Arbovirus Transmission in Australia from 2002 to 2017
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Source and Preparation
2.3. Case Definition
2.4. Data Analyses
2.4.1. Exploratory Data Analysis and Visualization
2.4.2. Seasonality of Arbovirus Cases
2.4.3. Space–Time Cluster Analysis
2.4.4. Collinearity Check and Correlation Analysis
2.4.5. Model Building and Evaluation
- (1)
- count~Transmission_Name, where ‘Transmission_Name’ is the variable defining the locally acquired arbovirus (dataset in long format),
- (2)
- count for each arbovirus (dataset in wide format),
- (3)
- counts of arboviruses in SA3s identified in the cluster analysis,
- (4)
- blood donation number,
- (5)
- blood donation number in SA3s identified in the cluster analysis.
2.5. Ethical Considerations
3. Results
3.1. Distribution of Arboviruses in Space and Time
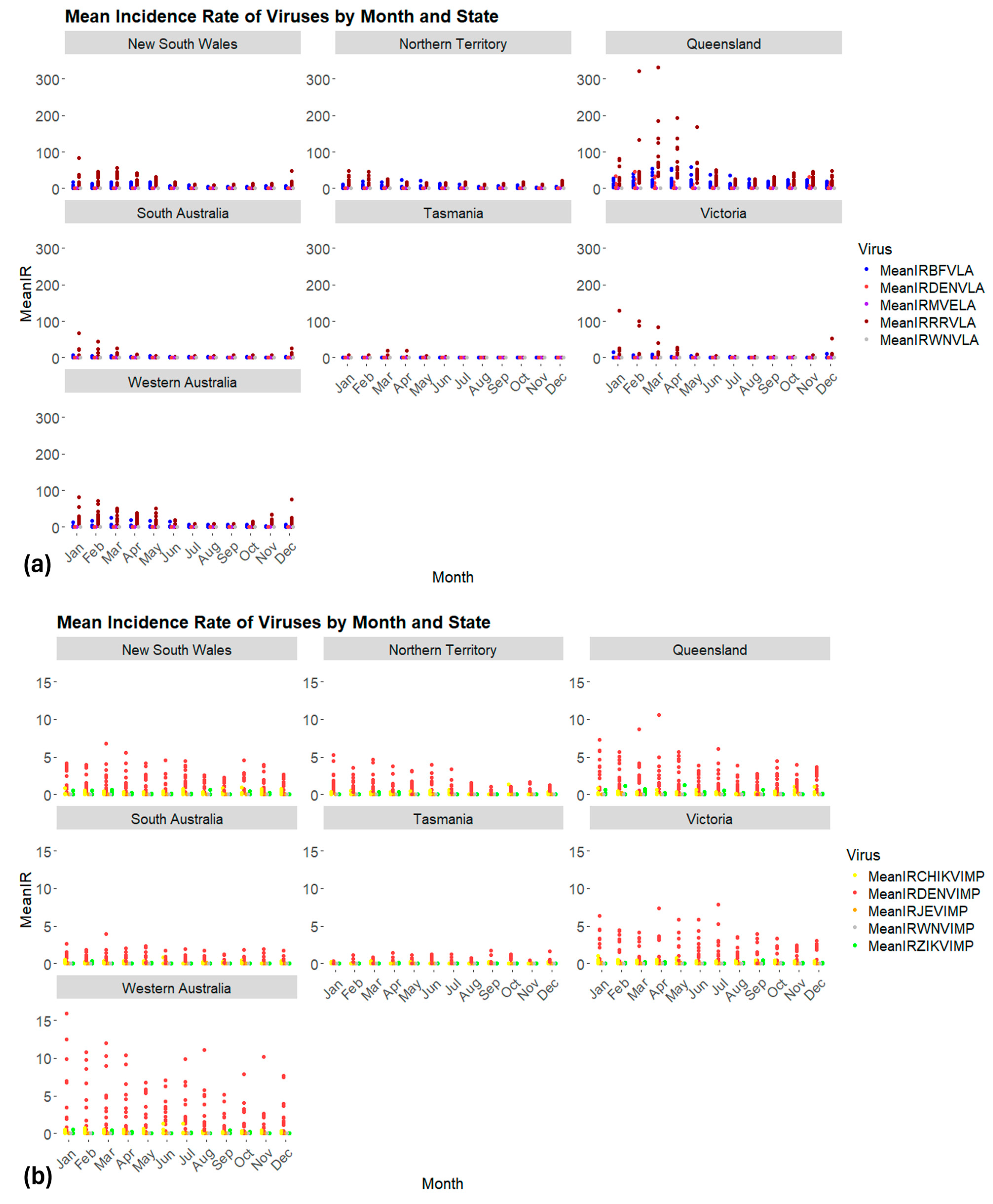
3.2. Seasonality of Locally Acquired and Imported Cases
3.3. Origin of Imported Cases
3.4. Spatiotemporal Clustering of Locally Acquired Arboviruses
3.5. Statistical Analyses
3.5.1. Arbovirus Transmission and Associations
- (i)
- For locally acquired MVEV and WNVKUN cases, the optimal model was a Poisson regression, with only the meteorological variables (defined as the ‘base model’).
