Rare Earth Element Content in Hair Samples of Children Living in the Vicinity of the Kola Peninsula Mining Site and Nervous System Diseases
Abstract
:Simple Summary
Abstract
1. Introduction
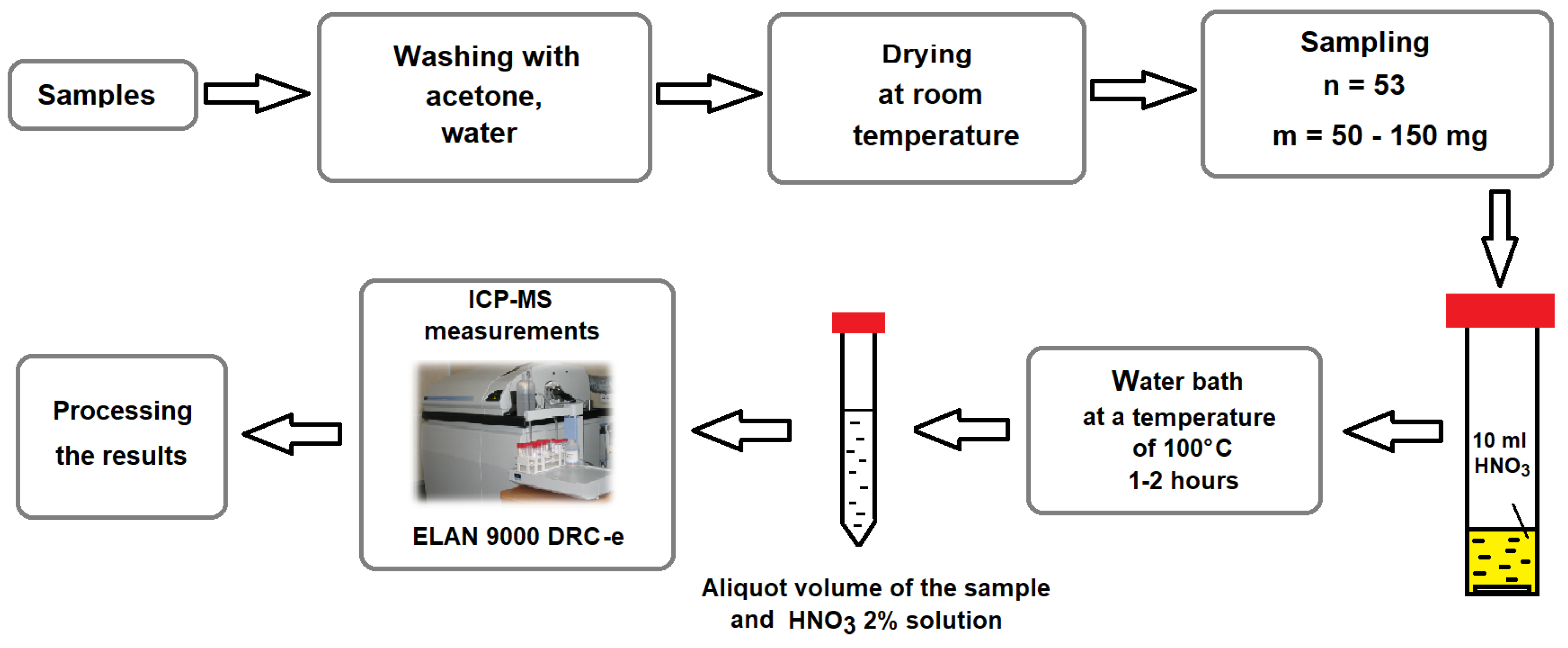
2. Experimental Procedure
2.1. Sample Preparation
2.2. Sample Analysis
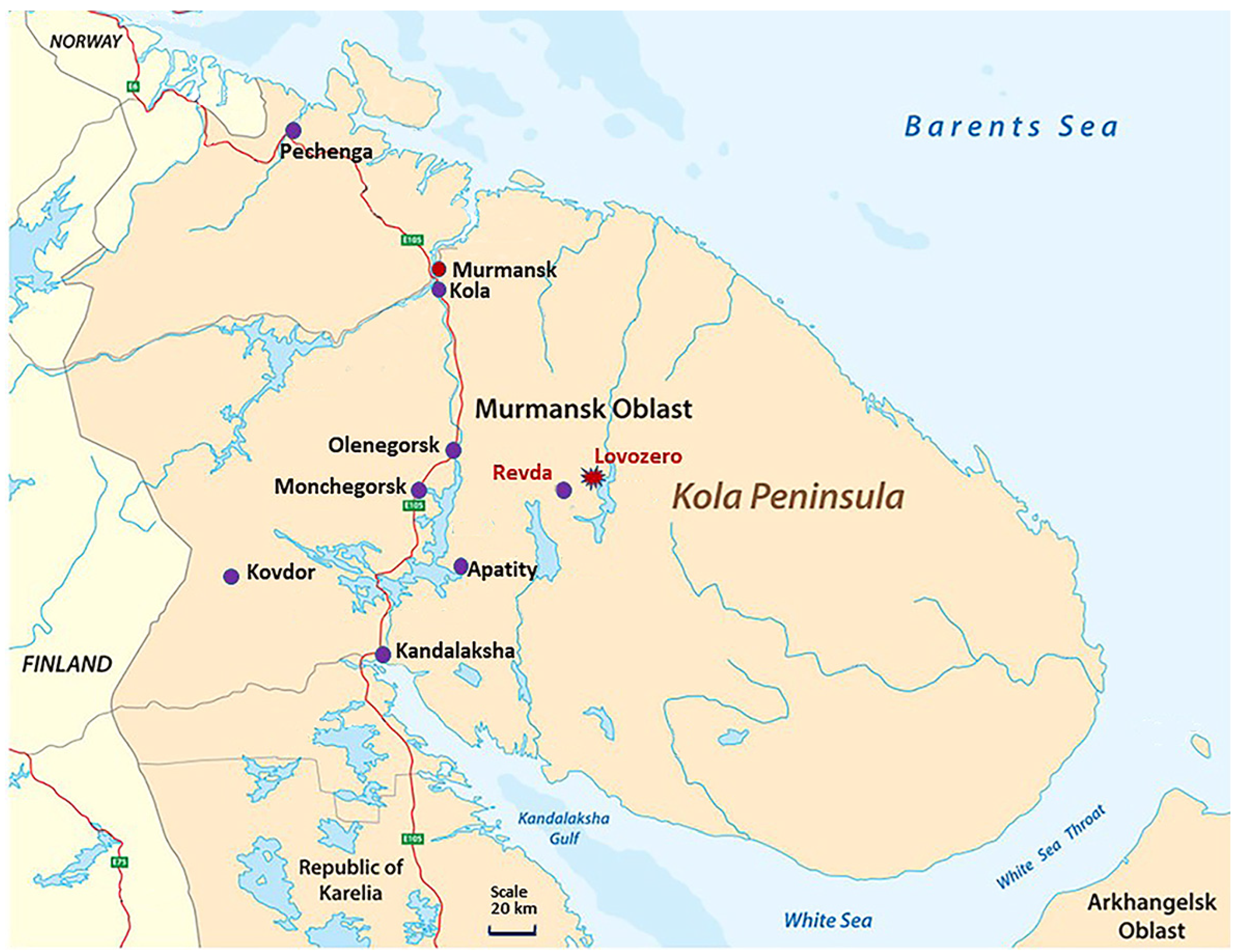
2.3. Comparison Areas
2.4. Analysis of the Population Morbidity in the Kola North
2.5. Statistical Data Processing
3. Results
3.1. REE Content in Children’s Hair Samples
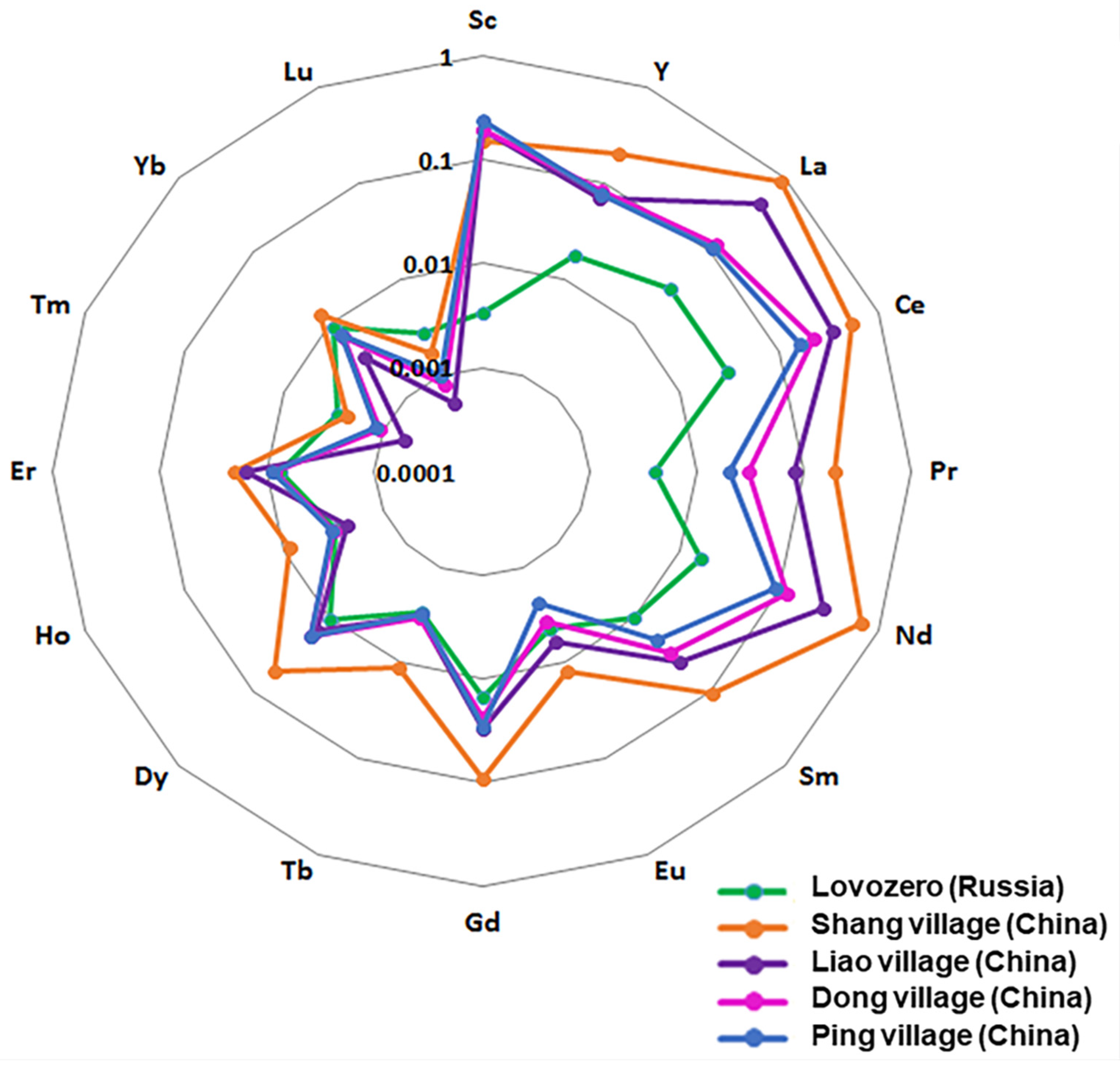
3.2. Comparison
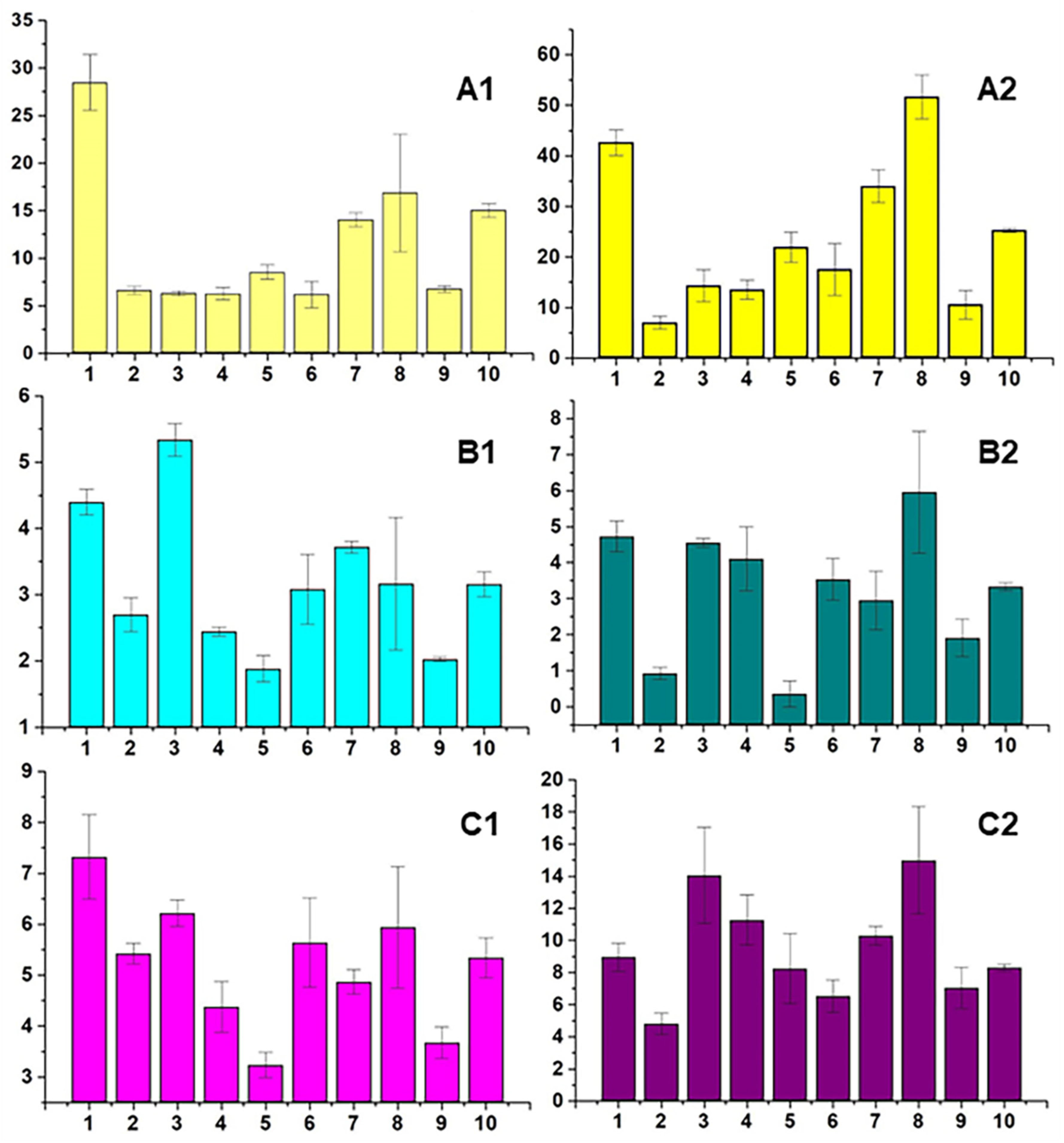
3.3. Nervous System Diseases
4. Discussion
4.1. Accumulation of REEs in the Brain
4.2. Nervous System Diseases and REEs
4.2.1. Episodic Paroxysmal Disorders (G40–G47)
4.2.2. Cerebral Palsy and Other Paralytic Syndromes (G80–G83)
4.2.3. Epilepsy and Status Epilepticus (G40–G41)
5. Advantages of This Study
6. Conclusions
7. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Yang, Y.; Wang, D.; Huang, L. Toxic Effects of Rare Earth Elements on Human Health: A Review. Toxics 2024, 12, 317. [Google Scholar] [CrossRef] [PubMed]
- Brouziotis, A.A.; Giarra, A.; Libralato, G.; Pagano, G.; Guida, M.; Trifuoggi, M. Toxicity of rare earth elements: An overview on human health impact. Front. Environ. Sci. 2022, 10, 948041. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Li, Z.; Liang, T.; Li, K.; Wang, P. Exposure of children to light rare earth elements through ingestion of various size fractions of road dust in REEs mining areas. Sci. Total. Environ. 2020, 743, 140432. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, Z.; Zhang, H.; Zhao, Y.; Chai, Z. Neurotoxicological evaluation of long-term lanthanum chloride exposure in rats. Toxicol. Sci. 2008, 103, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Xiao, H.; He, X.; Li, Z.; Li, F.; Liu, N.; Zhao, Y.; Huang, Y.; Zhang, Z.; Chai, Z. Neurotoxicological consequence of long-term exposure to lanthanum. Toxicol. Lett. 2006, 165, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Rim, K.T.; Koo, K.H.; Park, J.S. Toxicological Evaluations of rare earths and their health impacts to workers: A literature review. Saf. Health Work 2013, 4, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Rim, K.-T. Effects of rare earth elements on the environment and human health: A literature review. Toxicol. Environ. Health Sci. 2016, 8, 189–200. [Google Scholar] [CrossRef]
- Gwenzi, W.; Mangori, L.; Danha, C.; Chaukura, N.; Dunjana, N.; Sanganyado, E. Sources, behaviour, and environmental and human health risks of high-technology rare earth elements as emerging contaminants. Sci. Total. Environ. 2018, 636, 299–313. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; El-Hack, M.E.A.; Khafaga, A.F.; Noreldin, A.E.; Arif, M.; Chaudhry, M.T.; Losacco, C.; Abdeen, A.; Abdel-Daim, M.M. Impacts of rare earth elements on animal health and production: Highlights of cerium and lanthanum. Sci. Total. Environ. 2019, 672, 1021–1032. [Google Scholar] [CrossRef]
- Chung, C.; Deák, F.; Kavalali, E.T. Molecular substrates mediating lanthanide-evoked neurotransmitter release in central synapses. J. Neurophysiol. 2008, 100, 2089–2100. [Google Scholar] [CrossRef]
- Gaman, L.; Radoi, M.P.; Delia, C.E.; Luzardo, O.P.; Zumbado, M.; Rodríguez-Hernández, A.; Stoian, I.; Gilca, M.; Boada, L.D.; Henríquez-Hernández, L.A. Concentration of heavy metals and rare earth elements in patients with brain tumours: Analysis in tumour tissue, non-tumour tissue, and blood. Int. J. Environ. Health Res. 2019, 31, 741–754. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, J.; Yu, S.; Ding, Z.; Song, H.; Wang, Y.; Li, Y. Lanthanum Chloride Causes Neurotoxicity in Rats by Upregulating miR-124 Expression and Targeting PIK3CA to Regulate the PI3K/Akt Signaling Pathway. BioMed Res. Int. 2020, 2020, 5205142. [Google Scholar] [CrossRef]
