Glutathione Depletion Exacerbates Hepatic Mycobacterium tuberculosis Infection
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria and Chemicals
2.2. Aerosol Infection of Mice, Treatment, and Bacterial CFU Assay
2.3. Histology Staining of Liver Sections and Morphometry
2.4. Quantification of Glutathione Levels
2.5. Cytokine Measurement
2.6. Statistical Analysis
3. Results
3.1. rGSH Levels Are Decreased, and GSSG Levels Are Elevated in the Liver Post-DEM Treatment
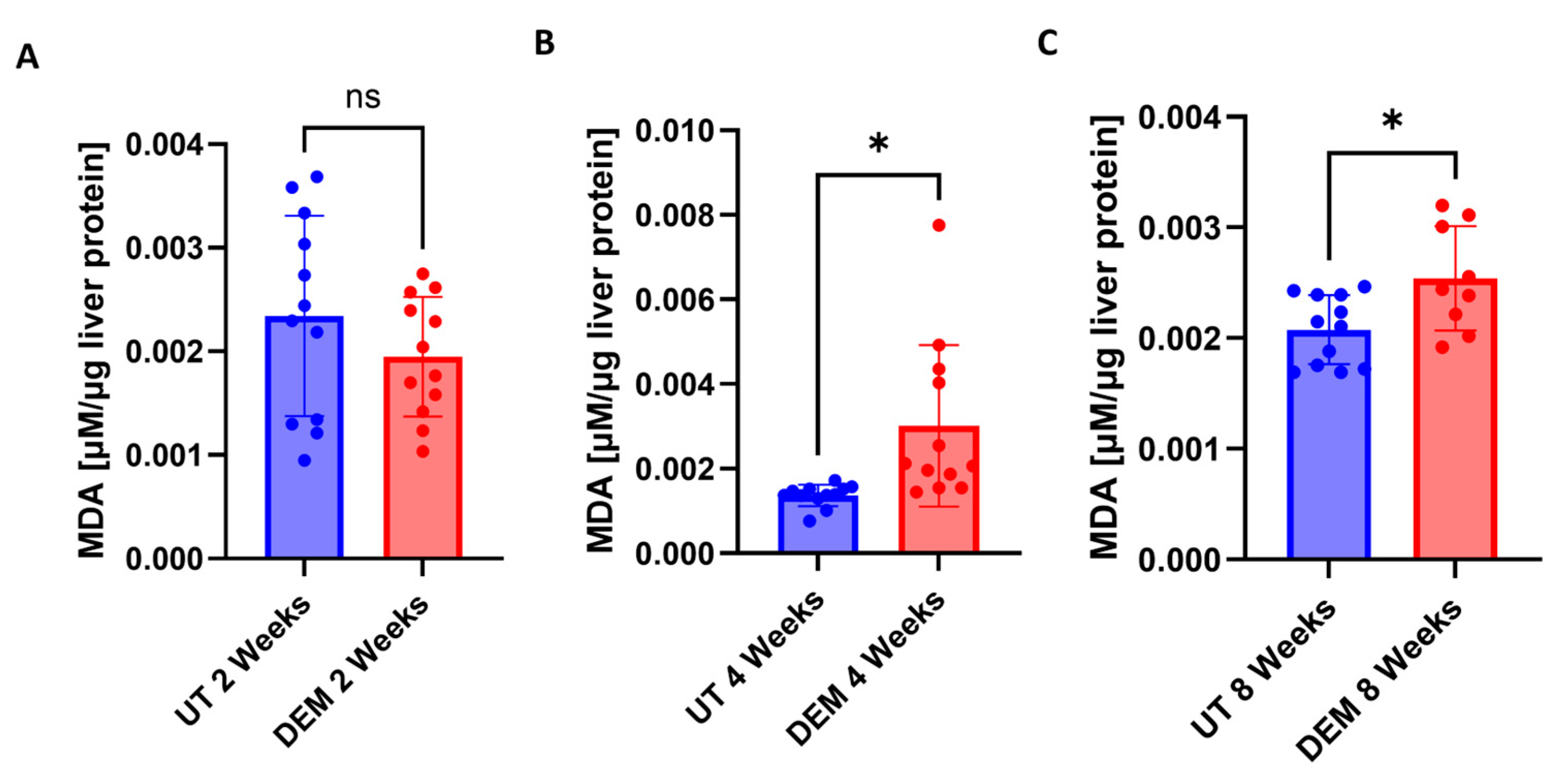
3.2. MDA Levels Are Increased in the Liver Post-DEM Treatment
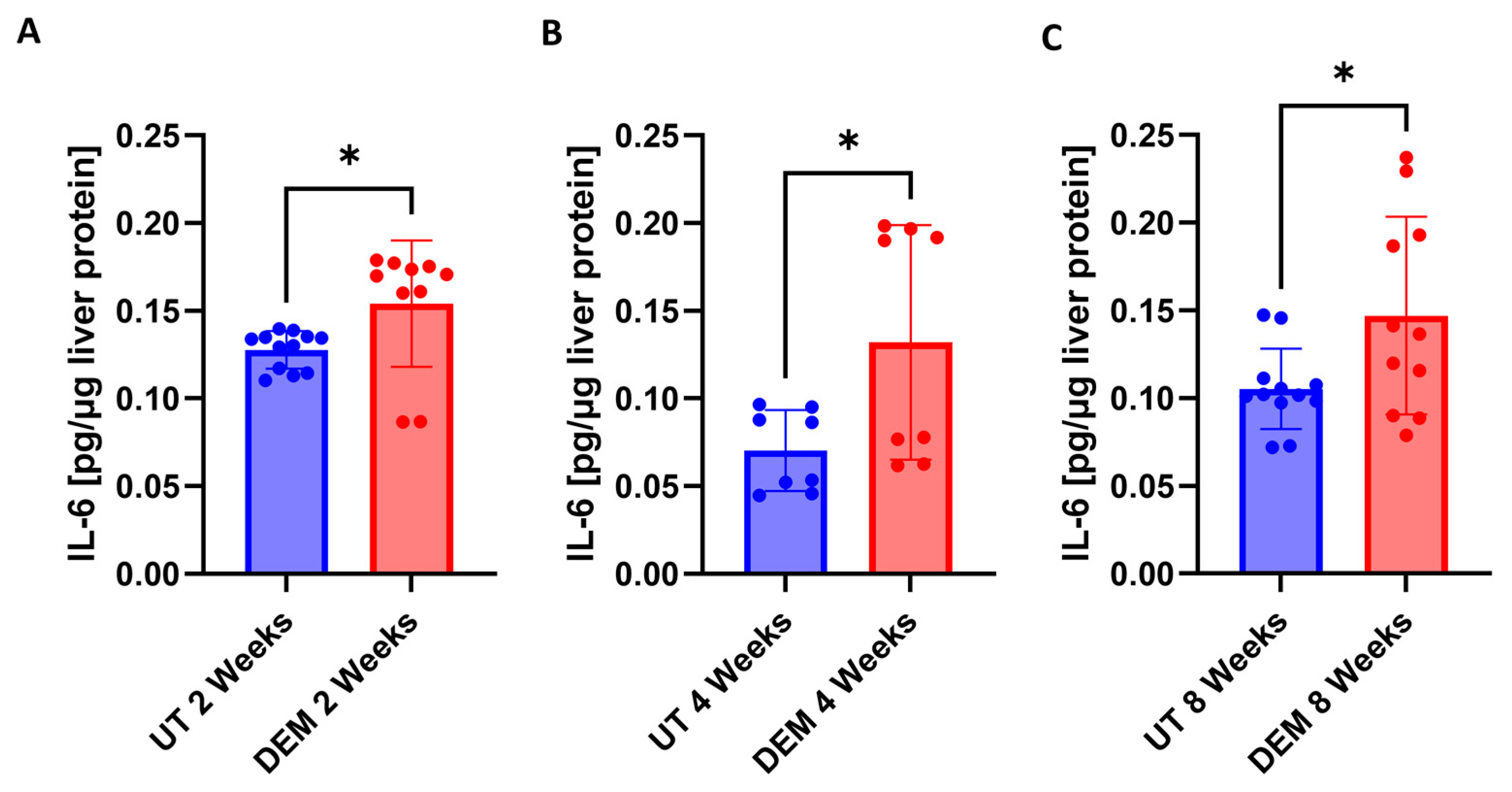
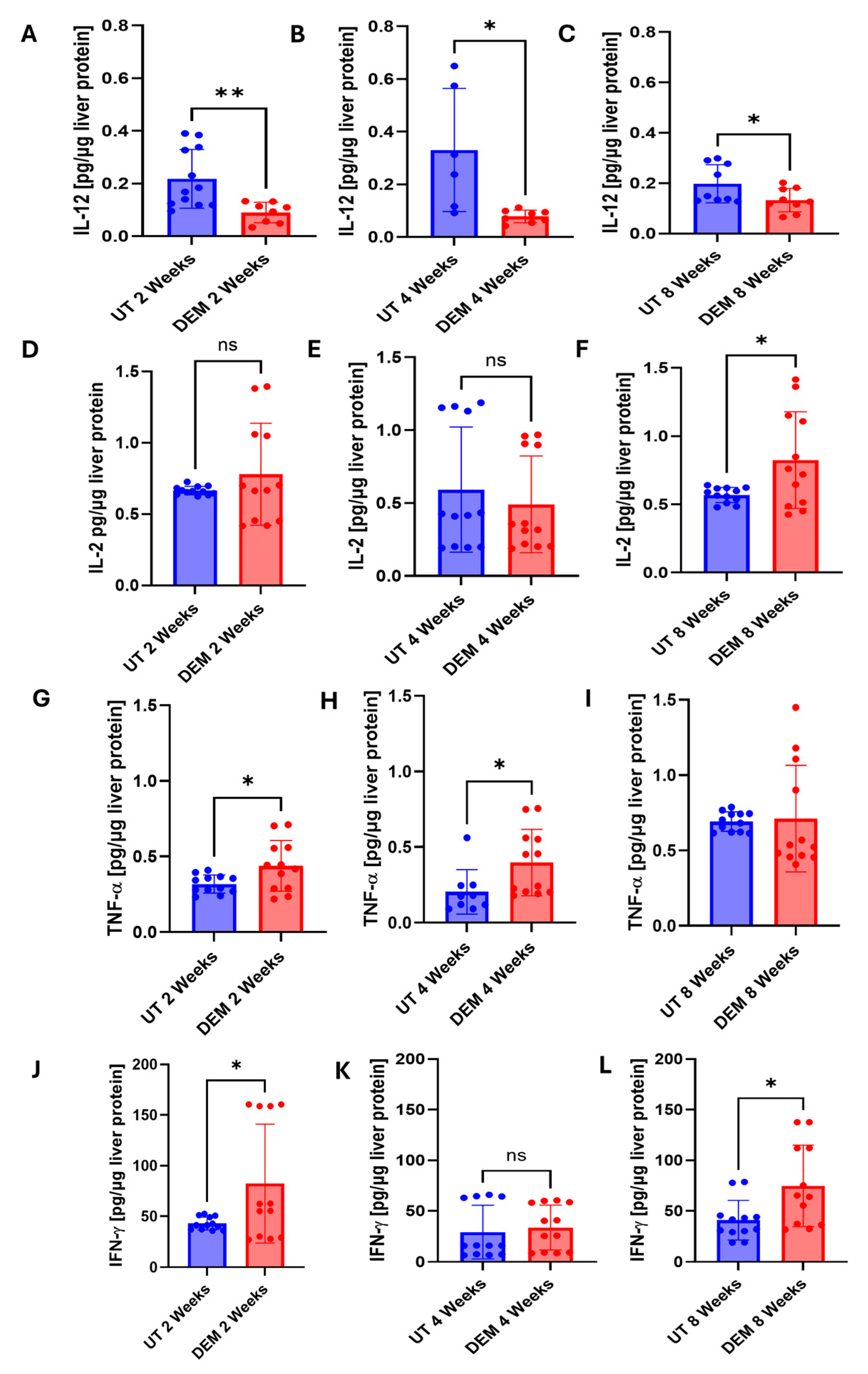
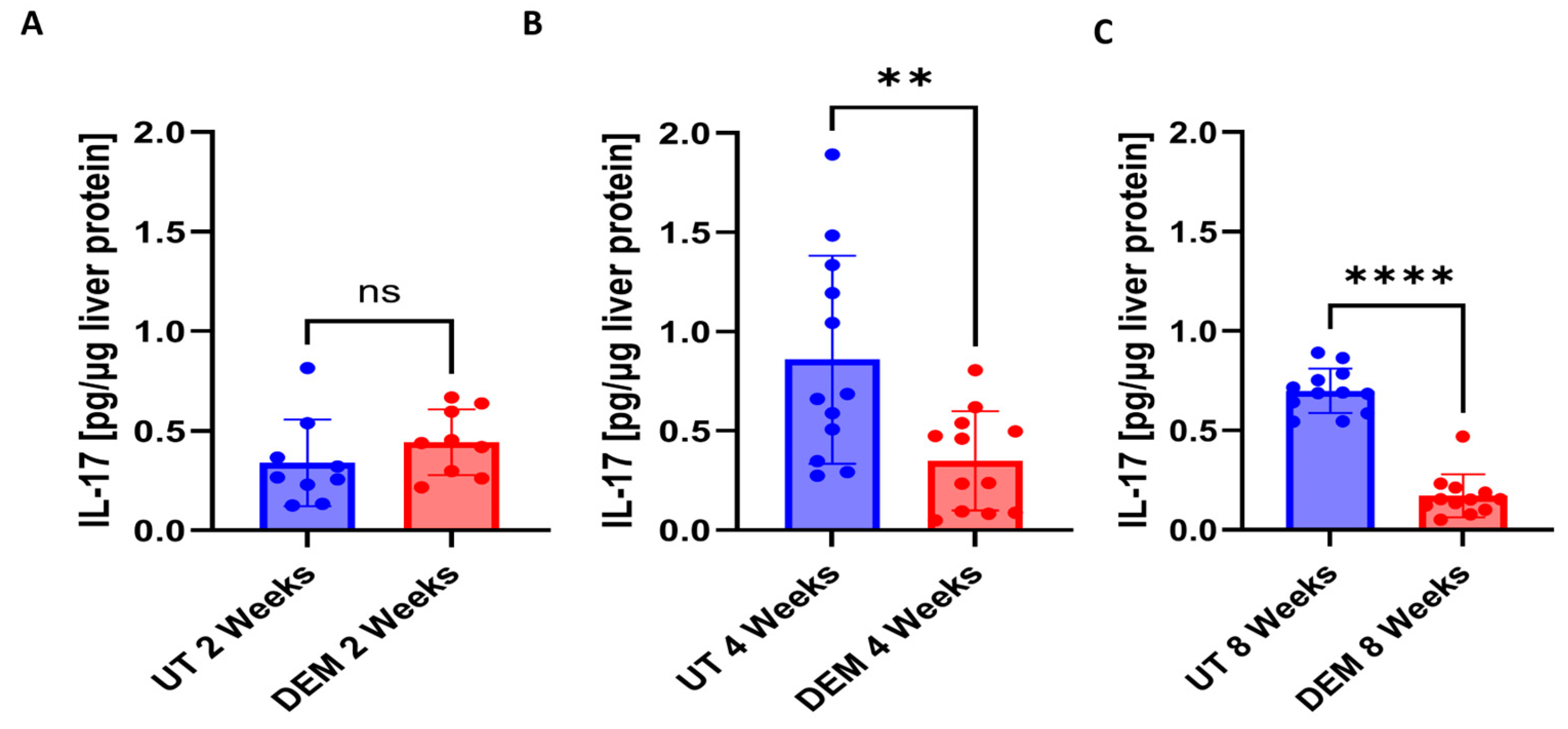
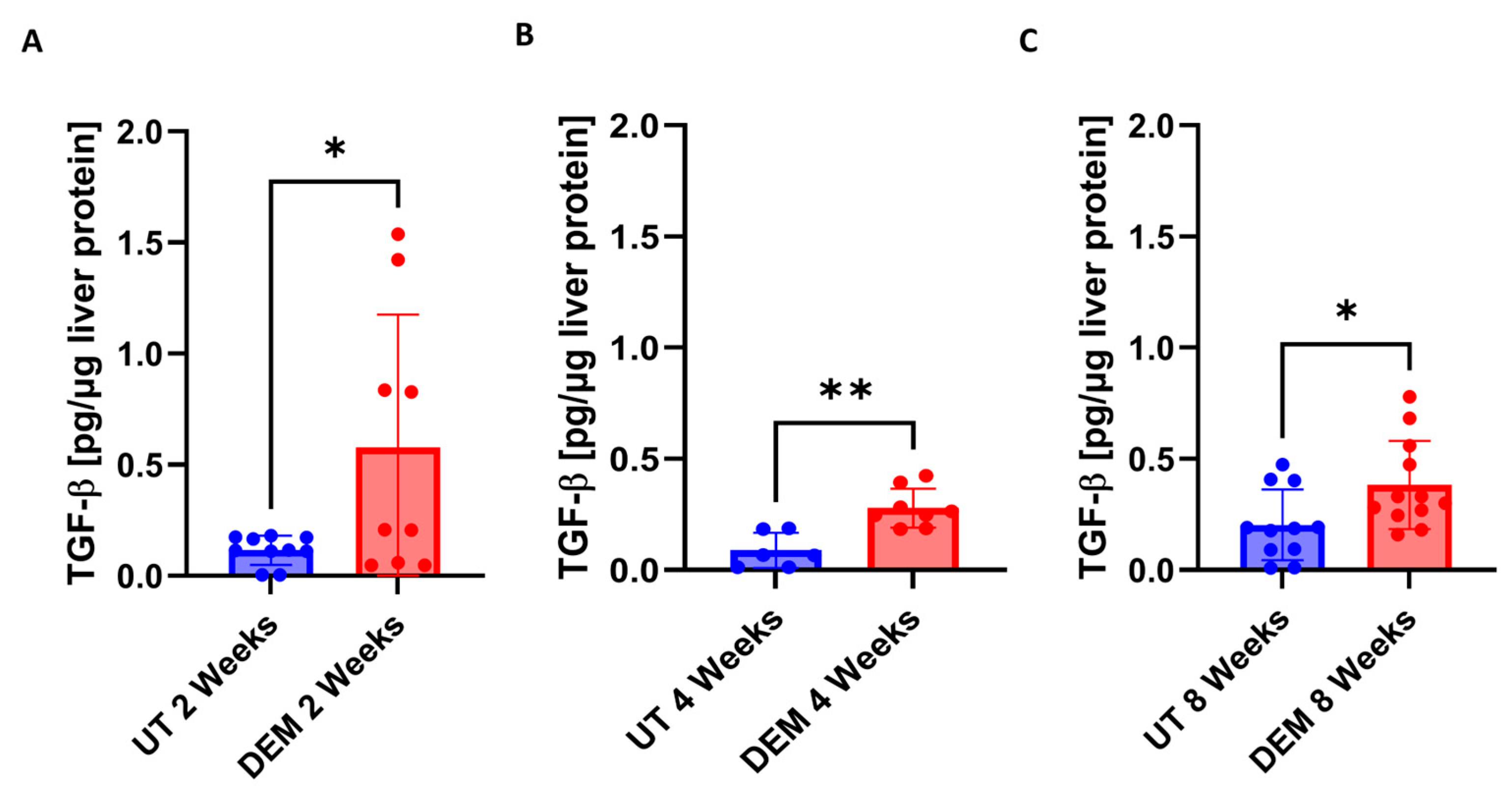
3.3. DEM Treatment Altered Cytokine Production in the Liver
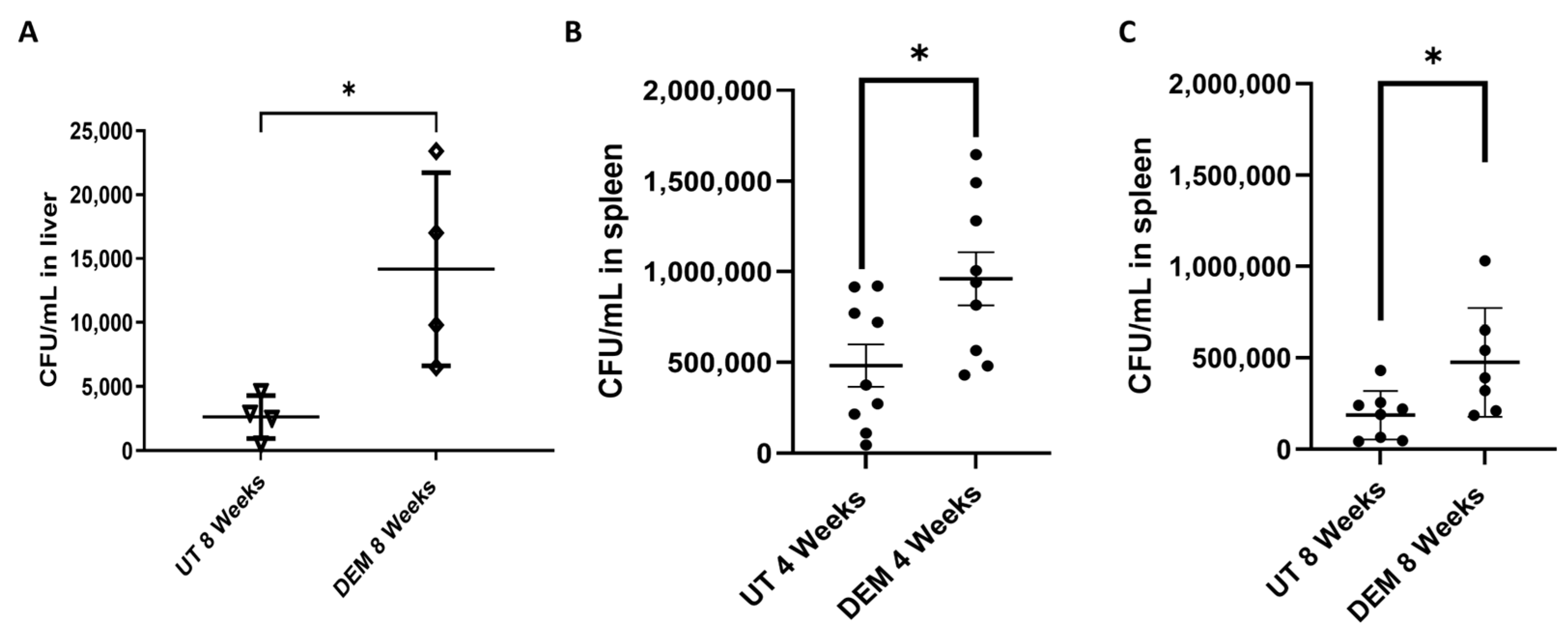
3.4. DEM Treatment Enhances M.tb Survival in the Liver and Spleen
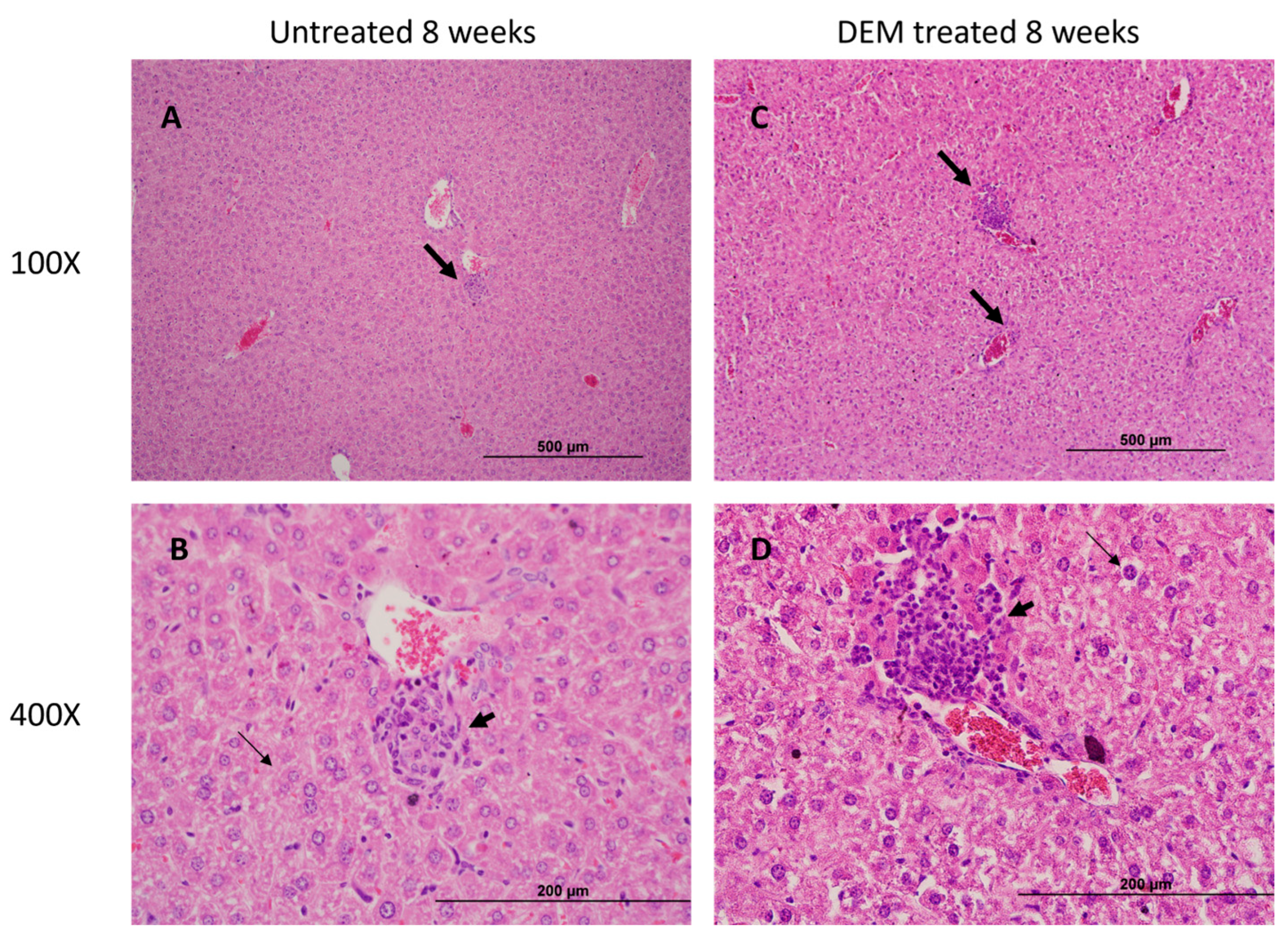
3.5. Granuloma Formation in the Liver Is Altered Post-DEM Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2023; World Health Organization: Geneva, Switzerland, 2023; Available online: https://iris.who.int/handle/10665/373828 (accessed on 20 November 2024).
- Narasimhan, P.; Wood, J.; MacIntyre, C.R.; Mathai, D. Risk factors for tuberculosis. Pulm. Med. 2013, 2013, 828939. [Google Scholar] [CrossRef] [PubMed]