- (ii)
- For locally acquired DENV cases, the optimal model was a negative binomial with a base model.
- (iii)
- For locally acquired BFV and RRV cases, the optimal model was the full model (base model, month–year, offset of log (population), and SA3s).
3.5.2. Blood Donation Numbers and Associations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gubler, D.J. The Global Threat of Emergent/Re-emergent Vector-Borne Diseases. In Vector Biology, Ecology and Control; Springer: New York, NY, USA, 2010; pp. 39–62. [Google Scholar] [CrossRef]
- Taylor-Salmon, E.; Hill, V.; Paul, L.M.; Koch, R.T.; Breban, M.I.; Chaguza, C.; Sodeinde, A.; Warren, J.L.; Bunch, S.; Cano, N.; et al. Travel surveillance uncovers dengue virus dynamics and introductions in the Caribbean. Nat. Commun. 2024, 15, 3508. [Google Scholar] [CrossRef] [PubMed]
- de Lima Cavalcanti, T.Y.V.; Pereira, M.R.; de Paula, S.O.; Franca, R.F.d.O. A Review on Chikungunya Virus Epidemiology, Pathogenesis and Current Vaccine Development. Viruses 2022, 14, 969. [Google Scholar] [CrossRef] [PubMed]
- Giovanetti, M.; Vazquez, C.; Lima, M.; Castro, E.; Rojas, A.; de la Fuente, A.G.; Aquino, C.; Cantero, C.; Fleitas, F.; Torales, J.; et al. Rapid Epidemic Expansion of Chikungunya Virus East/Central/South African Lineage, Paraguay. Emerg. Infect. Dis. 2023, 29, 1859–1863. [Google Scholar] [CrossRef] [PubMed]
- Giesen, C.; Herrador, Z.; Fernandez-Martinez, B.; Figuerola, J.; Gangoso, L.; Vazquez, A.; Gómez-Barroso, D. A systematic review of environmental factors related to WNV circulation in European and Mediterranean countries. One Health 2023, 16, 100478. [Google Scholar] [CrossRef] [PubMed]
- Noisumdaeng, P.; Dangsagul, W.; Sangsiriwut, K.; Prasertsopon, J.; Changsom, D.; Yoksan, S.; Ajawatanawong, P.; Buathong, R.; Puthavathana, P. Molecular characterization and geographical distribution of Zika virus worldwide from 1947 to 2022. Int. J. Infect. Dis. 2023, 136, 5–10. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Dengue Worldwide Overview. Available online: https://www.ecdc.europa.eu/en/dengue-monthly (accessed on 30 January 2024).
- European Centre for Disease Prevention and Control. Chikungunya Worldwide Overview. Available online: https://www.ecdc.europa.eu/en/chikungunya-monthly (accessed on 30 January 2024).