- Wei, J.; Wang, C.; Yin, S.; Pi, X.; Jin, L.; Li, Z.; Liu, J.; Wang, L.; Yin, C.; Ren, A. Concentrations of rare earth elements in maternal serum during pregnancy and risk for fetal neural tube defects. Environ. Int. 2020, 137, 105542. [Google Scholar] [CrossRef]
- Peng, R.L.; Pan, X.C.; Xie, Q. Relationship of the hair content of rare earth elements in young children aged 0 to 3 years to that in their mothers living in a rare earth mining area of Jiangxi. Chin. J. Prev. Med. 2003, 37, 20–22. (In Chinese) [Google Scholar]
- Zhu, W.F.; Xu, S.Q.; Zhang, H.; Shao, P.P.; Wu, D.S.; Yang, W.J.; Feng, J. Investigation of children intelligence quotient in REE mining area: Bio-effect study of REE mining area in South Jiangxi province. Chin. Sci. Bull. 1996, 41, 914–916. (In Chinese) [Google Scholar]
- Wang, Y.; Wang, D.; Yi, N.H.; Sheng, P.; Yang, B. Relationship between Rare Earth Elements, Lead and Intelligence of Children Aged 6 to 16 years: A Bayesian Structural Equation Modelling Method. Int. Arch. Nurs. Health Care 2019, 5, 123. [Google Scholar] [CrossRef]
- Fan, G.; Yuan, Z.; Zheng, H.; Liu, Z. Study on the effects of exposure to rare earth elements and health-responses in children aged 7–10 years. Wei Sheng Yan Jiu J. Hyg. Res. 2004, 33, 23–28. [Google Scholar]
- Zhu, W.; Xu, S.; Shao, P.; Zhang, H.; Wu, D.; Yang, W.; Feng, J. Bioelectrical activity of the central nervous system among populations in a rare earth element area. Biol. Trace Element Res. 1997, 57, 71–77. [Google Scholar] [CrossRef]
- Cao, Z.; Yang, M.; Gong, H.; Feng, X.; Hu, L.; Li, R.; Xu, S.; Wang, Y.; Xiao, H.; Zhou, A. Association between prenatal exposure to rare earth elements and the neurodevelopment of children at 24-months of age: A prospective cohort study. Environ. Pollut. 2024, 343, 123201. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Dawidziak, M.; Czlapka-Matyasik, M.; Wojciechowska, Z.; Proch, J.; Kowalski, R.; Niedzielski, P. Rare Earth Elements Accumulation in the Hair of Malagasy Children and Adolescents in Relation to Their Age and Nutritional Status. Int. J. Environ. Res. Public Health 2022, 19, 455. [Google Scholar] [CrossRef] [PubMed]
- Belisheva, N.K. Comparative Analysis of Morbidity and Elemental Composition of Hair Among Children Living on Different Territories of the Kola North. In Processes and Phenomena on the Boundary Between Biogenic and Abiogenic Nature; Frank-Kamenetskaya, O.V., Ed.; Lecture Notes in Earth System Sciences; Springer: Berlin/Heidelberg, Germany, 2020; pp. 803–827. [Google Scholar]
- Belisheva, N.; Martynova, A.; Mikhaylov, R. An Interdisciplinary Approach to Predicting the Effects of Transboundary Atmospheric Transport to Northwest European Neighboring States. KnE Soc. Sci. 2022, 158–171. [Google Scholar] [CrossRef]
- Mazukhina, S.; Krasavtseva, E.; Makarov, D.; Maksimova, V. Thermodynamic Modeling of Hypergene Processes in Loparite Ore Concentration Tailings. Minerals 2021, 11, 996. [Google Scholar] [CrossRef]
- Stovern, M.; Guzmán, H.; Rine, K.P.; Felix, O.; King, M.; Ela, W.P.; Betterton, E.A.; Sáez, A.E. Windblown Dust Deposition Forecasting and Spread of Contamination around Mine Tailings. Atmosphere 2016, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liang, T. Accumulation and fractionation of rare earth elements in atmospheric particulates around a mine tailing in Baotou, China. Atmospheric Environ. 2014, 88, 23–29. [Google Scholar] [CrossRef]
- Krasavtseva, E.; Maksimova, V.; Makarov, D.; Potorochin, E. Modelling of the Chemical Halo of Dust Pollution Migration in Loparite Ore Tailings Storage Facilities. Minerals 2021, 11, 1077. [Google Scholar] [CrossRef]