- Urdahl, K.B.; Shafiani, S.; Ernst, J.D. Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol. 2011, 4, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Nguyen, D.T.; Lyu, J.; Albers, A.E.; Bi, X.; Graviss, E.A. Risk factors for extrapulmonary dissemination of tuberculosis and associated mortality during treatment for extrapulmonary tuberculosis. Emerg. Microbes Infect. 2018, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Robertson, B.D.; Thwaites, G. The mechanisms and consequences of the extra-pulmonary dissemination of Mycobacterium tuberculosis. Tuberculosis 2010, 90, 361–366. [Google Scholar] [CrossRef]
- Seung, K.J.; Keshavjee, S.; Rich, M.L. Multidrug-Resistant Tuberculosis and Extensively Drug-Resistant Tuberculosis. Cold Spring Harb. Perspect. Med. 2015, 5, a017863. [Google Scholar] [CrossRef]
- Sekhar, R.V.; McKay, S.V.; Patel, S.G.; Guthikonda, A.P.; Reddy, V.T.; Balasubramanyam, A.; Jahoor, F. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care 2011, 34, 162–167. [Google Scholar] [CrossRef]
- Borges-Santos, M.D.; Moreto, F.; Pereira, P.C.M.; Ming-Yu, Y.; Burini, R.C. Plasma glutathione of HIV+ patients responded positively and differently to dietary supplementation with cysteine or glutamine. Nutrition 2012, 28, 753–756. [Google Scholar] [CrossRef]
- Muri, J.; Kopf, M. Redox regulation of immunometabolism. Nat. Rev. Immunol. 2021, 21, 363–381. [Google Scholar] [CrossRef] [PubMed]