- Mostashari, F.; Bunning, M.L.; Kitsutani, P.T.; Singer, D.A.; Nash, D.; Cooper, M.J.; Katz, N.; Liljebjelke, K.A.; Biggerstaff, B.J.; Fine, A.D.; et al. Epidemic West Nile encephalitis, New York, 1999: Results of a household-based seroepidemiological survey. Lancet 2001, 358, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Naeem, F.; Tabassum, S.; Afzaal, U.; Nazir, A.R.; Sabir, S.; Sah, S.S.; Mohanty, A.; Sah, R. Recurrent West. Nile virus outbreak in the United States in 2022: Current challenges and recommendations. J. Biosaf. Biosecurity 2023, 5, 146–152. [Google Scholar] [CrossRef]
- Russell, R.C. Arboviruses and their vectors in Australia: An update on the ecology and epidemiology of some mosquito-borne arboviruses. CAB Int. 1995, 83, 141–158. [Google Scholar]
- Klapsing, P.; MacLean, J.D.; Glaze, S.; McClean, K.L.; Drebot, M.A.; Lanciotti, R.S.; Campbell, G.L. Ross River Virus Disease Reemergence, Fiji, 2003–2004. Emerg. Infect. Dis. 2005, 11, 613–615. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Lindsay, M.D.; Coelen, R.J.; Broom, A.K.; Hall, R.A.; Smith, D.W. Arboviruses causing human disease in the Australasian zoogeographic region. Arch. Virol. 1994, 136, 447–467. [Google Scholar] [CrossRef]
- Jacups, S.P.; Whelan, P.I.; Currie, B.J. Ross River virus and Barmah Forest virus infections: A review of history, ecology, and predictive models, with implications for tropical Northern Australia. Vector-Borne Zoonotic Dis. 2008, 8, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Ong, O.; Skinner, E.B.; Johnson, B.J.; Old, J.M. Mosquito-borne viruses and non-human vertebrates in Australia: A review. Viruses 2021, 13, 265. [Google Scholar] [CrossRef]
- Flaxman, J.P.; Smith, D.W.; Mackenzie, J.S.; E Fraser, J.R.; Bass, S.P.; Hueston, L.; A Lindsay, M.D.; Cunningham, A.L. A comparison of the diseases caused by Ross River virus and Barmah Forest virus. Med. J. Aust. 1998, 169, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Lopes Marques, C.D.; Ranzolin, A.; Cavalcanti, N.G.; Branco Pinto Duarte, A.L. Arboviruses related with chronic musculoskeletal symptoms. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101502. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.A.; Do, K.-A.; Kay, B.H. Definition of Ross River Virus Vectors at Maroochy Shire, Australia. J. Med. Èntomol. 2000, 37, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Kay, B.H.; Carley, J.G.; Fanning, I.D.; Filippich, C. Quantitative studies of the vector competence of Aedes aegypti, Culex annulirostris and other mosquitoes (Diptera: Culicidae) with Murray Valley encephalitis and other Queensland arboviruses. J. Med. Èntomol. 1979, 16, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Viennet, E.; A Ritchie, S.; Faddy, H.M.; Williams, C.R.; Harley, D. Epidemiology of dengue in a high-income country: A case study in Queensland, Australia. Parasites Vectors 2014, 7, 379. [Google Scholar] [CrossRef]
- Gubler, D.J. Dengue and dengue hemorrhagic fever, Clin. Microbiol. Rev. 1998, 11, 480–496. Available online: http://cmr.asm.org/content/11/3/480.long (accessed on 25 March 2014). [CrossRef] [PubMed]
- Hoad, V.C.; Kiely, P.; Seed, C.R.; Viennet, E.; Gosbell, I.B. An Outbreak of Japanese Encephalitis Virus in Australia; What Is the Risk to Blood Safety? Viruses 2022, 14, 1935. [Google Scholar] [CrossRef]
- Selvey, L.A.; Dailey, L.; Lindsay, M.; Armstrong, P.; Tobin, S.; Koehler, A.P.; Markey, P.G.; Smith, D.W. The Changing Epidemiology of Murray Valley Encephalitis in Australia: The 2011 Outbreak and a Review of the Literature. PLoS Neglected Trop. Dis. 2014, 8, e2656. [Google Scholar] [CrossRef]
- Hall, R.A.; Scherret, J.H.; Mackenzie, J.S. Kunjin virus: An Australian variant of West Nile? Ann. N. Y. Acad. Sci. 2001, 951, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Prow, N.A.; Edmonds, J.H.; Williams, D.T.; Setoh, Y.X.; Bielefeldt-Ohmann, H.; Suen, W.W.; Hobson-Peters, J.; Hurk, A.F.v.D.; Pyke, A.T.; Hall-Mendelin, S.; et al. Virulence and Evolution of West Nile Virus, Australia, 1960–2012. Emerg. Infect. Dis. 2016, 22, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Frost, M.J.; Zhang, J.; Edmonds, J.H.; Prow, N.A.; Gu, X.; Davis, R.; Hornitzky, C.; Arzey, K.E.; Finlaison, D.; Hick, P.; et al. Characterization of Virulent West Nile Virus Kunjin Strain, Australia, 2011. Emerg. Infect. Dis. 2012, 18, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Australian Government, Department of Health and Aged Care. National Communicable Disease Surveillance Dashboard. 2024. Available online: https://nindss.health.gov.au/pbi-dashboard/ (accessed on 29 May 2024).