- Krasavtseva, E.A.; Maksimova, V.V.; Gorbacheva, T.T.; Makarov, D.V.; Alfertyev, N.L. Evaluation of soils and plants chemical pollution within the area affected by storages of loparite ore processing waste. Mine Surv. Subsurf. Use 2021, 112, 52–58. (In Russian) [Google Scholar]
- Krasavtseva, E.; Sandimirov, S.; Elizarova, I.; Makarov, D. Assessment of Trace and Rare Earth Elements Pollution in Water Bodies in the Area of Rare Metal Enterprise Influence: A Case Study—Kola Subarctic. Water 2022, 14, 3406. [Google Scholar] [CrossRef]
- Shin, S.-H.; Kim, H.-O.; Rim, K.-T. Worker Safety in the Rare Earth Elements Recycling Process From the Review of Toxicity and Issues. Saf. Health Work 2019, 10, 409–419. [Google Scholar] [CrossRef]
- Hao, Z.; Li, Y.; Li, H.; Wei, B.; Liao, X.; Liang, T.; Yu, J. Levels of rare Earth elements, heavy metals and uranium in a popu-lation living in Baiyun Obo, inner Mongolia, China: A pilot study. Chemosphere 2015, 128, 161–170. [Google Scholar] [CrossRef]
- Liang, Q.; Yin, H.; Li, J.; Zhang, L.; Hou, R.; Wang, S. Investigation of rare earth elements in urine and drinking water of children in mining area. Medicine 2018, 97, e12717. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Li, Y.; Li, H.; Yu, J.; Ye, B.; Liang, T. Rare earth elements in human hair from a mining area of China. Ecotoxicol. Environ. Saf. 2013, 96, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.-L.; Zhu, W.-Z.; Gao, Z.-H.; Meng, Y.-X.; Peng, R.-L.; Lu, G.-C. Distribution characteristics of rare earth elements in children’s scalp hair from a rare earths mining area in Southern China. J. Environ. Sci. Health Part A 2004, 39, 2517–2532. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-F.; Chen, Z.-Q. Distribution and fractionation of rare earth elements in soil–water system and human blood and hair from a mining area in southwest Fujian Province, China. Environ. Earth Sci. 2014, 72, 3599–3608. [Google Scholar] [CrossRef]
- Meryem, B.; Ji, H.; Gao, Y.; Ding, H.; Li, C. Distribution of rare earth elements in agricultural soil and human body (scalp hair and urine) near smelting and mining areas of Hezhang, China. J. Rare Earths 2016, 34, 1156–1167. [Google Scholar] [CrossRef]
- Edahbi, M.; Plante, B.; Benzaazoua, M. Environmental challenges and identification of the knowledge gaps associated with REE mine wastes management. J. Clean. Prod. 2018, 212, 1232–1241. [Google Scholar] [CrossRef]
- Billionnet, C.; Sherrill, D.; Annesi-Maesano, I. Estimating the health effects of exposure to multi-pollutant mixture. Ann. Epidemiol. 2012, 22, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Dominici, F.; Peng, R.D.; Barr, C.D.; Bell, M.L. Protecting human health from air pollution: Shifting from a single-pollutant to a multipollutant approach. Epidemiology 2010, 21, 187–194. [Google Scholar] [CrossRef]
- Cortes Toro, E.; De Goeij, J.; Bacso, J.; Cheng, Y.D.; Kinova, L.; Matsubara, J.; Niese, S.; Sato, T.; Wesenberg, G.; Muramatsu, Y.; et al. The Significance of Hair Mineral Analysis as a Means for Assessing Internal Body Badens of Environmental Pollutants: Results from an IAEA Co-ordinated Research Programme. J. Radioanal. Nucl. Chem. 1993, 167, 413–421. [Google Scholar] [CrossRef]
- Determination of chemical elements in biological media and preparations by inductively coupled plasma atomic emission spectrometry and inductively coupled plasma mass spectrometry: Guidelines; Federal Center for State Sanitary and Epidemiological Supervision of the Ministry of Health of the Russian Federation: Moscow, Russia, 2003; 56 p, ISBN 5—7508—0462—3. Available online: https://files.stroyinf.ru (accessed on 25 July 2024).
