Predicting the Impact of Climate Change on the Distribution of North China Leopards (Panthera pardus japonensis) in Gansu Province Using MaxEnt Modeling
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
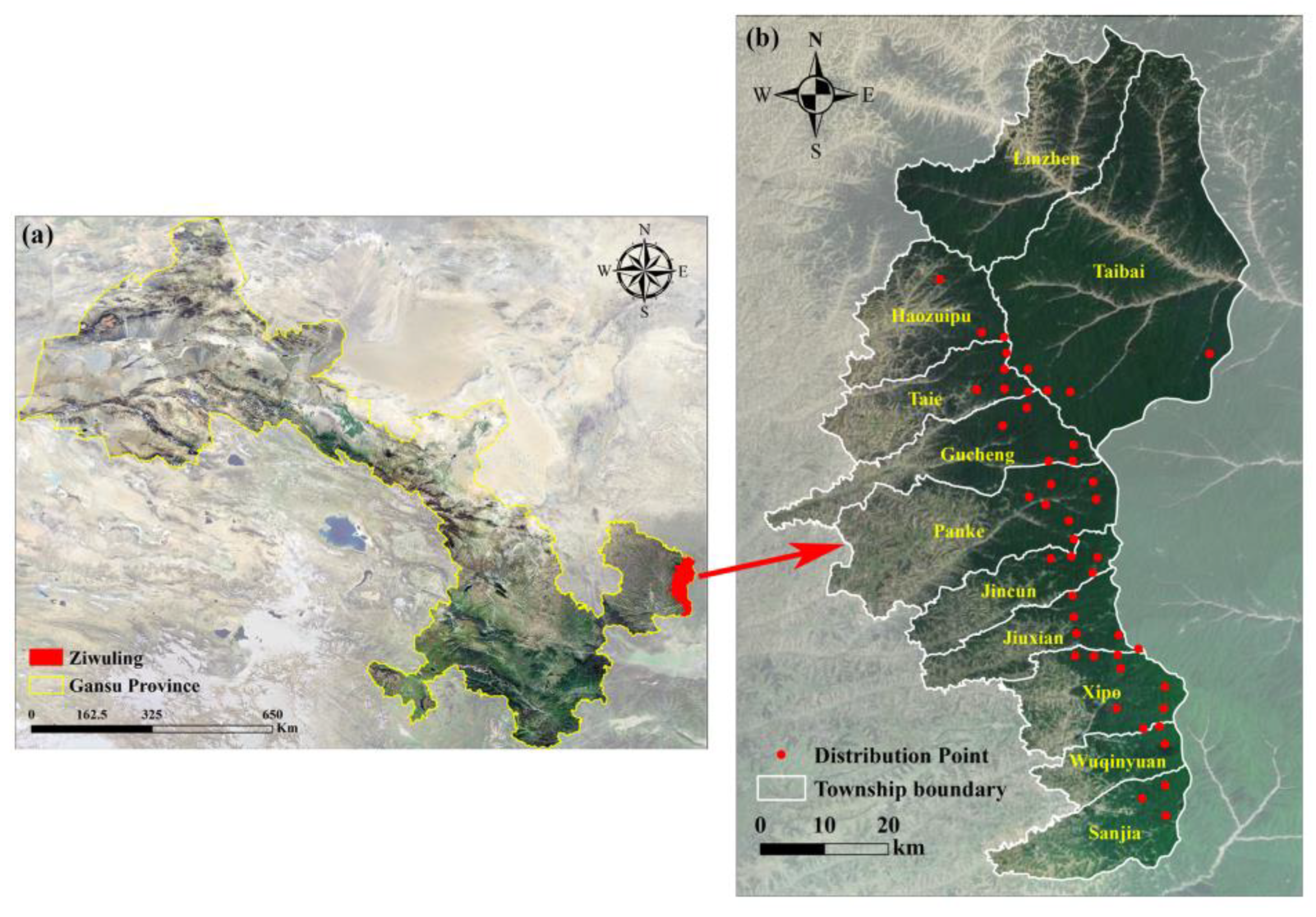
2.1. Study Area
2.2. Species Data
2.3. Environmental Data
2.4. Variable Selections
2.5. MaxEnt Model
3. Results
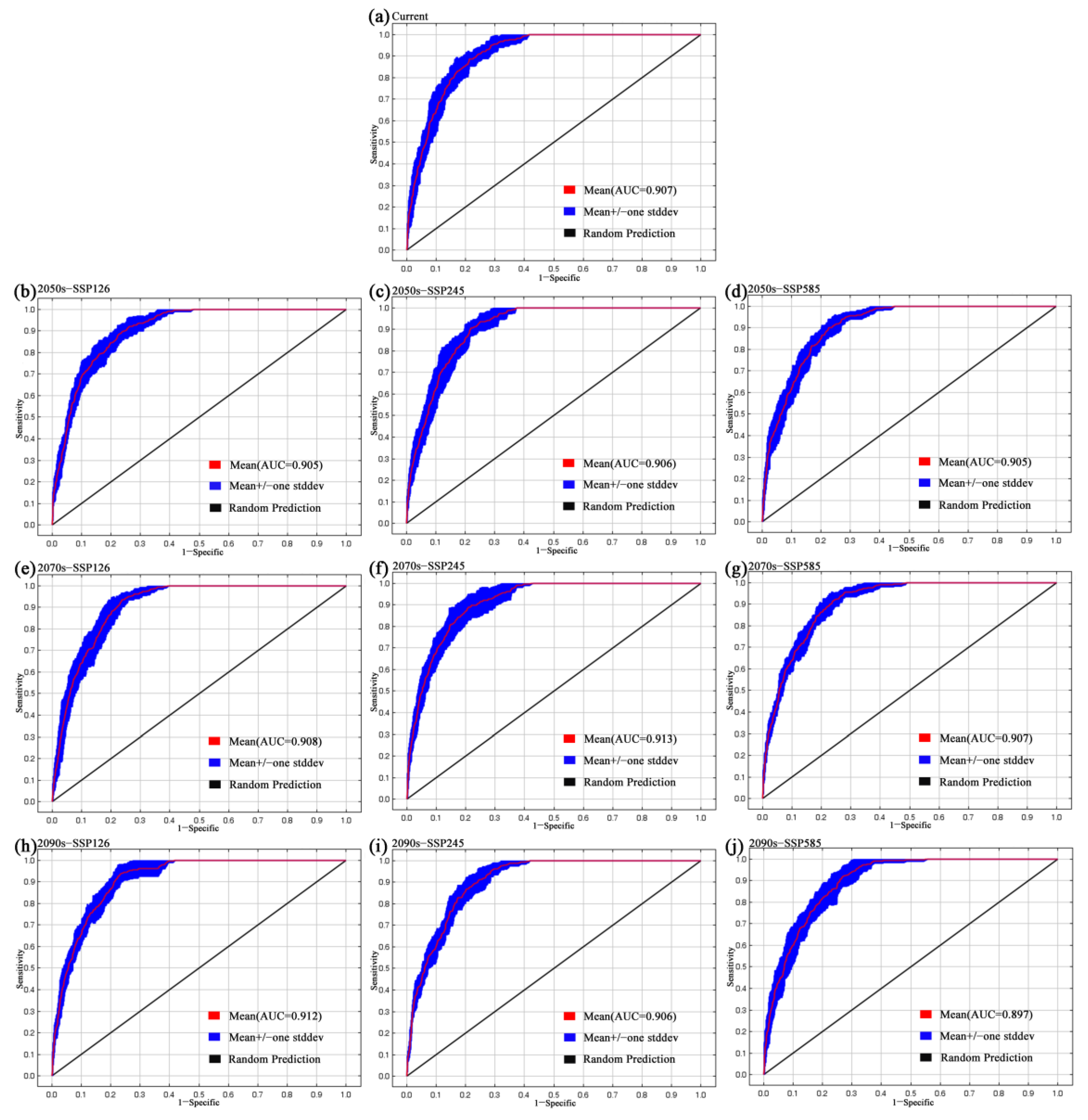
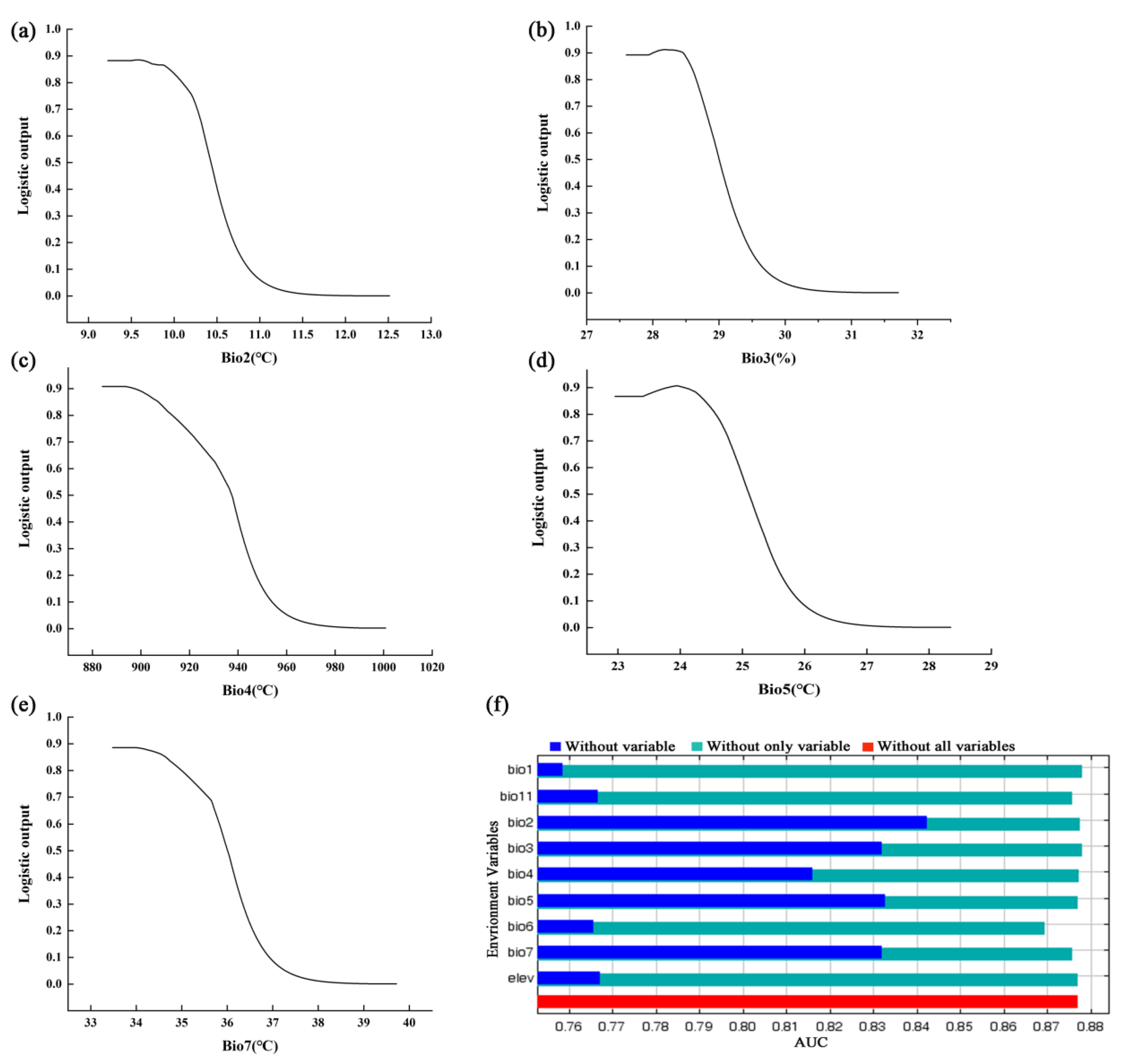
3.1. Model Performance and Variable Contributions
