [PubMed]
- Hur, K.C.; Louis, C.F. Regional distribution of the enzymes and substrates mediating the action of cAMP in the mammalian lens. Biochim. Biophys. Acta 1989, 1010, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Bizec, J.C.; Klethi, J.; Mandel, P. Modulation of adenylate cyclase activity in bovine lens epithelial cells. Ophthalmic Res. 1989, 21, 167–174. [Google Scholar] [CrossRef]
- Bizec, J.C.; Klethi, J.; Mandel, P. Calcium-dependent regulation of adenylate cyclase and phosphodiesterase activities in bovine lens: Involvement of lens calmodulin. Exp. Eye Res. 1985, 41, 239–247. [Google Scholar] [CrossRef]
- Shahidullah, M.; Mandal, A.; Delamere, N.A. A Role for Calcium-Activated Adenylate Cyclase and Protein Kinase A in the Lens Src Family Kinase and Na,K-ATPase Response to Hyposmotic Stress. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4447–4456. [Google Scholar] [CrossRef]
- Lee, Y.S.; Marmorstein, A.D. Control of Outflow Resistance by Soluble Adenylyl Cyclase. J. Ocul. Pharmacol. Ther. 2014, 30, 138–142. [Google Scholar] [CrossRef]
- Goh, Y.; Hotehama, Y.; Mishima, H.K. Characterization of ciliary muscle relaxation induced by various agents in cats. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1188–1192. [Google Scholar]
- Nii, H.; Ikeda, H.; Okada, K.; Yoshitomi, T.; Gregory, D.S. Circadian change of adenylate cyclase activity in rabbit ciliary processes. Curr. Eye Res. 2001, 23, 248–255. [Google Scholar] [CrossRef]
- Koçak, I.; Orgül, S.; Saruhan, A.; Haefliger, I.; Hendrickson, P.; Flammer, J. Measurement of intraocular pressure with a modern noncontact tonometer. Ophthalmologica 1998, 212, 81–87. [Google Scholar] [CrossRef]
- Mittag, T.W.; Tormay, A.; Podos, S.M. Vasoactive intestinal peptide and intraocular pressure: Adenylate cyclase activation and binding sites for vasoactive intestinal peptide in membranes of ocular ciliary processes. J. Pharmacol. Exp. Ther. 1987, 241, 230–235. [Google Scholar] [PubMed]
- Wu, R.; Ma, N.; Hu, Q. Effect of cAMP on short-circuit current in isolated human ciliary body. Chin. Med. J. 2013, 126, 2694–2698. [Google Scholar] [PubMed]
- Crook, R.B.; Polansky, J.R. Stimulation of Na+,K+,Cl− cotransport by forskolin-activated adenylyl cyclase in fetal human nonpigmented epithelial cells. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3374–3383. [Google Scholar]
- Caprioli, J.; Sears, M.; Bausher, L.; Gregory, D.; Mead, A. Forskolin lowers intraocular pressure by reducing aqueous inflow. Investig. Ophthalmol. Vis. Sci. 1984, 25, 268–277. [Google Scholar]
- Araie, M. Time change of rabbit aqueous flow under influence of adrenergic drugs. Exp. Eye Res. 1985, 41, 391–403. [Google Scholar] [CrossRef]
- Matsumoto, S.; Yamashita, T.; Araie, M.; Kametani, S.; Hosokawa, T.; Takase, M. The ocular penetration of topical forskolin and its effects on intraocular pressure, aqueous flow rate and cyclic AMP level in the rabbit eye. Jpn. J. Ophthalmol. 1990, 34, 428–435. [Google Scholar] [PubMed]
- Coakes, R.L.; Siah, P.B. Effects of adrenergic drugs on aqueous humour dynamics in the normal human eye. I. Salbutamol. Br. J. Ophthalmol. 1984, 68, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.; Okoro, E.; Ezuedu, C.; Bush, L.; Opere, C.A.; Ohia, S.E.; Njie-Mbye, Y.F. Effects of Hydrogen Sulfide-Releasing Compounds on Aqueous Humor Outflow Facility in Porcine Ocular Anterior Segments, Ex Vivo. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2017, 33, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, N.; Schroeder, A.; Erickson, K.A. Expression of adenylate cyclase subtypes II and IV in the human outflow pathway. Investig. Ophthalmol. Vis. Sci. 2000, 41, 998–1005. [Google Scholar]
- Busch, M.J.; Kobayashi, K.; Hoyng, P.F.; Mittag, T.W. Adenylyl cyclase in human and bovine trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3028–3034. [Google Scholar]