- Gyawali, N.; Taylor-Robinson, A.W.; Bradbury, R.S.; Potter, A.; Aaskov, J.G. Infection of Western Gray Kangaroos (Macropus fuliginosus) with Australian Arboviruses Associated with Human Infection. Vector-Borne Zoonotic Dis. 2020, 20, 33–39. [Google Scholar] [CrossRef]
- Stratton, M.D.; Ehrlich, H.Y.; Mor, S.M.; Naumova, E.N. A comparative analysis of three vector-borne diseases across Australia using seasonal and meteorological models. Sci. Rep. 2017, 7, 40186. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.R.; Busch, M.P. Transfusion-transmitted arboviruses. Vox Sang. 2010, 98, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Richarte, O.; de Salazar, M.I.O.; Giménez-Richarte, M.-P.; Collado, M.; Fernández, P.L.; Clavijo, C.; Navarro, L.; Arbona, C.; Marco, P.; Ramos-Rincon, J.-M. Transfusion-transmitted arboviruses: Update and systematic review. PLoS Neglected Trop. Dis. 2022, 16, e0010843. [Google Scholar] [CrossRef] [PubMed]
- Hoad, V.C.; Speers, D.J.; Keller, A.J.; Dowse, G.K.; Seed, C.R.; A Lindsay, M.D.; Faddy, H.M.; Pink, J. First reported case of transfusion-transmitted Ross River virus infection. Med. J. Aust. 2015, 202, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.C.; Sridhar, S.; Wong, S.C.; Chan, J.F.; Yip, C.C.; Chau, C.-H.; Au, T.W.; Hwang, Y.-Y.; Yau, C.S.; Lo, J.Y.; et al. Japanese Encephalitis Virus Transmitted Via Blood Transfusion, Hong Kong, China. Emerg. Infect. Dis. 2018, 24, 49–57. [Google Scholar] [CrossRef]
- Motta, I.J.; Spencer, B.R.; Cordeiro da Silva, S.G.; Arruda, M.B.; Dobbin, J.A.; Gonzaga, Y.B.; Arcuri, I.P.; Tavares, R.C.; Atta, E.H.; Fernandes, R.F.; et al. Evidence for Transmission of Zika Virus by Platelet Transfusion. N. Engl. J. Med. 2016, 375, 1101–1103. [Google Scholar] [CrossRef]
- Barjas-Castro, M.L.; Angerami, R.N.; Cunha, M.S.; Suzuki, A.; Nogueira, J.S.; Rocco, I.M.; Maeda, A.Y.; Vasami, F.G.; Katz, G.; Boin, I.F.; et al. Probable transfusion-transmitted Zika virus in Brazil. Transfusion 2016, 56, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- National Blood Authority Australia. Safety of Blood Products. Available online: https://blood.gov.au/safety-blood-products (accessed on 2 February 2024).
- Madzokere, E.T.; Hallgren, W.; Sahin, O.; Webster, J.A.; Webb, C.E.; Mackey, B.; Herrero, L.J. Integrating statistical and mechanistic approaches with biotic and environmental variables improves model predictions of the impact of climate and land-use changes on future mosquito-vector abundance, diversity and distributions in Australia. Parasites Vectors 2020, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Broom, A.; Whelan, P.I.; Brogan, B.; Currie, B.J.; Porigneaux, P.; Melville, L.; Jacups, S.P.; Smith, F.; Krause, V.L. Rainfall and vector mosquito numbers as risk indicators for mosquito-borne disease in central Australia. Commun. Dis. Intell. Q. Rep. 2003, 27, 110. [Google Scholar]
- Murphy, A.K.; Clennon, J.A.; Vazquez-Prokopec, G.; Jansen, C.C.; Frentiu, F.D.; Hafner, L.M.; Hu, W.; Devine, G.J. Spatial and temporal patterns of Ross River virus in south east Queensland, Australia: Identification of hot spots at the rural-urban interface. BMC Infect. Dis. 2020, 20, 722. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Mengersen, K.; Dale, P.; MacKenzie, J.S.; Toloo, G.; Wang, X.; Tong, S. Epidemiologic Patterns of Ross River Virus Disease in Queensland, Australia, 2001–2011. Am. J. Trop. Med. Hyg. 2014, 91, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Hurst, C.; Glass, K.; Harley, D.; Viennet, E. Spatial and Temporal Patterns of Ross River Virus in Queensland, 2001–2020. Trop. Med. Infect. Dis. 2021, 6, 145. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Oldenburg, B.; Mengersen, K.; Tong, S. Exploratory spatial analysis of social and environmental factors associated with the incidence of ross river virus in Brisbane, Australia. Am. J. Trop. Med. Hyg. 2007, 76, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hansen, A.; Cameron, S.; Bi, P. The geography of Ross River virus infection in South Australia, 2000–2013. Commun. Dis. Intell. 2020, 44, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.; Dear, K.; Harley, D.; McMichael, A. Analysis and Prediction of Ross River Virus Transmission in New South Wales, Australia. Vector-Borne Zoonotic Dis. 2014, 14, 422–438. [Google Scholar] [CrossRef]
