Effects of Low and High Doses of Deoxynivalenol on Growth Performance, Blood Biochemistry, Histology, Metabolites, and Microbial Community in Adult Rats
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Ethics Statements
2.2. Animals and Study Design
2.3. Blood Biochemical Analysis
2.4. Histological Analysis
2.5. DNA Preparation and Microbial Sequencing of Cecal and Fecal Contents
2.6. Preparation and Analysis of Metabolites in Different Tissues
2.7. Statistical Analysis
3. Results
3.1. Effects of DON on Growth Performance and Tissue Weight
3.2. Effects of DON on Blood Biochemistry
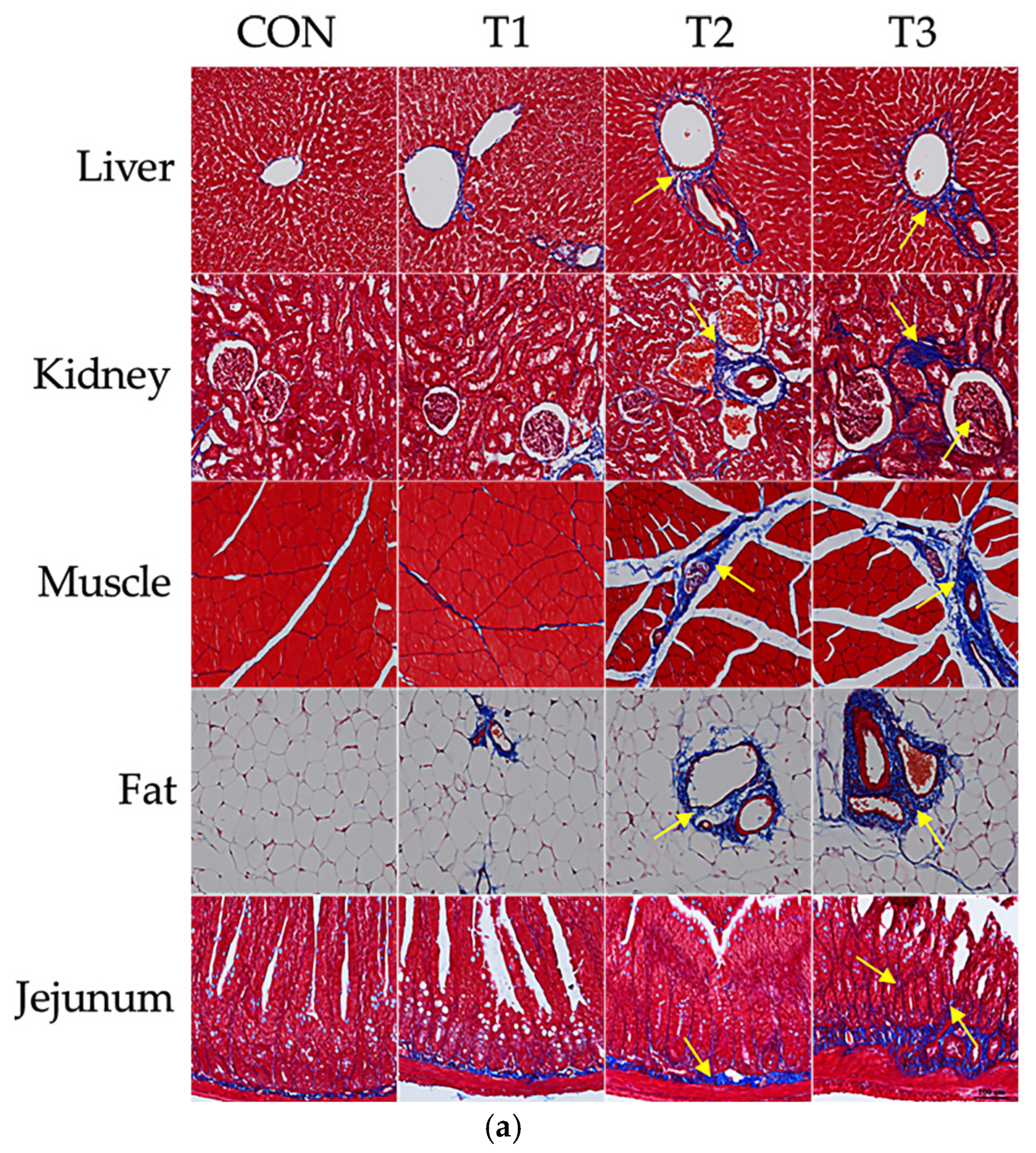
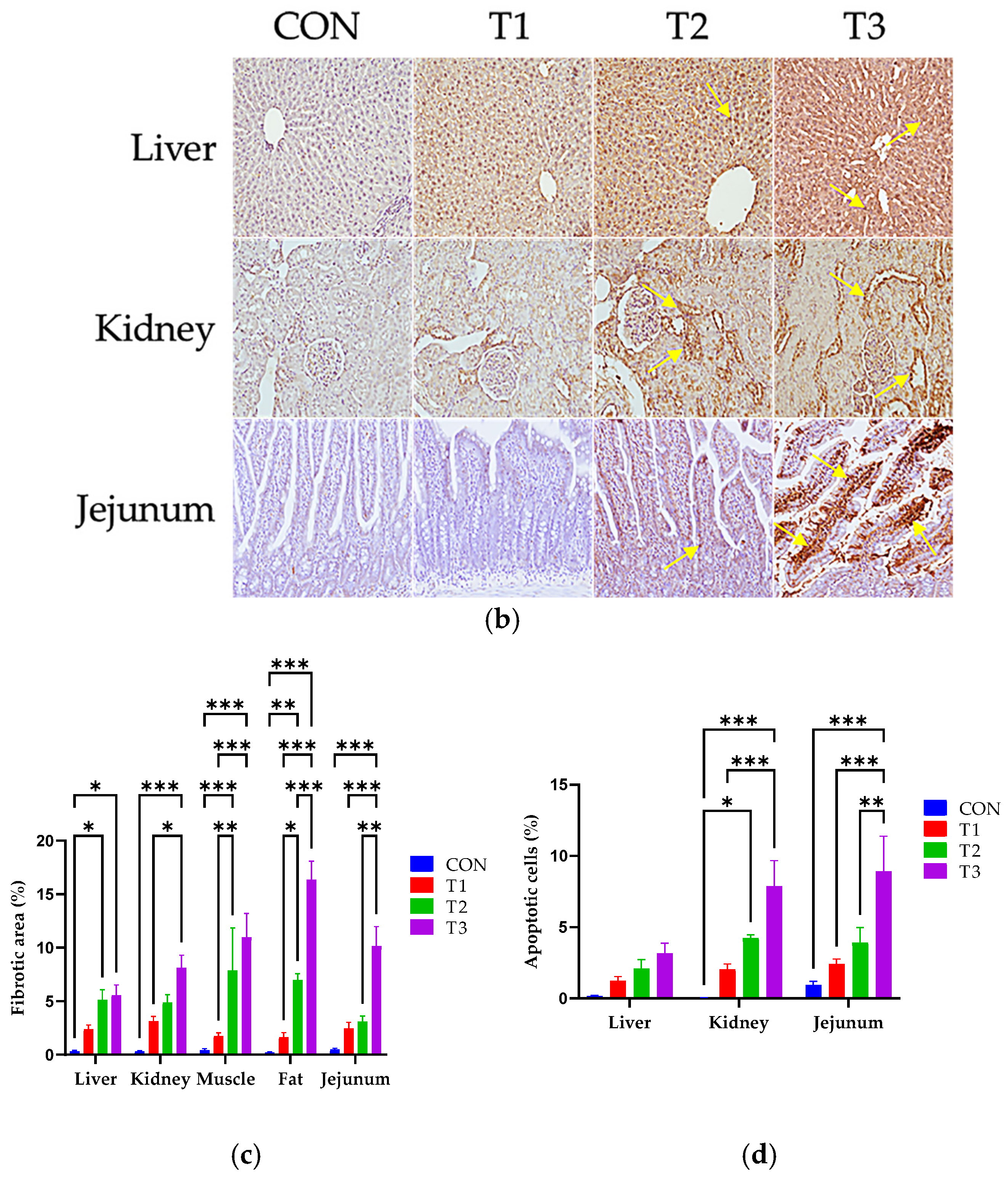
3.3. Effects of DON on Histological Changes
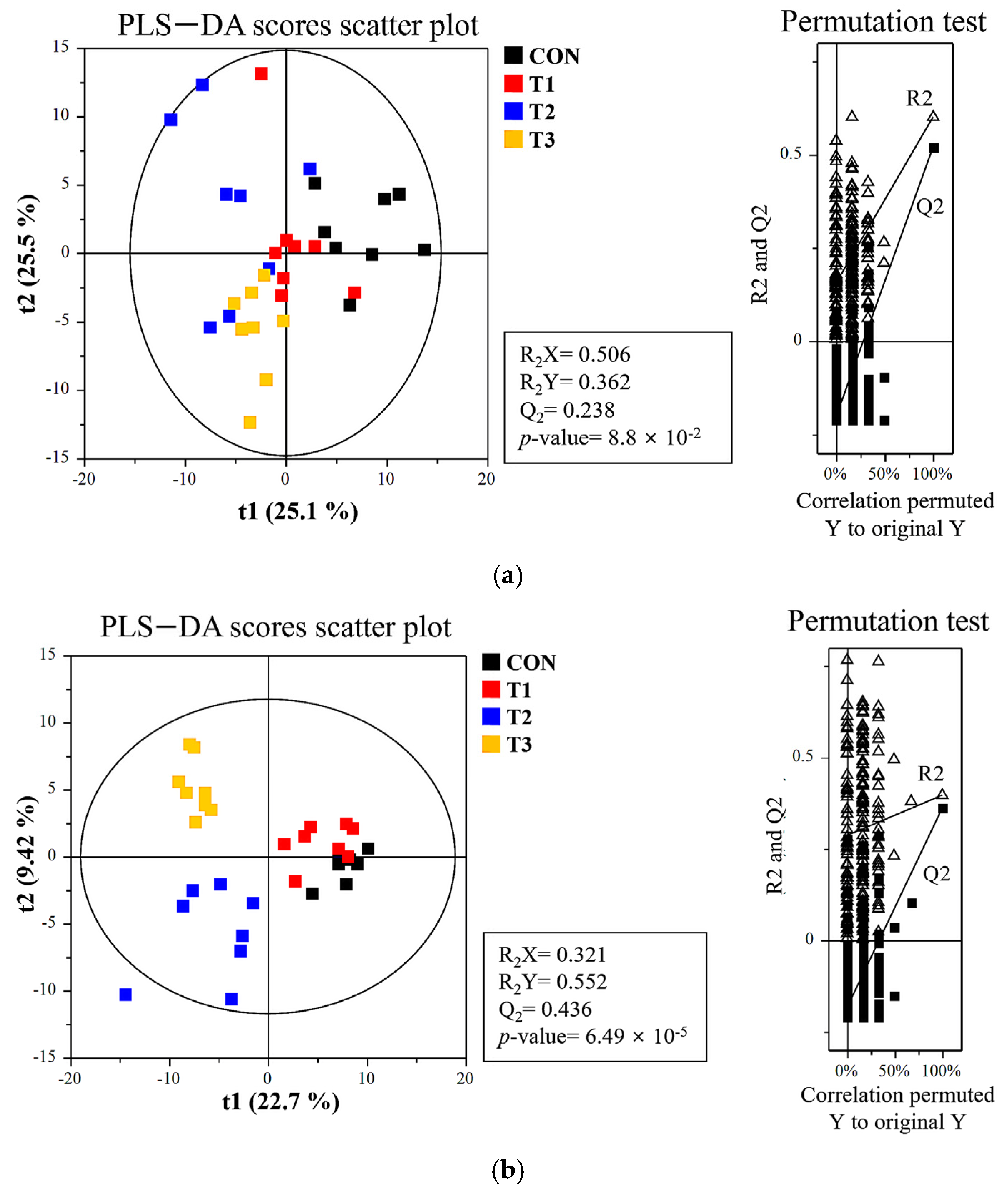
3.4. The Influence of DON on the Alpha and Beta Diversity of the Microbiome in Cecal and Fecal Samples
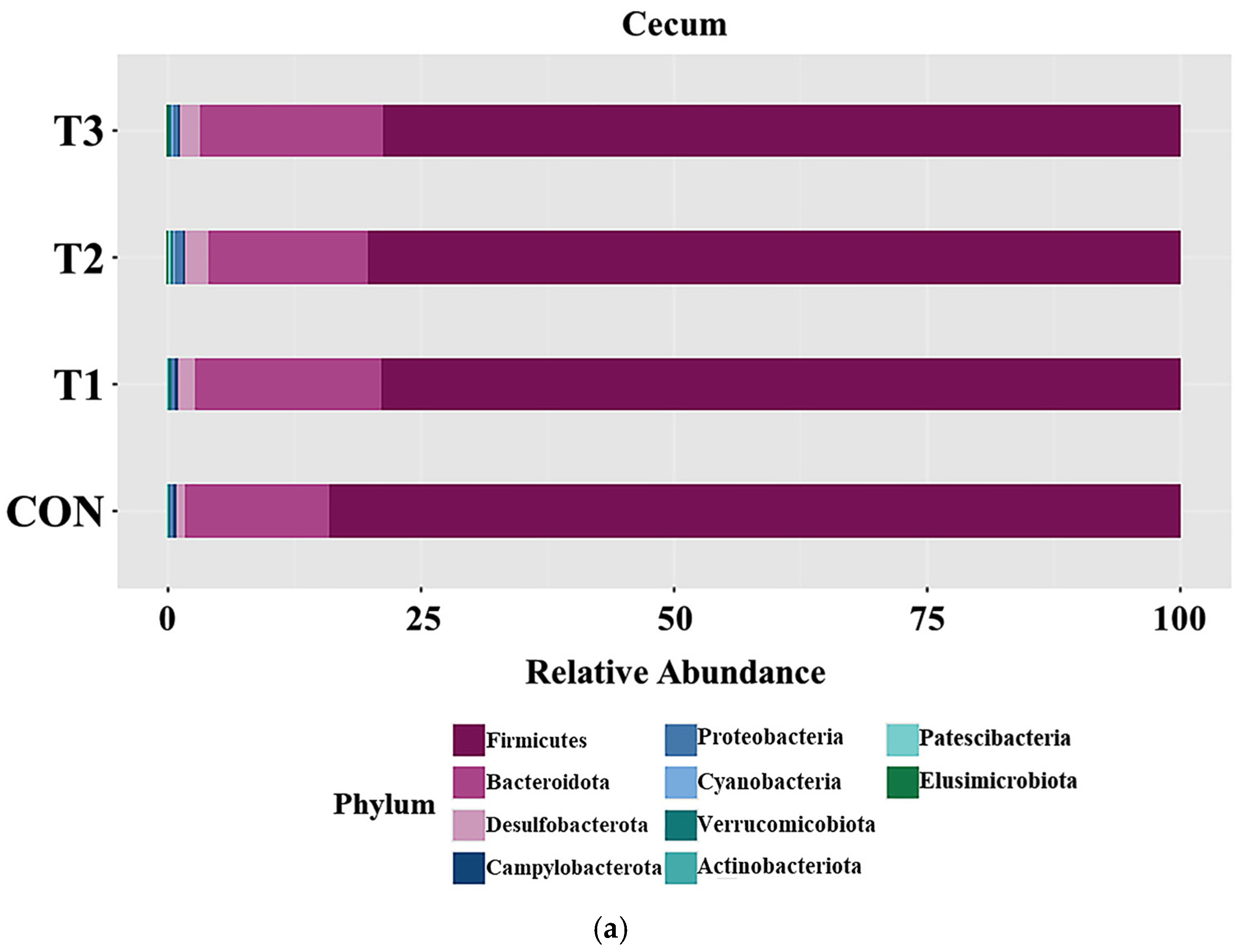
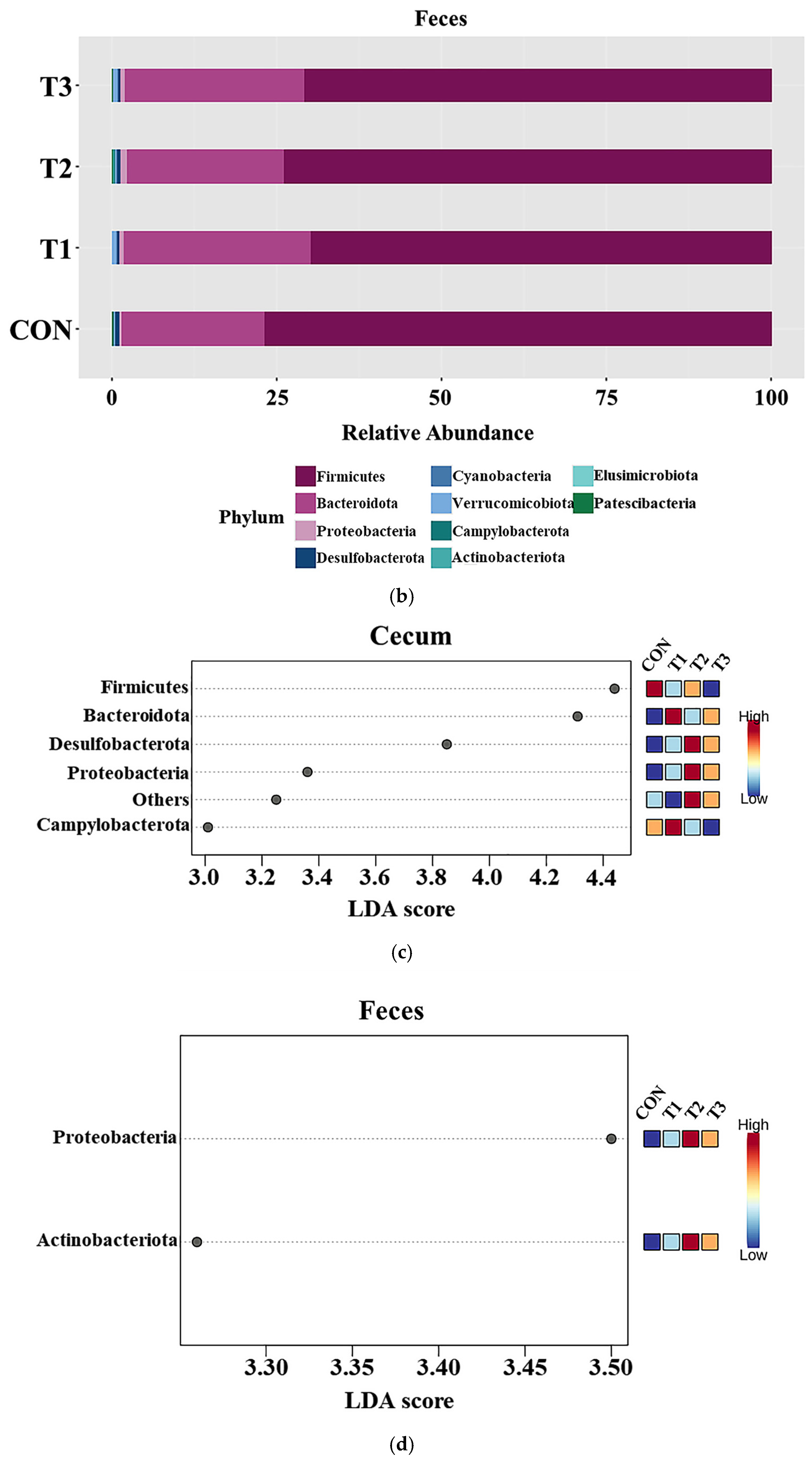
3.5. Effects of DON on the Phyla of the Cecal and Fecal Microbiota
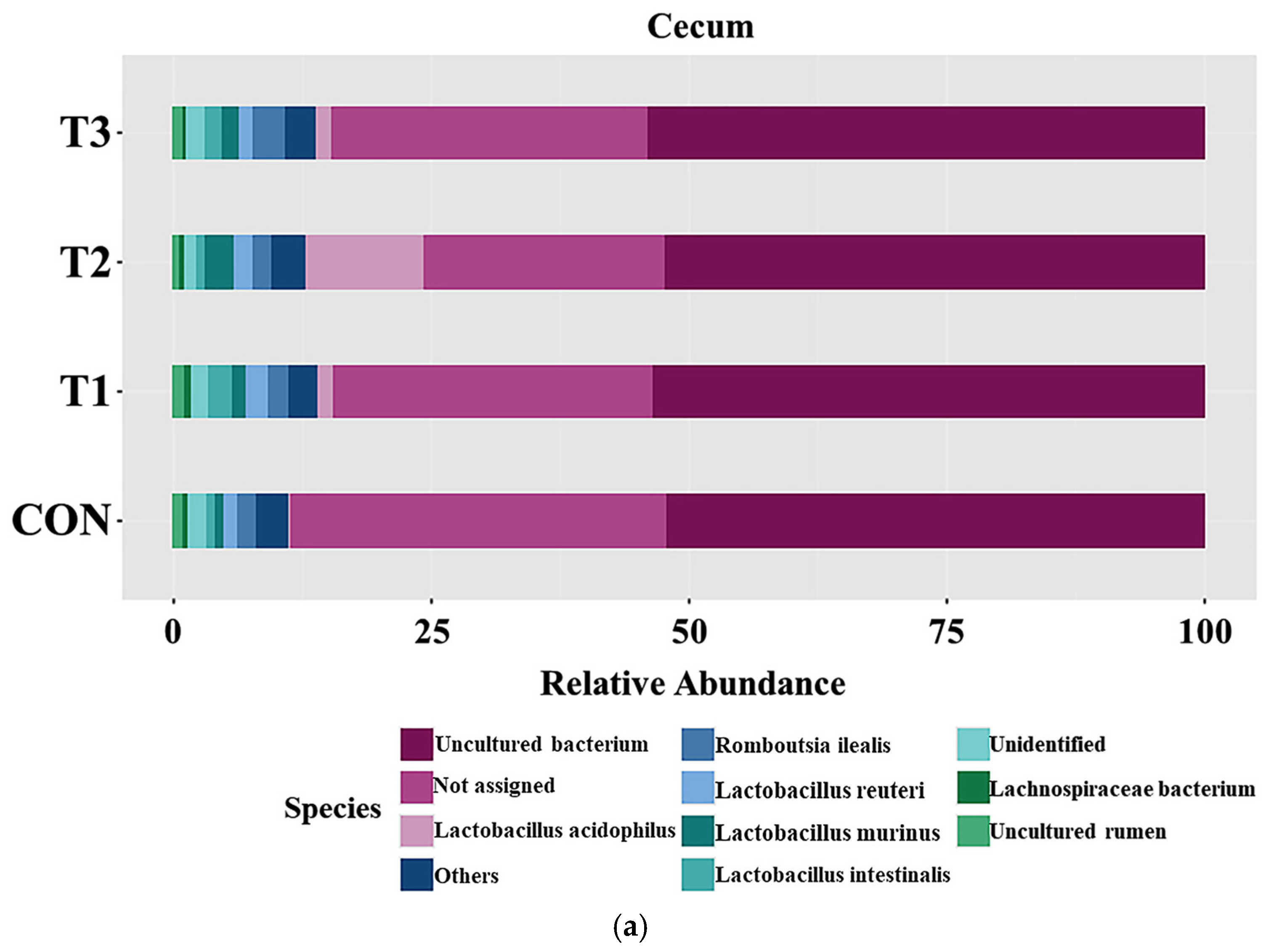
3.6. Effects of DON on the Cecal and Fecal Microbiota of the Species
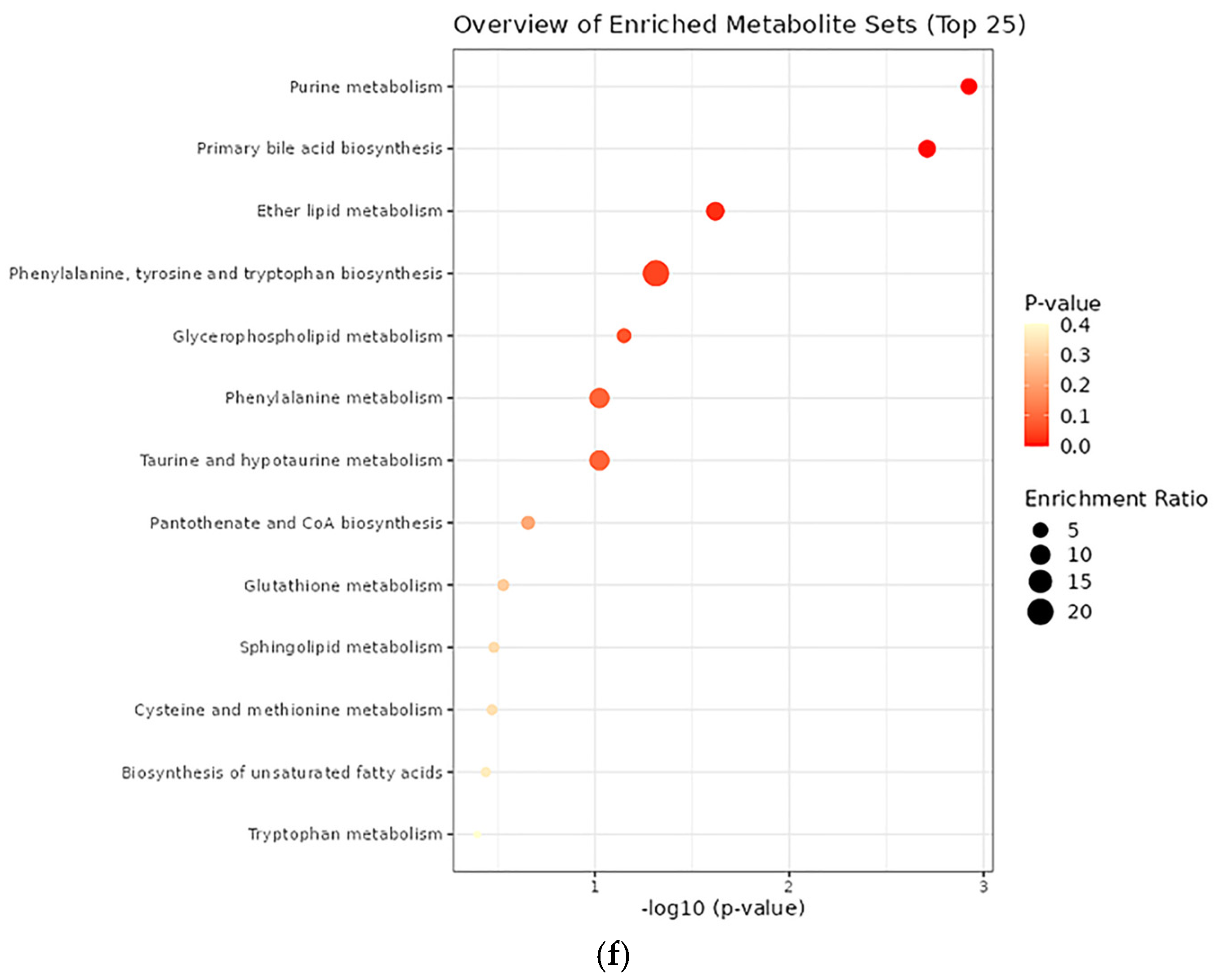
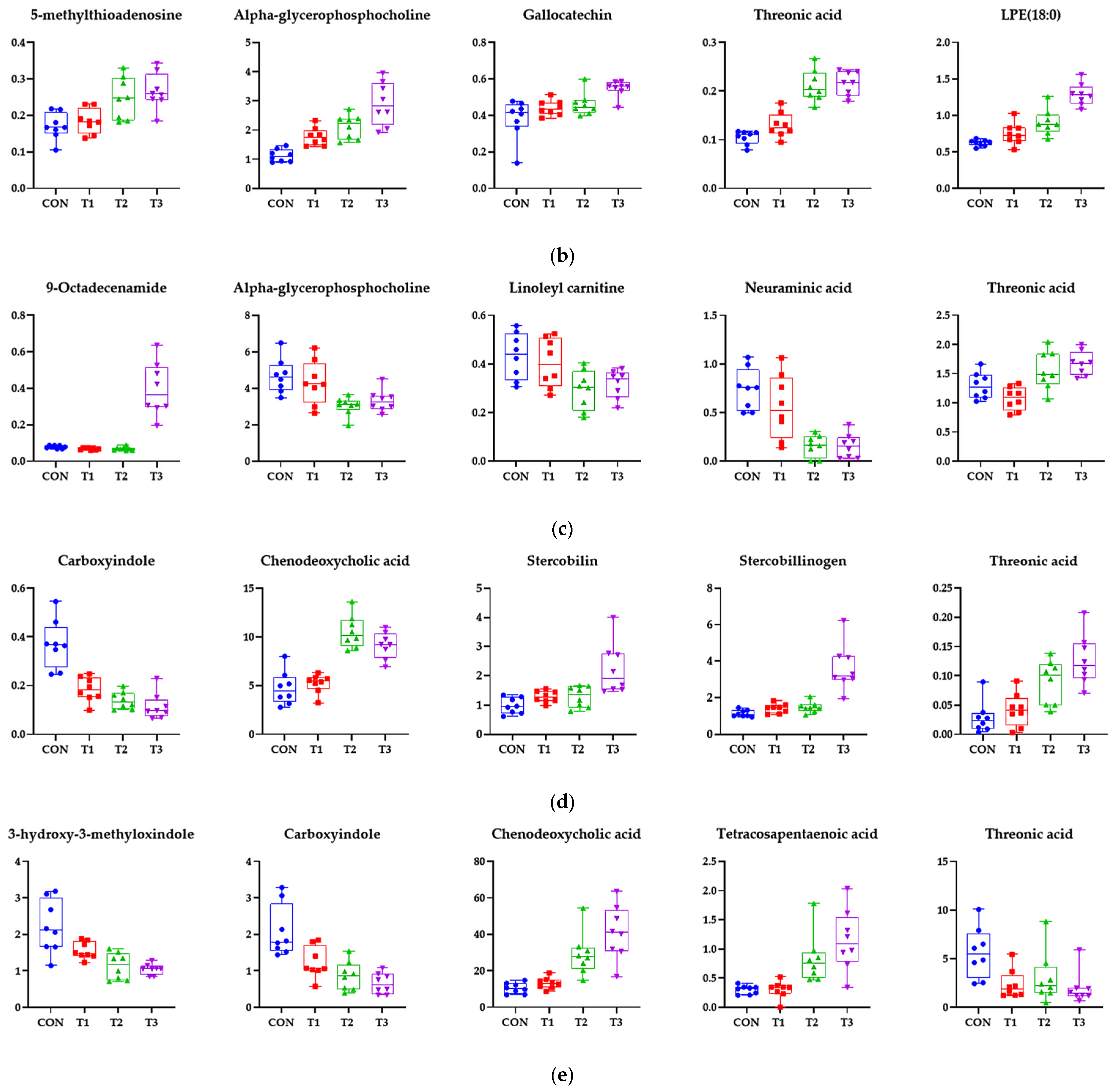