- Dijkstra, B.G.; Schneemann, A.; Hoyng, P.F. Flow after prostaglandin E1 is mediated by receptor-coupled adenylyl cyclase in human anterior segments. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2622–2626. [Google Scholar]
- Bhattacherjee, P.; Jacobs, N.; Coca-Prados, M.; Paterson, C. Identification of prostanoid receptors in rabbit non-pigmented ciliary epithelial cells. Exp. Eye Res. 1996, 62, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.S.; Kim, K.-Y.; Ju, W.-K. Role of cyclic AMP in the eye with glaucoma. BMB Rep. 2017, 50, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Shahidullah, M.; Mandal, A.; Wei, G.; Levin, L.R.; Buck, J.; Delamere, N.A. Nonpigmented ciliary epithelial cells respond to acetazolamide by a soluble adenylyl cyclase mechanism. Investig. Ophthalmol. Vis. Sci. 2014, 55, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, S.V.; Schreiner, R.; Ferreira, J.; Marmorstein, A.D.; Levin, L.R.; Buck, J. Carbonic Anhydrase Inhibitor Modulation of Intraocular Pressure Is Independent of Soluble Adenylyl Cyclase. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2023, 39, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Agarwal, P. Newer targets for modulation of intraocular pressure: Focus on adenosine receptor signaling pathways. Expert Opin. Ther. Targets 2014, 18, 527–539. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, N.; Beuve, A.; Townes-Anderson, E. Mislocalized opsin and cAMP signaling: A mechanism for sprouting by rod cells in retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6355–6369. [Google Scholar] [CrossRef]
- Nicol, X.; Bennis, M.; Ishikawa, Y.; Chan, G.C.-K.; Repérant, J.; Storm, D.R.; Gaspar, P. Role of the calcium modulated cyclases in the development of the retinal projections. Eur. J. Neurosci. 2006, 24, 3401–3414. [Google Scholar] [CrossRef]
- Ohia, S.E.; Opere, C.; Tang, L.; al-Zadjali, K. Role of cyclic AMP in prostaglandin mediated responses in the neural retina. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 1995, 11, 73–81. [Google Scholar] [CrossRef]
- Tremblay, F.; Abdel-Majid, R.; Neumann, P.E. Electroretinographic oscillatory potentials are reduced in adenylyl cyclase type I deficient mice. Vis. Res. 2002, 42, 1715–1725. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Luan, Y.; Zhu, K.; Hu, B.; Ma, B.; Chen, L.; Liu, X.; Lu, H.; Chen, X.; et al. Activation of Type 4 Metabotropic Glutamate Receptor Regulates Proliferation and Neuronal Differentiation in a Cultured Rat Retinal Progenitor Cell Through the Suppression of the cAMP/PTEN/AKT Pathway. Front. Mol. Neurosci. 2020, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.-J.; Guo, Y.; Ye, Y.; Hare, W.A. Presynaptic inhibition by α2 receptor/adenylate cyclase/PDE4 complex at retinal rod bipolar synapse. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 9432–9440. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chuang, A.Z.; O’Brien, J. Regulation of photoreceptor gap junction phosphorylation by adenosine in zebrafish retina. Vis. Neurosci. 2014, 31, 237–243. [Google Scholar] [CrossRef]
- Hattar, S.; Liao, H.W.; Takao, M.; Berson, D.M.; Yau, K.W. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science 2002, 295, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Varella, M.H.; Correa, D.F.; Campos, C.B.; Chiarini, L.B.; Linden, R. Protein kinases selectively modulate apoptosis in the developing retina in vitro. Neurochem. Int. 1997, 31, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Lakk, M.; Denes, V.; Gabriel, R. Pituitary Adenylate Cyclase-Activating Polypeptide Receptors Signal via Phospholipase C Pathway to Block Apoptosis in Newborn Rat Retina. Neurochem. Res. 2015, 40, 1402–1409. [Google Scholar] [CrossRef]