- Naish, S.; Hu, W.; Mengersen, K.; Tong, S. Spatio-Temporal Patterns of Barmah Forest Virus Disease in Queensland, Australia. PLoS ONE 2011, 6, e25688. [Google Scholar] [CrossRef]
- Naish, S.; Hu, W.; Mengersen, K.; Tong, S. Spatial and temporal clusters of Barmah Forest virus disease in Queensland, Australia. Trop. Med. Int. Health 2011, 16, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Knope, K.; Whelan, P.; Smith, D.; Johansen, C.; Moran, R.; Doggett, S.; Sly, A.; Hobby, M.; Wright, P.; Nicholson, J. Arboviral diseases and malaria in Australia, 2010–2011: Annual report of the National Arbovirus and Malaria Advisory Committee. Commun. Dis. Intell. Q. Rep. 2013, 37, E1–E20. Available online: https://search.informit.org/doi/full/10.3316/ielapa.335358772719959 (accessed on 6 July 2023). [PubMed]
- Fitzsimmons, G.J.; Wright, P.; Johansen, C.A.; Whelan, P.I. Arboviral diseases and malaria in Australia, 2007/08: Annual report of the National Arbovirus and Malaria Advisory Committee. Commun. Dis. Intell. Q. Rep. 2009, 33, 155–169. [Google Scholar] [PubMed]
- Vally, H.; Peel, M.; Dowse, G.K.; Cameron, S.; Codde, J.P.; Hanigan, I.; Lindsay, M.D. Geographic Information Systems used to describe the link between the risk of Ross River virus infection and proximity to the Leschenault estuary, WA. Aust. N. Z. J. Public Health 2012, 36, 229–235. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. 23/12/2010. Australian Statistical Geography Standard (ASGS): Volume 1—Main Structure and Greater Capital City Statistical Areas. July 2011. Available online: https://www.abs.gov.au/AUSSTATS/[email protected]/DetailsPage/1270.0.55.001July%202011 (accessed on 2 May 2014).
- Australian Bureau of Statistics. Regional Population. Available online: https://www.abs.gov.au/statistics/people/population/regional-population/2019-20#data-download (accessed on 2 July 2021).
- Australian Bureau of Statistics. Regional Population by Age and Sex. Available online: https://www.abs.gov.au/statistics/people/population/regional-population-age-and-sex/latest-release (accessed on 5 April 2021).
- Queensland Government. SILO. 2021. Available online: https://www.longpaddock.qld.gov.au/silo/ (accessed on 2 June 2021).
- Australian Government, Department of Health and Aged Care. CDNA Surveillance Case Definitions. Available online: https://www.health.gov.au/resources/collections/cdna-surveillance-case-definitions?utm_source=health.gov.au&utm_medium=redirect&utm_campaign=digital_transformation&utm_content=casedefinitions (accessed on 15 February 2022).
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 15 February 2021).
- QGIS—A Free and Open Source Geographic Information System. Available online: https://qgis.org (accessed on 18 February 2019).
- Kulldorff, M.; Heffernan, R.; Hartman, J.; Assunção, R.; Mostashari, F. A space–Time permutation scan statistic for disease outbreak detection. PLoS Med. 2005, 2, e59. [Google Scholar] [CrossRef] [PubMed]
- Kulldorff, M.; SaTScanTM User Guide for Version 10.1. July 2022. Available online: https://www.satscan.org/cgi-bin/satscan/register.pl/SaTScan_Users_Guide.pdf?todo=process_userguide_download (accessed on 12 March 2024).
- Hubbard, A.E.; Ahern, J.; Fleischer, N.L.; Van der Laan, M.; Lippman, S.A.; Jewell, N.; Bruckner, T.; Satariano, W.A. To GEE or not to GEE: Comparing population average and mixed models for estimating the associations between neighborhood risk factors and health. Epidemiology 2010, 21, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Bi, P.; Parton, K.A. Climate Variations and the Transmission of Ross River Virus Infection in Coastal and Inland Region of Queensland: An Analysis from Townsville and Toowoomba. Environ. Health 2002, 2, 28–34. Available online: https://researchoutput.csu.edu.au/ws/portalfiles/portal/8754504/PID14037.pdf (accessed on 15 January 2015).