3.7. Effects of DON on the Metabolome Profiles of Blood and Tissue
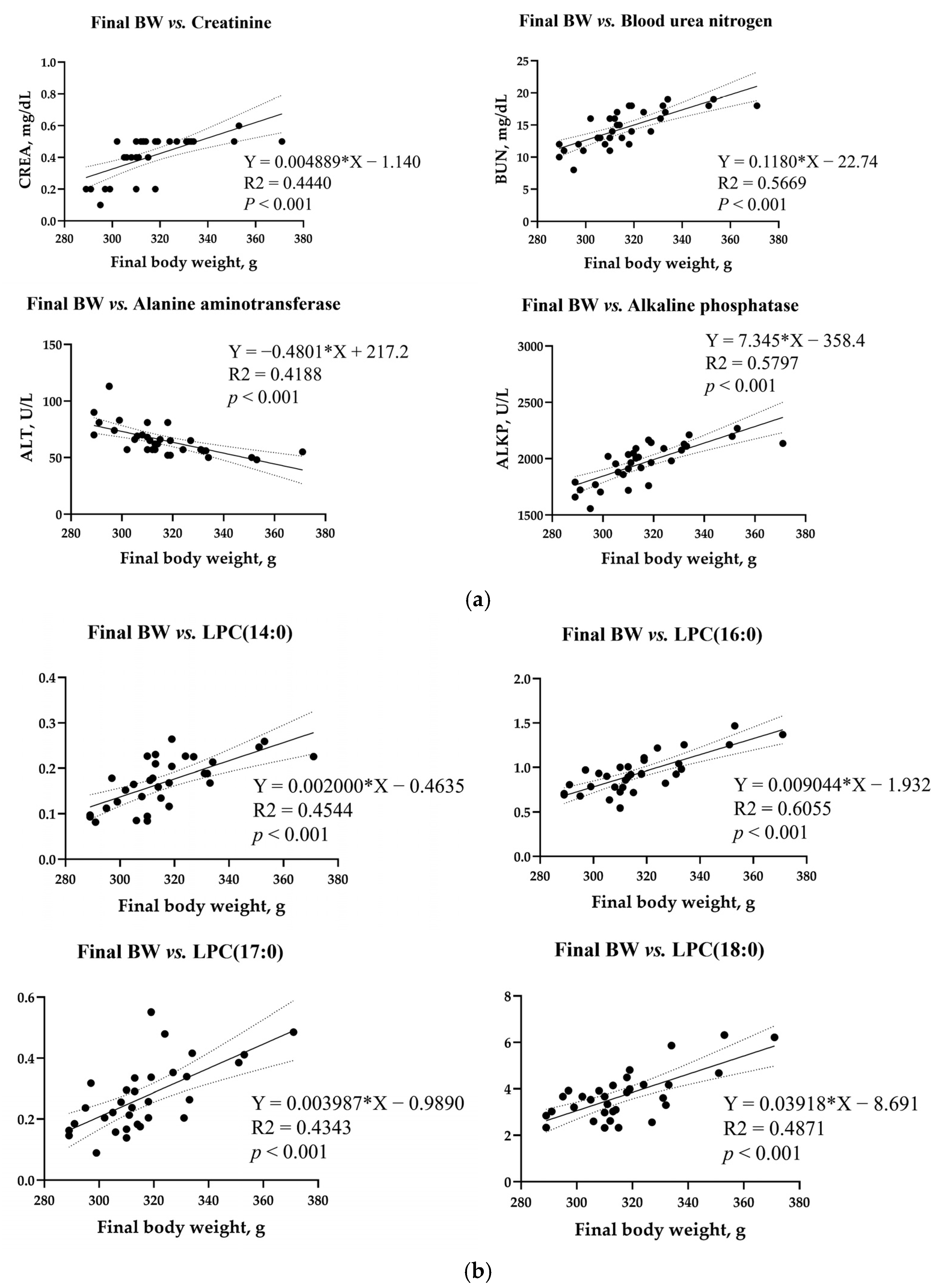
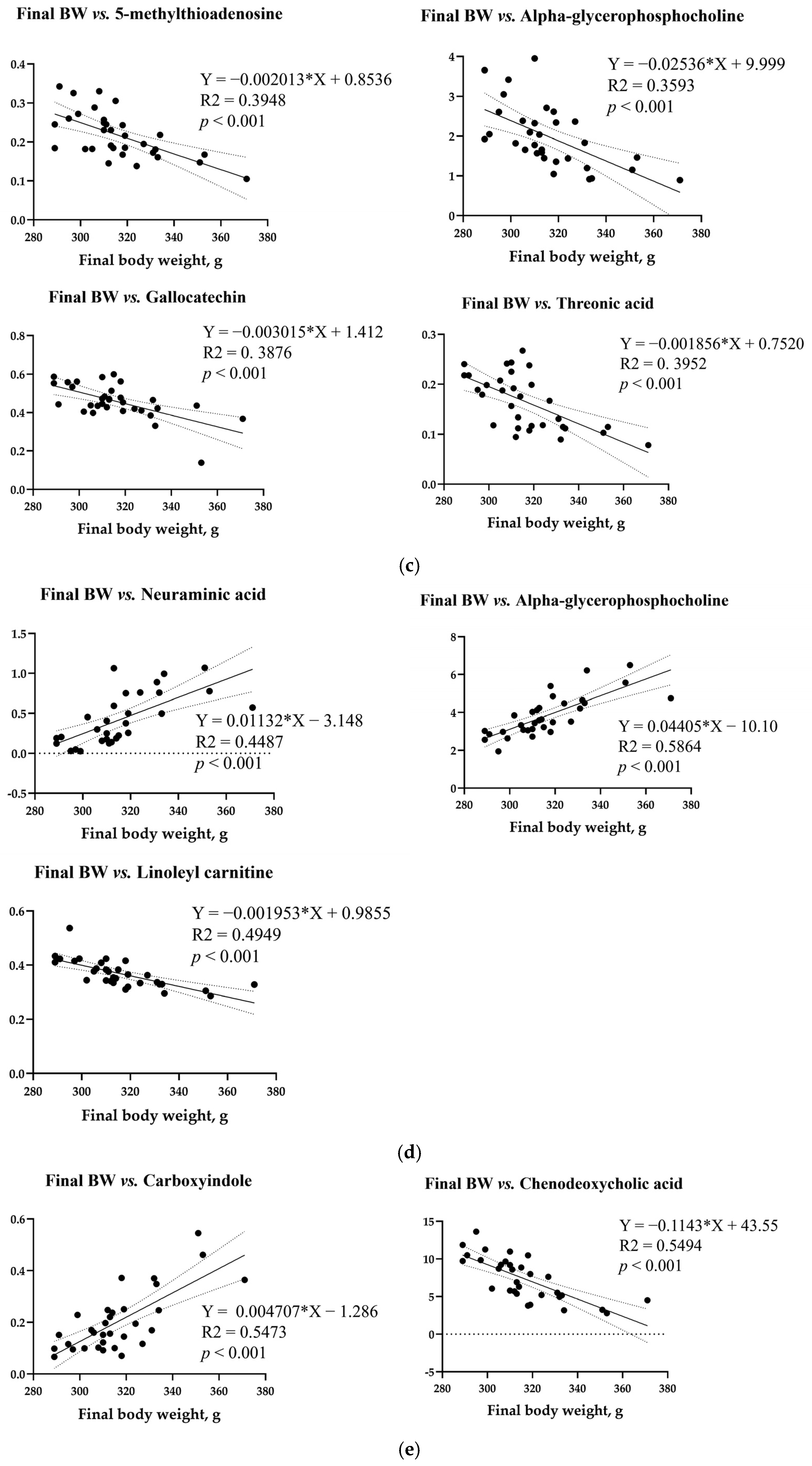
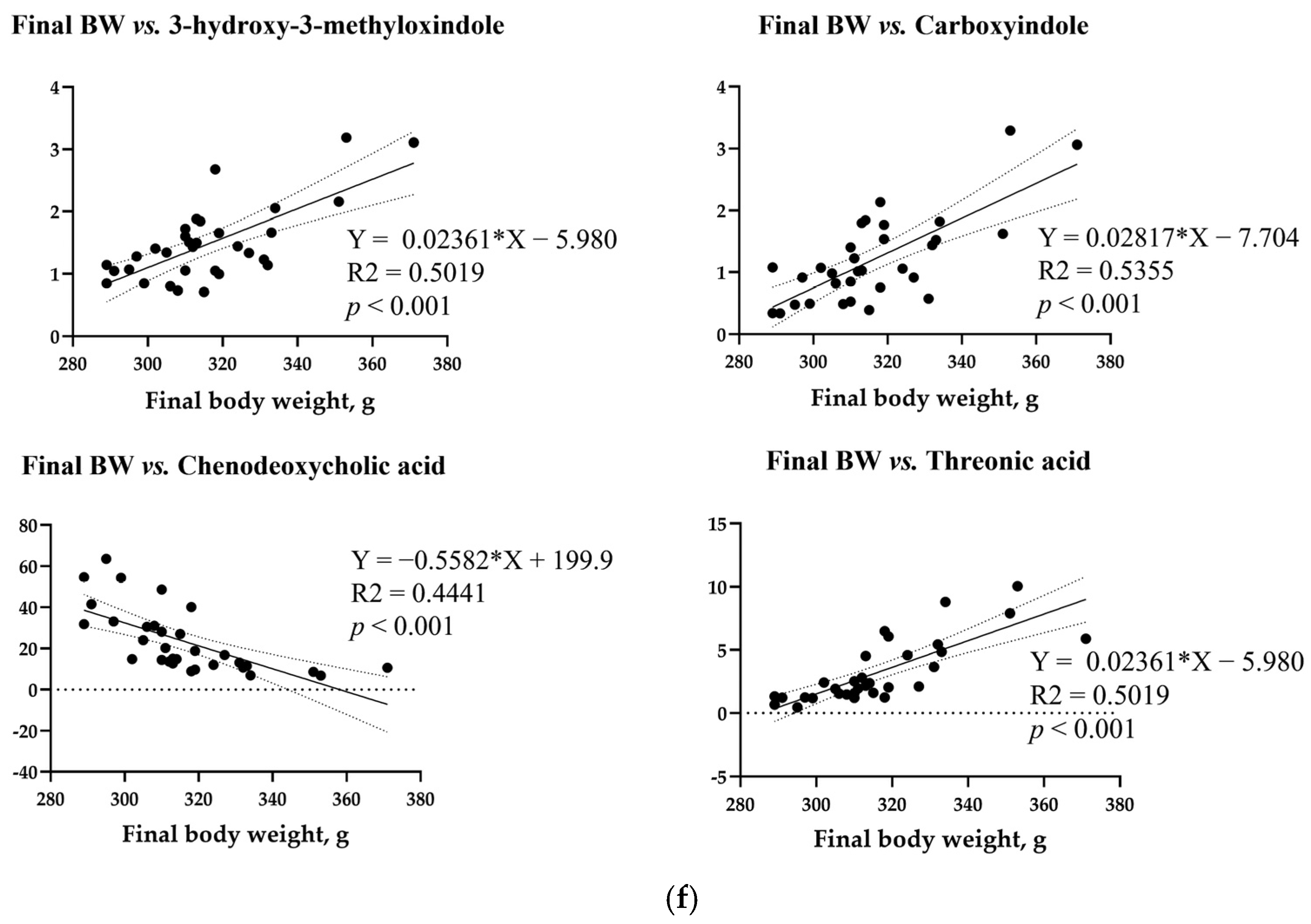
3.8. Simple Linear Regression Analysis
3.9. Functional Prediction for Cecal and Fecal Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | Abundance-based coverage estimator |
| ADFI | Average daily feed intake |
| ADG | Average daily gain |
| ALKP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| AMYL | Amylase |
| ANOVA | Analysis of variance |
| ASV | Amplicon sequence variants |
| BUN | Blood urea nitrogen |
| CON | Control |
| CREA | Creatinine |
| DON | Deoxynivalenol |
| EFSA | European Food Safety Authority |
| FCR | Feed conversion ratio |
| FDA | Food and Drug Administration |
| FDR | False discovery rate |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LDA | Linear discriminant analysis |
| LEfSe | LDA effect size |
| NBF | Neutral buffered formalin |
| PERMANOVA | Permutational multivariate analysis of variance |
| PLS-DA | Partial least squares discriminant analysis |
| SEM | Standard error of the mean |
| SMRT | Single Molecule Real-Time |
| T1 | 0.5 ppm DON |
| T2 | 50 ppm DON |