- Amaral, E.P.; Costa, D.L.; Namasivayam, S.; Riteau, N.; Kamenyeva, O.; Mittereder, L.; Mayer-Barber, K.D.; Andrade, B.B.; Sher, A. A major role for ferroptosis in Mycobacterium tuberculosis-induced cell death and tissue necrosis. J. Exp. Med. 2019, 216, 556–570. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amaral, E.P.; Foreman, T.W.; Namasivayam, S.; Hilligan, K.L.; Kauffman, K.D.; Barbosa Bomfim, C.C.; Costa, D.L.; Barreto-Duarte, B.; Gurgel-Rocha, C.; Santana, M.F.; et al. GPX4 regulates cellular necrosis and host resistance in Mycobacterium tuberculosis infection. J. Exp. Med. 2022, 219, e20220504. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, R.; Kolloli, A.; Kumar, R.; Owens, J.; Sasaninia, K.; Vaughn, C.; Singh, M.; Truong, E.; Kachour, N.; Beever, A.; et al. Effects of Glutathione Diminishment on the Immune Responses against Mycobacterium tuberculosis Infection. Appl. Sci. 2021, 11, 8274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Subbian, S.; Pandey, R.; Soteropoulos, P.; Rodriguez, G.M. Vaccination with an Attenuated Ferritin Mutant Protects Mice against Virulent Mycobacterium tuberculosis. J. Immunol. Res. 2015, 2015, 385402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bell, L.C.K.; Noursadeghi, M. Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nat. Rev. Microbiol. 2018, 16, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Magee, M.J.; Foote, M.; Ray, S.M.; Gandhi, N.R.; Kempker, R.R. Diabetes mellitus and extrapulmonary tuberculosis: Site distribution and risk of mortality. Epidemiol Infect. 