- Tong, S.; Dale, P.; Nicholls, N.; Mackenzie, J.S.; Wolff, R.; McMichael, A.J. Climate variability, social and environmental factors, and ross river virus transmission: Research development and future research needs. Environ. Health Perspect. 2008, 116, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J.C.; Learoyd, T.P.; Langendorf, B.J.; Logan, J.G. Japanese encephalitis: The vectors, ecology and potential for expansion. J. Travel. Med. 2018, 25, S16–S26. [Google Scholar] [CrossRef]
- Lima, M.F.; Cotton, J.; Marais, M.; Faggian, R. Modelling the risk of Japanese encephalitis virus in Victoria, Australia, using an expert-systems approach. BMC Infect. Dis. 2024, 24, 60. [Google Scholar] [CrossRef]
- Braddick, M.; O’brien, H.M.; Lim, C.K.; Feldman, R.; Bunter, C.; Neville, P.; Bailie, C.R.; Butel-Simoes, G.; Jung, M.-H.; Yuen, A.; et al. An integrated public health response to an outbreak of Murray Valley encephalitis virus infection during the 2022–2023 mosquito season in Victoria. Front. Public Health 2023, 11, 1256149. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Clements, A.; Williams, G.; Tong, S.; Mengersen, K. Spatial Patterns and Socioecological Drivers of Dengue Fever Transmission in Queensland, Australia. Environ. Health Perspect. 2012, 120, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Akter, R.; Naish, S.; Gatton, M.; Bambrick, H.; Hu, W.; Tong, S. Spatial and temporal analysis of dengue infections in Queensland, Australia: Recent trend and perspectives. PLoS ONE 2019, 14, e0220134. [Google Scholar] [CrossRef] [PubMed]
- Gatton, M.L.; Kelly-Hope, L.A.; Kay, B.H.; Ryan, P.A. Spatial-temporal analysis of Ross River virus disease patterns in Queensland, Australia. Am. J. Trop. Med. Hyg. 2004, 71, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.A.; Turley, A.P.; Wilson, G.; Hurst, T.P.; Retzki, K.; Brown-Kenyon, J.; Hodgson, L.; Kenny, N.; Cook, H.; Montgomery, B.L.; et al. Establishment of wMel Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland, Australia. Gates Open Res. 2020, 3, 1547. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Montgomery, B.L.; Popovici, J.; Iturbeormaetxe, I.; Johnson, P.H.; Muzzi, F.; Greenfield, M.; Durkan, M.; Leong, Y.S.; Dong, Y.; et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 2011, 476, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Jansen, C.C.; Shivas, M.A.; May, F.J.; Pyke, A.T.; Onn, M.B.; Lodo, K.; Hall-Mendelin, S.; McMahon, J.L.; Montgomery, B.L.; Darbro, J.M.; et al. Epidemiologic, Entomologic, and Virologic Factors of the 2014–2015 Ross River Virus Outbreak, Queensland, Australia. Emerg. Infect. Dis. 2019, 25, 2243–2252. [Google Scholar] [CrossRef] [PubMed]
- Mottram, P.; Kettle, D.S. Development and survival of immature Culex annulirostris mosquitoes in southeast Queensland. Med. Veter. Èntomol. 1997, 11, 181–186. [Google Scholar] [CrossRef]
- Russell, R.C. Age composition and overwintering of Culex annulirostris Skuse (Diptera: Culicidae) near Deniliquin, in the Murray Valley of New South Wales. Aust. J. Entomol. 1987, 26, 93–96. [Google Scholar] [CrossRef]
- Glass, K. Ecological mechanisms that promote arbovirus survival: A mathematical model of Ross River virus transmission. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 252–260. [Google Scholar] [CrossRef]
- Sohail, A.; Anders, K.L.; McGuinness, S.L.; Leder, K. The epidemiology of imported and locally acquired dengue in Australia, 2012–2022. J. Travel Med. 2024, 31, taae014. [Google Scholar] [CrossRef] [PubMed]
- Warrilow, D.; Northill, J.A.; Pyke, A.T. Sources of Dengue Viruses Imported into Queensland, Australia, 2002–2010. Emerg. Infect. Dis. 2012, 18, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Harapan, H.; Imrie, A. Movement of arboviruses between Indonesia and Western Australia. Microbiol. Aust. 2021, 42, 165–169. [Google Scholar] [CrossRef]