- Seregina, I.F.; Osipov, K.; Bolshov, M.A.; Filatova, D.G.; Lanskaya, S.Y. Matrix Interference in the Determination of Elements in Biological Samples by Inductively Coupled Plasma Mass Spectrometry and Methods for Its Elimination. J. Analyt. Chem. 2019, 74, 182–191. [Google Scholar] [CrossRef]
- Pashkevich, M.A.; Stryzhenok, A.V. Analysis of the landscape-geochemical situation in the area of the tailings management of ANOF-2 of JSC Apatit. Notes Min. Inst. 2013, 206, 155–159. (In Russian) [Google Scholar]
- Mikhailova, L.A.; Baranovskaya, N.V.; Bondarevich, E.A.; Vitkovsky, Y.A.; Zhornyak, L.V.; Epova, E.S.; Eryomin, O.V.; Nimaeva, B.V.; Ageeva, E.V. Determination of elemental homeostasis of the child population of Zabaikalsky Krai by the method of multi-element instrumental neutron activation analysis. Gigiena i Sanitariya. 2023, 102, 123–131. (In Russian) [Google Scholar]
- Baranovskaya, N.V.; Rikhvanov, L.P.; Ignatova, T.N.; Narkovich, D.V.; Denisova, O.A. The Human Geochemistry Essays: Monograph; Tomsk Polytechnic University Publishing House: Tomsk, Russia, 2015; p. 378. ISBN 978-5-4387-0581-9. [Google Scholar]
- Rodushkin, I.; Axelsson, M.D. Application of double focusing sector field ICP-MS for multielemental characterization of human hair and nails. Part II. A study of the inhabitants of northern Sweden. Sci. Total. Environ. 2000, 262, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Heuser, J.; Miledi, R. Effect of lanthanum ions on function and structure of frog neuromuscular junctions. Proc. R. Soc. London. Ser. B Biol. Sci. 1971, 179, 247–260. [Google Scholar] [CrossRef]
- Kajimoto, N.; Kirpekar, S.M. Effect of manganese and lanthanum on spontaneous release of acetylcholine at frog motor nerve terminals. Nat. New Biol. 1972, 235, 29–30. [Google Scholar] [CrossRef] [PubMed]
- Alnaes, E.; Rahamimoff, R. Dual Action of Praseodymium (Pr3+) on Transmitter Release at the Frog Neuromuscular Synapse. Nature 1974, 247, 478–479. [Google Scholar] [CrossRef]
- Haley, T.J.; Komesu, N.; Efros, M.; Koste, L.; Upham, H. Pharmacology and toxicology of praseodymium and neodymium chlorides. Toxicol. Appl. Pharmacol. 1964, 6, 614–620. [Google Scholar] [CrossRef]
- Haley, T.J.; Koste, L.; Komesu, N.; Efros, M.; Upham, H. Pharmacology and toxicology of dysprosium, holmium, and erbium chlorides*1. Toxicol. Appl. Pharmacol. 1966, 8, 37–43. [Google Scholar] [CrossRef]
- Haley, T.J.; Komesu, N.; Flesher, A.; Mavis, L.; Cawthorne, J.; Upham, H. Pharmacology and toxicology of terbium, thulium, and ytterbium chlorides. Toxicol. Appl. Pharmacol. 1963, 5, 427–436. [Google Scholar] [CrossRef]
- Haley, T.J.; Raymond, K.; Komesu, N.; Upham, H.C. Toxicological and pharmacological effects of gadolinium and samarium chlorides. Br. J. Pharmacol. Chemother. 1961, 17, 526–532. [Google Scholar] [CrossRef]
- Haley, T.J.; Komesu, N.; Mavis, L.; Cawthorne, J.; Upham, H. Pharmacology and toxicology of scandium chloride. J. Pharm. Sci. 1962, 51, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Li, F.; Zhang, Z.; Feng, L.; Li, Z.; Yang, J.; Chai, Z. Distribution of ytterbium-169 in rat brain after intravenous injection. Toxicol. Lett. 2005, 155, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Baranovskaya, N.V.; Mazukhina, S.I.; Panichev, A.M.; Vakh, E.A.; Tarasenko, I.A.; Seryodkin, I.V.; Ilenok, S.S.; Ivanov, V.V.; Ageeva, E.V.; Makarevich, R.A.; et al. Features of chemical elements migration in natural waters and their deposition in the form of neocrystallisations in living organisms (physico-chemical modeling with animal testing). Bull. Tomsk. Polytech. University. Geo Assets Eng. 2024, 335, 187–201. [Google Scholar] [CrossRef]
- Feng, L.; He, X.; Xiao, H.; Li, Z.; Li, F.; Liu, N.; Chai, Z.; Zhao, Y.; Zhang, Z. Ytterbium and trace element distribution in brain and organic tissues of offspring rats after prenatal and postnatal exposure to ytterbium. Biol. Trace Element Res. 2007, 117, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Yang, J.; Liu, Q.; Yu, F.; Wu, S.; Jin, C.; Lu, X.; Zhang, L.; Du, Y.; Xi, Q.; et al. Lanthanum chloride impairs spatial learning and memory and downregulates NF-κB signalling pathway in rats. Arch. Toxicol. 2013, 87, 2105–2117. [Google Scholar] [CrossRef]
- Iliff, J.J.; Lee, H.; Yu, M.; Feng, T.; Logan, J.; Nedergaard, M.; Benveniste, H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J. Clin. Investig. 2013, 123, 1299–1309. [Google Scholar] [CrossRef]
- Evans, C.H. Biochemistry of the Lanthanides; Springer: Boston, MA, USA, 1990. [Google Scholar]
- Przywara, D.A.; Bhave, S.V.; Bhave, A.; Chowdhury, P.S.; Wakade, T.D.; Wakade, A.R. Activation of K+ channels by lanthanum contributes to the block of transmitter release in chick and rat sympathetic neurons. J. Membr. Biol. 1992, 125, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, M.P.; Goldman, D.E. The action of certain polyvalent cations on the voltage-clamped lobster axon. J. Gen. Physiol. 1968, 51, 279–291. [Google Scholar] [CrossRef]
- Van Breemen, C.; De Weer, P. Lanthanum inhibition of 45Ca efflux from the squid giant axon. Nature 1970, 226, 760–761. [Google Scholar] [CrossRef]
- Basu, A.; Chakrabarty, K.; Chatterjee, G.C. Neurotoxicity of lanthanum chloride in newborn chicks. Toxicol. Lett. 1982, 14, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Chakrabarty, K.; Haldar, S.; Addya, S.; Chatterjee, G.C. The effects of lanthanum chloride administration in newborn chicks on glutamate uptake and release by brain synaptosomes. Toxicol. Lett. 1984, 20, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Miledi, R. Lanthanum Ions abolish the “calcium response” of nerve terminals. Nature 1971, 229, 410–411. [Google Scholar] [CrossRef] [PubMed]