2016, 144, 2209–2216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pacl, H.T.; Reddy, V.P.; Saini, V.; Chinta, K.C.; Steyn, A.J.C. Host-pathogen redox dynamics modulate Mycobacterium tuberculosis pathogenesis. Pathog. Dis. 2018, 76, fty036. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guerra, C.; Morris, D.; Sipin, A.; Kung, S.; Franklin, M.; Gray, D.; Tanzil, M.; Guilford, F.; Khasawneh, F.T.; Venketaraman, V. Glutathione and adaptive immune responses against Mycobacterium tuberculosis infection in healthy and HIV infected individuals. PLoS ONE 2011, 6, e28378. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lagman, M.; Ly, J.; Saing, T.; Kaur Singh, M.; Vera Tudela, E.; Morris, D.; Chi, P.T.; Ochoa, C.; Sathananthan, A.; Venketaraman, V. Investigating the causes for decreased levels of glutathione in individuals with type II diabetes. PLoS ONE 2015, 10, e0118436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tirmenstein, M.A.; Nicholls-Grzemski, F.A.; Zhang, J.G.; Fariss, M.W. Glutathione depletion and the production of reactive oxygen species in isolated hepatocyte suspensions. Chem. Biol. Interact. 2000, 127, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yang, Q.; Gao, S.; Liu, Z.; Kong, W.; Bian, X.; Li, Z.; Ye, J. IL-6 protects cardiomyocytes from oxidative stress at the early stage of LPS-induced sepsis. Biochem. Biophys. Res. Commun. 