- Knope, K.; Giele, C. Increasing notifications of dengue in Australia related to overseas travel, 1991 to 2012. Commun. Dis. Intell. Q. Rep. 2013, 37, E55. Available online: https://search.informit.org/doi/10.3316/ielapa.335414671633734 (accessed on 15 January 2015). [PubMed]
- Viennet, E.; Knope, K.; Faddy, H.M.; Williams, C.R.; Harley, D. Assessing the threat of chikungunya virus emergence in Australia. Commun. Dis. Intell. 2013, 37, E136–E143. [Google Scholar]
- Gardner, L.M.; Fajardo, D.; Waller, S.T.; Wang, O.; Sarkar, S. A Predictive spatial model to quantify the risk of air-travel-associated dengue importation into the United States and Europe. J. Trop. Med. 2012, 2012, 103679. [Google Scholar] [CrossRef] [PubMed]
- Viennet, E.; Mincham, G.; Frentiu, F.D.; Jansen, C.C.; Montgomery, B.L.; Harley, D.; Flower, R.L.; Williams, C.R.; Faddy, H.M. Epidemic Potential for Local Transmission of Zika Virus in 2015 and 2016 in Queensland, Australia. PLoS Curr. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Jansen, C.C.; Darbro, J.M.; Birrell, F.A.; Shivas, M.A.; Hurk, A.F.v.D. Impact of COVID-19 Mitigation Measures on Mosquito-Borne Diseases in 2020 in Queensland, Australia. Viruses 2021, 13, 1150. [Google Scholar] [CrossRef] [PubMed]
- Viennet, E.; Ritchie, S.A.; Williams, C.R.; Faddy, H.M.; Harley, D. Public health responses to and challenges for the control of dengue transmission in high-income countries: Four case studies. PLoS Neglected Trop. Dis. 2016, 10, e0004943. [Google Scholar] [CrossRef]
- Australian Bureau of Meteorology. Record-Breaking La Niña Events. 2012. Available online: http://www.bom.gov.au/climate/enso/history/La-Nina-2010-12.pdf (accessed on 15 January 2015).
- Australian Government, Bureau of Meteorology. What Is El Niño and How does It Impact Australia? 2020. Available online: http://www.bom.gov.au/climate/updates/articles/a008-el-nino-and-australia.shtml (accessed on 26 September 2021).
- McMichael, A.J.; Corvalán, C.F.; Ebi, K.L.; Githeko, A.K.; Scheraga, J.D.; Woodward, A. Climate Change and Human Health: Risks and Responses, Technical Report. 2003. Available online: https://iris.who.int/bitstream/handle/10665/42742/924156248X_eng.pdf?sequence=1 (accessed on 19 March 2014).
- Qian, W.; Viennet, E.; Glass, K.; Harley, D.; Hurst, C. Prediction of Ross River virus incidence using mosquito data in three cities of Queensland, Australia. Biology 2023, 12, 1429. [Google Scholar] [CrossRef]
- Hales, S.; Weinstein, P.; Woodward, A. Dengue fever epidemics in the South Pacific: Driven by El Nino Southern Oscillation? Lancet 1996, 348, 1664–1665. [Google Scholar] [CrossRef] [PubMed]
- Cutcher, Z.; Williamson, E.; Lynch, S.E.; Rowe, S.; Clothier, H.J.; Firestone, S.M. Predictive modelling of Ross River virus notifications in southeastern Australia. Epidemiol. Infect. 2016, 145, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Flies, E.J.; Weinstein, P.; Anderson, S.J.; Koolhof, I.; Foufopoulos, J.; Williams, C.R. Ross River Virus and the Necessity of Multiscale, Eco-epidemiological Analyses. J. Infect. Dis. 2017, 217, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Koolhof, I.S.; Bettiol, S.; Carver, S. Fine-temporal forecasting of outbreak probability and severity: Ross River virus in Western Australia. Epidemiol. Infect. 2017, 145, 2949–2960. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Donald, K.; McMichael, A.J. Climate variability and Ross River virus in 8 Australian cities, 1985–1996. Epidemiology 2001, 12, S33. [Google Scholar]
- Hu, W.; Nicholls, N.; Lindsay, M.; Dale, P.; McMichael, A.J.; Mackenzie, J.S.; Tong, S. Development of a predictive model for Ross River virus disease in Brisbane, Australia. Am. J. Trop. Med. Hyg. 2004, 71, 129–137. [Google Scholar] [CrossRef]
- Tong, S.; Hu, W.; McMichael, A.J. Climate variability and Ross River virus transmission in Townsville Region, Australia, 1985–1996. Trop. Med. Int. Health 2004, 9, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Bi, P.; Hiller, J.E.; Cameron, A.S.; Zhang, Y.; Givney, R. Climate variability and Ross River virus infections in Riverland, South Australia, 1992–2004. Epidemiol. Infect. 2009, 137, 1486–1493. [Google Scholar] [CrossRef]