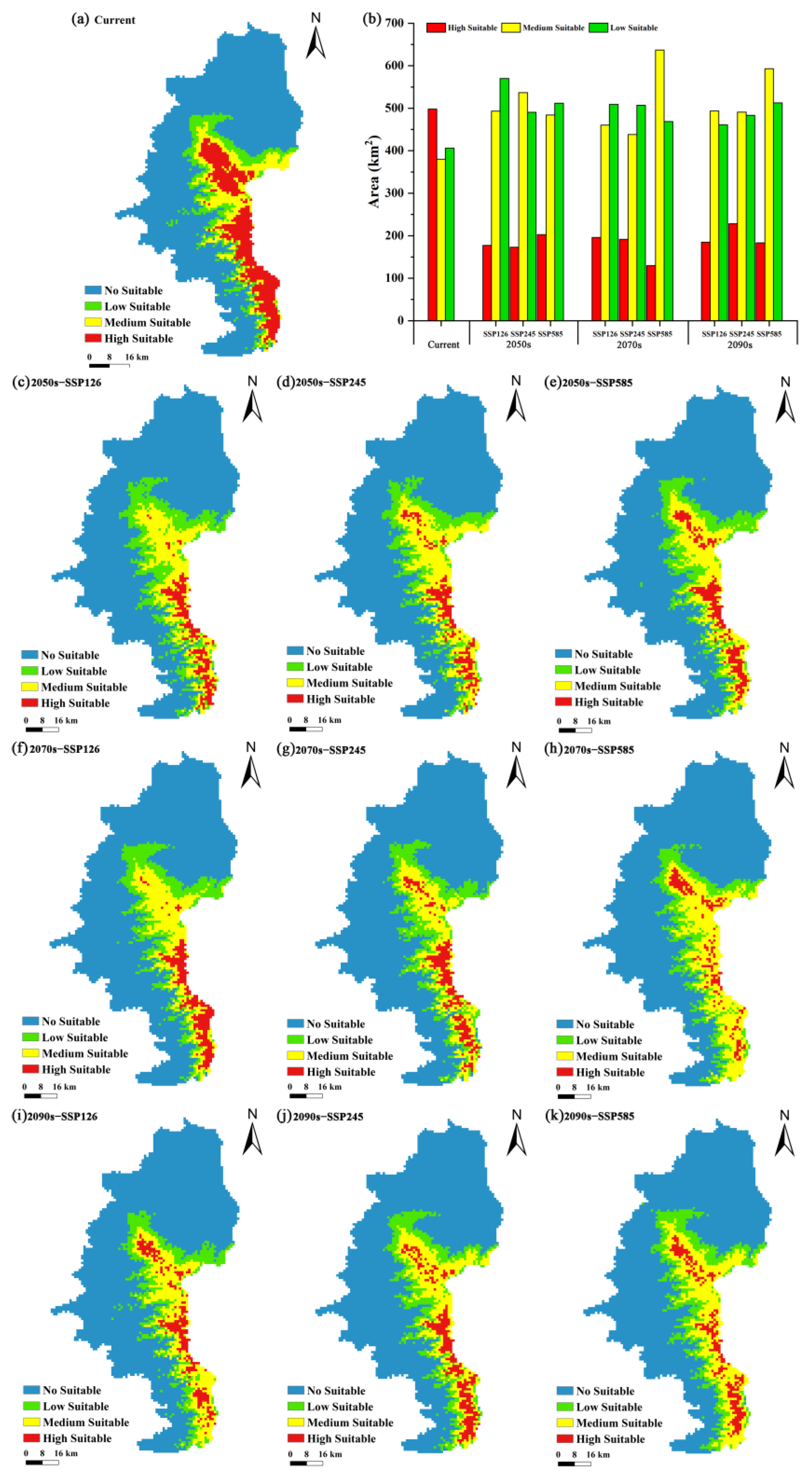
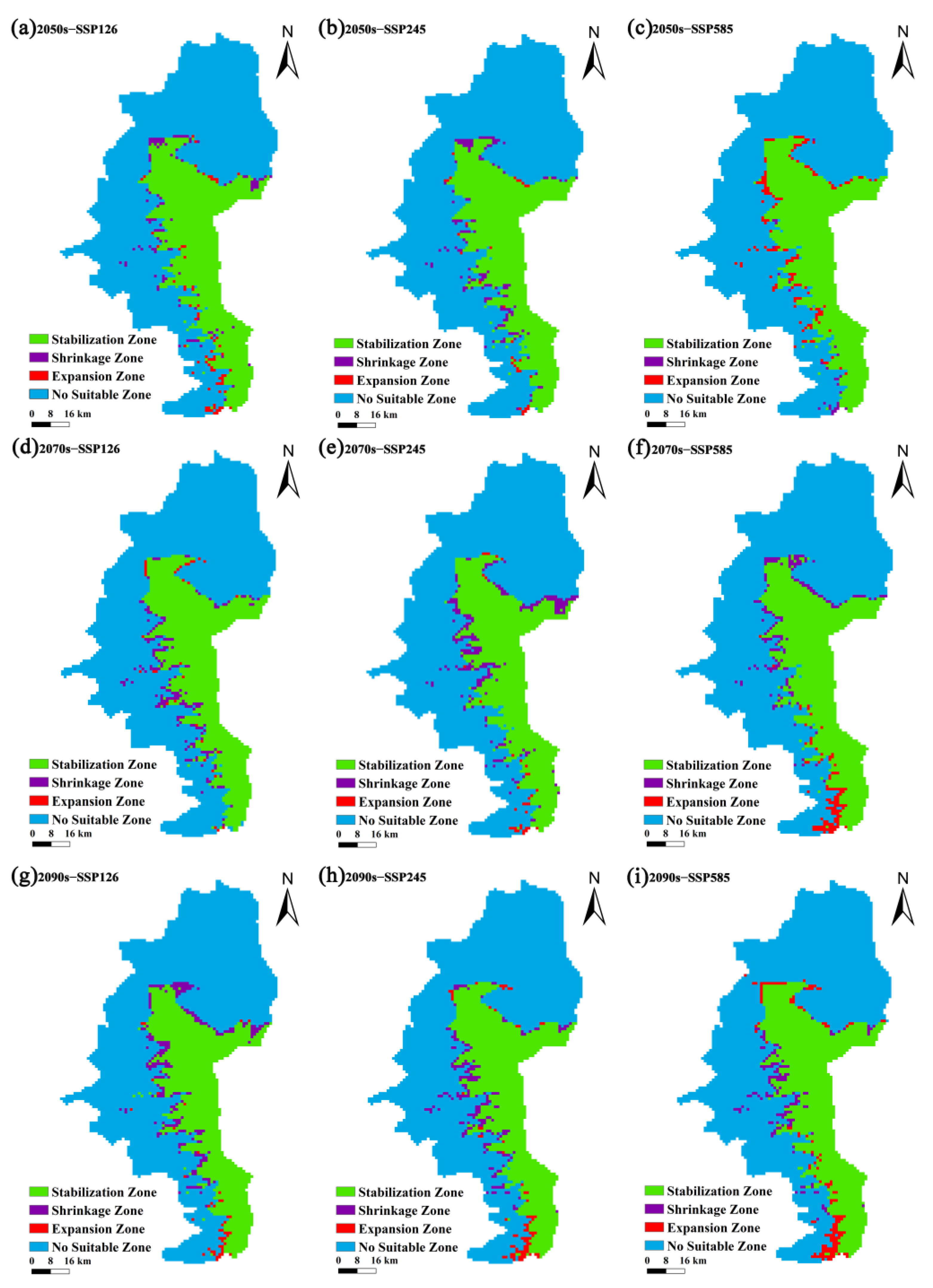
3.2. Current and Future Potential Suitable Areas for P. pardus japonensis and Their Spatiotemporal Changes
4. Discussion
4.1. Modeling Performance
4.2. Main Environmental Factors Affecting the Distribution of P. pardus japonensis
4.3. Changes in Potential Suitable Areas
4.4. Conservation Strategies for P. pardus japonensis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Booth, T.H.; Nix, H.A.; Busby, J.R.; Hutchinson, M.F. BIOCLIM: The first species distribution modelling package, its early applications and relevance to most current MAXENT studies. Divers. Distrib. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; De Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.C.; Hill, J.K.; Ohlemuller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Pauli, H.; Gottfried, M.; Dullinger, S.; Abdaladze, O.; Akhalkatsi, M.; Alonso, J.L.B.; Coldea, G.; Dick, J.; Erschbamer, B.; Calzado, R.F.; et al. Recent Plant Diversity Changes on Europe’s Mountain Summits. Science 2012, 336, 353–355. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 1–34. [Google Scholar]
- CMA. The Third National Assessment Report on Climate Change was Released; China Meteorological Administration. Available online: http://www.cma.gov.cn/2011xwzx/2011xqxxw/2011xqxyw/201412/t20141207_269047.html (accessed on 1 August 2024).