| T3 | 100 ppm DON |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick-end labeling |
| VIP | Variable importance projection |
References
- Holanda, D.M.; Kim, S.W. Mycotoxin occurrence, toxicity, and detoxifying agents in pig production with an emphasis on deoxynivalenol. Toxins 2021, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiang, J.; Mu, P.; Lin, R.; Wen, J.; Deng, Y. Toxicokinetics and metabolism of deoxynivalenol in animals and humans. Arch. Toxicol. 2022, 96, 2639–2654. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.M.; Solfrizzo, M.; De Girolamo, A.; Chulze, S.N.; Torres, A.M.; Visconti, A. LC–MS/MS characterization of the urinary excretion profile of the mycotoxin deoxynivalenol in human and rat. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 707–715. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Deoxynivalenol in food and feed: Occurrence and exposure. EFSA J. 2013, 11, 3379. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- Mishra, S.; Srivastava, S.; Dewangan, J.; Divakar, A.; Kumar Rath, S. Global occurrence of deoxynivalenol in food commodities and exposure risk assessment in humans in the last decade: A survey. Crit. Rev. Food Sci. Nutr. 2020, 60, 1346–1374. [Google Scholar] [CrossRef]
- Park, J.; Chang, H.; Kim, D.; Chung, S.; Lee, C. Long-term occurrence of deoxynivalenol in feed and feed raw materials with a special focus on South Korea. Toxins 2018, 10, 127. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, L.; Xu, Z.; Liu, X.; Chen, L.; Dai, J.; Karrow, N.A.; Sun, L. Occurrence of Aflatoxin B1, deoxynivalenol and zearalenone in feeds in China during 2018–2020. J. Anim. Sci. Biotechnol. 2021, 12, 74. [Google Scholar] [CrossRef]
- Ren, Z.H.; Deng, H.D.; Deng, Y.T.; Deng, J.L.; Zuo, Z.C.; Yu, S.M.; Shen, L.H.; Cui, H.M.; Xu, Z.W.; Hu, Y.C. Effect of the Fusarium toxins, zearalenone and deoxynivalenol, on the mouse brain. Environ. Toxicol. Pharmacol. 2016, 46, 62–70. [Google Scholar] [CrossRef]
- Miró-Abella, E.; Torrell, H.; Herrero, P.; Canela, N.; Arola, L.; Borrull, F.; Ras, R.; Fontanals, N. Monitoring and evaluation of the interaction between deoxynivalenol and gut microbiota in Wistar rats by mass spectrometry-based metabolomics and next-generation sequencing. Food Chem. Toxicol. 2018, 121, 124–130. [Google Scholar] [CrossRef]
- Clark, E.S.; Flannery, B.M.; Gardner, E.M.; Pestka, J.J. High sensitivity of aged mice to deoxynivalenol (vomitoxin)-induced anorexia corresponds to elevated proinflammatory cytokine and satiety hormone responses. Toxins 2015, 7, 4199–4215. [Google Scholar] [CrossRef]