- Qian, W.; Viennet, E.; Glass, K.; Harley, D. Epidemiological models for predicting Ross River virus in Australia: A systematic review. PLoS Neglected Trop. Dis. 2020, 14, e0008621. [Google Scholar] [CrossRef]
- Lindsay, M.; Mackenzie, J. Vector-borne viral diseases and climate change in the Australasian region: Major concerns and the public health response. In Climate Change and Human. Health in the Asia-Pacific Region; Curson, C.G.P., Jackson, E., Eds.; Australian Medical Association and Greenpeace International: Sydney, Australia, 1997; pp. 47–62. [Google Scholar]
- Naish, S.; Mengersen, K.; Tong, S. Spatial analysis of risk factors for transmission of the Barmah Forest virus in Queensland, Australia. Geospat. Health 2013, 8, 289–299. [Google Scholar] [CrossRef]
- Huang, X.; Clements, A.C.; Williams, G.; Milinovich, G.; Hu, W. A threshold analysis of dengue transmission in terms of weather variables and imported dengue cases in Australia. Emerg. Microbes Infect. 2013, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Akter, R.; Hu, W.; Gatton, M.; Bambrick, H.; Cheng, J.; Tong, S. Climate variability, socio-ecological factors and dengue transmission in tropical Queensland, Australia: A Bayesian spatial analysis. Environ. Res. 2020, 195, 110285. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Williams, G.; Clements, A.C.A.; Hu, W. Imported Dengue Cases, Weather Variation and Autochthonous Dengue Incidence in Cairns, Australia. PLoS ONE 2013, 8, e81887. [Google Scholar] [CrossRef] [PubMed]
- Akter, R.; Hu, W.; Gatton, M.; Bambrick, H.; Naish, S.; Tong, S. Different responses of dengue to weather variability across climate zones in Queensland, Australia. Environ. Res. 2020, 184, 109222. [Google Scholar] [CrossRef]
- Tong, S.; Bi, P.; Donald, K.; McMichael, A.J. Climate variability and Ross River virus transmission. J. Epidemiol. Community Health 2002, 56, 617–621. [Google Scholar] [CrossRef]
- Australian Government, Department of Health and Aged Care. Role and Functions of an Australian Centre for Disease Control. Prevention-Promotion-Protection, Consultation Paper, November 2022. Available online: https://www.cdc.gov.au/sites/default/files/2023-12/role-and-functions-of-an-australian-centre-for-disease-control.pdf (accessed on 13 February 2024).





| Virus | Imported | Locally Acquired |
|---|---|---|
| RRV | 0 | 76,009 |
| BFV | 0 | 23,390 |
| DENV | 12,448 | 2865 |
| MVEV | 0 | 31 |
| WNV | 6 | 13 (WNVKUN) |
| ZIKV | 132 | 0 |
| CHIKV | 693 | 0 |
| JEV | 13 | 0 |
| Virus | Country of Origin | Count | Total Count | Percentage |
|---|---|---|---|---|
| CHIKV | Indonesia | 241 | 693 | 34.77 |
| CHIKV | India | 134 | 693 | 19.33 |
| CHIKV | Samoa | 47 | 693 | 6.78 |
| DENV | Indonesia | 6545 | 12,303 | 53.19 |
| DENV | Thailand | 1387 | 12,303 | 11.27 |
| DENV | Philippines | 476 | 12,303 | 3.86 |
| JEV | Indonesia | 5 | 13 | 38.46 |
| JEV | Philippines | 2 | 13 | 15.38 |
| JEV | Thailand | 2 | 13 | 15.38 |
| WNV | Papua New Guinea | 1 | 6 | 16.66 |
| WNV | United States of America | 1 | 6 | 16.66 |
| WNV | Nauru | 1 | 6 | 16.66 |
| ZIKV | Tonga | 19 | 132 | 14.39 |
| ZIKV | Fiji | 15 | 132 | 11.36 |
| ZIKV | Mexico | 13 | 132 | 9.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viennet, E.; Frentiu, F.D.; McKenna, E.; Torres Vasconcelos, F.; Flower, R.L.P.; Faddy, H.M. Arbovirus Transmission in Australia from 2002 to 2017. Biology 2024, 13, 524. https://doi.org/10.3390/biology13070524
Viennet E, Frentiu FD, McKenna E, Torres Vasconcelos F, Flower RLP, Faddy HM. Arbovirus Transmission in Australia from 2002 to 2017. Biology. 2024; 13(7):524. https://doi.org/10.3390/biology13070524
Chicago/Turabian StyleViennet, Elvina, Francesca D. Frentiu, Emilie McKenna, Flavia Torres Vasconcelos, Robert L. P. Flower, and Helen M. Faddy. 2024. "Arbovirus Transmission in Australia from 2002 to 2017" Biology 13, no. 7: 524. https://doi.org/10.3390/biology13070524