- Osborne, R.H.; Bradford, H.F. the influence of sodium, potassium and lanthanum on amino acid release from spinal–medullary synaptosomes. J. Neurochem. 1975, 25, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Metral, S.; Bonneton, C.; Hort-Legrand, C.; Reynes, J. Dual action of erbium on transmitter release at the frog neuromuscular synapse. Nature 1978, 271, 773–775. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Q.; Zhang, L.; Wu, S.; Qi, M.; Lu, S.; Xi, Q.; Cai, Y. Lanthanum chloride impairs memory, decreases pCaMK IV, pMAPK and pCREB expression of hippocampus in rats. Toxicol. Lett. 2009, 190, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Heuser, J.E. The Structural Basis of Long-Term Potentiation in Hippocampal Synapses, Revealed by Electron Microscopy Imaging of Lanthanum-Induced Synaptic Vesicle Recycling. Front. Cell. Neurosci. 2022, 16, 920360. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, Z.; Hu, R.; Chen, J.; Hong, M.; Zhou, M.; Gong, X.; Wang, L.; Hong, F. Oxidative injury in the brain of mice caused by lanthanid. Biol. Trace Element Res. 2010, 142, 174–189. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, N.; Cheng, J.; Hu, R.; Gao, G.; Cui, Y.; Gong, X.; Wang, L.; Hong, F. Signal pathway of hippocampal apoptosis and cognitive impairment of mice caused by cerium chloride. Environ. Toxicol. 2011, 27, 707–718. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, Z.; Li, F.; Feng, L.; Li, Z.; Yang, J.; Chai, Z. Accumulation and distribution of samarium-153 in rat brain after intraperitoneal injection. Biol. Trace Element Res. 2005, 104, 033–040. [Google Scholar] [CrossRef]
- Kanda, T.; Ishii, K.; Kawaguchi, H.; Kitajima, K.; Takenaka, D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: Relationship with Increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014, 270, 834–841. [Google Scholar] [CrossRef]
- Kanda, T.; Nakai, Y.; Aoki, S.; Oba, H.; Toyoda, K.; Kitajima, K.; Furui, S. Contribution of metals to brain MR signal intensity: Review articles. Jpn. J. Radiol. 2016, 34, 258–266. [Google Scholar] [CrossRef]
- Kanda, T.; Nakai, Y.; Hagiwara, A.; Oba, H.; Toyoda, K.; Furui, S. Distribution and chemical forms of gadolinium in the brain: A review. Br. J. Radiol. 2017, 90, 20170115. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.J.; McDonald, J.S.; Kallmes, D.F.; Jentoft, M.E.; Paolini, M.A.; Murray, D.L.; Williamson, E.E.; Eckel, L.J. Gadolinium Deposition in Human Brain Tissues after Contrast-enhanced MR Imaging in Adult Patients without Intracranial Abnormalities. Radiology 2017, 285, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Assene, A.N.; Dieme, D.; Jomaa, M.; Côté, J.; Bouchard, M. Toxicokinetic study of scandium oxide in rats. Toxicol. Lett. 2024, 392, 56–63. [Google Scholar] [CrossRef]
- Yokel, R.A.; Au, T.C.; MacPhail, R.; Hardas, S.S.; Butterfield, D.A.; Sultana, R.; Goodman, M.; Tseng, M.T.; Dan, M.; Haghnazar, H.; et al. Distribution, elimination, and biopersistence to 90 Days of a systemically introduced 30 nm ceria-engineered nanomaterial in rats. Toxicol. Sci. 2012, 127, 256–268. [Google Scholar] [CrossRef]
- Jin, C.; Gao, L.; Li, Y.; Wu, S.; Lu, X.; Yang, J.; Cai, Y. Lanthanum damages learning and memory and suppresses astrocyte–neuron lactate shuttle in rat hippocampus. Exp. Brain Res. 2017, 235, 3817–3832. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Mao, H.; Liu, J.; Sun, W.; Wu, S.; Lu, X.; Jin, C.; Yang, J. Lanthanum Chloride Induces Axon Abnormality through LKB1-MARK2 and LKB1-STK25-GM130 Signaling Pathways. Cell. Mol. Neurobiol. 2022, 43, 1181–1196. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, P.; Liu, J.; Xue, Y. Effect of long-term intake of Y3+ in drinking water on gene expression in brains of rats. J. Rare Earths 2006, 24, 369–373. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, H.-J.; Qi, T.; Chen, D.-L.; Long, H.-M.; Liu, S.-H. Rare earths exposure and male infertility: The injury mechanism study of rare earths on male mice and human sperm. Environ. Sci. Pollut. Res. 2014, 22, 2076–2086. [Google Scholar] [CrossRef]
- Fahn, S.; Jankovic, J.; Hallett, M. Principles and Practice of Movement Disorders, 2nd ed.; Elsevier: Philadelphia, PA, USA, 2011. [Google Scholar]
- Delorme, C.; Giron, C.; Bendetowicz, D.; Méneret, A.; Mariani, L.-L.; Roze, E. Current challenges in the pathophysiology, diagnosis, and treatment of paroxysmal movement disorders. Expert Rev. Neurother. 2020, 21, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-J.; Xu, Z.-Y.; Wu, Y.-C.; Tan, E.-K. Paroxysmal movement disorders: Recent advances and proposal of a classification system. Park. Relat. Disord. 2019, 59, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Garone, G.; Capuano, A.; Travaglini, L.; Graziola, F.; Stregapede, F.; Zanni, G.; Vigevano, F.; Bertini, E.; Nicita, F. Clinical and Genetic Overview of Paroxysmal Movement Disorders and Episodic Ataxias. Int. J. Mol. Sci. 2020, 21, 3603. [Google Scholar] [CrossRef] [PubMed]
- Mark, M.D.; Maejima, T.; Kuckelsberg, D.; Yoo, J.W.; Hyde, R.A.; Shah, V.; Gutierrez, D.; Moreno, R.L.; Kruse, W.; Noebels, J.L.; et al. Delayed postnatal Loss of P/Q-type calcium channels recapitulates the absence epilepsy, dyskinesia, and ataxia phenotypes of genomic mutations. J. Neurosci. 2011, 31, 4311–4326. [Google Scholar] [CrossRef]
- Tan, G.-H.; Liu, Y.-Y.; Wang, L.; Li, K.; Zhang, Z.-Q.; Li, H.-F.; Yang, Z.-F.; Li, Y.; Li, D.; Wu, M.-Y.; et al. PRRT2 deficiency induces paroxysmal kinesigenic dyskinesia by regulating synaptic transmission in cerebellum. Cell Res. 2017, 28, 90–110. [Google Scholar] [CrossRef]