2022, 603, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.H.; Lee, H.; Park, C.; Hong, S.-H.; Hong, S.H.; Kim, G.-Y.; Cha, H.-J.; Kim, S.; Kim, H.-S.; Hwang, H.-J.; et al. Glutathione Induced Immune-Stimulatory Activity by Promoting M1-Like Macrophages Polarization via Potential ROS Scavenging Capacity. Antioxidants 2019, 8, 413. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Sternberg, P.; Cai, J. Essential roles of the PI3 kinase/Akt pathway in regulating Nrf2-dependent antioxidant functions in the RPE. Invest. Ophthalmol. Vis. Sci. 2008, 49, 1671–1678. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lyadova, I.V.; Panteleev, A.V. Th1 and Th17 Cells in Tuberculosis: Protection, Pathology, and Biomarkers. Mediat. Inflamm. 2015, 2015, 854507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dixon, L.J.; Barnes, M.; Tang, H.; Pritchard, M.T.; Nagy, L.E. Kupffer cells in the liver. Compr. Physiol. 2013, 3, 785–797. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Musrati, M.A.; De Baetselier, P.; Movahedi, K.; Van Ginderachter, J.A. Ontogeny, functions and reprogramming of Kupffer cells upon infectious disease. Front. Immunol. 2023, 14, 1238452. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhai, W.; Wu, F.; Zhang, Y.; Fu, Y.; Liu, Z. The Immune Escape Mechanisms of Mycobacterium Tuberculosis. Int. J. Mol. Sci. 2019, 20, 340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cronan, M.R. In the Thick of It: Formation of the Tuberculous Granuloma and Its Effects on Host and Therapeutic Responses. Front. Immunol. 2022, 13, 820134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, X.; Yan, W.; Zheng, H.; Du, Q.; Zhang, L.; Ban, Y.; Li, N.; Wei, F. Regulation of IL-10 and IL-12 production and function in macrophages and dendritic cells. F1000Research 2015, 4, F1000 Faculty Rev-1465. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herr, F.; Lemoine, R.; Gouilleux, F.; Meley, D.; Kazma, I.; Heraud, A.; Velge-Roussel, F.; Baron, C.; Lebranchu, Y. IL-2 phosphorylates STAT5 to drive IFN-γ production and activation of human dendritic cells. J. Immunol. 2014, 192, 5660–5670. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Torrado, E.; Cooper, A.M. IL-17 and Th17 cells in tuberculosis. Cytokine Growth Factor. Rev. 2010, 21, 455–462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- DiFazio, R.M.; Mattila, J.T.; Klein, E.C.; Cirrincione, L.R.; Howard, M.; Wong, E.A.; Flynn, J.L. Active transforming growth factor-β is associated with phenotypic changes in granulomas after drug treatment in pulmonary tuberculosis. Fibrogenesis Tissue Repair 2016, 9, 6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amatore, D.; Celestino, I.; Brundu, S.; Galluzzi, L.; Coluccio, P.; Checconi, P.; Magnani, M.; Palamara, A.T.; Fraternale, A.; Nencioni, L. Glutathione