- Qin, H.; Dong, G.; Zhang, Y.; Zhang, F.; Wang, M. Patterns of species and phylogenetic diversity of Pinus tabuliformis forests in the eastern Loess Plateau, China. Forest. Eco. Manag. 2017, 394, 42–51. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Wake, D.B. Climate change implicated in amphibian and lizard declines. Proc. Natl. Acad. Sci. USA 2007, 104, 8201–8202. [Google Scholar] [CrossRef]
- Lenoir, J.; Gegout, J.C.; Marquet, P.A.; Ruffray, P.D.; Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef]
- Mckenney, D.W.; Pedlar, J.H.; Lawrence, K.; Campbell, K.; Hutchinson, M.F. Potential impacts of climate change on the distribution of north american trees. Bioscience 2007, 57, 939–948. [Google Scholar] [CrossRef]
- Kumar, P. Assessment of impact of climate change on rhododendrons in Sikkim himalayas using maxent modelling: Limitations and challenges. Biodivers. Conserv. 2012, 21, 1251–1266. [Google Scholar] [CrossRef]
- Dadashi-Jourdehi, A.; Shams-Esfandabad, B.; Ahmadi, A.; Rezaei, H.R.; Toranj-Zar, H. Predicting the potential distribution of striped hyena Hyaena hyaena in Iran. Belg. J. Zool. 2020, 150. [Google Scholar] [CrossRef]
- Jacobson, A.P.; Gerngross, P.; Lemeris, J.R.; Schoonover, R.F.; Anco, C.; Breitenmoser-Würsten, C.; Durant, S.M.; Farhadinia, M.S.; Henschel, P.; Kamler, J.F.; et al. Leopard (Panthera pardus) status, distribution, and the research efforts across its range. Peerj 2016, 4, e1974. [Google Scholar] [CrossRef]
- Hayward, M.W.; Henschel, P.; O’Brien, J.; Hofmeyr, M.; Balme, G.; Kerley, G.I. Prey preferences of the leopard (Panthera pardus). J. Zool. 2006, 270, 298–313. [Google Scholar] [CrossRef]
- Athreya, V.; Odden, M.; Linnell, J.D.C.; Krishnaswamy, J.; Karanth, U. Big cats in our backyards: Persistence of large carnivores in a human dominated landscape in India. PLoS ONE 2013, 8, e57872. [Google Scholar] [CrossRef]
- Odden, M.; Athreya, V.R.; Rattan, S.K.; Linnell, J.D. Adaptable neighbours: Movement patterns of gps-collared leopards in human dominated landscapes in India. PLoS ONE 2014, 9, e112044. [Google Scholar] [CrossRef]
- Malhi, Y.; Doughty, C.E.; Galetti, M.; Smith, F.A.; Svenning, J.C.; Terborgh, J.W. Megafauna and ecosystem function from the Pleistocene to the Anthropocene. Proc. Natl. Acad. Sci. USA 2016, 113, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Hoeks, S.; Huijbregts, M.A.J.; Busana, M.; Harfoot, M.B.J.; Jens-Christian, S.; Santini, L. Mechanistic insights into the role of large carnivores for ecosystem structure and functioning. Ecography 2020, 43, 1752–1763. [Google Scholar] [CrossRef]
- Ripple, W.J.; Estes, J.A.; Beschta, R.L.; Wilmers, C.C.; Ritchie, E.G.; Hebblewhite, M.; Berger, J.; Elmhagen, B.; Letnic, M.; Nelson, M.P.; et al. Status and ecological effects of the world’s largest carnivores. Science 2014, 343, 1241484. [Google Scholar] [CrossRef] [PubMed]
- Sergio, F.; Caro, T.; Brown, D.; Clucas, B.; Hunter, J.; Ketchum, J.; McHugh, K.; Hiraldo, F. Top predators as conservation tools: Ecological rationale, assumptions, and efficacy. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 1–19. [Google Scholar] [CrossRef]
- Ritchie, E.G.; Johnson, C.N. Predator interactions, mesopredator release and biodiversity conservation. Ecol. Lett. 2009, 12, 982–998. [Google Scholar] [CrossRef] [PubMed]
- Estes, J.A.; Terborgh, J.; Brashares, J.S.; Power, M.E.; Berger, J.; Bond, W.J.; Carpenter, S.R.; Essington, T.E.; Holt, R.D.; Jackson, J.B.C.; et al. Trophic downgrading of planet earth. Science 2011, 333, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Laguardia, A.; Kamler, J.F.; Li, S.; Zhang, C.; Zhou, Z.; Shi, K. The current distribution and status of leopards Panthera pardus in China. Oryx 2017, 51, 153–159. [Google Scholar] [CrossRef]
- Jiang, Z.G.; Wu, Y.; Liu, S.Y.; Jiang, X.L.; Zhou, K.Y.; Hu, H.J. China’s Red List of Biodiversity· Vertebrates (Vol. I): Mammals (I, II and III); Science Press: Beijing, China, 2021; (In Chinese and in English). [Google Scholar]