- Sultan, F.; Hamodeh, S.; Baizer, J. The human dentate nucleus: A complex shape untangled. Neuroscience 2010, 167, 965–968. [Google Scholar] [CrossRef]
- Saab, C.Y.; Willis, W.D. The cerebellum: Organization, functions and its role in nociception. Brain Res. Rev. 2003, 42, 85–95. [Google Scholar] [CrossRef]
- Dum, R.P.; Strick, P.L. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J. Neurophysiol. 2003, 89, 634–639. [Google Scholar] [CrossRef]
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A report: The definition and classification of cerebral palsy. Dev. Med. Child. Neurol. Suppl. 2007, 109, 8–14. [Google Scholar]
- Graham, H.K.; Rosenbaum, P.; Paneth, N.; Dan, B.; Lin, J.-P.; Damiano, D.L.; Becher, J.G.; Gaebler-Spira, D.; Colver, A.; Reddihough, D.S.; et al. Cerebral palsy. Nat. Rev. Dis. Prim. 2016, 2, 15082. [Google Scholar] [CrossRef]
- Colver, A.; Fairhurst, C.; Pharoah, P.O.D. Cerebral palsy. Lancet 2014, 383, 1240–1249. [Google Scholar] [CrossRef]
- Pharoah, P.O.D. Prevalence and pathogenesis of congenital anomalies in cerebral palsy. Arch. Dis. Child.-Fetal Neonatal Ed. 2007, 92, F489–F493. [Google Scholar] [CrossRef]
- Patel, D.R.; Neelakantan, M.; Pandher, K.; Merrick, J. Cerebral palsy in children: A clinical overview. Transl. Pediatr. 2020, 9, S125–S135. [Google Scholar] [CrossRef]
- Copp, A.J.; Stanier, P.; DE Greene, N. Neural tube defects: Recent advances, unsolved questions, and controversies. Lancet Neurol. 2013, 12, 799–810. [Google Scholar] [CrossRef]
- Goyal, R.; Spencer, K.A.; Borodinsky, L.N. From Neural Tube Formation Through the Differentiation of Spinal Cord Neurons: Ion Channels in Action During Neural Development. Front. Mol. Neurosci. 2020, 13, 62. [Google Scholar] [CrossRef]
- McCobb, D.; Best, P.; Beam, K. The differentiation of excitability in embryonic chick limb motoneurons. J. Neurosci. 1990, 10, 2974–2984. [Google Scholar] [CrossRef]
- Zhang, M.; Ladas, T.P.; Qiu, C.; Shivacharan, R.S.; Gonzalez-Reyes, L.E.; Durand, D.M. Propagation of Epileptiform Activity Can Be Independent of Synaptic Transmission, Gap Junctions, or Diffusion and Is Consistent with Electrical Field Transmission. J. Neurosci. 2014, 34, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Greene, N.D.; Copp, A.J. Neural tube defects. Annu. Rev. Neurosci. 2014, 37, 221–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ren, A.; Yang, Z.; Pei, L.; Hao, L.; Xie, Q.; Jiang, Y. Correlation studies of trace elements in mother’s hair, venous blood and cord blood. Chin. J. Reprod. Health 2005, 4, 209–212. [Google Scholar]
- Das, T.; Sharma, A.; Talukder, G. Effects of lanthanum in cellular systems. Biol. Trace Element Res. 1988, 18, 201–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, J.; Xia, A.; Cheng, M.; Yang, Q.; Du, C.; Wei, H.; Huang, X.; Zhou, Q. Toxic effects of environmental rare earth elements on delayed outward potassium channels and their mechanisms from a microscopic perspective. Chemosphere 2017, 181, 690–698. [Google Scholar] [CrossRef]
- Pharoah, P.O.D. Causal hypothesis for some congenital anomalies. Twin Res. Hum. Genet. 2005, 8, 543–550. [Google Scholar] [CrossRef]
- Pharoah, P.; Dundar, Y. Monozygotic twinning, cerebral palsy and congenital anomalies. Hum. Reprod. Updat. 2009, 15, 639–648. [Google Scholar] [CrossRef]
- Rankin, J.; Cans, C.; Garne, E.; Colver, A.; Dolk, H.; Uldall, P.; Amar, E.; Krageloh-Mann, I. Congenital anomalies in children with cerebral palsy: A population-based record linkage study. Dev. Med. Child Neurol. 2010, 52, 345–351. [Google Scholar] [CrossRef]
- Bahtiyar, M.O.; Dulay, A.T.; Weeks, B.P.; Friedman, A.H.; Copel, J.A. Prevalence of congenital heart defects in monochorionic/diamniotic twin gestations. J. Ultrasound Med. 2007, 26, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Croen, L.A.; Grether, J.K.; Curry, C.J.; Nelson, K.B. Congenital abnormalities among children with cerebral palsy: More evidence for prenatal antecedents. J. Pediatr. 2001, 138, 804–810. [Google Scholar] [CrossRef]
- Garne, E.; Dolk, H.; Krägeloh-Mann, I.; Holst Ravn, S.; Cans, C. SCPE Collaborative Group. Cerebral palsy and congenital malformations. Eur. J. Paediatr. Neurol. 2008, 12, 82–88. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Wang, Y. Study on distributions and accumulations of rare earth element cerium (141Ce) in mice. J. Nanjing Agric. Univ. 2000, 23, 101–103. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTALNJNY200003025.htm (accessed on 14 June 2012). (In Chinese).
- Liu, Y.; Wu, M.; Zhang, L.; Bi, J.; Song, L.; Wang, L.; Liu, B.; Zhou, A.; Cao, Z.; Xiong, C.; et al. Prenatal exposure of rare earth elements cerium and ytterbium and neonatal thyroid stimulating hormone levels: Findings from a birth cohort study. Environ. Int. 2019, 133, 105222. [Google Scholar] [CrossRef]
- Liu, L.; Wang, L.; Ni, W.; Pan, Y.; Chen, Y.; Xie, Q.; Liu, Y.; Ren, A. Rare earth elements in umbilical cord and risk for orofacial clefts. Ecotoxicol. Environ. Saf. 2020, 207, 111284. [Google Scholar] [CrossRef]
- Wang, J.; Wu, T.; Ma, L.; Guo, Y.; Huang, Y.; Zheng, L. Action of Akt Pathway on La-Induced Hippocampal Neuron Apoptosis of Rats in the Growth Stage. Neurotox. Res. 2020, 38, 434–446. [Google Scholar] [CrossRef]
- Sun, W.; Yang, J.; Hong, Y.; Yuan, H.; Wang, J.; Zhang, Y.; Lu, X.; Jin, C.; Wu, S.; Cai, Y. Lanthanum Chloride Impairs Learning and Memory and Induces Dendritic Spine Abnormality by Down-Regulating Rac1/PAK Signaling Pathway in Hippocampus of Offspring Rats. Cell. Mol. Neurobiol. 2019, 40, 459–475. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef] [PubMed]
- Malenka, R. The long-term potential of LTP. Nat. Rev. Neurosci. 2003, 4, 923–926. [Google Scholar] [CrossRef]