- Gerez, J.R.; Verri, W.A.; Hohmann, M.S.; Flaiban, K.M.C.; Hasuda, A.L.; Gloria, E.M.; Bracarense, A.P.R.L. Animal performance and biochemical parameters are sex-dependent in peripubertal rats exposed to deoxynivalenol. Toxicon 2022, 220, 106944. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.Y.; Kim, J.; Kim, M.; Shim, S.H.; Park, C.; Jung, S.; Jung, H. Effects of increasing oral deoxynivalenol gavage on growth performance, blood biochemistry, metabolism, histology, and microbiome in rats. Biology 2024, 13, 836. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zhu, P.; Cui, F.; Pi, F.; Zhang, Y.; Sun, X. The disorder metabolic profiling in kidney and spleen of mice induced by mycotoxins deoxynivalenol through gas chromatography mass spectrometry. Chemosphere 2017, 180, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health Part B Crit. Rev. 2005, 8, 39–69. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Anim. Feed Sci. Technol. 2007, 137, 283–298. [Google Scholar] [CrossRef]
- Sprando, R.L.; Collins, T.F.; Black, T.N.; Olejnik, N.; Rorie, J.I.; Eppley, R.M.; Ruggles, D.I. Characterization of the effect of deoxynivalenol on selected male reproductive endpoints. Food Chem. Toxicol. 2005, 43, 623–635. [Google Scholar] [CrossRef]
- Collins, T.F.; Sprando, R.L.; Black, T.N.; Olejnik, N.; Eppley, R.M.; Hines, F.A.; Rorie, J.; Ruggles, D.I. Effects of deoxynivalenol (DON, vomitoxin) on in utero development in rats. Food Chem. Toxicol. 2006, 44, 747–757. [Google Scholar] [CrossRef]
- Yu, Z.; Morrison, M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. BioTechniques 2004, 36, 808–812. [Google Scholar] [CrossRef]
- Yang, S.; Li, L.; Yu, L.; Sun, L.; Li, K.; Tong, C.; Xu, W.; Cui, G.; Long, M.; Li, P. Selenium-enriched yeast reduces caecal pathological injuries and intervenes changes of the diversity of caecal microbiota caused by Ochratoxin-A in broilers. Food Chem. Toxicol. 2020, 137, 111139. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidance for Industry and FDA: Advisory Levels for Deoxynivalenol (DON) in Finished Wheat Products for Human Consumption and Grains and Grain By-Products Used for Animal Feed. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-and-fda-advisory-levels-deoxynivalenol-don-finished-wheat-products-human (accessed on 17 September 2018).