- Njaine, B.; Martins, R.A.P.; Santiago, M.F.; Linden, R.; Silveira, M.S. Pituitary adenylyl cyclase-activating polypeptide controls the proliferation of retinal progenitor cells through downregulation of cyclin D1. Eur. J. Neurosci. 2010, 32, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.S.; Costa, M.R.; Bozza, M.; Linden, R. Pituitary adenylyl cyclase-activating polypeptide prevents induced cell death in retinal tissue through activation of cyclic AMP-dependent protein kinase. J. Biol. Chem. 2002, 277, 16075–16080. [Google Scholar] [CrossRef]
- Santos, R.C.; Araujo, E.G. Cyclic AMP increases the survival of ganglion cells in mixed retinal cell cultures in the absence of exogenous neurotrophic molecules, an effect that involves cholinergic activity. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Medicas E Biol. 2001, 34, 1585–1593. [Google Scholar] [CrossRef]
- Gregory, C.Y.; Abrams, T.A.; Hall, M.O. cAMP production via the adenylyl cyclase pathway is reduced in RCS rat RPE. Investig. Ophthalmol. Vis. Sci. 1992, 33, 3121–3124. [Google Scholar]
- Cameron, E.G.; Nahmou, M.; Toth, A.B.; Heo, L.; Tanasa, B.; Dalal, R.; Yan, W.; Nallagatla, P.; Xia, X.; Hay, S.; et al. A molecular switch for neuroprotective astrocyte reactivity. Nature 2024, 626, 574–582. [Google Scholar] [CrossRef] [PubMed]
- da Encarnação, T.G.; Portugal, C.C.; Nogueira, C.E.; Santiago, F.N.; Socodato, R.; Paes-de-Carvalho, R. Dopamine Promotes Ascorbate Release from Retinal Neurons: Role of D1 Receptors and the Exchange Protein Directly Activated by cAMP type 2 (EPAC2). Mol. Neurobiol. 2018, 55, 7858–7871. [Google Scholar] [CrossRef] [PubMed]
- Paradis, H.; Werdyani, S.; Zhai, G.; Gendron, R.L.; Tabrizchi, R.; McGovern, M.; Jumper, J.M.; Brinton, D.; Good, W.V. Genetic Variants of the Beta-Adrenergic Receptor Pathways as Both Risk and Protective Factors for Retinopathy of Prematurity. Am. J. Ophthalmol. 2024, 263, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Palczewska, G.; Mustafi, D.; Golczak, M.; Dong, Z.; Sawada, O.; Maeda, T.; Maeda, A.; Palczewski, K. Systems pharmacology identifies drug targets for Stargardt disease–associated retinal degeneration. J. Clin. Investig. 2013, 123, 5119–5134. [Google Scholar] [CrossRef]
- Hurley, J.B. Retina Metabolism and Metabolism in the Pigmented Epithelium: A Busy Intersection. Annu. Rev. Vis. Sci. 2021, 7, 665–692. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.Y.; Shi, L.; Lin, C.-H.; Kim, A.J.; Ko, M.L.; Ko, G.Y.-P. A new role for AMP-activated protein kinase in the circadian regulation of L-type voltage-gated calcium channels in late-stage embryonic retinal photoreceptors. J. Neurochem. 2015, 135, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, S.S.; Haque, R.; Pozdeyev, N.; Jackson, C.R.; Iuvone, P.M. Temporal coupling of cyclic AMP and Ca/calmodulin-stimulated adenylyl cyclase to the circadian clock in chick retinal photoreceptor cells. J. Neurochem. 2006, 99, 1142–1150. [Google Scholar] [CrossRef]
- Ivanova, T.N.; Iuvone, P.M. Circadian rhythm and photic control of cAMP level in chick retinal cell cultures: A mechanism for coupling the circadian oscillator to the melatonin-synthesizing enzyme, arylalkylamine N-acetyltransferase, in photoreceptor cells. Brain Res. 2003, 991, 96–103. [Google Scholar] [CrossRef]
- Hwang, C.K.; Chaurasia, S.S.; Jackson, C.R.; Chan, G.C.-K.; Storm, D.R.; Iuvone, P.M. Circadian rhythm of contrast sensitivity is regulated by a dopamine-neuronal PAS-domain protein 2-adenylyl cyclase 1 signaling pathway in retinal ganglion cells. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 14989–14997. [Google Scholar] [CrossRef]
- de Andrade, M.J.O. Circadian Sensation and Visual Perception. In Circadian Rhythm—New Insights into Physiological and Pathological Implications; IntechOpen: London, UK, 2021; ISBN 978-1-80355-370-2. Available online: https://www.intechopen.com/chapters/79581 (accessed on 24 April 2024).