- Yuste, R.; Bonhoeffer, T. Genesis of dendritic spines: Insights from ultrastructural and imaging studies. Nat. Rev. Neurosci. 2004, 5, 24–34. [Google Scholar] [CrossRef]
- Tada, T.; Sheng, M. Molecular mechanisms of dendritic spine morphogenesis. Curr. Opin. Neurobiol. 2005, 16, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Allers, K.; Essue, B.M.; Hackett, M.L.; Muhunthan, J.; Anderson, C.S.; Pickles, K.; Scheibe, F.; Jan, S. The economic impact of epilepsy: A systematic review. BMC Neurol. 2015, 15, 245. [Google Scholar] [CrossRef]
- Pitkänen, A.; Engel, J., Jr. Past and present definitions of epileptogenesis and its biomarkers. Neurotherapeutics 2014, 11, 231–241. [Google Scholar] [CrossRef]
- Engel, T.; Brennan, G.P.; Soreq, H. Editorial: The molecular mechanisms of epilepsy and potential therapeutics. Front. Mol. Neurosci. 2022, 15, 1064121. [Google Scholar] [CrossRef]
- Jefferys, J.G. Advances in understanding basic mechanisms of epilepsy and seizures. Seizure 2010, 19, 638–646. [Google Scholar] [CrossRef]
- Fellin, T.; Pascual, O.; Gobbo, S.; Pozzan, T.; Haydon, P.G.; Carmignoto, G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 2004, 43, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, P.B.; Knappenberger, J.; Segal, M.; Bennett, M.V.L.; Charles, A.C.; Kater, S.B. ATP released from astrocytes mediates glial calcium waves. J. Neurosci. 1999, 19, 520–528. [Google Scholar] [CrossRef]
- Angulo, M.C.; Kozlov, A.S.; Charpak, S.; Audinat, E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J. Neurosci. 2004, 24, 6920–6927. [Google Scholar] [CrossRef]
- Halassa, M.M.; Fellin, T.; Haydon, P.G. The tripartite synapse: Roles for gliotransmission in health and disease. Trends Mol. Med. 2007, 13, 54–63. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Q.; Qi, M.; Lu, S.; Wu, S.; Xi, Q.; Cai, Y. Lanthanum chloride promotes mitochondrial apoptotic pathway in primary cultured rat astrocytes. Environ. Toxicol. 2011, 28, 489–497. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Parpura, V. Astrogliopathology in neurological, neurodevelopmental and psychiatric disorders. Neurobiol. Dis. 2016, 85, 254–261. [Google Scholar] [CrossRef]

| n = 53 | M | SD | Min | Max | Me | P25 | P75 | C. V. |
|---|---|---|---|---|---|---|---|---|
| Sc | 0.0536 | 0.1238 | 0.0010 | 0.5544 | 0.0010 | 0.0010 | 0.0010 | 230.9 |
| Y | 0.0204 | 0.0152 | 0.0090 | 0.0782 | 0.0157 | 0.0130 | 0.0193 | 74.6 |
| La | 0.0336 | 0.0222 | 0.0124 | 0.1288 | 0.0269 | 0.0198 | 0.0362 | 66.2 |
| Ce | 0.0351 | 0.0219 | 0.0053 | 0.1073 | 0.0297 | 0.0200 | 0.0412 | 62.3 |
| Pr | 0.0047 | 0.0019 | 0.0020 | 0.0131 | 0.0044 | 0.0038 | 0.0051 | 39.3 |
| Nd | 0.0161 | 0.0048 | 0.0089 | 0.0383 | 0.0154 | 0.0132 | 0.0174 | 29.7 |
| Sm | 0.0124 | 0.0069 | 0.0031 | 0.0548 | 0.0116 | 0.0092 | 0.0141 | 55.7 |
| Eu | 0.0049 | 0.0030 | 0.0017 | 0.0233 | 0.0045 | 0.0036 | 0.0054 | 61.9 |
| Gd | 0.0214 | 0.0024 | 0.0013 | 0.0630 | 0.0163 | 0.0101 | 0.0277 | 76.7 |
| Tb | 0.0032 | 0.0009 | 0.0015 | 0.0046 | 0.0032 | 0.0025 | 0.0040 | 27.3 |
| Dy | 0.0129 | 0.0199 | 0.0055 | 0.1531 | 0.0095 | 0.0080 | 0.0118 | 154.2 |
| Ho | 0.0031 | 0.0010 | 0.0015 | 0.0058 | 0.0033 | 0.0023 | 0.0038 | 30.3 |
| Er | 0.0077 | 0.0017 | 0.0032 | 0.0118 | 0.0076 | 0.0067 | 0.0091 | 22.4 |
| Tm | 0.0029 | 0.0009 | 0.0012 | 0.0050 | 0.0027 | 0.0023 | 0.0035 | 31.3 |
| Yb | 0.0121 | 0.0223 | 0.0037 | 0.1697 | 0.0091 | 0.0072 | 0.0103 | 184.0 |
| Lu | 0.0028 | 0.0012 | 0.0015 | 0.0089 | 0.0027 | 0.0022 | 0.0033 | 43.5 |
| Element | 1 | 2 | 3 | 4 | |||
|---|---|---|---|---|---|---|---|
| M ± SD | Me (Q25–Q75) | M ± SD | Me (Q25–Q75) | M ± SE | M (SD) ME | Range | |
| Na | 642 ± 599 | 431 (275–783) | 728 ± 1198 | 389(143–940) | 223 ± 17 | 147 (149) 94 | 17–670 |
| Ca | 287 ± 175 | 220 (186–349) | 960 ± 2844 | 228(100–673) | 1403 ± 90 | 750 (660) 590 | 113–2890 |
| Sc | 0.054 ±0.124 | 0.001 (0.001–0.001) | 0.02 ± 0.01 | 0.02(0.01–0.02) | 0.007 ± 0.0004 | 0.0014 (0.001) 0.0011 | 0.0004–0.0045 |
| Cr | 4.21 ±13.64 | 4.43 (1.32–6.5) | 5.23 ± 5.69 | 4.68(0.46–6.11) | 0.5 ± 0.07 | 0.167 (0.118) 0.131 | 0.046–0.527 |
| Fe | 24.5 ±18.6 | 23.0 (13.6–29.8) | 853 ± 872 | 793(300–1018) | 45 ± 3.7 | 9.6(4.4) 8.4 | 4.9–23 |
| Co | 0.030 ±0.043 | 0.008 (0.002–0.047) | 1.05 ± 1.42 | 0.66(0.53–0.9) | 0.07 ± 0.01 | 0.013 (0.011) 0.01 | 0.002–0.063 |
| Zn | 120 ±89 | 106 (77–130) | 210 ± 348 | 152(117–194) | 207 ± 8 | 142 (29) 144 | 68–198 |
| As | 0.61 ±0.70 | 0.45 (0.001–0.96) | 0.42 ± 1.02 | 0.24(0.16–0.5) | <0.8 | 0.085 (0.054) 0.067 | 0.034–0.319 |
| Se | 0.71 ± 0.28 | 0.68 (0.50–0.87) | - | - | 0.8 ± 0.03 | 0.83 (0.28) 0.79 | 0.48–1.84 |
| Br | - | - | 8.82 ± 17.23 | 3.96(2.07–7.91) | 6.5 ± 0.6 | 37 (33) 26 | 5.6–221 |
| Rb | 0.72 ± 0.79 | 0.48 (0.27–1.05) | 0.79 ± 1.24 | 0.3(0.2–0.81) | <3 | 0.093 (0.085) 0.06 | 0.012–0.482 |
| Sr | 1.38 ±1.65 | 0.59 (0.32–2.06) | 4.53 ± 1.23 | 5(2.5–5) | <15 | 1.2 (1) 0.97 | 0.14–5.54 |
| Ag | 0.28 ±0.16 | 0.23 (0.19–0.35) | 2.34 ± 17.67 | 0.11(0.05–0.26) | 0.3 ± 0.03 | 0.231 (0.298) 0.132 | 0.025–1.96 |
| Sb | 0.06 ±0.06 | 0.044 (0.028–0.07) | 0.11 ± 0.15 | 0.06(0.03–0.12) | 0.07 ± 0.01 | 0.022 (0.017) 0.017 | 0.007–0.122 |
| Cs | 0.014 ±0.022 | 0.01 (0.005–0.014) | 0.03 ± 0.14 | 0.005(0.001–0.02) | <0.05 | 0.00067 (0.00046) 0.00051 | 0.00017–0.0019 |
| Ba | 0.58 ±0.88 | 0.41 (0.21–0.60) | 4.07 ± 12.24 | 2(1.97–2.43) | <10 | 0.64 (0.49) 0.46 | 0.16–1.92 |
| Y | 0.020 ±0.015 | 0.016 (0.013–0.019) | - | - | - | - | - |
| La | 0.034 ±0.022 | 0.027(0.02–0.036) | 0.04 ± 0.09 | 0.02(0.01–0.02) | 0.05 ± 0.006 | 0.035 (0.046) 0.018 | 0.0046–0.106 |
| Ce | 0.035 ±0.022 | 0.03 (0.02–0.041) | 0.39 ± 1.79 | 0.1(0.05–0.1) | <0.08 | 0.039 (0.05) 0.019 | 0.007–0.164 |