- Jeong, J.; Kim, J.; Kim, M.; Lee, B.; Park, C.; Kim, M. Effects of Deoxynivalenol Contamination on Growth Performance, Blood Biochemistry, Histology, Metabolomics, and the Microbiota: A Subacute Dose Oral Toxicity Study in Rats. Int. J. Mol. Sci. 2025, 26, 3086. [Google Scholar] [CrossRef]
- Pestka, J.J.; Clark, E.S.; Schwartz-Zimmermann, H.E.; Berthiller, F. Sex is a determinant for deoxynivalenol metabolism and elimination in the mouse. Toxins 2017, 9, 240. [Google Scholar] [CrossRef] [PubMed]
- Wellington, M.O.; Bosompem, M.A.; Petracek, R.; Nagl, V.; Columbus, D.A. Effect of long-term feeding of graded levels of deoxynivalenol (DON) on growth performance, nutrient utilization, and organ health in finishing pigs and DON content in biological samples. J. Anim. Sci. 2020, 98, skaa378. [Google Scholar] [CrossRef]
- Pastorelli, H.; Van Milgen, J.; Lovatto, P.; Montagne, L. Meta-analysis of feed intake and growth responses of growing pigs after a sanitary challenge. Animal 2012, 6, 952–961. [Google Scholar] [CrossRef]
- Pinton, P.; Nougayrède, J.P.; Del Rio, J.C.; Moreno, C.; Marin, D.E.; Ferrier, L.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. The food contaminant deoxynivalenol decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharmacol. 2009, 237, 41–48. [Google Scholar] [CrossRef]
- Wu, L.; Liao, P.; He, L.; Ren, W.; Yin, J.; Duan, J.; Li, T. Growth performance, serum biochemical profile, jejunal morphology, and the expression of nutrient transporter genes in deoxynivalenol (DON)-challenged growing pigs. BMC Vet. Res. 2015, 11, 144. [Google Scholar] [CrossRef]
- Wan, S.; Sun, N.; Li, H.; Khan, A.; Zheng, X.; Sun, Y.; Fan, R. Deoxynivalenol damages the intestinal barrier and biota of the broiler chickens. BMC Vet. Res. 2022, 18, 311. [Google Scholar] [CrossRef]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of two mycotoxins deoxynivalenol and fumonisin on pig intestinal health. Porc. Health Manag. 2016, 2, 21. [Google Scholar] [CrossRef]
- Liang, Z.; Ren, Z.; Gao, S.; Chen, Y.; Yang, Y.; Yang, D.; Deng, J.; Zuo, Z.; Wang, Y.; Shen, L. Individual and combined effects of deoxynivalenol and zearalenone on mouse kidney. Environ. Toxicol. Pharmacol. 2015, 40, 686–691. [Google Scholar] [CrossRef]
- Ji, F.; Fu, S.J.; Guo, Z.Y.; Pang, H.; Ju, W.Q.; Wang, D.P.; Hua, Y.P.; He, X.S. Prognostic value of combined preoperative lactate dehydrogenase and alkaline phosphatase levels in patients with resectable pancreatic ductal adenocarcinoma. Medicine 2016, 95, e4065. [Google Scholar] [CrossRef]
- Qiang, Z.; Truong, M.; Meynen, K.; Murphy, P.A.; Hendrich, S. Efficacy of a mycotoxin binder against dietary fumonisin, deoxynivalenol, and zearalenone in rats. J. Agric. Food Chem. 2011, 59, 7527–7533. [Google Scholar] [CrossRef] [PubMed]
- Hasuda, A.L.; Person, E.; Khoshal, A.K.; Bruel, S.; Puel, S.; Oswald, I.P.; Bracarense, A.P.F.L.; Pinton, P. Deoxynivalenol induces apoptosis and inflammation in the liver: Analysis using precision-cut liver slices. Food Chem. Toxicol. 2022, 163, 112930. [Google Scholar] [CrossRef] [PubMed]
- Chaytor, A.C.; See, M.T.; Hansen, J.A.; de Souza, A.L.P.; Middleton, T.F.; Kim, S.W. Effects of chronic exposure of diets with reduced concentrations of aflatoxin and deoxynivalenol on growth and immune status of pigs. J. Anim. Sci. 2011, 89, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Bracarense, A.P.F.L.; Basso, K.M.; Da Silva, E.O.; Payros, D.; Oswald, I.P. Deoxynivalenol in the liver and lymphoid organs of rats: Effects of dose and duration on immunohistological changes. World Mycotoxin J. 2017, 10, 89–96. [Google Scholar] [CrossRef]
- Ruan, M.L.; Wang, J.; Xia, Z.Y.; Li, X.W.; Zhang, B.; Wang, G.L.; Wu, Y.Y.; Han, Y.; Deng, J.; Sun, L.H. An integrated mycotoxin-mitigating agent can effectively mitigate the combined toxicity of AFB1, DON and OTA on the production performance, liver and oviduct health in broiler breeder hens. Food Chem. Toxicol. 2023, 182, 114159. [Google Scholar] [CrossRef]
- Kang, R.; Li, R.; Dai, P.; Li, Z.; Li, Y.; Li, C. Deoxynivalenol induced apoptosis and inflammation of IPEC-J2 cells by promoting ROS production. Environ. Pollut. 2019, 251, 689–698. [Google Scholar] [CrossRef]
- Ji, X.; Tang, Z.; Zhang, F.; Zhou, F.; Wu, Y.; Wu, D. Dietary taurine supplementation counteracts deoxynivalenol-induced liver injury via alleviating oxidative stress, mitochondrial dysfunction, apoptosis, and inflammation in piglets. Ecotoxicol. Environ. Saf. 2023, 253, 114705. [Google Scholar] [CrossRef]
- Xu, X.; Yan, G.; Chang, J.; Wang, P.; Yin, Q.; Liu, C.; Liu, S.; Zhu, Q.; Lu, F. Astilbin ameliorates deoxynivalenol-induced oxidative stress and apoptosis in intestinal porcine epithelial cells (IPEC-J2). J. Appl. Toxicol. 2020, 40, 1362–1372. [Google Scholar] [CrossRef]
- Mishra, S.; Dwivedi, P.D.; Pandey, H.P.; Das, M. Role of oxidative stress in deoxynivalenol-induced toxicity. Food Chem. Toxicol. 2014, 72, 20–29. [Google Scholar] [CrossRef]
- Antar, S.A.; Ashour, N.A.; Marawan, M.E.; Al-Karmalawy, A.A. Fibrosis: Types, effects, markers, mechanisms for disease progression, and its relation with oxidative stress, immunity, and inflammation. Int. J. Mol. Sci. 2023, 24, 4004. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, X.; Zheng, S.; Wang, S.; Liu, H.; Xu, S. Subacute cadmium exposure promotes M1 macrophage polarization through oxidative stress-evoked inflammatory response and induces porcine adrenal fibrosis. Toxicology 2021, 461, 152899. [Google Scholar] [CrossRef] [PubMed]
- Ranneh, Y.; Ali, F.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A. Crosstalk between reactive oxygen species and pro-inflammatory markers in developing various chronic diseases: A review. Appl. Biol. Chem. 2017, 60, 327–338. [Google Scholar] [CrossRef]
- Meng, C.; Feng, S.; Hao, Z.; Dong, C.; Liu, H. Changes in gut microbiota composition with age and correlations with gut inflammation in rats. PLoS ONE 2022, 17, e0265430. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Beekmann, K.; Ringø, E.; Ivonne, M.C.M.; Rietjens, I.M.; Xing, F. Interaction between food-borne mycotoxins and gut microbiota: A review. Food Control 2021, 126, 107998. [Google Scholar] [CrossRef]
- Čoklo, M.; Maslov, D.R.; Kraljević Pavelić, S. Modulation of gut microbiota in healthy rats after exposure to nutritional supplements. Gut Microbes 2020, 12, 1779002. [Google Scholar] [CrossRef]
- Gureev, A.P.; Shaforostova, E.A.; Vitkalova, I.Y.; Sadovnikova, I.S.; Kalinina, Y.I.; Cherednichenko, V.R.; Reznikova, K.A.; Valuyskikh, V.V.; Popov, V.N. Long-term mildronate treatment increased Proteobacteria level in gut microbiome, and caused behavioral deviations and transcriptome change in liver, heart and brain of healthy mice. Toxicol. Appl. Pharmacol. 2020, 398, 115031. [Google Scholar] [CrossRef]
- Parnell, J.A.; Reimer, R.A. Prebiotic fibers dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR: LA-cp rats. Br. J. Nutr. 2012, 107, 601–613. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, Z.; Jiang, L.; Chen, Z.; Sun, H. Response of fecal bacterial flora to the exposure of fumonisin B1 in BALB/c mice. Toxins 2021, 13, 612. [Google Scholar] [CrossRef]
- Bensch, H.M.; Tolf, C.; Waldenström, J.; Lundin, D.; Zöttl, M. Bacteroidetes to Firmicutes: Captivity changes the gut microbiota composition and diversity in a social subterranean rodent. Anim. Microbiome 2023, 5, 9. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Han, F.; Wang, Y.; Han, Y.; Zhao, J.; Han, F.; Song, G.; Jiang, P.; Miao, H. Effects of whole-grain rice and wheat on composition of gut microbiota and short-chain fatty acids in rats. J. Agric. Food Chem. 2018, 66, 6326–6335. [Google Scholar] [CrossRef] [PubMed]