increase by the n-butanoyl glutathione derivative (GSH-C4) inhibits viral replication and induces a predominant Th1 immune profile in old mice infected with influenza virus. FASEB Bioadv. 2019, 1, 296–305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fraternale, A.; Brundu, S.; Magnani, M. Glutathione and glutathione derivatives in immunotherapy. Biol. Chem. 2017, 398, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.D.; Herzenberg, L.A.; Vasquez, K.; Waltenbaugh, C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc. Natl. Acad. Sci. USA 1998, 95, 3071–3076. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chung, J.Y.; Chan, M.K.; Li, J.S.; Chan, A.S.; Tang, P.C.; Leung, K.T.; To, K.F.; Lan, H.Y.; Tang, P.M. TGF-β Signaling: From Tissue Fibrosis to Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 7575. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Massagué, J.; Sheppard, D. TGF-β signaling in health and disease. Cell 2023, 186, 4007–4037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ryoo, I.G.; Ha, H.; Kwak, M.K. Inhibitory role of the KEAP1-NRF2 pathway in TGFβ1-stimulated renal epithelial transition to fibroblastic cells: A modulatory effect on SMAD signaling. PLoS ONE 2014, 9, e93265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hunter, L.; Ruedas-Torres, I.; Agulló-Ros, I.; Rayner, E.; Salguero, F.J. Comparative pathology of experimental pulmonary tuberculosis in animal models. Front. Vet. Sci. 2023, 10, 1264833. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaninia, K.; Mohan, A.S.; Badaoui, A.; Glassman, I.; Yoon, S.; Karapetyan, A.; Kolloli, A.; Kumar, R.; Ramasamy, S.; Subbian, S.; et al. Glutathione Depletion Exacerbates Hepatic Mycobacterium tuberculosis Infection. Biology 2025, 14, 131. https://doi.org/10.3390/biology14020131
Sasaninia K, Mohan AS, Badaoui A, Glassman I, Yoon S, Karapetyan A, Kolloli A, Kumar R, Ramasamy S, Subbian S, et al. Glutathione Depletion Exacerbates Hepatic Mycobacterium tuberculosis Infection. Biology. 2025; 14(2):131. https://doi.org/10.3390/biology14020131
Chicago/Turabian StyleSasaninia, Kayvan, Aishvaryaa Shree Mohan, Ali Badaoui, Ira Glassman, Sonyeol Yoon, Arshavir Karapetyan, Afsal Kolloli, Ranjeet Kumar, Santhamani Ramasamy, Selvakumar Subbian, and et al. 2025. "Glutathione Depletion Exacerbates Hepatic Mycobacterium tuberculosis Infection" Biology 14, no. 2: 131. https://doi.org/10.3390/biology14020131
APA StyleSasaninia, K., Mohan, A. S., Badaoui, A., Glassman, I., Yoon, S., Karapetyan, A., Kolloli, A., Kumar, R., Ramasamy, S., Subbian, S., & Venketaraman, V. (2025). Glutathione Depletion Exacerbates Hepatic Mycobacterium tuberculosis Infection. Biology, 14(2), 131. https://doi.org/10.3390/biology14020131