| Pr | 0.005 ±0.002 | 0.004 (0.004–0.005) | - | - | - | ||
| Nd | 0.016 ±0.005 | 0.015 (0.013–0.017) | 0.25 ± 0.46 | 0.09(0.09–0.22) | - | ||
| Sm | 0.0124 ±0.007 | 0.012 (0.009–0.014) | 0.006 ± 0.02 | 0.003(0–0.003) | 0.02 ± 0.003 | ||
| Eu | 0.005 ±0.003 | 0.004 (0.004–0.005) | 0.02 ± 0.07 | 0.004(0.001–0.01) | <0.03 | ||
| Gd | 0.0214 ±0.016 | 0.016 (0.010–0.028) | - | - | - | ||
| Tb | 0.003 ±0.001 | 0.003 (0.002–0.004) | 0.01 ± 0.02 | 0.005(0.005–0.02) | 0.01 ± 0.0003 | ||
| Dy | 0.013 ±0.02 | 0.01 (0.008–0.012) | - | - | - | ||
| Ho | 0.003 ±0.001 | 0.003 (0.002–0.004) | - | - | - | ||
| Er | 0.008 ±0.002 | 0.008 (0.007–0.009) | - | - | - | ||
| Tm | 0.003 ± 0.001 | 0.003 (0.002–0.004) | - | - | - | ||
| Yb | 0.012 ±0.022 | 0.009 (0.007–0.010) | 0.007 ± 0.009 | 0.005(0–0.009) | 0.03 ± 0.0005 | ||
| Lu | 0.003 ±0.001 | 0.003 (0.002–0.003) | 0.003 ± 0.01 | 0.001(0–0.003) | 0.002 ± 0.0001 | ||
| Hf | 0.004 ±0.005 | 0.004 (0.001–0.007) | 0.05 ± 0.12 | 0.037(0.001–0.05) | 0.02 ± 0.002 | 0.005 (0.008) 0.0017 | 0.0004–0.037 |
| Ta | 0.006 ±0.015 | 0.006 (0.001–0.001) | 0.02 ± 0.04 | 0.008(0–0.008 | <0.03 | 0.004 (0.003) 0.0031 | <0.002–0.020 |
| Au | 0.012 ±0.021 | 0.012 (0.001–0.017) | 0.03 ± 0.14 | 0.001(0–0.01) | 0.11 ± 0.03 | 0.03 (0.028) 0.017 | 0.003–0.200 |
| Hg | 0.42 ± 0.197 | 0.42 (0.31–0.5) | - | - | 0.4 ± 0.04 | 0.261 (0.145) 0.249 | 0.053–0.927 |
| Th | <0.001 <0.001 | <0.001 | 0.23 ± 0.29 | 0.14(0.05–0.27) | 0.02 ± 0.001 | 0.0013 (0.001) 0.001 | 0.0003–0.0044 |
| U | 0.005 ±0.003 | 0.005 (0.003–0.006) | 0.01 ± 0.01 | 0.01(0.01–0.016) | 0.33 ± 0.07 | 0.057 (0.065) 0.036 | 0.006–0.436 |
| L—G.M.(R) | L—M ± SE | M1—G.M.(R) | M2—G.M.(R) | C1—G.M.(R) | C2—G.M.(R) | |
|---|---|---|---|---|---|---|
| La * | 0.03 (0.01–0.13) | 0.033 ± 0.003 | 0.89 (0.17–6.93) | 0.45 (0.14–0.37) | 0.12 (0.05–0.30) | 0.11 (0.04–0.40) |
| Ce * | 0.03 (0.01–0.11) | 0.035 ± 0.003 | 0.53 (0.20–1.37) | 0.34 (0.16–0.82) | 0.22 (0.09–0.57) | 0.16 (0.07–0.44) |
| Pr * | 0.004 (0.00–0.01) | 0.005 ± 0.000 | 0.19 (0.04–1.55) | 0.08 (0.03–0.26) | 0.03 (0.01–0.09) | 0.02 (0.01–0.09) |
| Nd * | 0.016 (0.01–0.04) | 0.016 ± 0.001 | 0.67 (0.13–5.27) | 0.28 (0.09–0.95) | 0.12 (0.04–0.32) | 0.09 (0.04–0.32) |
| Sm * | 0.01 (0.00–0.05) | 0.012 ± 0.001 | 0.11 (0.02–0.91) | 0.04 (0.03–0.12) | 0.03 (0.01–0.06) | 0.02 (0.01–0.07) |
| Eu | 4.4 (1.70–23.33) | 4.9 ± 0.04 | 12.1 (2.9–95.0) | 6.0 (2.6–21.2) | 3.7 (1.8–8.7) | 2.4 (1.9–6.6) |
| Gd | 15.1 (1.29–63.22) | 21.4 ± 0.2 | 90.5 (19.4–645.6) | 30.6 (12.2–119.0) | 23.7 (8.3–59.3) | 29.5 (9.8–64.6) |
| Tb | 3.0 (1.51–4.65) | 3.17 ± 0.012 | 11.1(2.6–73.8) | 3.3 (1.4–13.2) | 3.4 (1.3–8.3) | 3.1 (1.6–9.7) |
| Dy | 10.3 (5.51–153.10) | 12.9 ± 0.27 | 53.5 (13.6–338.6) | 15.1(6.4–63.0) | 18.2 (7.5–44.1) | 17.8 (9.8–54.5) |
| Ho | 3.0 (1.53–5.83) | 3.14 ± 0.013 | 8.5 (2.2–55.63) | 2.3 (1.0–10.7) | 3.1 (1.2–7.3) | 3.2 (1.7–10.4) |
| Er | 7.5 (3.23–11.79) | 7.74 ± 0.024 | 20.7 (5.7–134.3) | 15.9 (2.7–27.3) | 8.5 (3.8–20.1) | 8.8 (5.2–28.9) |
| Tm | 2.8 (1.24–4.96) | 2.9 ± 0.012 | 2.3 (0.6–16.2) | 0.6 (0.3–3.1) | 1.1 (0.4–2.5) | 1.2(0.6–4.1) |
| Yb | 9.1 (3.68–169.73) | 12.1 ± 0.307 | 13.5 (4.4–90.5) | 3.6 (1.6–18.3) | 6.6 (3.6–15.0) | 7.1 (3.9–22.3) |
| Lu | 2.7 (1.47–8.93) | 2.8 ± 0.017 | 1.7 (0.4–13.3) | 0.5 (0.2–2.6) | 0.8 (0.4–2.0) | 1.0 (0.4–3.3) |
| Y | 17.5 (0.009–0.0078) * | 20.0 ± 2.0 | 203.5 (0.06–1.27) * | 71.2 (0.03–0.30) * | 82.3 (0.03–0.19) * | 76.5 (0.04–0.29) * |
| Sc | 3.4 (1.0–554.0) | 53.6 ± 17.0 | 154 | 195.5 | 194.7 | 243.1 |
| Lovozero Village, Russia | 4 Village, China | |||||
|---|---|---|---|---|---|---|
| M | P25 | P75 | M | P25 | P75 | |
| La | 0.0269 | 0.0198 | 0.0362 | 0.3907 | 0.2399 | 1.1505 |
| Ce | 0.0297 | 0.0200 | 0.0412 | 0.5958 | 0.3811 | 1.6591 |
| Pr | 0.0044 | 0.0038 | 0.0051 | 0.0489 | 0.0337 | 0.1271 |
| Nd | 0.0154 | 0.0132 | 0.0174 | 0.1646 | 0.1098 | 0.3574 |
| Sm | 0.0116 | 0.0092 | 0.0141 | 0.0226 | 0.0150 | 0.0364 |
| Eu | 0.0045 | 0.0036 | 0.0054 | 0.0060 | 0.0042 | 0.0097 |
| Gd | 0.0163 | 0.0101 | 0.0277 | 0.0184 | 0.0118 | 0.0296 |
| Tb | 0.0032 | 0.0025 | 0.0040 | 0.0020 | 0.0009 | 0.0030 |
| Dy | 0.0095 | 0.0080 | 0.0118 | 0.0112 | 0.0066 | 0.0151 |
| Ho | 0.0033 | 0.0023 | 0.0038 | 0.0020 | 0.0012 | 0.0027 |
| Er | 0.0076 | 0.0067 | 0.0091 | 0.0051 | 0.0028 | 0.0071 |
| Tm | 0.0027 | 0.0023 | 0.0035 | 0.0009 | 0.0005 | 0.0015 |
| Yb | 0.0091 | 0.0072 | 0.0103 | 0.0049 | 0.0030 | 0.0064 |
| Lu | 0.0027 | 0.0022 | 0.0033 | 0.0006 | 0.0003 | 0.0010 |
| Y | 0.0157 | 0.0130 | 0.0193 | 0.0497 | 0.0312 | 0.0672 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belisheva, N.K.; Drogobuzhskaya, S.V. Rare Earth Element Content in Hair Samples of Children Living in the Vicinity of the Kola Peninsula Mining Site and Nervous System Diseases. Biology 2024, 13, 626. https://doi.org/10.3390/biology13080626
Belisheva NK, Drogobuzhskaya SV. Rare Earth Element Content in Hair Samples of Children Living in the Vicinity of the Kola Peninsula Mining Site and Nervous System Diseases. Biology. 2024; 13(8):626. https://doi.org/10.3390/biology13080626
Chicago/Turabian StyleBelisheva, Natalia K., and Svetlana V. Drogobuzhskaya. 2024. "Rare Earth Element Content in Hair Samples of Children Living in the Vicinity of the Kola Peninsula Mining Site and Nervous System Diseases" Biology 13, no. 8: 626. https://doi.org/10.3390/biology13080626
APA StyleBelisheva, N. K., & Drogobuzhskaya, S. V. (2024). Rare Earth Element Content in Hair Samples of Children Living in the Vicinity of the Kola Peninsula Mining Site and Nervous System Diseases. Biology, 13(8), 626. https://doi.org/10.3390/biology13080626