- Kumar, B.; Babu, S.; Kumara, H.N. Predicting the potential distribution of the lesser-known endemic madras hedgehog Paraechinus nudiventris (order: Eulipotyphla, family: Erinaceidae) in southern india. Mammalia. 2018, 83, 470–478. [Google Scholar] [CrossRef]
- Wang, S.G.; Guo, Z.H.; Gu, B.J.; Li, T.T.; Su, Y.B.; Ma, B.C.; Guan, H.X.; Huang, Q.W.; Wang, F.; Zhang, Z.J. Habitat use of the North China leopard (Panthera pardus japonensis) in the Liupanshan Mountains and its implications for conservation planning. Biodivers. Sci. 2022, 30, 22342. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Duan, Y.Z.; Wang, H.T.; Wang, C.; Du, Z.Y. Potential distribution of endangered plant Helianthemum songaricum in China under climate change. J. Plant. Resour. Environ. 2020, 29, 55–68. [Google Scholar]
- Liu, Q.; Ye, J.; Kang, Z.; Yu, G.; Yang, C.; Li, J.; Tang, T. Reeve’s Muntjac (Muntiacus reevesi) Habitat Suitability Under Climate Change Scenarios in Hupingshan National Nature Reserve, China. Animals 2025, 15, 160. [Google Scholar] [CrossRef]
- Wang, Y.G.; Zhang, B.R.; Zhao, R. Influence of species interaction on species distribution simulation and modeling methods. Chin. J. Appl. Ecol. 2022, 33, 837–843. [Google Scholar]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.J.E.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Halvorsen, R. A strict maximum likelihood explanation of MaxEnt, and some implications for distribution modelling. Sommerfeltia 2013, 36, 1–132. [Google Scholar] [CrossRef]
- Ding, G.W.; Du, L.J. The current status of wild survival of North China leopard. Farmers Consult. 2020, 663, 240. [Google Scholar]
- Wang, T.J.; Li, J.P.; Tuo, J.H. North China leopard appears in Tuoliang National Nature Reserve, Hebei Province. Land Green. 2022, 5, 46–47. [Google Scholar]
- Han, Y.L. Population quantity investigation of Panthera pardus fontanierii of Tieqiao mountain in Shanxi. Shanxi For. Sci. Technol. 2018, 47, 18–19. [Google Scholar]
- Zhang, X.F. Continuous monitoring of Panthera pardus fontanierii population quantity in Tieqiao mountain nature reserve of Shanxi Province. Shanxi For. Sci. Technol. 2021, 50, 45–46. [Google Scholar]
- Yang, H.; Xie, B.; Zhao, G.; Gong, Y.; Mou, P.; Ge, J.; Feng, L.M. Elusive cats in our backyards: Persistence of the North Chinese leopard (Panthera pardus japonensis) in a human-dominated landscape in central China. Integr. Zool. 2021, 16, 67–83. [Google Scholar] [CrossRef]
- Song, D.Z.; Wang, B.P.; Jiang, J.Y.; Wan, S.P.; Cui, S.M.; Wang, T.M.; Feng, L.M. Using camera trap to monitor a North Chinese leopard (Panthera pardus japonesis) population and their main ungulate prey. Biodivers. Sci. 2014, 22, 733–736. [Google Scholar]
- Luo, W.H.; Gao, C.Y.; Li, J.Z.; Li, C.S.; Tang, Y.M.; Wang, J.; Jiang, G.S.; Hua, Y. Spatiotemporal coexistence of North Chinese Leopard (Panthera pardus japonesis) and prey in Tie Qiao Shan provincial nature reserve. Acta Ecol. Sinica 2020, 40, 5949–5956. [Google Scholar]
- Man, H.; Huang, B.X.; Qi, J.Z.; Jiang, G.S. North Chinese leopard habitat selection in Manghe national natural reserve. Chin. J. Wildl. 2022, 43, 585–594. [Google Scholar]
- Jin, T.T.; Cao, E.J.; Gong, J. Spatiotcmporal of variations of vegetation coverage and Its relationships with climate change and human activities in Ziwuling region during 2000–2018. Bull. Soil Water Conserv. 2022, 42, 335–343. [Google Scholar]
- Zhong, Z.K.; Wang, X.; Zhang, X.Y.; Zhang, W.; Xu, Y.D.; Ren, C.J.; Han, X.H.; Yang, G.H. Edaphic factors but not plant characteristics mainly alter soil microbial properties along a restoration chronosequence of Pinus tabulaeformis stands on Mt. Ziwuling, China. Forest Ecol. Manag. 2019, 453, 117625. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection. National Biodiversity Strategy and Action Plan (2011–2030); China Environmental Science Press: Beijing, China, 2011.