- Olli, K.; Saarinen, M.T.; Forssten, S.D.; Madetoja, M.; Herzig, K.H.; Tiihonen, K. Independent and combined effects of lactitol, polydextrose, and Bacteroides thetaiotaomicron on postprandial metabolism and body weight in rats fed a high-fat diet. Front. Nutr. 2016, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Cukovic-Cavka, S.; Likic, R.; Francetic, I.; Rustemovic, N.; Opacic, M.; Vucelic, B. Lactobacillus acidophilus as a cause of liver abscess in a NOD2/CARD15-positive patient with Crohn’s disease. Digestion 2006, 73, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Burnham, P.M.; Hendrixson, D.R. Campylobacter jejuni: Collective components promoting a successful enteric lifestyle. Nat. Rev. Microbiol. 2018, 16, 551–565. [Google Scholar] [CrossRef]
- Guo, L.; Shi, X.; Cao, F.; Hu, S.; Qian, W. Effects of dietary addition of mulberry leaf powder on blood metabolites and fecal microbiota composition in Hu sheep. Front. Anim. Sci. 2025, 5, 1469850. [Google Scholar] [CrossRef]
- Bhattamisra, S.K.; Yan, V.L.Y.; Lee, C.K.; Kuean, C.H.; Candasamy, M.; Liew, Y.K.; Sahu, P.S. Protective activity of geraniol against acetic acid and Helicobacter pylori-induced gastric ulcers in rats. J. Tradit. Complement. Med. 2019, 9, 206–214. [Google Scholar] [CrossRef]
- Harahap, I.A.; Schmidt, M.; Pruszyńska-Oszmałek, E.; Sassek, M.; Suliburska, J. Impact of Lactobacillus acidophilus and its combination with isoflavone products on calcium status, calcium transporters, and bone metabolism biomarkers in a post-menopausal osteoporotic rat model. Nutrients 2024, 16, 2524. [Google Scholar] [CrossRef]
- Gao, H.; Li, X.; Chen, X.; Hai, D.; Wei, C.; Zhang, L.; Li, P. The functional roles of Lactobacillus acidophilus in different physiological and pathological processes. J. Microbiol. Biotechnol. 2022, 32, 1226–1233. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, Y.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Protective effects of different Bacteroides vulgatus strains against lipopolysaccharide-induced acute intestinal injury, and their underlying functional genes. J. Adv. Res. 2021, 36, 27–37. [Google Scholar] [CrossRef]
- Hanson, T.; Constantine, E.; Nobles, Z.; Butler, E.; Renteria, K.M.; Teoh, C.M.; Koh, G.Y. Supplementation of vitamin D3 and fructooligosaccharides downregulates intestinal defensins and reduces the species abundance of Romboutsia ilealis in C57BL/6J mice. Nutrients 2024, 16, 2236. [Google Scholar] [CrossRef]
- Malvandi, A.M.; Shahba, S.; Mehrzad, J.; Lombardi, G. Metabolic disruption by naturally occurring mycotoxins in circulation: A focus on vascular and bone homeostasis dysfunction. Front. Nutr. 2022, 9, 915681. [Google Scholar] [CrossRef] [PubMed]
- Skrzypczak, N.; Przybylski, P. Structural diversity and biological relevance of benzenoid and atypical ansamycins and their congeners. Nat. Prod. Rep. 2022, 39, 1678–1704. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Metabolomics for investigating physiological and pathophysiological processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef]
- Zhu, Y.; Dwidar, M.; Nemet, I.; Buffa, J.A.; Sangwan, N.; Li, X.S.; Anderson, J.T.; Romano, K.A.; Fu, X.; Funabashi, M.; et al. Two distinct gut microbial pathways contribute to meta-organismal production of phenylacetylglutamine with links to cardiovascular disease. Cell Host Microbe 2023, 31, 18–32. [Google Scholar] [CrossRef]
- Sun, L.; Jia, H.; Li, J.; Yu, M.; Yang, Y.; Tian, D.; Zhang, H.; Zou, Z. Cecal gut microbiota and metabolites might contribute to the severity of acute myocardial ischemia by impacting the intestinal permeability, oxidative stress, and energy metabolism. Front. Microbiol. 2019, 10, 1745. [Google Scholar] [CrossRef]
- Awad, W.A.; Grenier, B.; Ruhnau, D.; Hess, C.; Schatzmayr, D.; Hess, M. Diametral influence of deoxynivalenol (DON) and deepoxy-deoxynivalenol (DOM-1) on the growth of Campylobacter jejuni with consequences on the bacterial transcriptome. BMC Microbiol. 2024, 24, 306. [Google Scholar] [CrossRef]
- Lima, W.G.; Martins-Santos, M.E.S.; Chaves, V.E. Uric acid as a modulator of glucose and lipid metabolism. Biochimie 2015, 116, 17–23. [Google Scholar] [CrossRef]
- Ciaula, A.D.; Garruti, G.; Baccetto, R.L.; Molina-Molina, E.; Bonfrate, L.; Wang, D.Q.-H.; Portincasa, P. Bile acid physiology. Ann. Hepatol. 2017, 16, s4–s14. [Google Scholar] [CrossRef]
- Fleishman, J.S.; Kumar, S. Bile acid metabolism and signaling in health and disease: Molecular mechanisms and therapeutic targets. Signal Transduct. Target Ther. 2024, 9, 97. [Google Scholar] [CrossRef]
- Peloquin, C.A.; Jaresko, G.S.; Yong, C.L.; Keung, A.C.; Bulpitt, A.E.; Jelliffe, R.W. Population pharmacokinetic modeling of isoniazid, rifampin, and pyrazinamide. Antimicrob. Agents Chemother. 1997, 41, 2670–2679. [Google Scholar] [CrossRef]
- He, X.; Zhou, H.X.; Fu, X.; Ni, K.D.; Lin, A.Z.; Zhang, L.T.; Yin, H.H.; Jiang, Q.; Zhou, X.; Meng, Y.W.; et al. Metabolomics study reveals increased deoxycholic acid contributes to deoxynivalenol-mediated intestinal barrier injury. Life Sci. 2024, 1, 122302. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bakker, W.; Zheng, W.; de Haan, L.; Rietjens, I.M.C.M.; Bouwmeester, H. Exposure to the mycotoxin deoxynivalenol reduces the transport of conjugated bile acids by intestinal Caco-2 cells. Arch. Toxicol. 2022, 96, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Baixauli, J.; Puigbò, P.; Abasolo, N.; Palacios-Jordan, H.; Foguet-Romero, E.; Suñol, D.; Galofré, M.; Caimari, A.; Baselga-Escudero, L.; Bas, J.M.D.; et al. Alterations in metabolome and microbiome associated with an early stress stage in male wistar rats: A multi-omics approach. Int. J. Mol. Sci. 2021, 22, 12931. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.A.; Beltrán, F.A.; Brauchi, S.; Concha, I.I. A metabolic switch in brain: Glucose and lactate metabolism modulation by ascorbic acid. J. Neurochem. 2009, 110, 423–440. [Google Scholar] [CrossRef]
- Jia, X.; Suzuki, Y.; Naito, H.; Yetti, H.; Kitamori, K.; Hayashi, Y.; Kaneko, R.; Nomura, M.; Yamori, Y.; Zaitsu, K.; et al. A possible role of chenodeoxycholic acid and glycine-conjugated bile acids in fibrotic steatohepatitis in a dietary rat model. Dig. Dis. Sci. 2014, 59, 1490–1501. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.; Kim, J.; Lee, B.; Park, C.; Kim, M. Effects of Low and High Doses of Deoxynivalenol on Growth Performance, Blood Biochemistry, Histology, Metabolites, and Microbial Community in Adult Rats. Biology 2025, 14, 429. https://doi.org/10.3390/biology14040429
Jeong J, Kim J, Lee B, Park C, Kim M. Effects of Low and High Doses of Deoxynivalenol on Growth Performance, Blood Biochemistry, Histology, Metabolites, and Microbial Community in Adult Rats. Biology. 2025; 14(4):429. https://doi.org/10.3390/biology14040429
Chicago/Turabian StyleJeong, Jinyoung, Junsik Kim, Boram Lee, Cheolju Park, and Minseok Kim. 2025. "Effects of Low and High Doses of Deoxynivalenol on Growth Performance, Blood Biochemistry, Histology, Metabolites, and Microbial Community in Adult Rats" Biology 14, no. 4: 429. https://doi.org/10.3390/biology14040429
APA StyleJeong, J., Kim, J., Lee, B., Park, C., & Kim, M. (2025). Effects of Low and High Doses of Deoxynivalenol on Growth Performance, Blood Biochemistry, Histology, Metabolites, and Microbial Community in Adult Rats. Biology, 14(4), 429. https://doi.org/10.3390/biology14040429