- Jackson, C.R.; Chaurasia, S.S.; Hwang, C.K.; Iuvone, P.M. Dopamine D4 receptor activation controls circadian timing of the adenylyl cyclase 1/cyclic AMP signaling system in mouse retina. Eur. J. Neurosci. 2011, 34, 57–64. [Google Scholar] [CrossRef]
- Fukuhara, C.; Liu, C.; Ivanova, T.N.; Chan, G.C.-K.; Storm, D.R.; Iuvone, P.M.; Tosini, G. Gating of the cAMP signaling cascade and melatonin synthesis by the circadian clock in mammalian retina. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Tosini, G.; Baba, K.; Hwang, C.K.; Iuvone, P.M. Melatonin: An underappreciated player in retinal physiology and pathophysiology. Exp. Eye Res. 2012, 103, 82–89. [Google Scholar] [CrossRef] [PubMed]
- The epidemiology of dry eye disease: Report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 2007, 5, 93–107. [CrossRef] [PubMed]
- Hodges, R.R.; Zoukhri, D.; Sergheraert, C.; Zieske, J.D.; Dartt, D.A. Identification of vasoactive intestinal peptide receptor subtypes in the lacrimal gland and their signal-transducing components. Investig. Ophthalmol. Vis. Sci. 1997, 38, 610–619. [Google Scholar]
- Said, S.I.; Mutt, V. A peptide fraction from lung tissue with prolonged peripheral vasodilator activity. Scand. J. Clin. Lab. Investig. Suppl. 1969, 107, 51–56. [Google Scholar]
- Gilbard, J.P.; Dartt, D.A.; Rood, R.P.; Rossi, S.R.; Gray, K.L.; Donowitz, M. Increased tear secretion in pancreatic cholera: A newly recognized symptom in an experiment of nature. Am. J. Med. 1988, 85, 552–554. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson, P.; Vilkelyte, V.; Woronkowicz, M.; Tavakoli, M.; Skopinski, P.; Roberts, H. Adenylyl Cyclase in Ocular Health and Disease: A Comprehensive Review. Biology 2024, 13, 445. https://doi.org/10.3390/biology13060445
Thompson P, Vilkelyte V, Woronkowicz M, Tavakoli M, Skopinski P, Roberts H. Adenylyl Cyclase in Ocular Health and Disease: A Comprehensive Review. Biology. 2024; 13(6):445. https://doi.org/10.3390/biology13060445
Chicago/Turabian StyleThompson, Polly, Virginija Vilkelyte, Malgorzata Woronkowicz, Mitra Tavakoli, Piotr Skopinski, and Harry Roberts. 2024. "Adenylyl Cyclase in Ocular Health and Disease: A Comprehensive Review" Biology 13, no. 6: 445. https://doi.org/10.3390/biology13060445
APA StyleThompson, P., Vilkelyte, V., Woronkowicz, M., Tavakoli, M., Skopinski, P., & Roberts, H. (2024). Adenylyl Cyclase in Ocular Health and Disease: A Comprehensive Review. Biology, 13(6), 445. https://doi.org/10.3390/biology13060445