- Wang, T.M.; Feng, L.M.; Yang, H.T.; Han, B.Y.; Zhao, Y.H.; Li, J.; Lü, X.Y.; Zou, L.; Li, T.; Xiao, W.H.; et al. A science-based approach to guide Amur leopard recovery in China. Biol. Conser. 2016, 210, 47–55. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, P.; Lin, F.; Yang, W.Y.; Gaisberger, H.; Christopher, K.; Zheng, Y.Q. MaxEnt modelling for predicting the potential distribution of a near threatened rosewood species (Dalbergia cultrata Graham ex Benth). Ecol. Eng. 2019, 141, 105612. [Google Scholar] [CrossRef]
- Meinshausen, M.; Nicholls, Z.R.J.; Lewis, J.; Gidden, M.J.; Wang, R.H.J. The shared socio-economic pathway (SSP) greenhouse gas concentrations and their extensions to 2500. Geosci. Model Dev. 2020, 13, 3571–3605. [Google Scholar] [CrossRef]
- Ismaili, R.R.R.; Peng, X.; Li, Y.; Ali, A.; Ahmad, T.; Rahman, A.U.; Ahmad, S.; Shi, K. Modeling Habitat Suitability of Snow Leopards in Yanchiwan National Reserve, China. Animals 2024, 14, 1938. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.L.; Qian, C.; Huang, A.N.; Guo, R.X.; Kuang, X.Y. Evaluation of AMIP models from CMIP6 in simulating winter surface air temperature trends over Eurasia during 1998–2012 based on dynamical adjustment. Clim. Dynam. 2023, 60, 17–31. [Google Scholar] [CrossRef]
- Li, Q.; Qi, Y.; Wang, Q.; Wang, D.L. Prediction of the Potential Distribution of Vaccinium uliginosum in China Based on the Maxent Niche Model. Horticulturae 2022, 8, 1202. [Google Scholar] [CrossRef]
- Roshani; Rahaman, M.H.; Masroor, M.; Sajjad, H.; Saha, T.K. Assessment of habitat suitability and potential corridors for Bengal Tiger (Panthera tigris tigris) in Valmiki Tiger Reserve, India, using MaxEnt model and Least-Cost modeling approach. Environ. Model. Assess. 2024, 29, 405–422. [Google Scholar] [CrossRef]
- Hou, J.L.; Xiang, J.G.; Li, D.L.; Liu, X.H. Prediction of potential suitable distribution areas of Quasipaa spinosa in China based on MaxEnt optimization model. Biology 2023, 12, 366. [Google Scholar] [CrossRef]
- Li, X.; Huang, Y.Y.; Ruan, T.; Wei, W. Maxent model-based evaluation of habitat suitability of Chinese red panda in Qionglai mountains. J. Guizhou Norm. Univ. Nat. Sci. 2022, 40, 34–39. [Google Scholar]
- Hill, M.P.; Hoffmann, A.A.; McColl, S.A.; Umina, P.A. Distribution of cryptic blue oat mite species in Australia: Current and future climate conditions. Agric. For. Entomol. 2012, 14, 127–137. [Google Scholar] [CrossRef]
- Wang, R.L.; Li, Q.; Feng, C.H.; Shi, Z.P. Predicting potential ecological distribution of Locusta migratoria tibetensis in China using MaxEnt ecological niche modeling. Acta Ecol. Sin. 2017, 37, 8556–8566. [Google Scholar]
- Xu, D.P.; Zhou, Z.H.; Wang, R.L.; Ye, M.; Pu, B. Modeling the distribution of Zanthoxylum armatum in China with MaxEnt modeling. Glob. Ecol. Conserv. 2019, 19, e00691. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Jackson, C.R.; Robertson, M.P. Predicting the potential distribution of an endangered cryptic subterranean mammal from few occurrence records. J. Nat. Conserv. 2011, 19, 87–94. [Google Scholar] [CrossRef]
- Clark, J.T.; Fei, S.; Liang, L.; Rieske, L.K. Mapping eastern hemlock: Comparing classification techniques to evaluate susceptibility of a fragmented and valued resource to an exotic invader, the hemlock woolly adelgid. For. Ecol. Manag. 2012, 266, 216–222. [Google Scholar] [CrossRef]
- Majumder, A.; Suryan, T.; Rizvi, T.; Tripathi, R.M.; Nag, S. Assessment of the population and suitable habitat for a leopard (Panthera pardus) in the urban landscapes of Central India. Eur. J. Wildl. Res. 2024, 70, 120. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Fourcade, Y.; Besnard, A.G.; Secondi, J. Paintings predict the distribution of species, or the challenge of selecting environmental predictors and evaluation statistics. Glob. Ecol. Biogeogr. 2018, 27, 245–256. [Google Scholar] [CrossRef]
- Nolan, C.; Overpeck, J.T.; Allen, J.R.M.; Anderson, P.M.; Betancourt, J.L.; Binney, H.A.; Brewer, S.; Bush, M.B.; Chase, B.M.; Cheddadi, R.; et al. Past and future global transformation of terrestrial ecosystems under climate change. Science 2018, 361, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Tape, K.D.; Christie, K.; Carroll, G.; O’Donnell, J.A. Novel wildlife in the Arctic:the influence of changing riparian ecosystems and shrub habitat expansion on snowshoe hares. Glob. Change Biol. 2016, 22, 208–219. [Google Scholar] [CrossRef]
- Allen, M.R.; Barros, V.R.; Broome, J.; CHRIST, R.; Church, J.; Clarke, L.; Cramer, W.; Dasgupta, P.; DUBASH, N.; Edenhofer, O.; et al. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Stirling, I.; Derocher, A.E. Effects of climate warming on polar bears: A review of the evidence. Glob. Change Biol. 2012, 18, 2694–2706. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.F. Study on the Distribution and Changes of Leopards (Panthera pardus) in China in the Past 300 Years; Northeast Forestry University: Heilongjiang, China, 2020. [Google Scholar]
- Gethöffer, F.; Keuling, O.; Maistrelli, C.; Ludwig, T.; Siebert, U. Heavy Youngsters-Habitat and climate factors lead to a significant increase in body weight of wild boar females. Animals 2023, 13, 898. [Google Scholar] [CrossRef] [PubMed]
| Code | Bioclimatic Variables | Unit | PC (%) | PI (%) |
|---|---|---|---|---|
| Bio1 | Annual Mean Temperature | °C | 2.40 | 5.40 |
| Bio2 | Mean Diurnal Range | °C | 25.50 | 8.70 |
| Bio3 | Isothermality | % | 20.90 | 29.80 |
| Bio4 | Temperature Seasonality | °C | 10.40 | 8.10 |
| Bio5 | Max Temperature of Warmest Month | °C | 7.00 | 6.60 |
| Bio6 | Min Temperature of Coldest Month | °C | 5.40 | 22.10 |
| Bio7 | Temperature Annual Range | °C | 14.50 | 3.60 |
| Bio11 | Mean Temperature of Coldest Quarter | °C | 13.60 | 14.70 |
| Ele | Elevation | m | 0.50 | 0.90 |
| Circumstances | Low Suitability | Medium Suitability | High Suitability | All | ||||
|---|---|---|---|---|---|---|---|---|
| Area (km2) | Percentage (%) | Area (km2) | Percentage (%) | Area (km2) | Percentage (%) | Area (km2) | Percentage (%) | |
| Current | 406.25 | 6.57 | 379.86 | 6.14 | 497.92 | 8.05 | 1284.03 | 20.76 |
| 2050s-SSP126 | 570.14 | 9.22 | 493.06 | 7.97 | 177.08 | 2.86 | 1240.28 | 20.05 |
| 2050s-SSP245 | 490.28 | 7.93 | 536.81 | 8.68 | 172.92 | 2.80 | 1200.01 | 19.40 |
| 2050s-SSP585 | 511.81 | 8.27 | 484.03 | 7.82 | 202.78 | 3.28 | 1198.62 | 19.38 |
| 2070s-SSP126 | 509.03 | 8.23 | 460.42 | 7.44 | 195.83 | 3.17 | 1165.28 | 18.84 |
| 2070s-SSP245 | 506.94 | 8.19 | 438.19 | 7.08 | 191.67 | 3.10 | 1136.8 | 18.38 |
| 2070s-SSP585 | 468.75 | 7.58 | 636.81 | 10.29 | 129.86 | 2.10 | 1235.42 | 19.97 |
| 2090s-SSP126 | 461.11 | 7.45 | 493.75 | 7.98 | 184.72 | 2.99 | 1139.58 | 18.42 |
| 2090s-SSP245 | 483.33 | 7.81 | 490.97 | 7.94 | 228.47 | 3.69 | 1202.77 | 19.44 |
| 2090s-SSP585 | 512.5 | 8.28 | 593.06 | 9.59 | 183.33 | 2.96 | 1288.89 | 20.83 |
| Circumstances | Area (km2) | Rate of Change (%) | ||||
|---|---|---|---|---|---|---|
| EZ | SZ | Change | EZ | SZ | Change | |
| 2050-126 | 53.47 | 59.72 | 6.25 | 3.33 | 3.72 | 0.39 |
| 2050-245 | 75.00 | 21.53 | 53.47 | 4.67 | 1.34 | 3.33 |
| 2050-585 | 88.19 | 70.83 | 17.36 | 5.50 | 4.41 | 1.08 |
| 2070-126 | 50.00 | 59.03 | 9.03 | 3.12 | 3.68 | 0.56 |
| 2070-245 | 38.89 | 100.00 | 61.11 | 2.42 | 6.23 | 3.81 |
| 2070-585 | 65.97 | 47.92 | 18.05 | 4.11 | 2.99 | 1.12 |
| 2090-126 | 68.75 | 29.86 | 38.89 | 4.28 | 1.86 | 2.42 |
| 2090-245 | 75.00 | 67.36 | 7.64 | 4.67 | 4.20 | 0.48 |
| 2090-585 | 66.67 | 77.08 | 10.41 | 4.15 | 4.80 | 0.65 |
| Environmental Variables | Suitable Range | Optimal Value |
|---|---|---|
| Bio1: Annual Mean Temperature (°C) | 6.87–8.16 | 7.70 |
| Bio2: Mean Diurnal Range (°C) | 9.23–10.43 | 9.57 |
| Bio3: Isothermality (%) | 27.60–29.00 | 28.45 |
| Bio4: Temperature Seasonality (°C) | 884.00–937.42 | 884 |
| Bio5: Max Temperature of Warmest Month (°C) | 22.95–25.10 | 23.94 |
| Bio6: Min Temperature of Coldest Month (°C) | −11.1–−10.27 | −10.46 |
| Bio7: Temperature Annual Range (°C) | 33.48–36.01 | 33.48 |
| Bio11: Mean Temperature of Coldest Quarter (°C) | −4.17–−3.57 | −3.82 |
| Elevation (m) | 1454.14–1723.06 | 1694.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Gao, W.; Han, Y.; Zhou, T. Predicting the Impact of Climate Change on the Distribution of North China Leopards (Panthera pardus japonensis) in Gansu Province Using MaxEnt Modeling. Biology 2025, 14, 126. https://doi.org/10.3390/biology14020126
Yang Y, Gao W, Han Y, Zhou T. Predicting the Impact of Climate Change on the Distribution of North China Leopards (Panthera pardus japonensis) in Gansu Province Using MaxEnt Modeling. Biology. 2025; 14(2):126. https://doi.org/10.3390/biology14020126
Chicago/Turabian StyleYang, Yongqiang, Wenjie Gao, Yapeng Han, and Tianlin Zhou. 2025. "Predicting the Impact of Climate Change on the Distribution of North China Leopards (Panthera pardus japonensis) in Gansu Province Using MaxEnt Modeling" Biology 14, no. 2: 126. https://doi.org/10.3390/biology14020126
APA StyleYang, Y., Gao, W., Han, Y., & Zhou, T. (2025). Predicting the Impact of Climate Change on the Distribution of North China Leopards (Panthera pardus japonensis) in Gansu Province Using MaxEnt Modeling. Biology, 14(2), 126. https://doi.org/10.3390/biology14020126






